Introduction
Gastric cancer (GC) remains the fifth most common
cancer and the third leading cause of cancer-related deaths
worldwide (1). Surgical resection
of the primary tumor in the stomach is by far the most effective
treatment, yet less than half of patients with GC are diagnosed and
undergo radical resection at an early stage, which is associated
with a 5-year survival rate of up to 90% (2,3). In
addition, patients with advanced GC who undergo radical resection
have a higher recurrence rate and lower 5-year survival rate (~20%)
compared with early-stage GC (2,3). The
use of sensitive methods and specific tumor biomarkers for early
detection of GC and monitoring the risk of recurrence can markedly
improve the prognosis of patients with GC. However, the existent
biomarkers for GC are mostly non-specific, as they are also
elevated in other malignant tumors and certain benign diseases
(4). Therefore, it is necessary to
find novel biomarkers to improve the early diagnosis rate of
GC.
MicroRNAs (miRs/miRNAs), which are 18–22 nucleotides
in length, are considered a large class of evolutionarily conserved
non-coding RNAs, which can regulate target genes and serve critical
roles in a number of essential cell processes, including
proliferation, differentiation, development, survival and death
(5). Previous studies have
demonstrated that miRNAs serve a key role in the occurrence and
development of cancer (6–11). Numerous cancer types, including
liver cell carcinoma, papillary thyroid carcinoma, lung cancer,
glioblastoma, breast cancer, colorectal cancer and lymphoma, have
been demonstrated to exhibit dysregulation of miRNAs to promote or
inhibit tumor progression, which suggests that miRNAs could be
potential biomarkers of cancer (7–11).
Dysregulation of miR-153 has previously been
observed in several common human cancer types, with evidence
indicating that miR-153 can serve as an oncogene in colorectal
cancer, hepatocellular carcinoma and prostate cancer (12–14) or
as a tumor suppressor gene in glioma, nasopharyngeal cancer, breast
cancer and laryngeal carcinoma (15–19).
Therefore, the functional role of miR-153 in cancer development and
progression is cancer type-specific. In addition, the clinical role
of miR-153 in GC and its expression in the serum of patients with
GC have not been elucidated clearly. The present study aimed to
investigate miR-153 expression in tissues and serum samples of
patients with GC, and to identify the association between miR-153
expression and clinicopathological characteristics of patients with
GC.
Materials and methods
Cells and cell culture
The GES-1 human gastric epithelial cell line and the
HGC-27, AGS and MKN-28 human GC cell lines were obtained from BeNa
Culture Collection; Beijing Beina Chunglian Institute of
Biotechnology (Beijing, China). MKN-28 cells were authenticated by
BeNa Culture Collection; Beijing Beina Chunglian Institute of
Biotechnology by short tandem repeat profiling. All cells were
cultured in RPMI-1640 medium (HyClone; Cytiva) with 10% FBS
(Biological Industries; Sartorius AG) at 37°C in a humidified
incubator with 5% CO2.
Patient tissue and blood sample
preparation
In the present study, clinical tissues of 20
patients (age range, 40–77 years) and blood samples of 59 patients
with GC (age range, 40–77 years) and 9 healthy controls (HCs; age
range, 38–72 years) were collected from Weihai Municipal Hospital,
Shandong University (Weihai, China) between January 2021 and
January 2022. Clinicopathological data are presented in Table I. Strict inclusion and exclusion
criteria were established for patients with GC. The inclusion
criteria were: i) 18–80 years old; and ii) patients were diagnosed
by endoscopic biopsy or surgery according to the World Health
Organization pathological classification (20). The exclusion criteria were as
follows: i) History of other malignant tumors; ii) history of any
kind of preoperative treatment, including radiotherapy,
chemotherapy and immunotherapy; and iii) severe liver and kidney
diseases such as liver failure and kidney failure, endocrine and
metabolic diseases such as diabetes and hypertension, and other
connective tissue diseases such as systemic lupus erythematosus.
After screening, a total of 59 patients with GC were enrolled and
GC tissues and matched adjacent non-cancerous tissues were obtained
from surgical resected gastric tissues from 20 of these patients
and immediately snap-frozen in dry ice and stored at −80°C.
 | Table I.Demographic data of all patients. |
Table I.
Demographic data of all patients.
|
|
| Serum samples |
|---|
|
|
|
|
|---|
| Clinicopathological
features | Tissue samples of
patients with GC (n=20) | Patients with GC
(n=59) | Healthy controls
(n=9) |
|---|
| Sex |
|
|
|
|
Male | 14 | 34 | 5 |
|
Female | 6 | 25 | 4 |
| Age, years | 63.851±9.438 | 64.118±7.704 | 56.453±11.396 |
| Histology |
|
|
|
| Well,
moderate | 13 | 33 | - |
| Poor,
signet | 7 | 26 | - |
| Tumor size, cm |
|
|
|
|
<4 | 8 | 34 | - |
| ≥4 | 12 | 25 | - |
| Lymph node
metastasis |
|
|
|
|
Absent | 7 | 40 | - |
|
Present | 13 | 19 | - |
| TNM stage |
|
|
|
| I,
II | 11 | 43 | - |
| III,
IV | 9 | 16 | - |
Blood samples were obtained from 59 patients with GC
(25 patients with early-stage GC, including high-grade
intraepithelial neoplasia and low-grade intraepithelial neoplasia,
and 34 patients with advanced GC stages) and 9 HCs. HCs were
identified by clinical manifestations, disease history and blood
test results. The inclusion criteria for HCs were: i) 18–80 years
old; ii) no sex restriction; and iii) volunteers who were in good
physical condition. The exclusion criterion was history of disease
and surgery. Blood samples were centrifuged at 900 × g (iCEN-24R;
Hangzhou Allsheng Instruments Co., Ltd.) at 20°C for 10 min for
serum separation and stored at −80°C prior to small RNA isolation.
