Introduction
Non-small cell lung cancer (NSCLC) is a leading
cause of cancer-related mortality worldwide (1), and the advanced disease is difficult
to cure. Although systemic therapy with cytotoxic agents was a
standard therapy for advanced NSCLC, the identification of driver
gene mutation and development of kinase inhibitors has improved the
prognosis of patients with driver gene mutated NSCLC. In the subset
of patients with NSCLC harboring EGFR mutations, it has been
reported that treatment with epidermal growth factor
receptor-tyrosine kinase inhibitors (EGFR-TKI) conferred a longer
progression-free survival (PFS) than treatment with cytotoxic
agents (2–4). A longer overall survival (OS) was also
reported in patients who received EGFR-TKI therapy than in those
who did not, suggesting an OS benefit of EGFR-TKIs in patients with
EGFR-mutant NSCLC (5).
Furthermore, it has been shown that osimertinib, a
third-generation EGFR-TKI, is effective in overcoming the acquired
resistance to EGFR-TKI therapy that is associated with the EGFR
exon 20 T790M mutation (6), which
is a major mechanism underlying resistance to first- and
second-generation EGFR-TKIs (7).
Osimertinib also yielded a longer PFS and OS than first-generation
EGFR-TKIs in previously untreated patients with EGFR-mutant NSCLC
(8,9). Therefore, osimertinib is an important
therapeutic alternative for patients with EGFR-mutant NSCLC.
Programmed death-ligand 1 (PD-L1) is an immune
checkpoint molecule, and the interaction between PD-1 and PD-L1
inactivates T-cell immunity (10).
The PD-L1 expression level in tumors has been correlated with the
efficacy of immune checkpoint inhibitor therapy in patients with
nonsquamous cell NSCLC (11). In
addition, PD-L1 expression is also expected to be a biomarker of
the efficacy of EGFR-TKIs in patients with EGFR-mutant NSCLC. PFS
after initiation of treatment with first- and second-generation
EGFR-TKIs was reported to be shorter in cases of EGFR-mutant NSCLC
showing increased tumor PD-L1 expression (12–16).
Subsequently, increased tumor expression of PD-L1 was shown to be
associated with a shorter PFS after osimertinib therapy (17–20).
Furthermore, tumor PD-L1 expression has been associated with a
lower detection rate of secondary EGFR exon 20 T790M mutation
developing after first- and second-generation EGFR-TKI therapy
(12–14), which resulted in a shorter duration
of treatment with EGFR-TKIs in patients with PD-L1-positive
EGFR-mutant NSCLC (21).
Based on these reports, determining the most
appropriate treatment strategy for PD-L1-positive EGFR-mutant NSCLC
patients may be a clinical issue. Previous clinical trials have
shown that combined EGFR-TKI plus vascular endothelial growth
factor (VEGF) inhibitor (22,23) or
cytotoxic agent (24,25) therapy conferred a longer PFS than
treatment with first-generation EGFR-TKI alone. It has also been
reported that tumor cell growth and survival may be relatively less
dependent on EGFR signaling in cases of PD-L1-positive EGFR-mutant
NSCLC, and it may be advisable to recommend the aforementioned
combined therapy (14).
The present retrospective study was conducted to
analyze survival after the initiation of treatment with first- or
second-generation EGFR-TKI monotherapy, osimertinib monotherapy,
and combined therapy in patients with PD-L1-positive EGFR-mutant
NSCLC.
Patients and methods
Patients
Data from patients with PD-L1-positive EGFR-mutant
NSCLC who had received EGFR-TKI monotherapy or combined EGFR-TKI
plus VEGF inhibitor/cytotoxic therapy (hereafter referred to as
combined therapy) at one of the 12 participating institutions were
retrospectively analyzed. The patient inclusion criteria were
established as follows: i) patients who had been cytologically or
histopathologically diagnosed with NSCLC; ii) patients with tumors
confirmed as harboring common EGFR mutations in clinical practice;
iii) patients in whom tumor PD-L1 positivity was confirmed using
the 22C3 antibody, with a tumor proportion score (TPS) of ≥1%; and
iv) patients who had received EGFR-TKI therapy, including EGFR-TKI
monotherapy or combined therapy, between January 2015 and June
2021. The exclusion criteria were determined as follows: i) NSCLC
patients with tumors confirmed as harboring uncommon EGFR
mutations; ii) patients for whom information on tumor PD-L1
expression was unavailable; and iii) patients who had received
treatment with EGFR-TKIs prior to the study period. The present
study was conducted following the principles outlined in the
Declaration of Helsinki and Ethical Guidelines for Medical and
Biological Research Involving Human Subjects (Ministry of Health,
Labour and Welfare, Japan). The need to obtain informed consent
from the patients was waived under approval from the Ethics
Committee, University of Toyama (approval number: R2022070), and we
disclosed information about the study to the patients.
