Introduction
Lung cancer is one of the most common causes of
cancer-related deaths in men and women worldwide (1,2).
Tobacco smoke (TS) is associated with multiple types of cancer, but
especially lung cancer (3,4). Epidemiological studies have revealed
a link between TS and lung cancer initiation and development
(5–7). TS accounts for 80% of female and 90%
of male lung cancer cases (8,9).
Lung cancer contributes to >3,000 deaths per day and is the
leading cause of cancer-related deaths in men and women (2). However, the molecular pathogenesis of
TS-induced lung cancer still remains largely unknown.
Lung cancer is a multicentric and multistep
phenomenon, which sequentially accumulates molecular and genetic
abnormalities (9). Abnormal cell
proliferation and epithelial-mesenchymal transition (EMT) help in
developing lung cancer and can be activated by carcinogens
(10–12). It has been demonstrated that
exposure of cells or mice to TS accelerates the EMT process, which
is characterized by changes in the expression of EMT markers,
including decreased E-cadherin, and increased vimentin and
N-cadherin (13–15). In addition, TS-induced EMT
initiates early-stage carcinogenesis (16–18).
Cancer is a group of diseases characterized by abnormal cell
proliferation, and abnormal cell proliferation is a key step that
may promote the occurrence and development of cancer (19–22).
Studies have suggested that exposure to TS induces abnormal cell
proliferation accompanied by changes in the expression of PCNA or
Ki-67 (23–25). To the best of our knowledge, the
molecular mechanism of TS-induced abnormal pulmonary cell
proliferation and EMT is unclear. However, further investigations
may provide strategies for early treatment and intervention in lung
cancer.
MAPKs control cellular processes, such as
proliferation, apoptosis, angiogenesis, cell motility and
differentiation (26,27). Therefore, MAPKs can contribute to
tumorigenesis (28–30). p38 is a member of the MAPK family,
participating in the occurrence and development of TS-induced lung
cancer by regulating the EMT process (30–36).
However, the functional mechanism of p38 in lung tissues is not
clear.
Dietary phytochemicals are potentially anticancerous
and flavonoids have been reported to inhibit cancer progression
(37). Hesperidin is a citrus
flavone, which is the abundant polyphenol in citrus fruits and is
commonly used in Traditional Chinese Medicine (37,38).
Hesperidin exerts a range of biological and pharmacological
activities, including antioxidant, anti-inflammatory and anticancer
effects, with minimal or no side effects (39–41).
Hesperidin is anticancerous for tumors, such as breast, gastric and
lung tumors. The anticancer activity of hesperidin has been well
studied (37,39,42,43).
However, limited work has been conducted on its potential to treat
TS-induced abnormal cell proliferation and EMT in lung tissues.
The present study examined the regulation of the p38
pathway in TS-induced abnormal lung cell proliferation and EMT. The
preventive effects of hesperidin were determined by examining the
lung tissues of treated mice. The findings may provide a novel
avenue for determining the pathogenesis and early interventions of
TS-induced lung tumorigenesis.
Materials and methods
Chemicals and reagents
Phosphorylated p38 (catalogue number, 4511T; 1:500),
phosphorylated c-Fos (catalogue number, 5348T; 1:1,000), p38
(catalogue number, 8690T; 1:1,000), c-Fos (catalogue number, 2250T;
1:1,000), E-cadherin (catalogue number, 3195T; 1:1,000) and
N-cadherin (catalogue number, 13116T; 1:1,000) antibodies were
purchased from Cell Signaling Technology, Inc. Vimentin (catalogue
number, MB65651; 1:1,000), proliferating cell nuclear antigen
(PCNA) (catalogue number, MB0156; 1:500) and GAPDH (catalogue
number, BS65483M; 1:2,000) antibodies were purchased from Bioworld
Technology, Inc. Horseradish peroxidase-conjugated secondary
antibodies were purchased from Invitrogen; Thermo Fisher
Scientific, Inc. (catalogue numbers, 31430 and 31460; 1:2,000).
Primers for Vimentin, E-cadherin, N-cadherin, PCNA and GAPDH
(Table I) were synthesized by
Invitrogen; Thermo Fisher Scientific, Inc. SB203580 was purchased
from MilliporeSigma. The sources of other materials are indicated
throughout the text.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Primer sequence
(5′-3′) |
|---|
| E-cadherin | Forward:
CAGGTCTCCTCATGGCTTTGC |
|
| Reverse:
CTTCCGAAAAGAAGGCTGTCC |
| PCNA | Forward:
CAAGAAGGTGTTGGAGGCA |
|
| Reverse:
TCGCAGCGGTAGGTGTC |
| Vimentin | Forward:
CCTTGACATTGAGATTGCCA |
|
| Reverse:
GTATCAACCAGAGGGAGTGA |
| N-cadherin | Forward:
TCAGGCGTCTGTAGAGGCTT |
|
| Reverse:
ATGCACATCCTTCGATAAGACTG |
| GAPDH | Forward:
AGGTCGGTGTGAACGGATTTG |
|
| Reverse:
TGTAGACCATGTAGTTGAGGTCA |
Mice and exposure to TS
Male 8-week-old BALB/c mice weighing 18–22 g (n=60)
were purchased from the Animal Research Center of Jiangsu
University (Zhenjiang, China). Mice were acclimated for 1 week
prior to TS exposure. The mice were housed in polypropylene cages
at 22±0.5°C and 40–60% humidity with 12 h light/dark cycles at the
Animal Care Facility of Jiangsu University (Zhenjiang, China).