The study was approved by the Ethics Committee of Weihai Municipal
Hospital (approval number 2020010; Weihai, China) and written
informed consent was obtained from all patients and HCs.
Reverse transcription-quantitative PCR
(RT-qPCR)
Small RNAs were extracted from cells and tissues
using RNAiso for Small RNA (Takara Bio, Inc.) according to the
manufacturer's protocol. The synthetic cel-miR-39-3p standard RNA
(Guangzhou RiboBio Co., Ltd.) was adopted as the external
reference. Small RNAs were isolated from the serum with the
Serum/Plasma miRNA Extraction Kit (HaiGene Biotech Co., Ltd.)
according to the manufacturer's instructions. The optical density
(OD) of the extracted RNA was measured with a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.) at 260 and 280
nm. Both the tissue and serum small RNAs (OD260/280
>1.8) were then converted into the reverse transcription product
using a HiFiScript cDNA Synthesis Kit (cat. no. CW2569M; CoWin
Biosciences) using specific primers (confidential sequences; cat.
no. F02002; Suzhou GenePharma Co., Ltd.) according to the
manufacturer's instructions. miRNA expression was then examined
using the UltraSYBR Mixture kit Low ROX (CoWin Biosciences) on the
thermocycler ABI 7500 (Thermo Fisher Scientific, Inc.). For the
determination of serum miRNA expression, miR-39 was used as the
external reference, while U6 was used as the internal reference for
the determination of tissue miRNA expression. The sequences of
miR-153 and U6 primers (Suzhou GenePharma Co., Ltd.) were as
follows: hsa-miR-153-3p forward, 5′-AACGAACTTGCATAGTCACAAAAG-3′ and
reverse, 5′-TATGGTTTTGACGACTGTGTGAT-3′; and U6 forward,
5′-CAGCACATATACTAAAATTGGAACG-3′ and reverse,
5′-ACGAATTTGCGTGTCATCC-3′. The sequence of the primers for miR-39
is confidential and the design method has been patented (cat. no.
MQPS0000071-1-100; Guangzhou RiboBio Co., Ltd.). qPCR was performed
at 95°C for 10 min, followed by 40 cycles of denaturation for 15
sec and annealing/elongation at 60°C for 1 min. All reactions were
repeated at least three times and the 2−ΔΔCq method was
used to analyze the relative expression (21).
miRNA sequencing
To examine the potential significance of miRNAs in
GC, we sent several pairs of tissues for sequencing. We required OD
260/280 >1.8 and a complete sample band visible on the agarose
gel electrophoresis image to verify the quality of samples.
Finally, the miRNA profiles in three GC tissues and matched
adjacent non-tumor tissues were determined by miRNA sequencing with
the Hiseq Rapid SBS Kit V2 (50 cycle) (cat. no. FC-402-4022;
Illumina, Inc.) and Hiseq Rapid SR Cluster Kit V2 (cat. no.
GD-402-4002; Illumina, Inc.). Total RNA was isolated from cells
using a Magzol Reagent kit (cat. no. R4801; Magen Biotechnology
Co., Ltd.) according to the manufacturer's protocol. The quantity
and integrity of the RNA yield was assessed by using the K5500
(Beijing Kaiao Technology Development Co., Ltd.) and the Agilent
2200 TapeStation (Agilent Technologies, Inc.). Briefly, total RNA
was ligated with 3′ and 5′ adapters. Subsequently, the
adapter-ligated RNAs were subjected to RT-PCR and amplified with 12
cycles. The sequences of the primers were forward,
5′-AATGATACGGCGACCACCGAGATCTACACGTTCAGAGTTCTACAGTCCGA-3′ and
reverse,
5′-CAAGCAGAAGACGGCATACGAGATCGTGATGTGACTGGAGTTCCTTGGCACCCGAGAATTCCA-3′.
PCR products were size-selected using an 8% agarose gel according
to instructions of the NEBNext® Multiplex Small RNA
Library Prep Set for Illumina® (cat. no. E7560;
Illumina, Inc.). The purified library products were evaluated using
the Agilent 2200 TapeStation and a Qubit fluorometer (Thermo Fisher
Scientific, Inc.). The loading concentrations of the final library
(Table II) were measured using a
Qubit dsDNA HS Assay Kit (cat. no. Q32854; Invitrogen; Thermo
Fisher Scientific, Inc.) to quantify the concentration in ng/µl
first, and then converted to molar concentrations using the
formula. The sequencing was outsourced to Guangzhou RiboBio Co.,
Ltd. and was performed with an Illumina instrument (Illumina, Inc.;
single-end; 50 bp).
 | Table II.Final library concentrations. |
Table II.
