Introduction
Lung cancer represents a fatal malignancy accounting
for the largest number of cancer-related deaths worldwide,
accompanied by an estimated 2 million new cases and 1.8 million
deaths annually (1,2). Lung adenocarcinoma (LUAD), a highly
heterogeneous malignancy, is the most prevalent histological
subtype of lung cancer occupying ~50% of reported cases (3,4). In
recent years, despite the progress of multimodal treatment
strategies, including surgical resection, chemoradiotherapy,
molecular targeted therapy and emerging immunotherapies, the
overall survival time of patients with lung cancer has not
substantially improved (5). This
has been mainly attributed to the deficiency of effective
biomarkers for diagnosis and treatment. Hence, it is of urgent
significance to identify more molecular mechanisms associated with
LUAD development, so as to improve clinical diagnosis and
treatment.
Motor neuron and pancreas homeobox 1 (MNX1), also
termed HLXB9 or HB9, is located on chromosome 7q36.3 and is a
homeodomain-containing transcriptional factor (6). MNX1 was initially discovered by
Harrison et al (7) in
pancreatic and lymphoid tissues, followed by the confirmation of
the involvement of MNX1 in pancreatic and invertebrate development
and motor neuronal differentiation (8–10).
Subsequently, dysregulation of MNX1 was ascertained to be
associated with Currarino syndrome, permanent neonatal diabetes in
a consanguineous family and necrotizing enterocolitis of neonates
(11–13). Emerging evidence has identified MNX1
as a novel oncogene that is upregulated in prostate, colorectal,
cervical and breast cancer (14–17).
However, the role of MNX1 in LUAD remains to be elucidated.
The coiled-coil domain (CCD), a structural motif
identified and widely expressed in proteins, is associated with
multiple biological functions, including gene expression
regulation, membrane fusion, cell division and drug metabolism
(18). Several reports have
confirmed the connection between CCD-containing proteins (CCDC) and
cancer. For example, CCDC43, CCDC137 and CCDC106 were distinctly
upregulated in gastric cancer, hepatocellular carcinoma and lung
cancer, respectively and have an association with the malignant
phenotypes (19–21). CCDC34, also termed NY-REN-41, RAMA3
or L15, is located on chromosomes 11p14.1 and chromosome 11 is
regarded as one of the most disease- and gene-rich chromosomes in
the human genome. CCDC34 was first discovered in hamartoma of the
retinal pigment epithelium (22).
Accumulating studies have illustrated the dysregulation of CCDC34
in hepatocellular carcinoma, colorectal cancer and bladder cancer,
where it was associated with tumor cell growth, invasion and
apoptosis (23–25). However, to the best of the authors'
knowledge, whether CCDC34 is also involved in LUAD has yet to be
reported.
Notably, it was predicted on the JASPAR website
(https://jaspar.genereg.net/) that MNX1
may bind to the CCDC34 promoter, indicating a possible interaction
between MNX1 and CCDC34. Based on this, the present study explored
the role and function of MNX1 and CCDC34 in LUAD and further
investigated their regulatory mechanism, proposing potential
targets for the treatment of LUAD.
Materials and methods
Bioinformatic analysis
The Gene Expression Profiling Interactive Analysis
(GEPIA, http://gepia.cancer-pku.cn/) cancer
database was mined to assess the expression level of MNX1 and
CCDC34 in LUAD tumor (n=483) and normal tissues (n=347). Meanwhile,
the overall survival curves of patients with LUAD were plotted
using the Kaplan-Meier method with log-rank test.
Cell culture
Human bronchial epithelial cells, BEAS-2B (cat. no.
BNCC359274) and the human LUAD cells, PC-9 (cat. no. BNCC340767),
were purchased from BeNa Culture Collection. Human LUAD cell lines,
A549 (cat. no. CL-0016) and H1975 (cat. no. CL-0298), were provided
by Procell Life Science & Technology Co. Ltd. and the H3255
cell line (cat. no. Bio-106227) was provided by Biobw Biotechnology
Co., Ltd. All cell lines were maintained in Dulbecco's Modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C in a humidified atmosphere incubator with 5%
CO2.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from A549 cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions and quantified. A
total of 1 µg of RNA was reverse transcribed into cDNA using M-MLV
Reverse Transcriptase (Promega Corporation) according to the
manufacturer's protocol. The qPCR procedure was conducted utilizing
SYBR-Green (Takara Bio, Inc.) on a ABI7500 quantitative PCR
instrument (Thermo Fisher Scientific, Inc.) in line with the
manufacturer's guidelines. The thermocycling conditions were as
follows: 94°C for 5 min, followed by 40 cycles at 94°C for 15 sec,
60°C for 25 sec and 72°C for 30 sec. The sequences of primers used
were as followed: MNX1, forward, 5′-GCCTAAGATGCCCGACTTCAAC-3′,
reverse, 5′-CGCGACAGGTACTTGTTGAGCT-3′; CCDC34, forward,
5′-ACAGAAACAGGTGCGCTTACC-3′, reverse, 5′-CAGCCGGTCACGTTCTTCTTT-3′;
β-actin, forward, 5′-AGCGAGCATCCCCCAAAGTT-3′, reverse,
5′-GGGCACGAAGGCTCATCATT-3′. Gene expression was calculated by
2−ΔΔCq (26) and
normalized to the housekeeping gene β-actin. All experiments
were repeated three times.
Western blotting
Total protein was extracted using
radioimmunoprecipitation assay (Beyotime Institute of
Biotechnology) and protein concentration was quantified using the
bicinchoninic acid method (Beyotime Institute of Biotechnology).
Subsequently, the denatured proteins (30 µg/lane) were resolved on
12% SDS-PAGE gels and transferred onto PVDF membranes
(MilliporeSigma). After being blocked with 5% skimmed milk at room
temperature for 2 h, the membranes were probed overnight with
primary antibodies against MNX1 (dilution 1:1,000; cat. no. 41983;
Cell Signaling Technology, Inc.), Ki67 (dilution 1:1,000; cat. no.
ab16667; Abcam), proliferating cell nuclear antigen (PCNA; dilution
1:3,000; cat. no. ab29; Abcam), LaminB1 (dilution 1:10,000; cat.
no. ab16048; Abcam), MMP2 (dilution 1:1,000; cat. no. 10373-2-AP;
Proteintech Group, Inc.), MMP9 (dilution 1:1,000; cat. no.
10375-1-AP; Proteintech Group, Inc.), Cyclin B1 (dilution 1:50,000;
cat. no. ab32053; Abcam), p-Histone H2A.X (dilution 1:1,000; cat.
no. 9718; Cell Signaling Technology, Inc.), Bcl-2 (dilution
1:1,000; cat. no. ab196495; Abcam), Bax (dilution 1:1,000; cat. no.
ab32503; Abcam), cleaved-caspase3 (dilution 1:1,000; cat. no. 9661;
Cell Signaling Technology, Inc.), cleaved-caspase9 (dilution
1:1,000; cat. no. 7237; Cell Signaling Technology, Inc.), CCDC34
(dilution 1:1,000; cat. no. ab122396; Abcam) and GAPDH (dilution
1:2,500; cat. no. ab9485; Abcam) at 4°C overnight, followed by
incubation with goat anti-rabbit (dilution 1:3,000; cat. no.
ab6721; Abcam) or goat anti-mouse (dilution 1:2,000; cat. no.
Ab6789; Abcam) secondary antibodies conjugated to horseradish
peroxidase for 2 h at room temperature. The target bands were
examined using a Bio-Rad gel imaging system (Bio-Rad Laboratories)
with an ECL reagent (MilliporeSigma). Finally, the blots were
semi-quantified with ImageJ software (version 1.52; National
Institutes of Health).