Water and a normal diet were provided ad libitum. All of the
mouse experiments were approved by the Animal Care and Use
Committee of Jiangsu University and efforts were made to minimize
suffering and distress. Mice in the TS group (n=18) were exposed in
the smoking apparatus (Beijing Huironghe Technology Co., Ltd.) for
6 h daily for 12 weeks. The filtered air (FA) control group (n=18)
mice were exposed to filtered air in the smoking apparatus. After
TS exposure, mice in each group were provided with water and a
normal diet. Filter-less 3R4F Kentucky reference cigarettes
(containing 9.4 mg tar and 0.76 mg nicotine per cigarette) were
used as the TS source. In the TS + DMSO group (n=12), mice were
injected with sterile DMSO (catalogue number, D2650;
MilliporeSigma) and exposed to TS, while in the TS + SB203580 group
(n=6), mice were injected with SB203580 (1 mg/kg body weight) and
exposed to TS. SB203580 was dissolved in sterile DMSO and
administered intraperitoneally every other day. In the TS +
hesperidin group (n=6), mice were exposed to TS and received 30
mg/kg hesperidin (catalogue number, HY-15337; MedChemExpress) every
other day by gavage, and a normal diet. SB203580 and hesperidin
were dissolved in DMSO, and further diluted in 0.9% saline to the
final concentration. TS was generated by a smoke machine, which
pumped the smoke from the burning cigarette at a constant rate (5
min/cigarette). Smoke was delivered to the whole-body exposure
chambers with total particulate matter (TPM) of 85
mg/m3. The exposures were monitored and characterized
as: Carbon monoxide (16.75±2.47 ppm) and TPM (0 mg/m3)
for the control group; and carbon monoxide (181.05±14.79 ppm) and
TPM (84.83±5.19 mg/m3) for the TS exposure group. Animal
health and behavior were monitored twice a week and the experiment
lasted for 12 weeks. There was no accidental death of mice during
the experiment, and all mice were euthanized at the end of the
experiment. Mice were sacrificed by cervical dislocation and death
was confirmed by the sound of cervical spine fracture and the
absence of breathing (44).
Western blot analysis
Lung tissues were homogenized using a full automatic
sample rapid grinding instrument (Shanghai Jingxin Industrial
Development, Co., Ltd.) in lysis buffer containing 1X protease
inhibitor cocktail (Pierce; Thermo Fisher Scientific, Inc.) and
centrifuged at 12,000 × g at 4°C for 15 min. Protein concentration
was determined by bicinchoninic acid assay. Equal amounts of
proteins (60 µg) were fractionated by electrophoresis via 7.5–10%
SDS-PAGE and transferred to a PVDF membrane (MilliporeSigma). The
membrane was blocked using 5% non-fat milk at 25°C for 1 h and
incubated overnight with monoclonal antibody at 4°C. The membranes
were washed with tris-buffered saline with 0.1% Tween-20 and probed
with horseradish peroxidase-conjugated secondary antibody diluted
in 5% skimmed milk. GAPDH served as the loading control. The
membranes were developed with ECL kit (catalogue number, E412-02;
Vazyme Biotech Co., Ltd.). For densitometric analyses, protein
bands on the blots were measured with ImageJ 1.8.0.345 (National
Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from the lung tissues of the mice was
isolated using TRIzol™ (Gibco; Thermo Fisher Scientific, Inc.). A
total of 2 µg RNA was reverse transcribed into cDNA using AMV
Reverse Transcriptase (Promega Corporation) and the
HiScript® III 1st Strand cDNA Synthesis Kit (catalogue
number, R312-01; Vazyme Biotech Co., Ltd.) was used according to
the manufacturer's protocol. qPCR was performed using
AceQ® qPCR SYBR Green Master Mix (Vazyme Biotech Co.,
Ltd.) and a StepOnePlus™ Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling conditions were
as follows: 95°C for 5 min, followed by 40 cycles at 95°C for 15
sec, 60°C for 15 sec, 72°C for 20 sec and a 65–95°C drawing
dissociation curve (45). GAPDH
expression was used as the normalization control. Fold-changes in
the expression of each gene were calculated using the
2−ΔΔCq (46). The
primers used are shown in Table
I.
Immunohistochemistry
Immunohistochemistry was performed according a
previously reported method (47).
Briefly, tissues were fixed in 4% buffered formalin at room
temperature for 24 h. 5-µm paraffin-embedded continuous sections
were de-waxed in xylene and rehydrated in graded alcohol. Next, the
endogenous peroxidase activity was quenched by incubating the
slices in 3% (v/v) H2O2 in methanol.
Antigen-retrieval was performed by incubating the sections in
citrate buffer (pH 6.0) and the non-specific binding was blocked
using 5% bovine serum albumin at 37°C for 30 min. After incubation
overnight with E-cadherin (catalogue number, 3195T; 1:200; Cell
Signaling Technology, Inc.) and Vimentin (catalogue number, MB9006;
1:100; Bioworld Technology, Inc.) at 4°C, the sections were
subsequently washed with phosphate-buffered solution, and then
incubated with biotinylated immunoglobulin G and SABC (catalogue
number, SA1020; Wuhan Boster Biological Technology, Ltd.) for 1 h.
Image acquisition was performed with a light microscope (Nikon
Solar Eclipse Ti-S; Nikon Corporation).