Final library concentrations.
|
|
| Testing result |
|
|---|
|
|
|
|
|
|---|
| No. | Samples | Main peak size,
bp | Mass concentration,
ng/µl | Molar
concentration, ×103 pmol/l | Test
conclusion |
|---|
| 1 | GC-1 | 168 | 7.66 | 70.3 | Pass |
| 2 | Adjacent-1 | 163 | 2.71 | 25.6 | Pass |
| 3 | GC-2 | 167 | 13.4 | 123 | Pass |
| 4 | Adjacent-2 | 163 | 2.74 | 25.9 | Pass |
| 5 | GC-3 | 165 | 3.12 | 29 | Pass |
| 6 | Adjacent-3 | 160 | 4.62 | 44.5 | Pass |
Bioinformatics analysis
The raw reads were processed by filtering out reads
containing adapters, poly ‘N’, low quality reads and reads of
<17 nucleotides using FastQC (version 0.11.2; http://www.bioinformatics.babraham.ac.uk/projects/fastqc/)
to obtain clean reads. Mapping of clean reads to the reference
genome (hg19; http://www.ncbi.nlm.nih.gov/data-hub/genome/GCF_000001405.25/)
was completed using Bowtie (version 1.1.1; http://sourceforge.net/projects/bowtie-bio/).
MiRDeep2 (version miRDeep2.0.0.8; http://github.com/rajewsky-lab/mirdeep2/) was used to
identify known mature miRNAs based on miRBase22 (www.miRBase.org) and to predict novel miRNAs.
Databases, including Rfam12.1 (http://ftp.ebi.ac.uk/pub/databases/Rfam/12.1/) and
pirnabank (http://pirnabank.ibab.ac.in/), were used to identify
ribosomal RNA, transfer RNA, small nuclear RNA, small nucleolar RNA
and piwi-interacting RNA using BLAST (version 2.2.30+; http://ftp.ncbi.nlm.nih.gov/blast/executables/blast+/2.2.30/).
The miRNA expression was calculated as reads per million values.
Differential expression between two sets of samples was calculated
using the edgeR algorithm (version 33.20.9; http://www.bioconductor.org/packages/release/bioc/html/edgeR.html)
according to the criteria of log2(fold change)≥1 and P<0.05. The
heat map of the top 10 upregulated and downregulated miRNAs with
the highest absolute fold-change values was analyzed using pheatmap
(version 1.0.12; http://www.rdocumentation.org/packages/pheatmap/versions/1.0.12/topics/pheatmap)
and R (version 3.5.0; http://www.r-project.org/).
Fluorescence in situ hybridization
(FISH)
FISH for miR-153 was performed on paraffin sections
using a Fluorescent In Situ Hybridization Kit (cat. no.
F43501; Suzhou GenePharma Co., Ltd.) and the
CY3®-labeled locked nucleic acid (LNA) probe (Suzhou
GenePharma Co., Ltd.) according to the manufacturer's protocol. The
probe sequence (5′-3′) was GAT CAC T+TTT GTG AC+TAT GCA A, where +
means LNA modification. First, the dissected tissues were fixed
with 10% formalin for 48 h at room temperature. After rinsing the
tissues with running tap water and dehydrating these in 70, 95 and
100% ethanol successively, these were incubated in a 65°C paraffin
bath twice for 30 min each. Subsequently, the paraffin-embedded
tissues were sectioned into 4-µm-thick slices. Briefly, the
paraffin sections were dewaxed and rehydrated using a graded series
of ethanol (100, 95, 90, 80 and 70%) for 10 min each at room
temperature, and digested with proteinase K (GenePharma) for 20 min
at 37°C. After washing three times with 100 µl 2X Buffer C for 1
min each at room temperature, each sample was denatured with 100 µl
0.1% 2X Buffer C and 0.7% Buffer D for 8 min at 78°C. Each sample
was incubated successively with 70, 80, 90 and 100% ethanol for 2
min at room temperature. After drying at room temperature,
hybridization was performed overnight at 37°C with 100 µl denatured
5′ CY3-labeled LNA probe (2 µM) targeted at miR-153 in 1X Buffer E
(The probe with Buffer E was preincubated for denaturation at 73°C
for 5 min). To remove excess probes, one wash was performed with
100 µl 0.1% 2X Buffer C and 0.5% Buffer D for 15 min at 43°C,
followed by two washes with 100 µl 2X Buffer C for 10 min at 37°C,
and one wash with PBS for 10 min at room temperature. The sections
were counterstained with DAPI at room temperature for 20 min after
washing, and were observed under a fluorescence microscope (Nikon
ECLIPSE Ti; Nikon Corporation) and analyzed using ImageJ software
(version 1.8.0.112; National Institutes of Health).
Cell transfection
Cells (2×105 cells per well) were
inoculated in a 6-well plate at 37°C in a humidified atmosphere
containing 5% CO2 and transfected with synthetic miR-153
mimics (sense, 5′-UUGCAUAGUCACAAAAGUGAUC-3′; antisense,
5′-UCACUUUUGUGACUAUGCAAUU-3′), inhibitors
(5′-GAUCACUUUUGUGACUAUGCAA-3′), scrambled mimics negative controls
(NCs; sense, 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) or scrambled inhibitor NCs (InNCs;
5′-CAGUACUUUUGUGUAGUACAA-3′), which were all purchased from
Shanghai GenePharma Co., Ltd., at a concentration of 100 nM using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following incubation at 37°C for 5–8 h, the
transfection solution was removed. Functional experiments were
conducted 24–48 h post-transfection. The transfection efficiency
was assessed by RT-qPCR.