Cell transfection
Short hairpin (sh) RNA (pGPU6 plasmid) targeting
MNX1 (sh-MNX1-1, forward,
5′-CCGGGCAGGAAGCGGAGAAACAGAACTCGAGTTCTGTTTCTCCGCTTCCTGCTTTTTG-3′
and reverse,
5′-AATTCAAAAAGCAGGAAGCGGAGAAACAGAACTCGAGTTCTGTTTCTCCGCTTCCTGC-3′;
sh-MNX1-2, forward,
5′-CCGGCGAGGACGACGAGGACCATTTCTCGAGAAATGGTCCTCGTCGTCCTCGTTTTTG-3′
and reverse,
5′-AATTCAAAAACGAGGACGACGAGGACCATTTCTCGAGAAATGGTCCTCGTCGTCCTCG-3′),
pcDNA3.1 vector expressing MNX1 (oe-MNX1) or CCDC34 (oe-CCDC34) and
the negative control (NC) of shRNA (sh-NC,
5′-CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTTG-3′)
and pcDNA3.1 (oe-NC) were obtained from Shanghai GenePharma Co.,
Ltd. A549 cells (1×105) were aliquoted into 6-well
plates. Upon achieving 60–70% confluency, cells were transfected
with sh-MNX1-1/2 (50 nM), sh-NC (50 nM), oe-MNX1 (40 nM), oe-CCDC34
(40 nM) or oe-NC (40 nM) plasmids using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48 h
in accordance with the manufacturer's protocol. Thereafter, the
cells were harvested for detecting transfection efficacy using
RT-qPCR and western blotting.
Cell proliferation assays
Cell proliferation ability was assessed using Cell
Counting Kit-8 (CCK-8) and colony formation assays. For the CCK-8
assay, A549 cells were aliquoted into 96-well plates
(1×104 cells/well). After incubation at 37°C and 5%
CO2 for 24, 48 and 72 h, 10 µl of CCK-8 solution
(Nanjing KeyGen Biotech Co., Ltd.) was added to each well for a
further 2 h incubation. The absorbance at 450 nm was measured using
a spectrophotometer (Synergy H1; Omega Bio-Tek, Inc.). For the
colony formation assay, A549 cells were aliquoted in 6-well plates
(1×103 cells/well). Following incubation for 2 weeks, 4%
paraformaldehyde was utilized to fix the colonies for 15 min at
room temperature, followed by staining with 1% crystal violet for
20 min at room temperature. The colonies (>50 cells) were
observed and counted using ImageJ software (version 1.52; National
Institutes of Health).
Cell migration assay
A549 cells (5×104) were aliquoted into
96-well plates for culturing until cell confluency achieved 100%. A
straight wound was generated using a 200 µl pipette tip. The plates
were washed with PBS to wipe out the deciduous cells and serum-free
medium was applied for the further incubation for 48 h at 37°C.
Images of the wounds were captured at 0 and 48 h under a light
microscope (Nikon Corporation).
Cell invasion assay
A 24-well plate containing 8-µm pore inserts
precoated with Matrigel (Corning, Inc.) for 30 min at 37°C was used
for the cell invasion assay. A549 cells (5×104) were
aliquoted into the upper chamber and allowed to invade towards the
lower chamber, which contained the complete medium supplemented
with 10% FBS. Following incubation at 37°C for 48 h, the invaded
cells were fixed with 4% paraformaldehyde for 15 min at room
temperature, stained with 1% crystal violet for 20 min at room
temperature and were finally observed under a light microscope
(Nikon Corporation).
Flow cytometry assay
Cell cycle and apoptosis were evaluated via flow
cytometry analysis. For the cell cycle assay, A549 cells were
washed with PBS and fixed in 70% ethanol at 4°C for 1 h.
Subsequently, cells were stained with 2 mg/ml propidium iodide
(40X; MilliporeSigma) supplemented with 10 mg/ml RNase (100X;
Thermo Fisher Scientific, Inc.) at room temperature for 30 min away
from light, followed by detection with a FACS C6 flow cytometer
(Becton, Dickinson and Company) and analysis with FlowJo v7.6
software (FlowJo LLC). For the cell apoptosis assay, cells were
washed with pre-cooled PBS and then resuspended in binding buffer
(1X), followed by staining with propidium iodide and Annexin V,
according to the instructions of an Annexin V-FITC apoptosis kit
(Dalian Meilun Biology Technology Co., Ltd.). Finally, the cell
apoptotic rate (the percentage of early + late apoptotic cells) was
examined using the aforementioned flow cytometer.
Luciferase reporter assay
The wild-type (WT) and mutant (MUT) sequences of the
CCDC34 promoter were inserted into pGL3 luciferase reporter vectors
(Promega Corporation) to construct CCDC34-WT and CCDC34-MUT
vectors. A549 cells (1×105) were aliquoted into 6-well
plates and incubated at 37°C overnight. Cells were transfected with
oe-NC or oe-MNX1 and co-transfected with CCDC34-WT or CCDC34-MUT
vectors using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
After 48 h of transfection, the luciferase activity was detected
using a dual-luciferase reporter system (Promega Corporation) and
normalized to Renilla luciferase activity.
Chromatin immunoprecipitation (ChIP)
assay
A549 cells (2×106 cells/assay) were
cross-linked with 1% formaldehyde for 10 min at 37°C and quenched
with glycine, followed by washing with ice-cold PBS, and cell lysis
with SDS lysis buffer (Upstate Biotechnology, Inc.). The lysates
were sonicated (20 kHz) into chromatin fragments with a 10-sec on
and 10-sec off mode for 12 cycles on ice. Subsequently, 2% of the
supernatant served as the input, and the remaining 100 µl of the
supernatants were cultured overnight with 60 µl A/G agarose beads
(Cell Signaling Technology, Inc.) at 4°C, followed by incubation
with anti-MNX1 antibody (dilution 1:50; cat. no. 41983; Cell
Signaling Technology, Inc.) or the negative control IgG (dilution
1:50; cat. no. 2729; Cell Signaling Technology, Inc.) at 4°C
overnight. The precipitated DNA was purified and then detected by
RT-qPCR as aforementioned.
Animal experiments
A total of 12 BALB/c nude male mice (4–5 weeks old;
weight, 18–22 g) were provided by Shanghai SLAC Laboratory Animal
Co., Ltd. and housed under specific pathogen-free conditions at
23–25°C, controlled humidity of 50–70%, under a 12-h light/dark
cycle, and with free access to food and water. After acclimation
for 1 week, all mice were randomly assigned into two groups (n=6
per group): i) sh-NC group; A549 cells (2×106 cells)
infected with lentivirus containing sh-NC were subcutaneously
injected into the left flank of mice; and ii) sh-MNX1 group; A549
cells (2×106 cells) infected with lentivirus containing
sh-MNX1 were subcutaneously injected into the left flank of mice.
Animal body weight and tumor size were monitored every 3 days and
the tumor volumes were calculated using the formula: (length ×
width2)/2. After continuous feeding for 21 days (the
longest length of the tumor did not exceed 1.5 cm at this time
point), all mice were sacrificed by administering an
intraperitoneal injection of pentobarbital sodium (50 mg/kg body
weight) and rapid cervical dislocation. Tumors were extracted for
weighing and were stored at −80°C for western blot analysis. All
animal experiments were implemented in accordance with the
guidelines and protocols for animal care and were approved by the
Ethics Committee of Mianyang Central Hospital [approval no.
S2021097 (01)].