Statistical analysis
Statistical analysis was performed using SPSS 16.0
(SPSS, Inc.). The data of three repeated experiments are presented
as the mean ± standard deviation. One-way ANOVA, followed by
Tukey's post hoc test, was used to analyze the statistical
differences among multiple groups. The differences between two
groups were analyzed using an unpaired t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
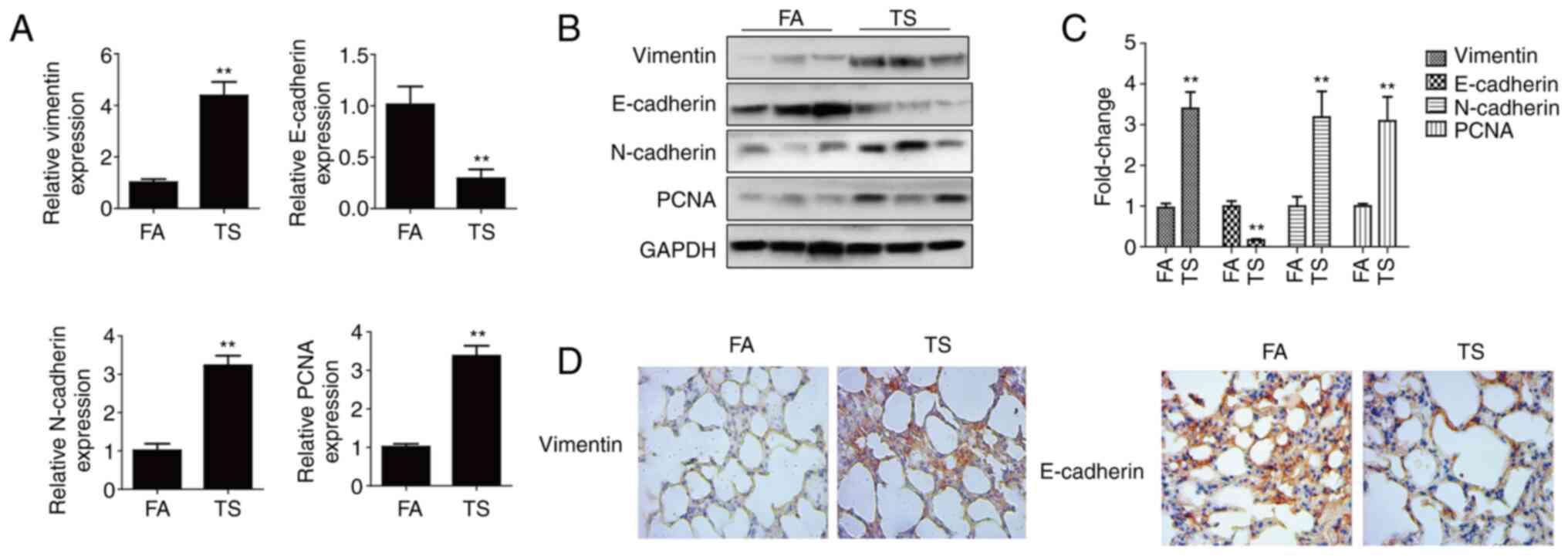
TS-induced abnormal EMT and cell
proliferation in mouse lung tissues
TS is the dominant risk factor for lung cancer
(3,4). Abnormal EMT and cell proliferation
initiate TS-induced lung cancer (16,25).
The altered EMT and cell proliferation marker (E-cadherin,
vimentin, N-cadherin and PCNA) levels were screened in mouse lung
tissues after 12 weeks of TS exposure. The RT-qPCR results showed a
reduction in E-cadherin mRNA levels after TS exposure compared with
the levels in the FA control group, whereas vimentin, N-cadherin
and PCNA levels were elevated (Fig.
1A). The western blotting results revealed that TS reduced
E-cadherin protein expression compared with that in the FA group,
but increased vimentin, N-cadherin and PCNA expression (Fig. 1B and C). In order to further
clarify that smoking can induce EMT in lung tissue of mice,
vimentin and E-cadherin were detected by immunohistochemistry. It
was demonstrated that TS increased vimentin expression but
decreased E-cadherin expression compared with that in the FA group
(Fig. 1D). Therefore, TS exposure
induced abnormal EMT and cell proliferation in mouse lung
tissues.
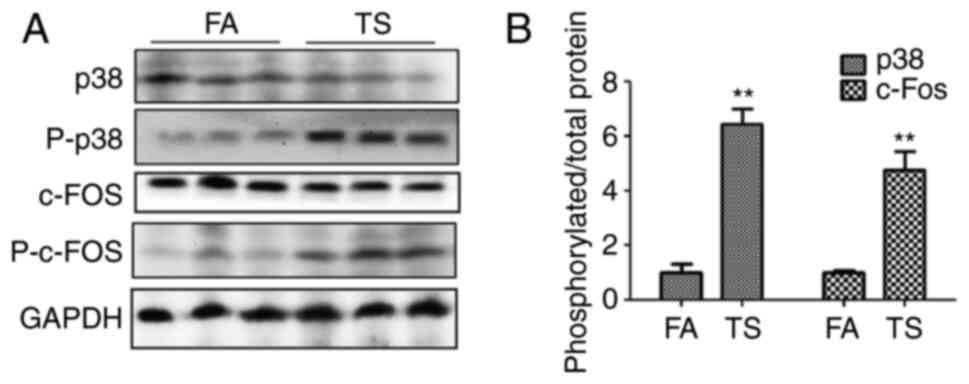
TS-mediated abnormal EMT and cell
proliferation are inhibited by p38 pathway inhibition
To determine whether the abnormal lung EMT and
proliferation processes triggered by TS are associated with the p38
pathway, the levels of p38, phosphorylated p38 and phosphorylated
c-fos in mouse lung tissues were investigated. The western blotting
results revealed an increase in phosphorylated p38 and
phosphorylated c-Fos levels upon TS exposure compared with that in
the FA group (Fig. 2A). In
addition, the ratio of phosphorylated protein to total protein was
also evaluated, and TS was shown to increase the ratio of
phosphorylated p38 and phosphorylated c-Fos compared with the FA
group (Fig. 2B).
To further verify the role of p38 pathway, BALB/c
mice were treated with SB203580. After 12 weeks of treatment with
SB203580, the upregulation of phosphorylated p38 and phosphorylated
c-Fos induced by TS was significantly inhibited, as demonstrated by
western blotting (Fig. 3A). In
addition, the ratio of phosphorylated protein to total protein
evaluated by density analysis showed that SB203580 reduced the
proportion of the TS-induced increase in phosphorylated p38 and
phosphorylated c-Fos (Fig.
3B).