Wound-healing assay
Migration was evaluated using a wound healing assay.
Briefly, AGS and GES-1 cells (2×105 cells per well) were
inoculated into a 24-well plate and cultured to 90% confluence.
Subsequently, a line-shaped wound was created using a sterile
pipette tip, and cells were washed using PBS. The cells were then
incubated in RPMI-1640 without FBS at 37°C for 48 h. Images of cell
migration were captured at 0, 24 and 48 h under an inverted light
microscope (magnification, ×10) and analyzed using the ImageJ
(version 1.8.0.112; National Institutes of Health). The percentage
of wound gap closure was calculated as follows: Wound healing rate
(migration area)=(A0-An)/A0, where
A0 represents the initial wound area, and An represents
the remaining area of the wound at the timepoint examined.
Apoptosis assay
Cells were collected, resuspended and stained using
an Annexin V-FITC apoptosis detection kit [including propidium
iodide (PI); cat. no. C1062L; Beyotime Institute of Biotechnology]
according to the manufacturer's instructions. Next, cells were
detected using a BD FACSAria™ II cell sorter (BD Biosciences) and
analyzed using BD FACSDiva software (version 8.0; BD
Biosciences).
5-ethynyl-2-deoxyuridine (EdU)
proliferation assay
This assay was performed using the BeyoClickTM EdU
Cell Proliferation Kit with Alexa Fluor 488 (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. AGS cells
(1×106 cells per well) were inoculated in a 6-well plate
and labeled with 10 µM EdU reagent at 37°C for 2 h. Subsequently,
the AGS cells were fixed in 4% paraformaldehyde for 15 min at room
temperature and permeabilized with 0.3% Triton X-100 for 15 min.
The cells were washed three times using PBS, and cultured with 0.5
ml Click Reaction Mixture for 30 min at room temperature without
light. Subsequently, cells were stained with 1 ml DAPI (5 µg/ml)
for 10 min at room temperature, and were imaged using a
fluorescence microscope (Nikon ECLIPSE Ti; Nikon Corporation) and
analyzed using ImageJ software (version 1.8.0.112; National
Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS
(version 24.0; IBM Corp.) and GraphPad Prism (version 7.0;
Dotmatics). All experiments were performed independently at least
three times and all values are presented as the mean ± SD. An
unpaired or paired Student's t-test was used for comparisons
between two groups. Comparisons among multiple groups were analyzed
using one-way ANOVA followed by Dunnett's or Tukey's post-hoc
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-153 expression in GC and matched
adjacent non-tumor tissues
To screen differentially regulated miRNAs, human
miRNA sequencing was conducted in three pairs of GC tissues and
matched adjacent tissues. In total, 194 miRNAs were aberrantly
expressed with fold change ≥2.0 and P<0.05 after normalization.
Among them, 124 miRNAs were upregulated and 70 miRNAs were
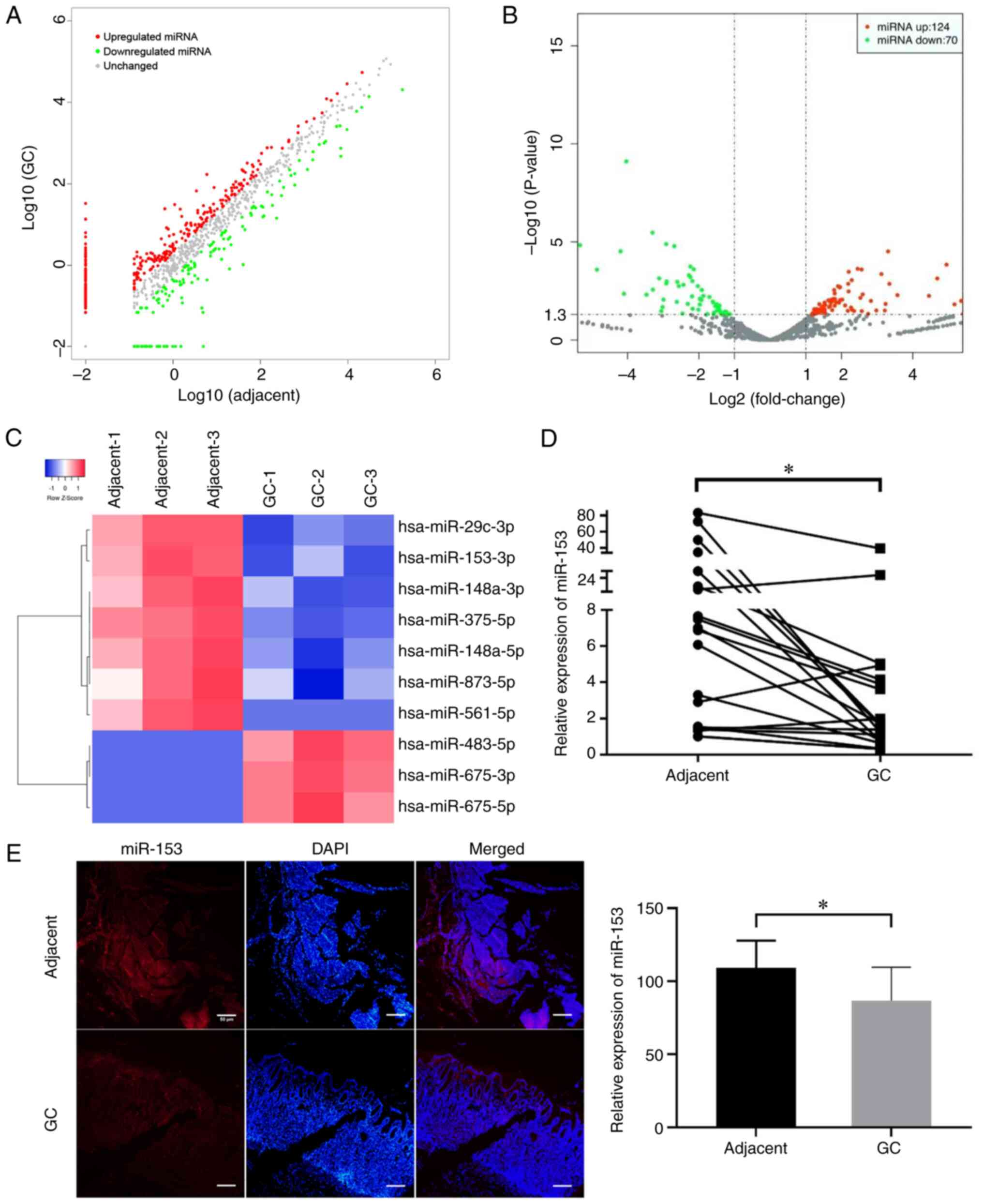
downregulated. The scatter (Fig.