Statistical analysis
The results analyzed by GraphPad Prism version 8.0
(Dotmatics) were presented as mean ± SD. Comparison analysis was
implemented using Student's unpaired t-test between two groups and
using one-way ANOVA analysis with Tukey's post hoc test among more
than two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
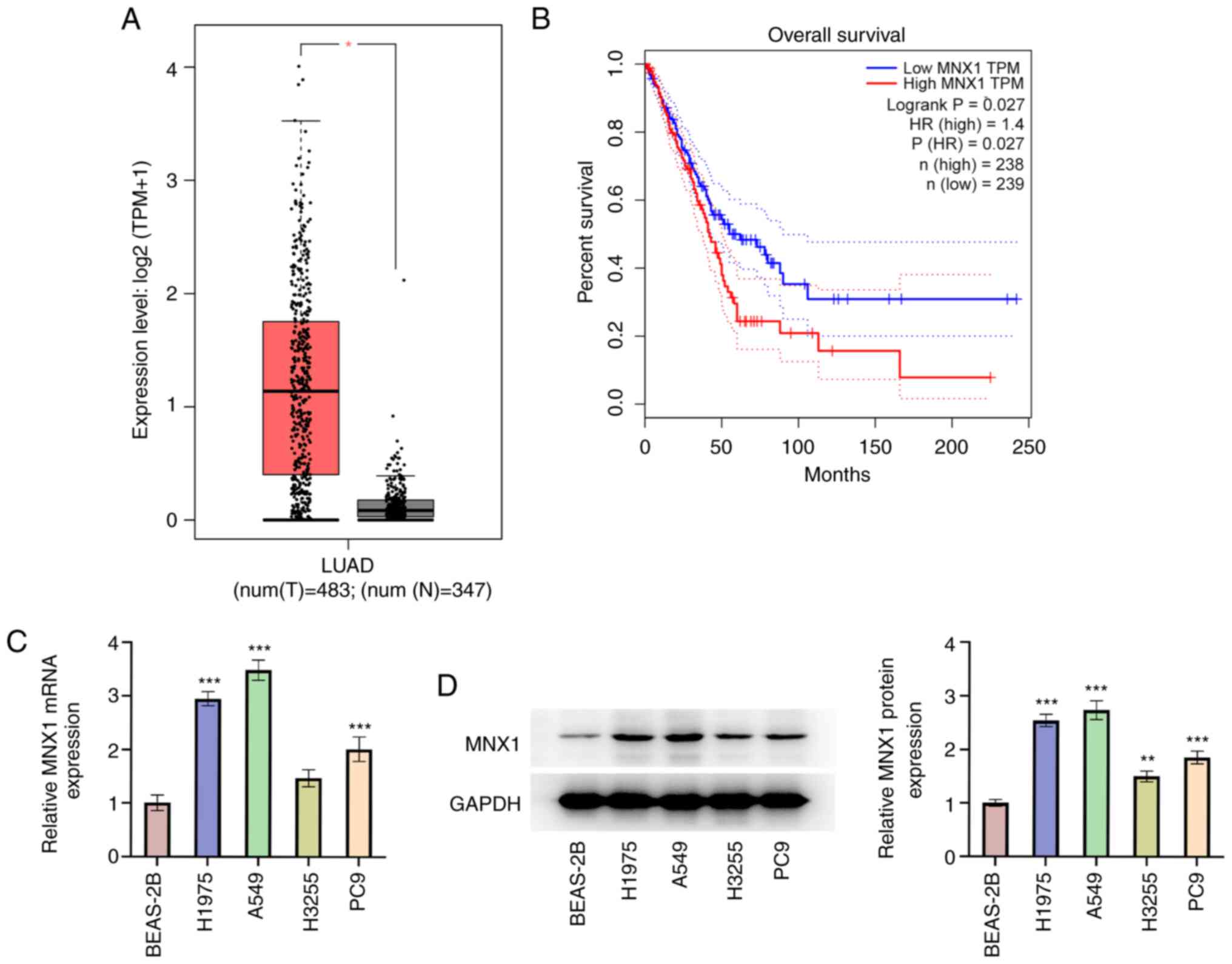
MNX1 is upregulated in LUAD
First, it was evident from the analysis of datasets
from the GEPIA database that the MNX1 expression level in LUAD
tumor tissues was significantly higher than that in normal tissues
(Fig. 1A). Meanwhile, patients with
LUAD with a high level of MNX1 were associated with a poor overall
survival (Fig. 1B). Next, the
expression level of MNX1 in human bronchial epithelial cells
(BEAS-2B) and human LUAD cell lines (PC-9, A549, H1975 and H3255)
was examined. It is shown in Fig. 1C
and D that LUAD cell lines, especially the A549 cell line,
possessed a relatively high level of MNX1 compared with the BEAS-2B
cell line, further confirming the aberrant upregulation of MNX1 in
LUAD.
MNX1 knockdown represses
proliferation, migration and invasion of A549 cells
To clarify the specific role of MNX1 in LUAD, cell
transfection with MNX1 shRNA was implemented in A549 cells.
Following transfection with sh-MNX1-1 or sh-MNX1-2, both the mRNA
and protein expression levels of MNX1 were notably downregulated
(Fig. 2A and B). Due to a higher
transfection efficacy, sh-MNX1-1 was selected for subsequent
experiments. Compared with the sh-NC group, sh-MNX1 not only
repressed the proliferation ability of A549 cells, evidenced by the
decreased cell viability and number of colonies, but also
diminished the migration and invasion abilities of A549 cells
(Fig. 2C-G). In addition, a marked
decrease in the level of proliferation-related proteins (Ki67 and
PCNA) and invasion-related proteins (MMP2 and MMP9) in the sh-MNX1
group (Fig. 2H) was observed,
further confirming the antiproliferative and anti-invasive
properties of the MNX1 knockdown.
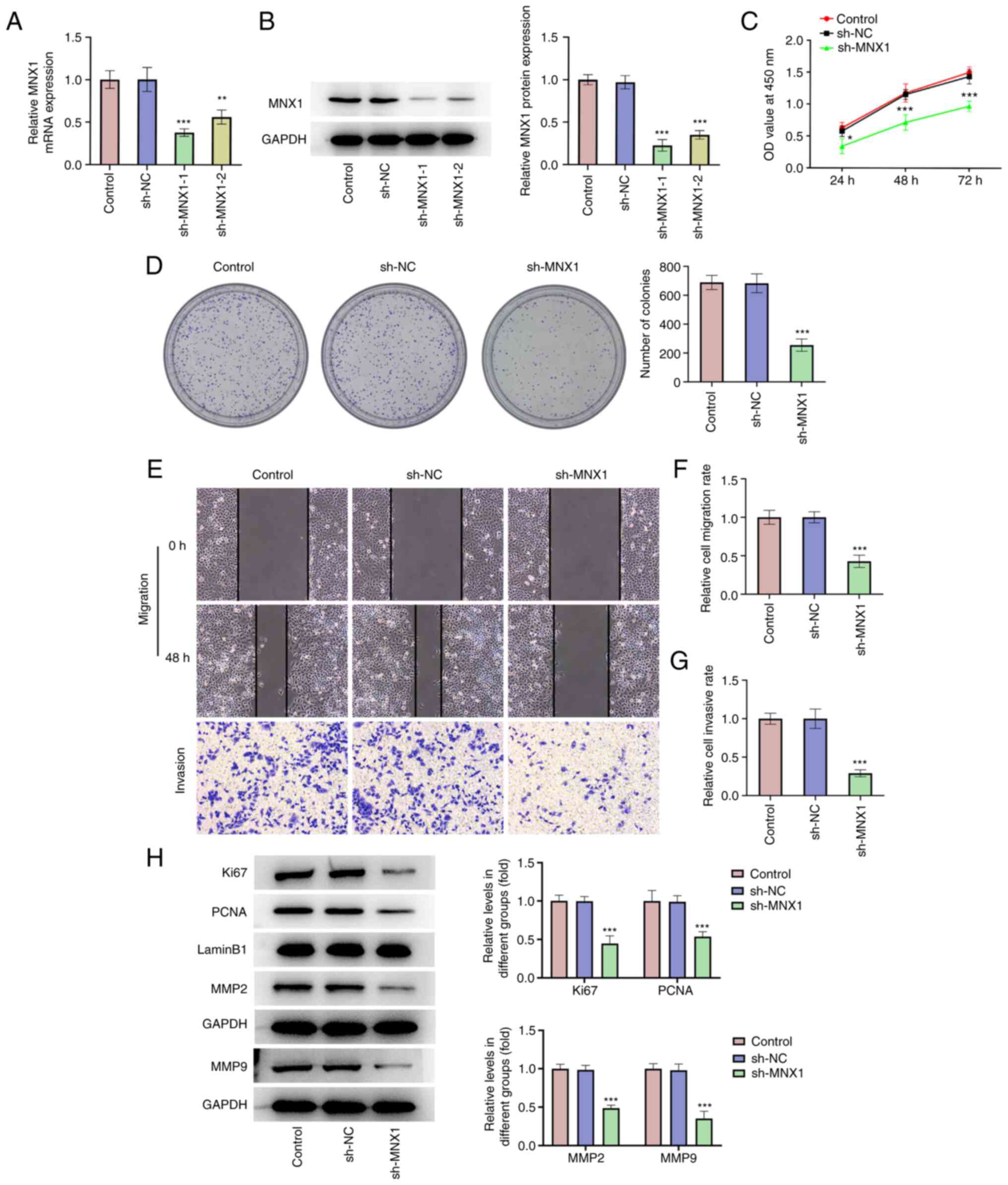 | Figure 2.MNX1 knockdown represses
proliferation, migration and invasion of A549 cells. Following
transfection with sh-NC or sh-MNX1-1/sh-MNX1-2 in A549 cells, (A)
the mRNA level and (B) protein expression of MNX1 were examined
using reverse transcription-quantitative PCR and western blotting,
respectively. (C) CCK-8 assay was used to examine cell viability.