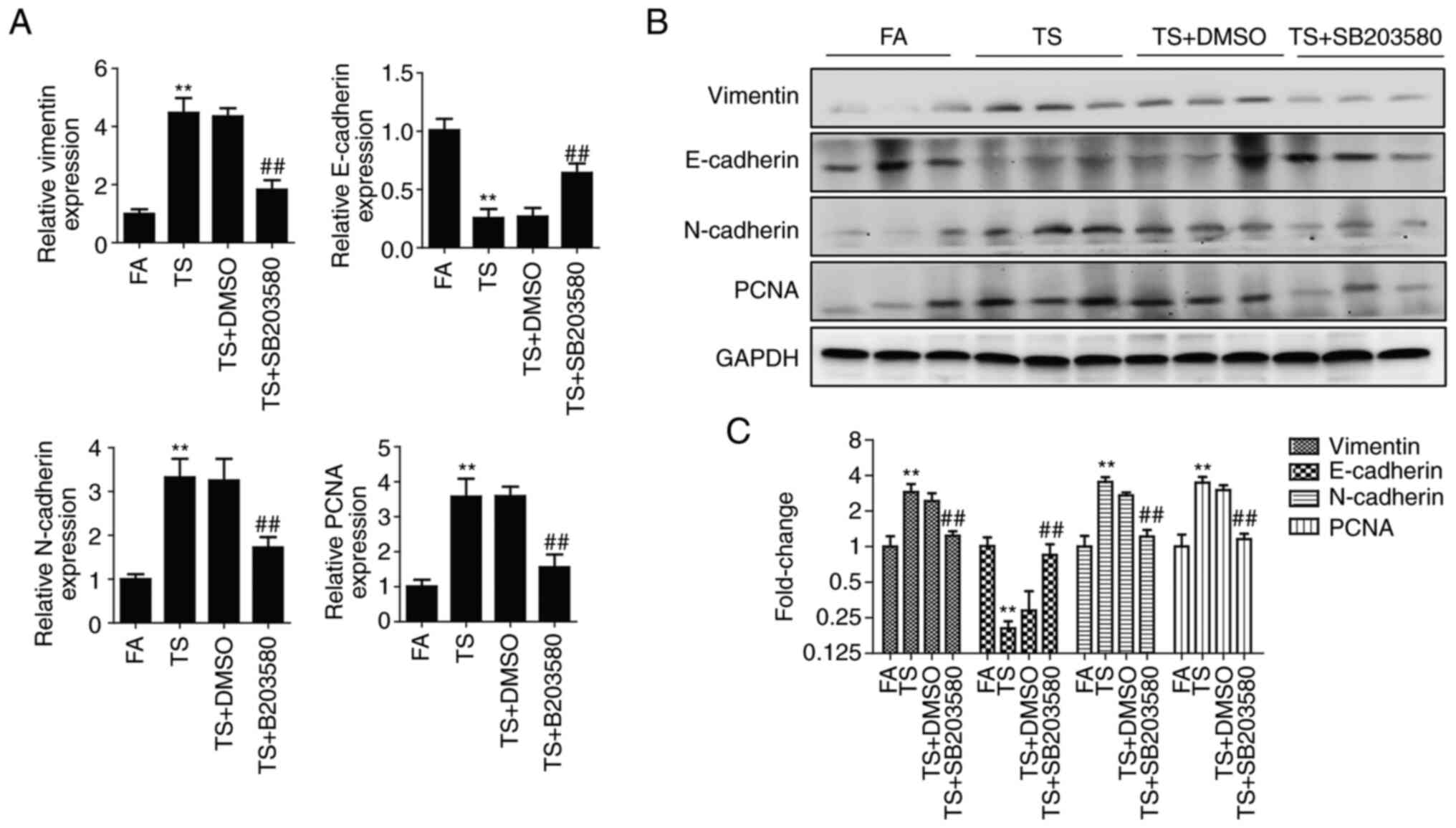
The expression of EMT and proliferation markers was
also detected after 12 weeks treatment. These results showed that
alterations in the levels of EMT and proliferation markers induced
by TS were significantly suppressed by inhibition of the p38
pathway in mouse lung tissues (Fig.
4). Therefore, TS-mediated abnormal EMT and cell proliferation
were inhibited by the inhibition of the p38 pathway as shown in the
in vivo experiments in mice.
Hesperidin inhibits abnormal EMT and
cell proliferation in mouse lung tissues elicited by TS
BALB/c mice were administered with hesperidin and
exposed to TS for 12 weeks. The downregulation of E-cadherin was
reduced, and the upregulation of vimentin, N-cadherin and PCNA was
reduced compared with that in the TS group (Fig. 5). The preventive effects of
hesperidin on TS-mediated abnormal EMT and cell proliferation in
mouse lung tissues were evident.