1A) and volcano (Fig. 1B) plots
showed the variation in miRNA expression between GC and matched
adjacent tissues. In addition, a heat map shows the top 10 miRNAs
with the highest absolute fold-change values (Fig. 1C). The literature was reviewed and
it was revealed that dysregulation of miR-153 has previously been
observed in several common human cancer types, including colorectal
cancer, hepatocellular carcinoma and breast cancer (12–19);
however, the role of miR-153 in GC remains unclear. The results
demonstrated that miR-153-3p was one of the most downregulated
miRNAs in GC tissues. miR-153 expression was examined in 20 pairs
of GC and matched adjacent non-tumor stomach tissues by RT-qPCR and
normalized to an endogenous control (U6). miR-153 expression was
downregulated in 17 out of 20 GC tissues compared with matched
adjacent non-tumor tissues (Fig.
1D), and the result was further confirmed by FISH (Fig. 1E). Therefore, it was concluded that
miR-153 expression in GC was markedly lower than that in paired
adjacent tissues, suggesting that miR-153 may function as a tumor
suppressor in GC.
miR-153 expression in cell lines and
culture media
RT-qPCR was performed to evaluate the expression
levels of miR-153 in the three GC cell lines (HGC-27, AGS and
MKN28) and GES-1 cells, and the culture media. miR-153 expression
was lower in GC cell lines (Fig.
2A) and GC cell culture media (Fig.
2B) compared with GES-1 cells and GES-1 culture media,
respectively. Therefore, we hypothesized that miR-153 may be
secreted outside the cells to serve its role.
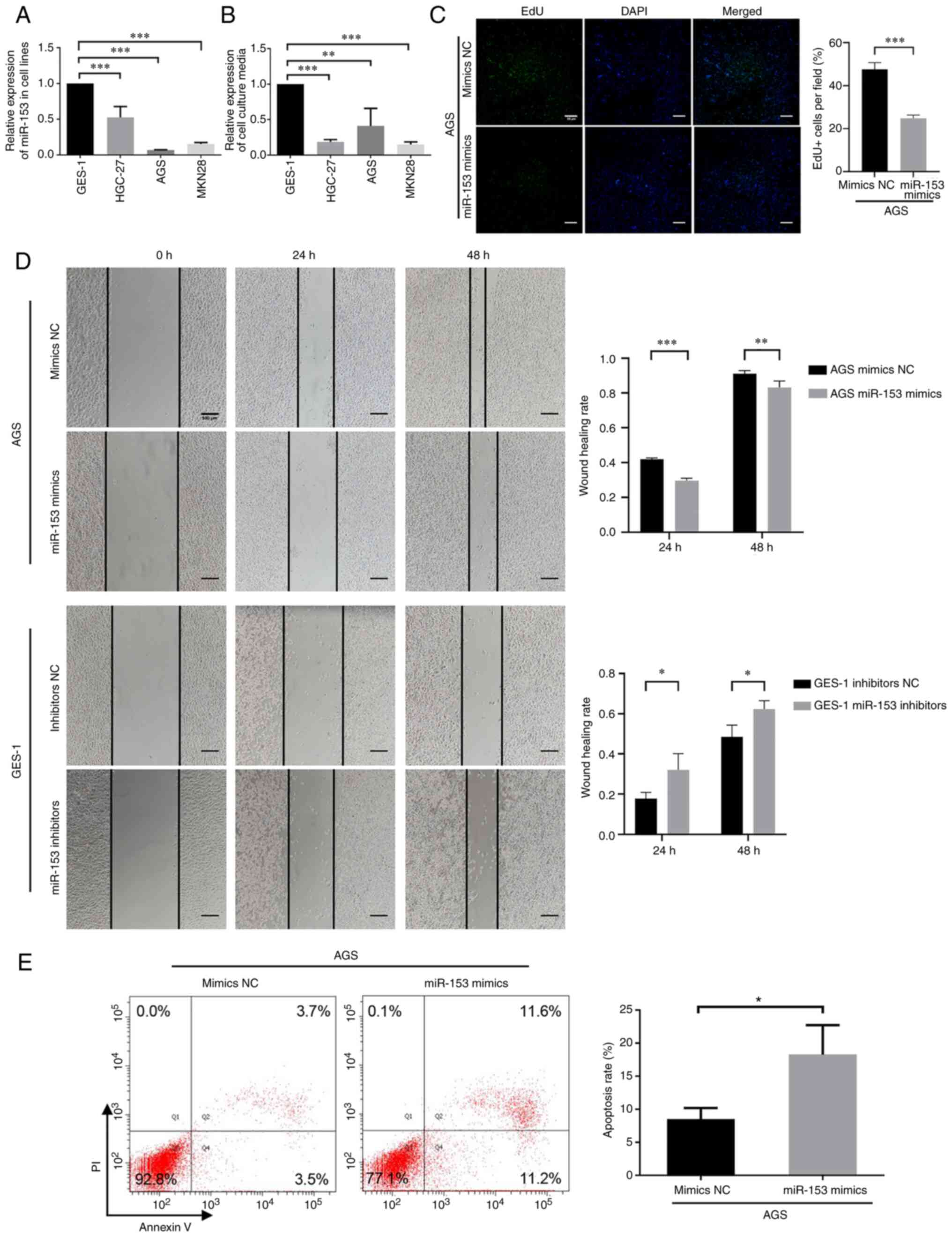 | Figure 2.Expression and function of miR-153 in
GC cells. miR-153 expression was downregulated in both (A) GC cell
lines and (B) their culture media. P-values in (A and B) were
obtained using one-way ANOVA followed by Dunnett's post-hoc test.
In terms of the function of miR-153 in GC cells, miR-153 could
inhibit GC cell proliferation and migration, and increase apoptosis
in vitro. (C) Representative images of EdU cell
proliferation assay (magnification, ×20; scale bar, 50 µm) and the
quantification bar plot of the percentage of EdU-positive cells
transfected with mimics NC or miR-153 mimics. (D) Wound healing
(scale bar, 500 µm) and (E) apoptosis assays were performed to
evaluate the migration and apoptosis of AGS cells, respectively.
P-values in (C-E) were obtained using an unpaired t-test.