(D) Cell colony formation assay was conducted to assay formed
colonies. (E-G) Wound-healing and Transwell assays were performed
to examine cell migration and invasion, respectively.
Magnification, ×100. (H) Protein expression of Ki67, PCNA, MMP2 and
MMP9 was examined using western blotting. *P<0.05, **P<0.01,
***P<0.001 vs. sh-NC. MNX1, Motor neuron and pancreas homeobox
1; sh, short hairpin RNA; NC, negative control (empty vector);
CCK-8, Cell Counting Kit-8; PCNA, proliferating cell nuclear
antigen. |
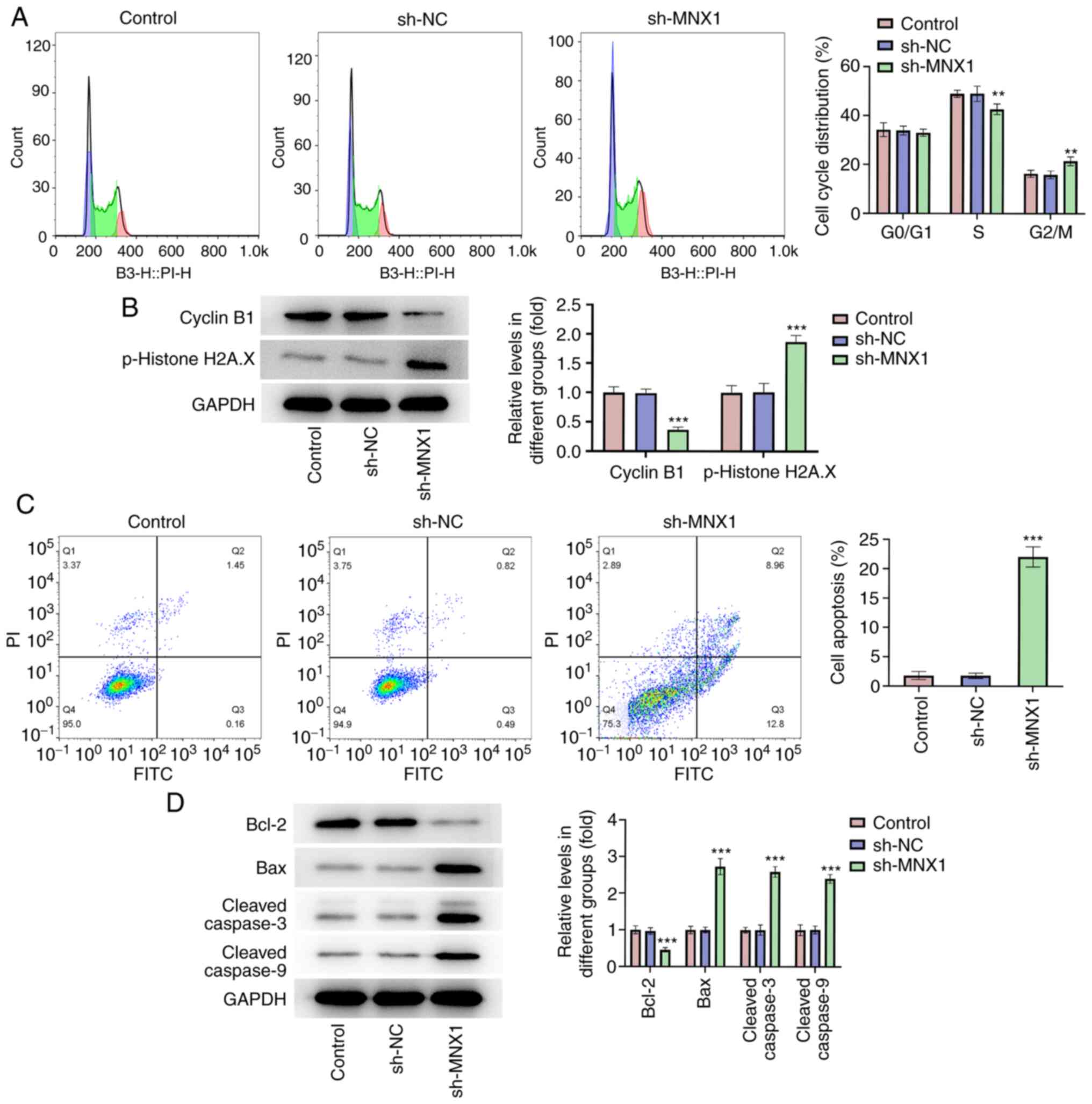
MNX1 knockdown hinders cell cycle
progression and promotes apoptosis of A549 cells
In addition, according to flow cytometry analysis,
it was observed that the proportion of cells in S phase was
decreased in the sh-MNX1 group compared with the sh-NC group, while
cell cycle arrest in G2/M was increased, suggesting that
MNX1 knockdown may hinder cell cycle progression (Fig. 3A). Cyclin B1 accumulates steadily
during G2 phase to promote G2/M transition.
Phosphorylated (p)-histone H2A.X is a DNA double strand break
marker related to checkpoint signaling (27). In the present study, it was observed
that MNX1 knockdown lowered the protein levels of cyclin B1 and
p-histone H2A.X (Fig. 3B). This
further demonstrated that MNX1 knockdown resulted in a delay in
transition at G2/M phase, thereby stalling cell cycle
progression. Furthermore, a prominent elevation of cell apoptosis
rate was observed in the sh-MNX1 group, accompanied with
downregulation of Bcl-2 and upregulation of Bax, cleaved-caspase3
and cleaved-caspase9 (Fig. 3C and
D), suggesting that MNX1 knockdown also facilitated cell
apoptosis of A549 cells.