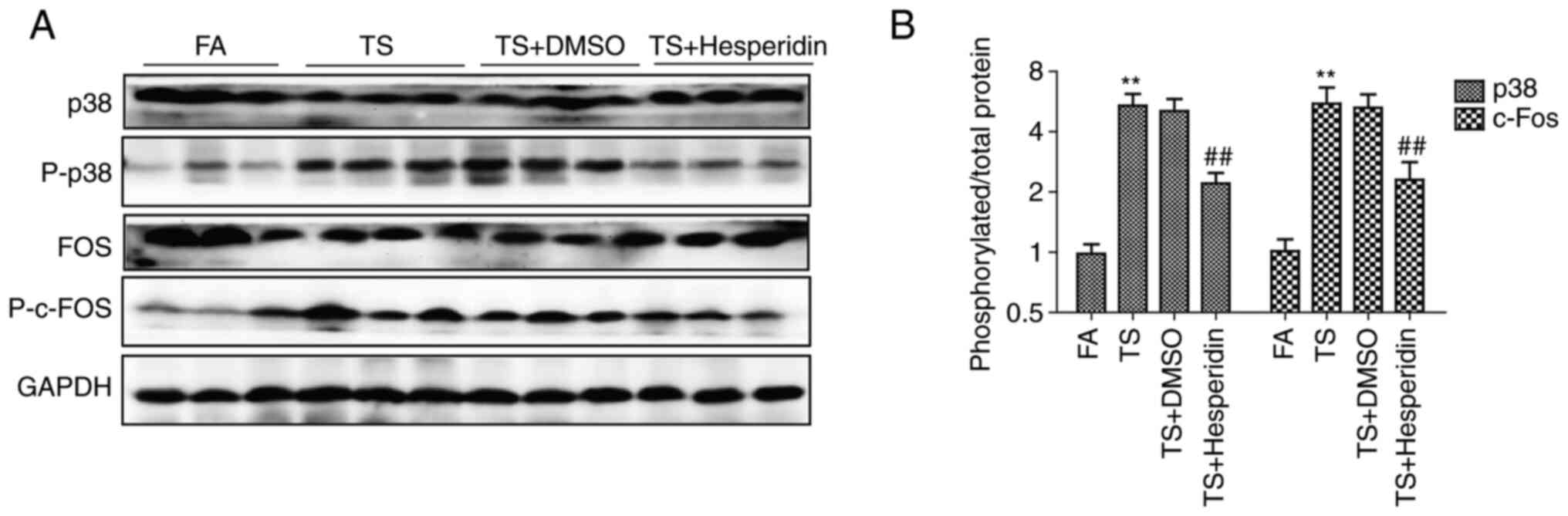
The effect of hesperidin on TS-induced abnormal
pulmonary EMT and cell proliferation through its effects on the p38
pathway was also studied. It was found that hesperidin (30 mg/kg)
reversed the increased expression of phosphorylated p38 and
phosphorylated c-fos induced by TS (Fig. 6A). In addition, the ratio of
phosphorylated protein to total protein evaluated by density
analysis showed that hesperidin reduced the phosphorylation level
of p38 and c-Fos compared with that in the TS group (Fig. 6B).
Discussion
TS is the leading cause of lung cancer and promotes
initiation and progression of pulmonary tumorigenesis (5–7,9,48).
The underlying molecular mechanism of TS causing lung cancer
remains unclear. The present study focused on TS-induced abnormal
EMT and cell proliferation in mouse lungs. The study demonstrated
that the p38 pathway regulates TS-associated abnormal pulmonary EMT
and cell proliferation. The data presented in the present study
indicated that hesperidin suppressed the p38 pathway to prevent
TS-induced abnormal pulmonary EMT and cell proliferation. The
findings provide insights into the molecular mechanisms of
TS-mediated pulmonary tumorigenesis, and provide potential targets
for lung cancer intervention.
It is well known that both normal cells and cancer
cells can proliferate, but the proliferation of cancer cells is
abnormal and uncontrolled (48,49).
Antiproliferative activity can inhibit the proliferation of all
cells, while antitumor activity only targets cancer cells with
abnormal proliferation and has little effect on normal cells. EMT
is a common cellular process where cells lose epithelial properties
and acquire mesenchymal properties (50,51).
Normal cells can acquire EMT properties, which may be an important
feature in the carcinogenic process (51). The tumor cell EMT process is a
strategy of ‘immune escape’ and a means to improve invasion and
metastasis (52,53). Carcinogens can stimulate abnormal
cell proliferation and EMT, leading to lung cancer (10–12).
TS-induced abnormal EMT and cell proliferation regulate early
events in cancer (54–56). In the present study, the change in
the expression levels of EMT and proliferation markers indicated
abnormal EMT and cell proliferation in the lungs of mice exposed to
TS. This was demonstrated through western blot analysis and RT-qPCR
where reduced levels of E-cadherin, and increased levels of
vimentin, N-cadherin and PCNA were observed. Immunohistochemical
staining also revealed increased vimentin expression and decreased
E-cadherin expression.
A number of signaling pathways control abnormal EMT
and cell proliferation, including the Wnt/β-catenin, MAPK and NF-κB
signaling pathways (57–59). The MAPK pathway regulates
physiological processes and pathologies, such as cell
proliferation, apoptosis, inflammation, cell motility,
differentiation and tumorigenesis (60,61).
p38 is an important member of the MAPK family and participates in
the development of cancer by regulating EMT and abnormal cell
proliferation (30,31,62).
The present study demonstrated that TS-mediated abnormal pulmonary
EMT and cell proliferation were associated with the upregulation of
phosphorylated p38 and phosphorylated c-Fos.
The role of the p38 pathway in abnormal pulmonary
EMT and cell proliferation has been previously studied. In these
studies, mice were treated with SB203580, which inhibited p38
activation (63,64). In the present study, SB203580
inhibited the upregulation of phosphorylated p38 and phosphorylated
c-Fos induced by TS. The suppressed p38 pathway inhibited
TS-mediated abnormal pulmonary EMT and cell proliferation, as shown
by elevated E-cadherin levels and decreased vimentin, N-cadherin
and PCNA levels.
Dietary phytochemicals, such as hesperidin, are
considered to contribute to cancer prevention (37,38).
The safety of hesperidin and its anticancer activity have been
previously demonstrated (41,65,66).
The intervention of hesperidin in TS-induced abnormal pulmonary EMT
and cell proliferation is through the p38 pathway, where
phosphorylated p38 and phosphorylated c-Fos are attenuated by
hesperidin.
The results of the present study illustrated that
the p38 pathway positively regulated TS-induced abnormal pulmonary
EMT and proliferation. The interventive effects of hesperidin were
demonstrated, which may aid the understanding of the mechanisms and
chemoprevention of TS-induced lung cancer.
Acknowledgements
The authors would like to thank Professor Caiyun
Zhong (Nanjing Medical University, Nanjing, China) for providing
guidance on mouse model construction and research design.
Funding
The present study was supported by the project of Social
Development in Zhenjiang (grant no. SH2021045), the Foundation for
Excellent Young Teachers of Jiangsu University (grant no.