*P<0.05; **P<0.01; ***P<0.001. NC, negative control; GC,
gastric cancer; miRNA/miR, microRNA; EdU,
5-ethynyl-2-deoxyuridine. |
miR-153 inhibits GC cell proliferation
and migration, and increases apoptosis in vitro
The AGS GC cell line was transfected with miR-153
mimics to investigate the role of miR-153 in GC. miR-153 expression
was increased in AGS cells transfected with miR-153 mimics
(Fig. S1A) and decreased in GES-1
cells transfected with miR-153 inhibitors (Fig. S1B). EdU assays were conducted and
revealed that miR-153 upregulation significantly reduced
EdU-positive AGS cells (Fig. 2C).
Furthermore, wound healing assays were conducted to determine the
effect of miR-153 mimics transfection in AGS cells and miR-153
inhibitors transfection in GES-1 cells on migration. A marked
reduction in migration of AGS cells was observed when miR-153 was
upregulated compared with the mimics NC group. On the other hand,
the downregulation of miR-153 resulted in significant promotion of
migration of GES-1 cells compared with the inhibitors NC group
(Fig. 2D). The effect of miR-153 on
GC cell apoptosis was examined using an apoptosis assay.
Upregulation of miR-153 resulted in a significant increase in the
apoptosis of AGS cells (Fig. 2E).
These results demonstrated that miR-153 could inhibit GC cell
proliferation and migration, and increase apoptosis in
vitro.
Clinical significance of serum
expression levels of miR-153 in patients with GC and HCs
The serum expression levels of miR-153 in 59
patients with GC (25/59 in early GC stage) and 9 HCs were examined
by RT-qPCR. The serum expression levels of miR-153 were
downregulated in patients with GC compared with HCs (Fig. 3A). Lower miR-153 expression was
identified in patients with advanced GC compared with patients with
early GC (Fig. 3B). Serum miR-153
was expressed at significantly lower levels in patients with GC
with larger tumor size (≥4 cm), poor differentiation and signet
histology, lymph node metastasis and advanced tumor stage [TNM
stage (22) III and IV] compared
with patients with smaller tumor size (<4 cm), well and moderate
differentiation, no lymph node metastasis and TNM stage I and II,
respectively, revealing that the downregulation of miR-153 may
contribute to GC aggressiveness and poor prognosis (Table III).
 | Table III.Association between
clinicopathological features and miR-153 expression in the serum of
59 patients with GC. |
Table III.