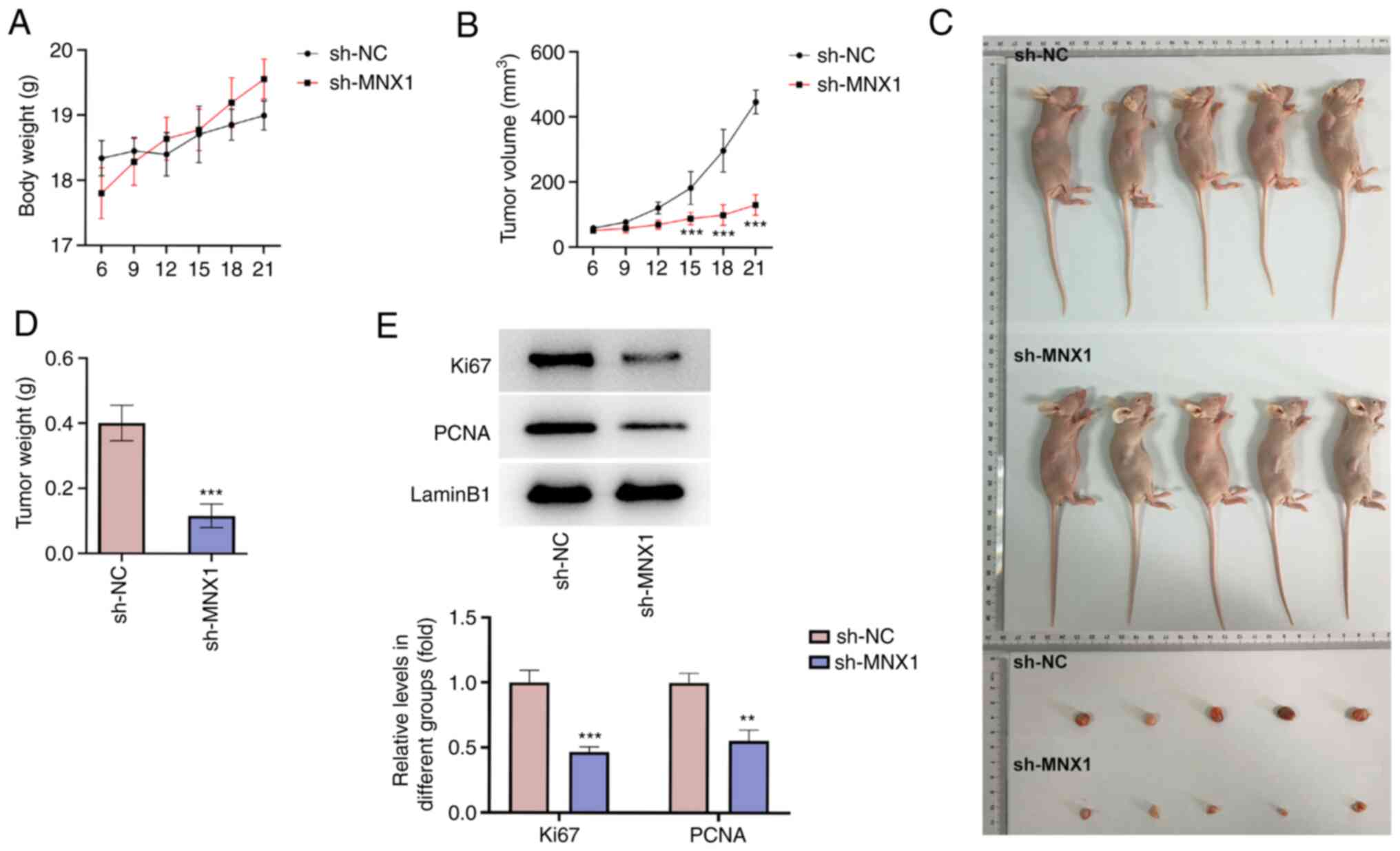
MNX1 knockdown inhibits tumor growth
in vivo
To verify the antitumor activity of MNX1 in LUAD, an
in vivo animal model was established. The daily monitoring
of the animals in the study demonstrated that the body weight of
the mice in the sh-NC and sh-MNX1 groups increased steadily,
without a significant difference between the two groups (Fig. 4A). However, the tumor volume in the
sh-MNX1 group was markedly smaller than that in the sh-NC group
(Fig. 4B). After sacrificing the
mice, the tumors were extracted and examined and a smaller tumor
size and a lower tumor weight were observed in the sh-MNX1 group
(Fig. 4C and D). Meanwhile, the
protein expression level of Ki67 and PCNA in tumor tissues was
markedly decreased in the sh-MNX1 group (Fig. 4E).
MNX1 binds to the CCDC34 promoter and
transcriptionally regulates CCDC34 expression
Next, the expression profile of CCDC34 in LUAD was
examined. As assessed by the GEPIA database, CCDC34 was highly
expressed in LUAD tumor tissues (Fig.
5A), which was consistent with the following results where the
expression level of CCDC34 in A549 cells was higher than that in
BEAS-2B cells (Fig. 5B and C).
Subsequently, to understand the connection between MNX1 and CCDC34,
a successful transfection of a MNX1 overexpression vector was first
verified (Fig. 5D and E). The
expression level of CCDC34 was subsequently demonstrated to be
upregulated following MNX1 overexpression, whereas it was
downregulated following MNX1 knockdown (Fig. 5F and G). These results demonstrated
that MNX1 may regulate CCDC34 expression in A549 cells. Moreover,
based on the prediction from the JASPAR website, MNX1 may bind to
the CCDC34 promoter (Fig. 5H). This
prediction was verified by luciferase reporter assay and ChIP assay
where MNX1 overexpression significantly increased the luciferase
activity of in CCDC34-WT group and CCDC34 was enriched in anti-MNX1
group compared to the anti-IgG group (Fig. 5I and J).
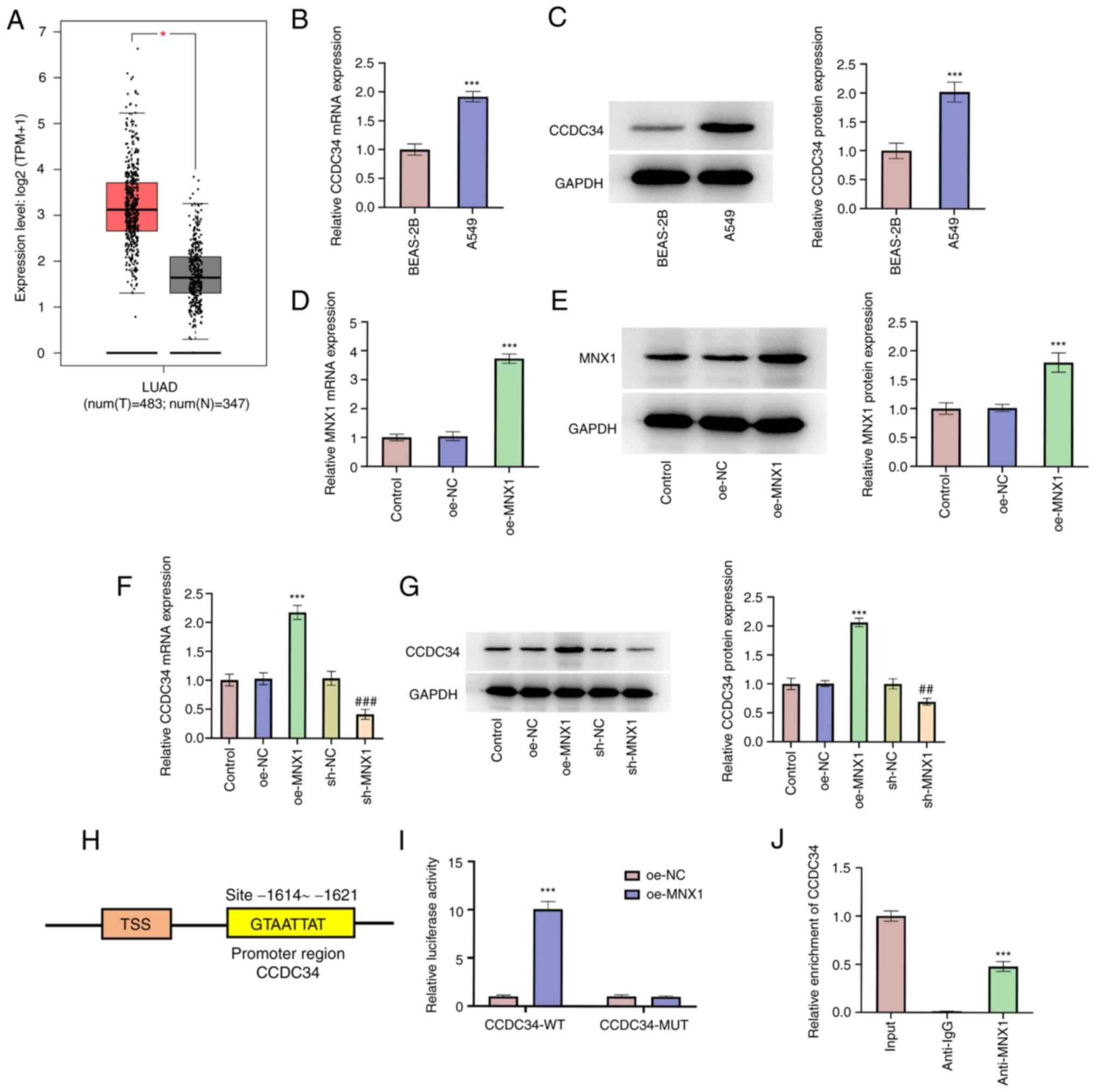 | Figure 5.MNX1 binds to CCDC34 promoter and
transcriptionally regulates CCDC34 expression. (A) GEPIA database
exhibited the CCDC34 expression level in tumor tissues of LUAD.