5521280013), and Zhenjiang Key Laboratory of High Technology
Research on Exosomes Foundation and Transformation Applications
(grant no. SS2018003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL, YW and YZ designed the study and wrote, revised
the manuscript. YZ and YX performed the experiments. XZ and YX
analyzed the data. All authors read and approved the final
manuscript. ZL and YW confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Mice were handled as per the guidelines of the
Animal Care and Welfare Committee of Jiangsu University (Zhenjiang,
China). The study protocol was approved by the Committee on the
Ethics of Animal Experiments of Jiangsu University (Zhenjiang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang Z, Sun S, Lee M, Maslov AY, Shi M,
Waldman S, Marsh A, Siddiqui T, Dong X, Peter Y, et al: Single-cell
analysis of somatic mutations in human bronchial epithelial cells
in relation to aging and smoking. Nat Genet. 54:492–498. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pfeifer GP: Smoke signals in the DNA of
normal lung cells. Nature. 578:224–226. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nasim F, Sabath BF and Eapen GA: Lung
cancer. Med Clin North Am. 103:463–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bade BC and Dela Cruz CS: Lung Cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Chen SJ, Hsu SW, Zhang J, Li JM,
Yang DC, Gu S, Pinkerton KE and Chen CH: MARCKS cooperates with
NKAP to activate NF-kB signaling in smoke-related lung cancer.
Theranostics. 11:4122–4136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen J, Fu JH, Zhang W and Guo M: Lung
carcinoma signaling pathways activated by smoking. Chin J Cancer.
30:551–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang Z, Xie W, Wu R, Geng H, Zhao L, Xie
C, Li X, Huang C, Zhu J, Zhu M, et al: ERK5 negatively regulates
tobacco smoke-induced pulmonary epithelial-mesenchymal transition.
Oncotarget. 6:19605–19618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pastushenko I, Mauri F, Song Y, Cock F,
Meeusen B, Swedlund B, Impens F, Van Haver D, Opitz M, Thery M, et
al: Fat1 deletion promotes hybrid EMT state, tumour stemness and
metastasis. Nature. 589:448–455. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banerjee P, Xiao GY, Tan X, Zheng VJ, Shi
L, Rabassedas MNB, Guo HF, Liu X, Yu J, Diao L, et al: The EMT
activator ZEB1 accelerates endosomal trafficking to establish a
polarity axis in lung adenocarcinoma cells. Nat Commun.
12:63542021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adachi Y, Ito K, Hayashi Y, Kimura R, Tan
TZ, Yamaguchi R and Ebi H: Epithelial-to-mesenchymal transition is
a cause of both intrinsic and acquired resistance to KRAS G12C
inhibitor in KRAS G12C-mutant non-small cell lung cancer. Clin
Cancer Res. 26:5962–5973. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chu S, Ma L, Wu Y, Zhao X, Xiao B and Pan
Q: C-EBPβ mediates in cigarette/IL-17A-induced bronchial
epithelial-mesenchymal transition in COPD mice. BMC Pulm Med.
21:3762021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen TY, Liu CH, Chen TH, Chen MR, Liu SW,
Lin P and Lin KM: Conditioned media of adipose-derived stem cells
suppresses sidestream cigarette smoke extract induced cell death
and epithelial-mesenchymal transition in lung epithelial cells. Int
J Mol Sci. 22:120692021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu L, Chen J, Li M, Tang L, Wu R, Jin L
and Liang Z: β-carotene reverses tobacco smoke-induced gastric EMT
via Notch pathway in vivo. Oncol Rep. 39:1867–1873.
2018.PubMed/NCBI
|
|
16
|
Xie C, Zhu J, Huang C, Yang X, Wang X,
Meng Y, Geng S, Wu J, Shen H, Hu Z, et al: Interleukin-17A mediates
tobacco smoke-induced lung cancer epithelial-mesenchymal transition
through transcriptional regulation of ΔNp63α on miR-19. Cell Biol
Toxicol. 38:273–289. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie C, Zhu J, Yang X, Huang C, Zhou L,
Meng Z, Li X and Zhong C: TAp63α is involved in tobacco
smoke-induced lung cancer EMT and the anti-cancer activity of
curcumin via miR-19 transcriptional suppression. Front Cell Dev
Biol. 9:6454022021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su X, Chen J, Lin X, Chen X, Zhu Z, Wu W,
Lin H, Wang J, Ye J and Zeng Y: FERMT3 mediates cigarette
smoke-induced epithelial-mesenchymal transition through
Wnt/β-catenin signaling. Respir Res. 22:2862021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gandhi GR, Antony PJ, Lana MJMP, da Silva
BFX, Oliveira RV, Jothi G, Hariharan G, Mohana T, Gan RY, Gurgel
RQ, et al: Natural products modulating interleukins and other
inflammatory mediators in tumor-bearing animals: A systematic
review. Phytomedicine. 100:1540382022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan LTO and Trio-Ranche FKC: Atypical
lymphoid proliferation of the orbit. GMS Ophthalmol Cases.
12:Doc062022.PubMed/NCBI
|
|
21
|
Mondal P, Mohapatra S, Bhunia D, Gharai
PK, Mukherjee N, Gupta V and Ghosh S and Ghosh S: Designed hybrid
anticancer nuclear-localized peptide inhibits aggressive cancer
cell proliferation. RSC Med Chem. 13:196–201. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu JY, Chen YJ, Fu XQ, Li JK, Chou JY, Yin
CL, Bai JX, Wu Y, Wang XQ, Li AS, et al: Chrysoeriol suppresses
hyperproliferation of rheumatoid arthritis fibroblast-like
synoviocytes and inhibits JAK2/STAT3 signaling. BMC Complement Med
Ther. 22:732022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Irie H, Ozaki M, Chubachi S, Hegab AE,
Tsutsumi A, Kameyama N, Sakurai K, Nakayama S, Kagawa S, Wada S, et
al: Short-term intermittent cigarette smoke exposure enhances
alveolar type 2 cell stemness via fatty acid oxidation. Respir Res.