Association between
clinicopathological features and miR-153 expression in the serum of
59 patients with GC.
| Clinicopathological
features | All patients with
GC, n (n=59) | miR-153 expression,
mean ± SD | t | P-value |
|---|
| Age, years |
|
| 0.441 | 0.661 |
|
<65 | 32 | 0.717±0.964 |
|
|
|
≥65 | 27 | 0.610±0.886 |
|
|
| Sex |
|
| −1.700 | 0.095 |
|
Male | 34 | 0.496±0.784 |
|
|
|
Female | 25 | 0.903±1.055 |
|
|
| Histology |
|
| 2.614 | 0.013a |
| Well,
moderate | 33 | 0.909±1.162 |
|
|
| Poor,
signet | 26 | 0.363±0.272 |
|
|
| Tumor size, cm |
|
| 2.602 | 0.013a |
|
<4 | 34 | 0.895±1.141 |
|
|
| ≥4 | 25 | 0.361±0.308 |
|
|
| Lymph node
metastasis |
|
| −2.303 | 0.025a |
|
Absent | 40 | 0.805±1.080 |
|
|
|
Present | 19 | 0.381±0.298 |
|
|
| TNM stage |
|
| 2.022 | 0.048a |
| I,
II | 43 | 0.766±1.051 |
|
|
| III,
IV | 16 | 0.406±0.311 |
|
|
Discussion
In addition to the multiple genetic and epigenetic
changes of protein-coding genes in GC, accumulating evidence
indicates that dysregulation of miRNAs serves an important role in
the development of GC, including cell proliferation, apoptosis,
migration and invasion (2,3). As a member of the miRNA family,
several studies have demonstrated that miR-153 contributes to
suppression or promotion of cancer cell proliferation, invasion and
migration in different cancer types, including breast cancer,
ovarian cancer and malignant melanoma (17,23,24);
however, the molecular mechanism remains unclear.
In the present study, miR-153 expression was
significantly downregulated in GC tissues and cell lines compared
with matched adjacent non-tumor tissues and GES-1 cells. It was
observed that miR-153 could inhibit GC cell proliferation and
migration, and increase apoptosis in vitro, thus miR-153
downregulation may be a key process in the progression of human GC.
Furthermore, miR-153 expression in cell culture media was
investigated and a similar trend was observed compared with that of
the cell lines, indicating that miR-153 might act as a potent serum
clinical diagnostic marker in GC. Based on this finding, the serum
expression of miR-153 was detected, and it was shown that it was
downregulated in patients with GC and in advanced GC compared with
early GC. Accordingly, lower expression of miR-153 was
significantly associated with advanced clinical stages of GC and
thus poor prognosis. In addition to this, serum miR-153 was
expressed at markedly lower levels in patients with GC with poor
differentiation, larger tumor sizes, lymph node metastasis and
advanced tumor stage. Although the difference of serum miR-153
between TNM stage III/IV and I/II was small (P=0.048), a larger
sample size could confirm this finding. These data indicated that
miR-153 may be a potential biomarker for the prediction of the
prognosis of patients with GC.
Studies have found that epithelial-mesenchymal
transition (EMT) serves an important role in the migration and
invasion of GC, which leads to a poor prognosis (25–28).
Cellular EMT is characterized by loss of cell polarity and
intracellular junctions, as well as acquisition of mesenchymal
properties. As a result, GC cells migrate and invade more
frequently than normal cells (26).
By targeting snail family transcriptional repressor 1 (SNAI1) and
zinc finger E-box binding homeobox 2, miR-153 is a novel regulator
of EMT, suggesting its potential therapeutic value in reducing
cancer metastasis (27).
Additionally, overexpression of miR-153 significantly reduces GC
cell proliferation, migration and invasion (27). According to Zhang et al
(28), miR-153 overexpression
reduced SNAI1 expression and inhibited EMT of GC cells as evidenced
by upregulated E-cadherin and downregulated vimentin levels.
Subsequently, miR-153 upregulation inhibited MKN-45 GC cell
migration and invasion, while knockdown promoted the migration and
invasion of SGC-7901 GC cells (28).
In conclusion, the serum expression of miR-153 was
downregulated in patients with GC, especially in advanced clinical
stages. Furthermore, lower expression of miR-153 was associated
with larger tumor sizes, poor differentiation, lymph node
metastasis and advanced tumor TNM stage, which are associated with
poor prognosis. As a result, the findings of the present study
suggest that miR-153 may serve as a tumor biomarker and prognostic
indicator of patients with GC, as well as a therapeutic target.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Science Foundation
of China (grant no. 82102398), Shandong Provincial Natural Science
Foundation, China (grant nos. ZR2020QC073 and ZR2021MH409),
Shandong Provincial Key Research and Development Program, China
(grant no. 2019GSF108190), Shandong Province Medical and Health
Science and Technology Development Plan, China (grant nos.
2018WS106 and 202103030841), and Weihai Science and Technology
Development Plan, Shandong (grant no. 2017GNS11).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the National Genomic Data Center,
China National Center for Bioinformation/Beijing Institute of
Genomics, Chinese Academy of Sciences repository (https://ngdc.cncb.ac.cn/gsa-human/; accession no.
HRA003675).
Authors' contributions
XG and YC were involved in the conception and design
of the study. TL, DG, XX and PL retrieved literature and analyzed
data. TL and DG wrote the manuscript, designed the figures and
revised the manuscript critically. XX, PW, YZ, LL, YQ and FL were
involved in acquisition of data, and analysis and interpretation of
data. DG and PL helped with the discussion and corrected the text.
TL and DG confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weihai Municipal Hospital (Weihai, China) and written
informed consent was obtained from all patients and HCs.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao X, Li X and Yuan H: microRNAs in
gastric cancer invasion and metastasis. Front Biosci (Landmark Ed).