*P<0.05. Human bronchial epithelial cells BEAS-2B and human LUAD
cell lines A549 were applied to examine the (B) mRNA level and (C)
protein expression of CCDC34 using RT-qPCR and western blotting,
respectively. ***P<0.001 vs. BEAS-2B. A549 cells were
transfected with oe-NC or oe-MNX1 and the (D) mRNA level and (E)
protein expression of MNX1 using reverse RT-qPCR and western
blotting, respectively. A549 cells were transfected with oe-MNX1 or
sh-MNX1 or their negative controls and the (F) mRNA level and (G)
protein expression of CCDC34 using RT-qPCR and western blotting,
respectively. ***P<0.001 vs. oe-NC; ##P<0.01,
###P<0.001 vs. sh-NC. (H) As predicted from JASPAR
website, MNX1 might bind to CCDC34 promoter. (I) Luciferase
reporter assay was performed to verify the interaction between MNX1
and CCDC34 promoter and the luciferase activity was examined using
dual-luciferase reporter system. ***P<0.001 vs. oe-NC. (J) ChIP
assay was used to verify the interaction between MNX1 and CCDC34
promoter. ***P<0.001 vs. anti-IgG. MNX1, Motor neuron and
pancreas homeobox 1; CCDC34, coiled-coil domain-containing 34;
LUAD, Lung adenocarcinoma; GEPIA, Gene Expression Profiling
Interactive Analysis; sh, short hairpin RNA; oe, overexpression;
NC, negative control (empty vector); RT-qPCR, Reverse
transcription-quantitative PCR; ChIP, chromatin
immunoprecipitation. |
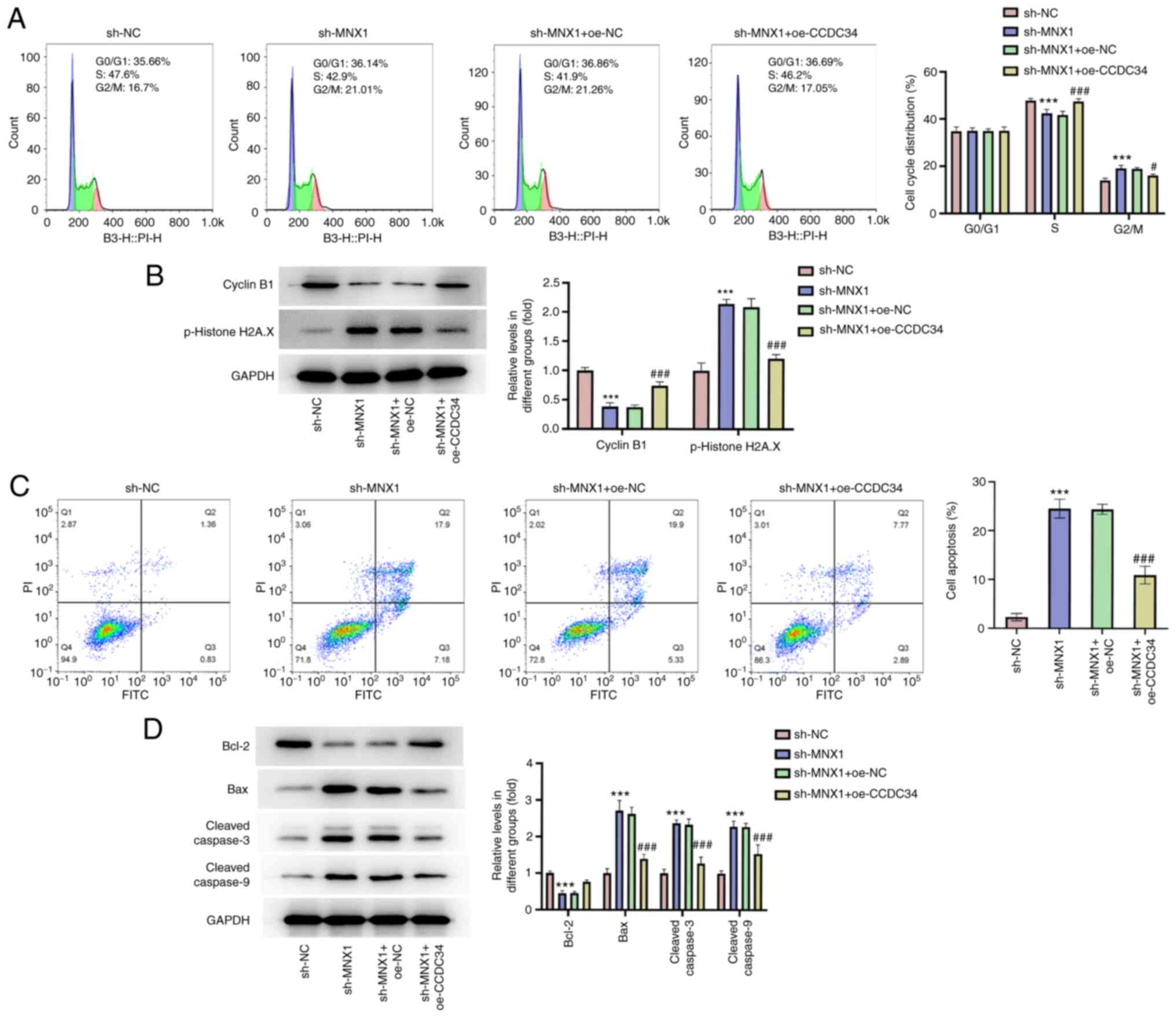
CCDC34 overexpression abolishes the
inhibitory effect of MNX1 knockdown on the malignant phenotypes of
A549 cells
To further establish the regulatory role of
MNX1/CCDC34 in LUAD, the successful transfection of a CCDC34
overexpression vector was first verified (Fig. 6A and B). Then, A549 cells were
transfected with sh-MNX1 alone or co-transfected with
oe-NC/oe-CCDC34. A series of in vitro assays including
CCK-8, colony formation, wound-healing and Transwell assays
revealed that the inhibitory effects of MNX1 knockdown on cell
proliferation, migration and invasion abilities in A549 cells were
partly abolished by CCDC34 overexpression (Fig. 6C-G), which was also verified by the
restored protein expression of Ki67, PCNA, MMP2 and MMP9 in the
sh-MNX1 + oe-CCDC34 group compared with the sh-MNX1 + oe-NC group
(Fig. 6H). Additionally, CCDC34
overexpression may facilitate cell cycle progression, which was
hindered by MNX1 knockdown, through diminishing cell cycle arrest
in G2/M phase, accompanied by the restored protein
levels of cyclin B1 and p-histone H2A.X (Fig. 7A and B). Meanwhile, the elevated
apoptosis rate induced by MNX1 knockdown was also diminished by
CCDC34 overexpression, as well as the upregulation of Bcl-2 and
downregulation of Bax, cleaved-caspase3 and cleaved-caspase9
(Fig. 7C and D).