23:412022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu W, Wang L, Deng G, Gu X, Tang Z, Li S,
Jin W, Yang J, Guo X and Li Q: Knockdown of long noncoding RNA MIAT
attenuates cigarette smoke-induced airway remodeling by
downregulating miR-29c-3p-HIF3A axis. Toxicol Lett. 357:11–19.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geng H, Zhao L, Liang Z, Zhang Z, Xie D,
Bi L, Wang Y, Zhang T, Cheng L, Yu D and Zhong C: Cigarette smoke
extract-induced proliferation of normal human urothelial cells via
the MAPK/AP-1 pathway. Oncol Lett. 13:469–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rovida E and Tusa I: Targeting MAPK in
cancer 2.0. Int J Mol Sci. 23:57022022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caeser R, Hulton C, Costa E, Durani V,
Little M, Chen X, Tischfield SE, Asher M, Kombak FE, Chavan SS, et
al: MAPK pathway activation selectively inhibits ASCL1-driven small
cell lung cancer. iScience. 24:1032242021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang B, Zhuang R, Luo X, Yin L, Pang C,
Feng T, You H, Zhai Y, Ren Y, Zhang L, et al: Prevalence of
metabolically healthy obese and metabolically obese but normal
weight in adults worldwide: A meta-analysis. Horm Metab Res.
47:839–845. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okada T, Sinha S, Esposito I, Schiavon G,
López-Lago MA, Su W, Pratilas CA, Abele C, Hernandez JM, Ohara M,
et al: The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT
by restraining Ras-MAPK signalling. Nat Cell Biol. 17:81–94. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang J, Park JH, Kong JS, Kim MJ, Lee SS,
Park S and Myung JK: PINX1 promotes malignant transformation of
thyroid cancer through the activation of the AKT/MAPK/β-catenin
signaling pathway. Am J Cancer Res. 11:5485–5495. 2021.PubMed/NCBI
|
|
31
|
Kumar D, Patel SA, Hassan MK, Mohapatra N,
Pattanaik N and Dixit M: Reduced IQGAP2 expression promotes EMT and
inhibits apoptosis by modulating the MEK-ERK and p38 signaling in
breast cancer irrespective of ER status. Cell Death Dis.
12:3892021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu N, Zhang XJ, Zou H, Zhang YY, Xia JW,
Zhang P, Zhang YZ, Li J, Dong L, Wumaier G and Li SQ: PTPL1
suppresses lung cancer cell migration via inhibiting TGF-β1-induced
activation of p38 MAPK and Smad 2/3 pathways and EMT. Acta
Pharmacol Sin. 42:1280–1287. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shu L, Chen S, Lin S, Lin H, Shao Y, Yao
J, Qu L, Zhang Y, Liu X, Du X, et al: The pseudomonas aeruginosa
secreted protein PA3611 promotes bronchial epithelial cell
epithelial-mesenchymal transition via integrin αvβ6-mediated
TGF-β1-induced p38/NF-κB pathway activation. Front Microbiol.
12:7637492022.PubMed/NCBI
|
|
34
|
Li S, Wang H, Ma R and Wang L: Schisandrin
B inhibits epithelial-mesenchymal transition and stemness of
large-cell lung cancer cells and tumorigenesis in xenografts via
inhibiting the NF-κB and p38 MAPK signaling pathways. Oncol Rep.
45:1152021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang Z, Wu R, Xie W, Zhu M, Xie C, Li X,
Zhu J, Zhu W, Wu J, Geng S, et al: Curcumin reverses tobacco
smoke-induced epithelial-mesenchymal transition by suppressing the
MAPK pathway in the lungs of mice. Mol Med Rep. 17:2019–2025.
2018.PubMed/NCBI
|
|
36
|
Saxena A, Walters MS, Shieh JH, Shen LB,
Gomi K, Downey RJ, Crystal RG and Moore MAS: Extracellular vesicles
from human airway basal cells respond to cigarette smoke extract
and affect vascular endothelial cells. Sci Rep. 11:61042021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu Q, Lai Y, Zhang H, Ren K, Liu W, An Y,
Yao J and Fan H: Hesperetin inhibits TGF-β1-induced migration and
invasion of triple negative breast cancer MDA-MB-231 cells via
suppressing Fyn/Paxillin/RhoA pathway. Integr Cancer Ther.
21:153473542210869002022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ricci A, Gallorini M, Del Bufalo D,
Cataldi A, D'Agostino I, Carradori S and Zara S: Negative
modulation of the angiogenic cascade induced by allosteric kinesin
Eg5 inhibitors in a gastric adenocarcinoma in vitro model.
Molecules. 27:9572022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang SW, Sheng H, Zheng F and Zhang F:
Hesperetin promotes DOT1L degradation and reduces histone H3K79
methylation to inhibit gastric cancer metastasis. Phytomedicine.
84:1534992021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang J, Wu D, Vikash, Song J, Wang J, Yi
J and Dong W: Hesperetin induces the apoptosis of gastric cancer
cells via activating mitochondrial pathway by increasing reactive
oxygen species. Dig Dis Sci. 60:2985–2995. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Semis HS, Kandemir FM, Kaynar O, Dogan T
and Arikan SM: The protective effects of hesperidin against
paclitaxel-induced peripheral neuropathy in rats. Life Sci.
287:1201042021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou L, Gu W, Kui F, Gao F, Niu Y, Li W,
Zhang Y, Guo L, Wang J, Guo Z and Du G: The mechanism and candidate
compounds of aged citrus peel (chenpi) preventing chronic
obstructive pulmonary disease and its progression to lung cancer.
Food Nutr Res. 65:2021. View Article : Google Scholar
|
|
43
|
Kong W, Ling X, Chen Y, Wu X, Zhao Z, Wang
W, Wang S, Lai G and Yu Z: Hesperetin reverses
P-glycoprotein-mediated cisplatin resistance in DDP-resistant human
lung cancer cells via modulation of the nuclear factor-κB signaling
pathway. Int J Mol Med. 45:1213–1224. 2020.PubMed/NCBI
|
|
44
|
Hu G, Cao C, Deng Z, Li J, Zhou X, Huang Z
and Cen C: Effects of matrine in combination with cisplatin on
liver cancer. Oncol Lett. 21:662021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang B, Gong A, Shi H, Bie Q, Liang Z, Wu
P, Mao F, Qian H and Xu W: Identification of a novel YAP-14-3-3ζ
negative feedback loop in gastric cancer. Oncotarget.