18:803–810. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu G, Jiang C, Li D, Wang R and Wang W:
MiRNA-34a inhibits EGFR-signaling-dependent MMP7 activation in
gastric cancer. Tumour Biol. 35:9801–9806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuoka T and Yashiro M: Biomarkers of
gastric cancer: Current topics and future perspective. World J
Gastroenterol. 24:2818–2832. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang G, Yan J, Gu Y, Qiao M, Fan R, Mao Y
and Tang X: Construction of short tandem target mimic (STTM) to
block the functions of plant and animal microRNAs. Methods.
58:118–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muhammad S, Kaur K, Huang R, Zhang Q, Kaur
P, Yazdani HO, Bilol MU, Zheng J, Zheng L and Wang XS: MicroRNAs in
colorectal cancer: Role in metastasis and clinical perspectives.
World J Gastroenterol. 20:17011–17019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan B, Manley J, Lee J and Singh SR: The
emerging roles of microRNAs in cancer metabolism. Cancer Lett.
356((2 Pt A)): 301–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Romero-Cordoba SL, Salido-Guadarrama I,
Rodriguez-Dorantes M and Hidalgo-Miranda A: miRNA biogenesis:
Biological impact in the development of cancer. Cancer Biol Ther.
15:1444–1455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang WT and Chen YQ: Circulating miRNAs in
cancer: From detection to therapy. J Hematol Oncol. 7:862014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faam B, Ghaffari MA, Ghadiri A and Azizi
F: Epigenetic modifications in human thyroid cancer. Biomed Rep.
3:3–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Pickard K, Jenei V, Bullock MD,
Bruce A, Mitter R, Kelly G, Paraskeva C, Strefford J, Primrose J,
et al: miR-153 supports colorectal cancer progression via
pleiotropic effects that enhance invasion and chemotherapeutic
resistance. Cancer Res. 73:6435–6447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hua HW, Jiang F, Huang Q, Liao Z and Ding
G: MicroRNA-153 promotes Wnt/β-catenin activation in hepatocellular
carcinoma through suppression of WWOX. Oncotarget. 6:3840–3847.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bi CW, Zhang GY, Bai Y, Zhao B and Yang H:
Increased expression of miR-153 predicts poor prognosis for
patients with prostate cancer. Medicine (Baltimore). 98:e167052019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao W, Yin CY, Jiang J, Kong W, Xu H and
Zhang H: MicroRNA-153 suppresses cell invasion by targeting SNAI1
and predicts patient prognosis in glioma. Oncol Lett. 17:1189–1195.
2019.PubMed/NCBI
|
|
16
|
Guo G, Zhang Y, Hu L and Bian X:
MicroRNA-153 affects nasopharyngeal cancer cell viability by
targeting TGF-β2. Oncol Lett. 17:646–651.
2019.PubMed/NCBI
|
|
17
|
Wang J, Liang S and Duan X: Molecular
mechanism of miR-153 inhibiting migration, invasion and
epithelial-mesenchymal transition of breast cancer by regulating
transforming growth factor beta (TGF-β) signaling pathway. J Cell
Biochem. 120:9539–9546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang B, Fu T and Zhang L: MicroRNA-153
suppresses human laryngeal squamous cell carcinoma migration and
invasion by targeting the SNAI1 gene. Oncol Lett. 16:5075–5083.
2018.PubMed/NCBI
|
|
19
|
Wu X, Li L, Li Y and Liu Z: MiR-153
promotes breast cancer cell apoptosis by targeting HECTD3. Am J
Cancer Res. 6:1563–1571. 2016.PubMed/NCBI
|
|
20
|
Najtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sano T, Coit DG, Kim HH, Roviello F,
Kassab P, Wittekind C, Yamamoto Y and Ohashi Y: Proposal of a new
stage grouping of gastric cancer for TNM classification:
International Gastric Cancer Association staging project. Gastric
Cancer. 20:217–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou J, Xie M, Shi Y, Luo B, Gong G, Li J,
Wang J, Zhao W, Zi Y, Wu X and Wen J: MicroRNA-153 functions as a
tumor suppressor by targeting SET7 and ZEB2 in ovarian cancer
cells. Oncol Rep. 34:111–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luan W, Shi Y, Zhou Z, Xia Y and Wang J:
circRNA_0084043 promote malignant melanoma progression via
miR-153-3p/Snail axis. Biochem Biophys Res Commun. 502:22–29. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murai T, Yamada S, Fuchs BC, Fujii T,
Nakayama G, Sugimoto H, Koike M, Fujiwara M, Tanabe KK and Kodera
Y: Epithelial-to-mesenchymal transition predicts prognosis in
clinical gastric cancer. J Surg Oncol. 109:684–689. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang ZY and Ge HY: Micrometastasis in
gastric cancer. Cancer Lett. 336:34–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Q, Sun Q, Zhang J, Yu J, Chen W and
Zhang Z: Downregulation of miR-153 contributes to
epithelial-mesenchymal transition and tumor metastasis in human
epithelial cancer. Carcinogenesis. 34:539–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Z, Sun J, Bai Z, Li H, He S, Chen R
and Che X: MicroRNA-153 acts as a prognostic marker in gastric
cancer and its role in cell migration and invasion. Onco Targets
Ther. 8:357–364. 2015.PubMed/NCBI
|