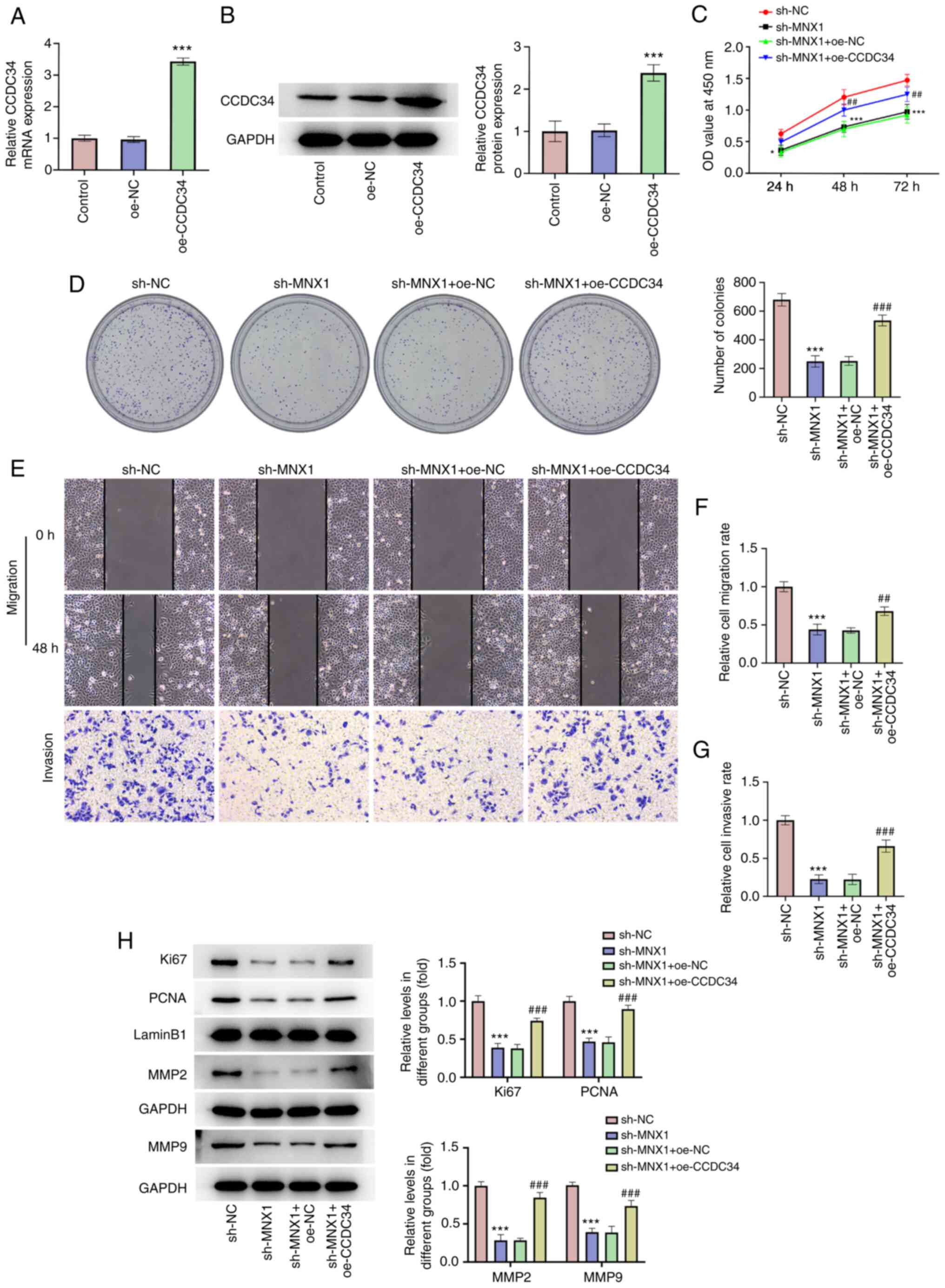 | Figure 6.CCDC34 overexpression abolishes the
inhibitory effect of MNX1 knockdown on proliferation, migration and
invasion in A549 cells. Following transfection with oe-NC or
oe-CCDC34 in A549 cells, (A) the mRNA level and (B) protein
expression of CCDC34 were examined using reverse
transcription-quantitative PCR and western blotting, respectively.
***P<0.001 vs. oe-NC. (C) CCK-8 assay was used to examine cell
viability. (D) Cell colony formation assay was conducted to assay
formed colonies. (E-G) Wound-healing and Transwell assays were
performed to examine cell migration and invasion, respectively.
Magnification, ×100. (H) Protein expression of Ki67, PCNA, MMP2 and
MMP9 was examined using western blot. *P<0.05, ***P<0.001 vs.
sh-NC. ##P<0.01, ###P<0.001 vs.
sh-MNX1+oe-NC. CCDC34, coiled-coil domain-containing 34; MNX1,
Motor neuron and pancreas homeobox 1; sh, short hairpin RNA; oe,
overexpression; NC, negative control (empty vector); PCNA,
proliferating cell nuclear antigen. |
Discussion
LUAD, representing the most prevalent subtype of
lung cancer, typically has high incidence and fatality rates
(28). Despite improvements in LUAD
therapeutic strategies, the outcomes remain suboptimal and the
5-year overall survival rate is <20% (29). Identification of reliable diagnostic
or prognostic biomarkers is beneficial to the development of
specific therapeutic targets in the field of cancer gene therapy,
to improve the overall survival of patients (30).
MNX1 exerts an oncogenic role in multiple types of
cancer such as colorectal cancer and cervical cancer and the
epigenetic silencing of which suppresses proliferation, migration
and invasion of cancer cells in vitro and restricted tumor
growth in vivo, while MNX1 overexpression may accelerate
tumorigenicity (15,16,31).
However, studies supporting the oncogenic role of MNX1 in LUAD are
few. In the present study, using bioinformatic analysis and the
clinicopathological data of LUAD, it was revealed for the first
time, to the best of the authors' knowledge, that patients with
LUAD had a relatively high level of MNX1 and a patient with a high
level of MNX1 was associated with a poor survival outcome.
Consistently, the subsequent in vitro and in vivo
assays confirmed that MNX1 knockdown hindered the malignant
phenotypes of LUAD cells, such as proliferation, migration and
invasion and restricted tumor growth in a LUAD animal model. The
data suggested that an abnormally high level of MNX1 in tumor
tissues may be a critical contributor to the poor prognosis of
patients with LUAD and that MNX1 may serve as a potential candidate
for therapeutic targets supporting the treatment of LUAD.
Transcription factors, a class of DNA-interacting
proteins, have been reported to be involved in a wide spectrum of
human diseases, such as cancer, in recent years. Of note,
transcription factors occupy ~20% of all identified oncogenes to
date, becoming targets for cancer treatment (32). Coincidently, MNX1 encodes a homeobox
transcription factor that promotes motor neuron differentiation and
pancreatic development (33). In
addition, MNX1 has been demonstrated to directly bind upstream of
the TrkB gene to activate its expression, thereby suppressing
adhesion and apoptosis of glioma cells and subsequently
facilitating the development of gliomas (34). In the present study, it was
demonstrated that MNX1 may transcriptionally activate CCDC34
expression through binding to its promoter region. Previously
published evidence revealed CCDC34 as a newly identified oncogene
in several types of cancer. For instance, Geng et al
(24) discovered an upregulated
level of CCDC34 in colorectal cancer tissues compared with
paracancerous tissues and CCDC34 silencing resulted in a decreased
tumor invasion ability and an increase in cancer cell apoptosis.
Gong et al (25) also
uncovered the abnormal upregulation of CCDC34 in bladder cancer and
demonstrated that the knockdown of CCDC34 may prevent bladder
cancer cells from proliferating and migrating and may increase cell
apoptosis and induce cell cycle arrest in G2/M phase
through inactivation of the MEK-ERK1/2-MAPK pathway. In the present
study, it was revealed that the antineoplastic effect of MNX1
knockdown on the malignant phenotypes of LUAD cells in vitro
were weakened following CCDC34 overexpression, highlighting the
critical role of the MNX1/CCDC34 axis during the progression of
LUAD.
However, there are some limitations in the present
study. Detection of clinical samples would be beneficial to
validate the findings, which is now planned for future work.
Meanwhile, the molecular mechanisms underlying MNX1/CCDC34 axis
during the progression of LUAD still deserves to be further
investigated.