8:71894–71910. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lu L, Chen J, Tang H, Bai L, Lu C, Wang K,
Li M, Yan Y, Tang L, Wu R, et al: EGCG suppresses ERK5 activation
to reverse tobacco smoke-triggered gastric epithelial-mesenchymal
transition in BALB/c mice. Nutrients. 8:3802016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lu L, Liang Q, Shen S, Feng L, Jin L and
Liang ZF: Tobacco smoke plays an important role in initiation and
development of lung cancer by promoting the characteristics of
cancer stem cells. Cancer Manag Res. 12:9735–9739. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Intlekofer AM and Finley LWS: Metabolic
signatures of cancer cells and stem cells. Nat Metab. 1:177–188.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liang Z, Wu R, Xie W, Xie C, Wu J, Geng S,
Li X, Zhu M, Zhu W, Zhu J, et al: Effects of curcumin on tobacco
smoke-induced hepatic MAPK pathway activation and
epithelial-mesenchymal transition in vivo. Phytother Res.
31:1230–1239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liang Z, Lu L, Mao J, Li X, Qian H and Xu
W: Curcumin reversed chronic tobacco smoke exposure induced
urocystic EMT and acquisition of cancer stem cells properties via
Wnt/β-catenin. Cell Death Dis. 8:e30662017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Terry S, Savagner P, Ortiz-Cuaran S,
Mahjoubi L, Saintigny P, Thiery JP and Chouaib S: New insights into
the role of EMT in tumor immune escape. Mol Oncol. 11:824–846.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jang HR, Shin SB, Kim CH, Won JY, Xu R,
Kim DE and Yim H: PLK1/vimentin signaling facilitates immune escape
by recruiting Smad2/3 to PD-L1 promoter in metastatic lung
adenocarcinoma. Cell Death Differ. 28:2745–2764. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bai X, Wei H, Liu W, Coker OO, Gou H, Liu
C, Zhao L, Li C, Zhou Y, Wang G, et al: Cigarette smoke promotes
colorectal cancer through modulation of gut microbiota and related
metabolites. Gut. 71:2439–2450. 2022.PubMed/NCBI
|
|
55
|
Jia Y, Zhang Q, Liu Z, Pan P, Jia Y, Zhu
P, Jiao Y, Kang G and Ma X: The role of α5-nicotinic acetylcholine
receptor/NLRP3 signaling pathway in lung adenocarcinoma cell
proliferation and migration. Toxicology. 469:1531202022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Agraval H, Sharma JR, Prakash N and Yadav
UCS: Fisetin suppresses cigarette smoke extract-induced epithelial
to mesenchymal transition of airway epithelial cells through
regulating COX-2/MMPs/β-catenin pathway. Chem Biol Interact.
351:1097712022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang J, Chang Y, Xia H, Xu L and Wei X:
HIST1H2BN induced cell proliferation and EMT phenotype in prostate
cancer via NF-κB signal pathway. Genes Genomics. 43:1361–1369.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xueqin T, Jinhong M and Yuping H: Inhibin
subunit beta A promotes cell proliferation and metastasis of breast
cancer through Wnt/β-catenin signaling pathway. Bioengineered.
12:11567–11575. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang M, Jin M, Li K, Liu H, Yang X, Zhang
X, Zhang B, Gong A and Bie Q: TRAF6 promotes gastric cancer cell
self-renewal, proliferation, and migration. Stem Cells Int.
2020:32961922020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Drosten M and Barbacid M: Targeting the
MAPK pathway in KRAS-driven tumors. Cancer Cell. 37:543–550. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee S, Rauch J and Kolch W: Targeting MAPK
signaling in cancer: Mechanisms of drug resistance and sensitivity.
Int J Mol Sci. 21:11022020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Arora A, Bhuria V, Singh S, Pathak U,
Mathur S, Hazari PP, Roy BG, Sandhir R, Soni R, Dwarakanath BS and
Bhatt AN: Amifostine analog, DRDE-30, alleviates radiation induced
lung damage by attenuating inflammation and fibrosis. Life Sci.
298:1205182022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xie X, Deng T, Duan J, Xie J, Yuan J and
Chen M: Exposure to polystyrene microplastics causes reproductive
toxicity through oxidative stress and activation of the p38 MAPK
signaling pathway. Ecotoxicol Environ Saf. 190:1101332020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sanit J, Prompunt E, Adulyaritthikul P,
Nokkaew N, Mongkolpathumrat P, Kongpol K, Kijtawornrat A, Petchdee
S, Barrère-Lemaire S and Kumphune S: Combination of metformin and
p38 MAPK inhibitor, SB203580, reduced myocardial
ischemia/reperfusion injury in non-obese type 2 diabetic
Goto-Kakizaki rats. Exp Ther Med. 18:1701–1714. 2019.PubMed/NCBI
|
|
65
|
Yamamoto S, Lee S, Ariyasu T, Endo S,
Miyata S, Yasuda A, Harashima A, Ohta T, Kumagai-Τakei N, Ito T, et
al: Ingredients such as trehalose and hesperidin taken as
supplements or foods reverse alterations in human T cells, reducing
asbestos exposure-induced antitumor immunity. Int J Oncol.
58:22021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Deng J, Liu L, Li L, Sun J and Yan F:
Hesperidin delays cell cycle progression into the G0/G1 phase via
suspension of MAPK signaling pathway in intrahepatic
cholangiocarcinoma. J Biochem Mol Toxicol. 36:e229812022.
View Article : Google Scholar : PubMed/NCBI
|