In conclusion, to the best of the authors'
knowledge, the present study demonstrated for the first time that
MNX1 knockdown may repress the malignant phenotypes of LUAD cells
in vitro and restrict tumor growth in vivo, revealing
MNX1 as a potential oncogene in LUAD. In addition, the results
identified that MNX1 may transcriptionally activate CCDC34
expression by binding directly to its promoter region. The
antitumor activity of MNX1 knockdown was partly hindered by CCDC34
overexpression. Overall, the central theme of the findings of the
present study provides evidence of the association between the
MNX1/CCDC34 axis and LUAD progression and provides novel insights
of potential therapeutic targets for LUAD treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by Sichuan Medical Association
Venous Thromboembolism Prevention and Treatment (Hengrui) Special
Research Project (grant no. 2019HR59).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX designed the experiments; JW, CY, HL, JZ and LL
obtained the data; JW, CY and HL analyzed and interpreted the data;
JW and CY drafted the manuscript and WX revised the manuscript. JW
and WX confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were implemented in
accordance with the guidelines and protocols for animal care and
were approved by the Ethics Committee of Mianyang Central Hospital
[approval no. S2021097 (01)].
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. Apr 5–2021.(Epub ahead of
print). View Article : Google Scholar
|
|
3
|
Sivakumar S, Lucas FAS, McDowell TL, Lang
W, Xu L, Fujimoto J, Zhang J, Futreal PA, Fukuoka J, Yatabe Y, et
al: Genomic landscape of atypical adenomatous hyperplasia reveals
divergent modes to lung adenocarcinoma. Cancer Res. 77:6119–6130.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu F, He L, Zhan X, Chen J, Xu H, Huang X,
Li Y, Zheng X, Lin L and Chen Y: DNA methylation-based lung
adenocarcinoma subtypes can predict prognosis, recurrence, and
immunotherapeutic implications. Aging (Albany NY). 12:25275–25293.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen D, Wang R, Yu C, Cao F, Zhang X, Yan
F, Chen L, Zhu H, Yu Z and Feng J: FOX-A1 contributes to
acquisition of chemoresistance in human lung adenocarcinoma via
transactivation of SOX5. EBioMedicine. 44:150–161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leotta CG, Federico C, Brundo MV, Tosi S
and Saccone S: HLXB9 gene expression, and nuclear location during
in vitro neuronal differentiation in the SK-N-BE neuroblastoma cell
line. PLoS One. 9:e1054812014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harrison KA, Druey KM, Deguchi Y, Tuscano
JM and Kehrl JH: A novel human homeobox gene distantly related to
proboscipedia is expressed in lymphoid and pancreatic tissues. J
Biol Chem. 269:19968–19975. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woolfe A, Goodson M, Goode DK, Snell P,
McEwen GK, Vavouri T, Smith SF, North P, Callaway H, Kelly K, et
al: Highly conserved non-coding sequences are associated with
vertebrate development. PLoS Biol. 3:e72005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Habener JF, Kemp DM and Thomas MK:
Minireview: Transcriptional regulation in pancreatic development.
Endocrinology. 146:1025–1034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vult von Steyern F, Martinov V, Rabben I,
Nja A, de Lapeyriere O and Lomo T: The homeodomain transcription
factors Islet 1 and HB9 are expressed in adult alpha and gamma
motoneurons identified by selective retrograde tracing. Eur J
Neurosci. 11:2093–2102. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonnefond A, Vaillant E, Philippe J,
Skrobek B, Lobbens S, Yengo L, Huyvaert M, Cavé H, Busiah K,
Scharfmann R, et al: Transcription factor gene MNX1 is a novel
cause of permanent neonatal diabetes in a consanguineous family.
Diabetes Metab. 39:276–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Zeng L, Zheng W, Li X and Lin B:
Increased expression of microRNA-141-3p improves necrotizing
enterocolitis of neonates through targeting MNX1. Front Pediatr.
8:3852020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Merello E, De Marco P, Ravegnani M,
Riccipetitoni G, Cama A and Capra V: Novel MNX1 mutations and
clinical analysis of familial and sporadic Currarino cases. Eur J
Med Genet. 56:648–654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Das M: MNX1: A novel prostate cancer
oncogene. Lancet Oncol. 17:e5212016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu B, Wu Y, Luo J, Zhang Q, Huang J, Li
Q, Xu L, Lu E and Ren B: MNX1 promotes malignant progression of
cervical cancer via repressing the transcription of
p21cip1. Front Oncol. 10:13072020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang X, Pan Q, Lu Y, Jiang X, Zhang S and
Wu J: MNX1 promotes cell proliferation and activates Wnt/β-catenin
signaling in colorectal cancer. Cell Biol Int. 43:402–408. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian T, Wang M, Zhu Y, Zhu W, Yang T, Li
H, Lin S, Dai C, Deng Y, Song D, et al: Expression, clinical
significance, and functional prediction of MNX1 in breast cancer.
Mol Ther Nucleic Acids. 13:399–406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McFarlane AA, Orriss GL and Stetefeld J:
The use of coiled-coil proteins in drug delivery systems. Eur J
Pharmacol. 625:101–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Wu X, Dai W, Li J, Xiang L, Tang
W, Lin J, Zhang W, Liu G, Yang Q, et al: The CCDC43-ADRM1 axis
regulated by YY1, promotes proliferation and metastasis of gastric
cancer. Cancer Lett. 482:90–101. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai L, Yang ZX, Liu JS, Wang DS and Yu HC:
Prognostic significance of CCDC137 expression and its association
with immune infiltration in hepatocellular carcinoma. Dis Markers.
2022:56386752022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Zheng Q, Wang C, Zhou H, Jiang G,
Miao Y, Zhang Y, Liu Y, Li Q, Qiu X and Wang E: CCDC106 promotes
non-small cell lung cancer cell proliferation. Oncotarget.
8:26662–26670. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kutsche K, Glauner E, Knauf S, Pomarino A,
Schmidt M, Schröder B, Nothwang H, Schüler H, Goecke T, Kersten A,
et al: Cloning and characterization of the breakpoint regions of a
chromosome 11;18 translocation in a patient with hamartoma of the
retinal pigment epithelium. Cytogenet Cell Genet. 91:141–147. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin Z, Qu S, Peng W, Yang P, Zhang R,
Zhang P, Guo D, Du J, Wu W, Tao K and Wang J: Up-Regulated CCDC34
contributes to the proliferation and metastasis of hepatocellular
carcinoma. Onco Targets Ther. 13:51–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geng W, Liang W, Fan Y, Ye Z and Zhang L:
Overexpression of CCDC34 in colorectal cancer and its involvement
in tumor growth, apoptosis and invasion. Mol Med Rep. 17:465–473.
2018.PubMed/NCBI
|
|
25
|
Gong Y, Qiu W, Ning X, Yang X, Liu L, Wang
Z, Lin J, Li X and Guo Y: CCDC34 is up-regulated in bladder cancer
and regulates bladder cancer cell proliferation, apoptosis and
migration. Oncotarget. 6:25856–25867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giglia-Mari G, Zotter A and Vermeulen W:
DNA damage response. Cold Spring Harb Perspect Biol. 3:a0007452011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi J, Hua X, Zhu B, Ravichandran S, Wang
M, Nguyen C, Brodie SA, Palleschi A, Alloisio M, Pariscenti G, et
al: Somatic genomics and clinical features of lung adenocarcinoma:
A retrospective study. PLoS Med. 13:e10021622016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su L, Zhao J, Su H, Wang Y, Huang W, Jiang
X and Gao S: CircRNAs in lung adenocarcinoma: Diagnosis and
therapy. Curr Gene Ther. 22:15–22. 2022.PubMed/NCBI
|
|
31
|
Chen M, Wu R, Li G, Liu C, Tan L, Xiao K,
Ye Y and Qin Z: Motor neuron and pancreas homeobox 1/HLXB9 promotes
sustained proliferation in bladder cancer by upregulating CCNE1/2.
J Exp Clin Cancer Res. 37:1542018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lambert M, Jambon S, Depauw S and
David-Cordonnier MH: Targeting transcription factors for cancer
treatment. Molecules. 23:14792018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harrison KA, Thaler J, Pfaff SL, Gu H and
Kehrl JH: Pancreas dorsal lobe agenesis and abnormal islets of
Langerhans in Hlxb9-deficient mice. Nat Genet. 23:71–75. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang L, Chen S, Zhao D, Yan J, Chen J,
Yang C and Zheng G: MNX1 reduces sensitivity to anoikis by
activating TrkB in human glioma cells. Mol Med Rep. 18:3271–3279.
2018.PubMed/NCBI
|