Statistical analysis
The endpoints of the present study were PFS, OS, and
duration of treatment with EGFR-TKIs. PFS was calculated from the
day of treatment initiation until the day on which any progressive
disease was confirmed according to the Response Evaluation Criteria
in Solid Tumors version 1.1, clinical judgment of progression, or
death from any cause was confirmed, and PFS was censored at the
last visit without any events. After the discontinuation of
EGFR-TKI therapy because of adverse events, if subsequent therapy
was initiated before any event was confirmed, PFS was censored on
the initiation day of the subsequent treatment. However, any switch
between first- and second-generation EGFR-TKIs was not considered a
change in treatment. OS was calculated from the initiation day of
EGFR-TKI therapy until the day on which death was confirmed, and OS
was censored at the last visit prior to death. The duration of
treatment with EGFR-TKIs was defined as the sum of the PFS after
the initial EGFR-TKI therapy and subsequent osimertinib therapy
after acquiring the secondary T790M mutation. The association
between the treatment option and risk of progression or death was
analyzed using the Cox proportional hazards model, with sex,
performance status (PS), EGFR mutation status, PD-L1 expression
level, and the presence or absence of brain metastasis set as
independent variables. Kaplan-Meier curves were plotted to evaluate
PFS and OS and analyzed by the log-rank test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using the JMP statistical
software package version 15.0.0 (SAS, Cary, NC, USA).
Results
Table I shows the
patient characteristics and the therapeutic regimens. Data from a
total of 263 patients with PD-L1-positive EGFR-mutant NSCLC were
included. The majority of the NSCLC patients (249, 94.7%) were
diagnosed with adenocarcinoma. Tumor PD-L1 expression was evaluated
using tissue specimens in 262 cases and a pleural cell block in 1
patient. The TPS was determined to be 1–49% in 182 (69.2%) patients
and ≥50% in 81 (30.8%) patients. Brain metastases were detected in
90 (34.2%) patients at the time of treatment initiation. Of these
patients, 40 received local therapy for brain metastases, which
involved surgery, whole-brain irradiation, or stereotactic
radiotherapy. Of the 263 patients, 111 (42.2%), 132 (50.2%), and 20
(7.6%) received first- or second-generation EGFR-TKI monotherapy,
osimertinib monotherapy, and combined therapy, respectively.
Disease progression was confirmed in 89, 81, and 11 patients who
had received first- or second-generation EGFR-TKI monotherapy,
osimertinib monotherapy, and combined therapy, respectively.
Secondary T790M mutation was detected in 35 patients, all of whom
were subsequently treated with osimertinib.
 | Table I.Patient characteristics and
therapeutic regimens (n=263). |
Table I.
Patient characteristics and
therapeutic regimens (n=263).
| Characteristic | Value |
|---|
| Median age, years
(range) | 71 (29–92) |
| Age, n (%) |
|
| <75
years | 175 (66.5) |
| ≥75
years | 88 (33.5) |
| Sex, n (%) |
|
|
Male | 92 (35.0) |
|
Female | 171 (65.0) |
| PS, n (%) |
|
|
0-1 | 231 (87.8) |
| ≥2 | 32 (12.2) |
| Histology, n
(%) |
|
|
Adenocarcinoma | 249 (94.7) |
|
Others | 14 (5.3) |
| EGFR, n (%) |
|
| del
19 | 132 (50.2) |
|
L858R | 131 (49.8) |
| PD-L1, n (%) |
|
|
1–49% | 182 (69.2) |
|
≥50% | 81 (30.8) |
| Brain metastasis, n
(%) |
|
|
Yes | 90 (34.2) |
| No | 173 (65.8) |
| Therapeutic
regimen, n (%) |
|
|
Gefitinib | 50 (19.0) |
|
Erlotinib | 16 (6.1) |
|
Afatinib | 45 (17.1) |
|
Osimertinib | 132 (50.2) |
|
Erlotinib + bevacizumab | 6 (2.3) |
|
Erlotinib + ramucirumab | 1 (0.4) |
|
Gefitinib + platinum
doublet | 9 (3.4) |
|
Osimertinib + platinum
doublet | 4 (1.5) |
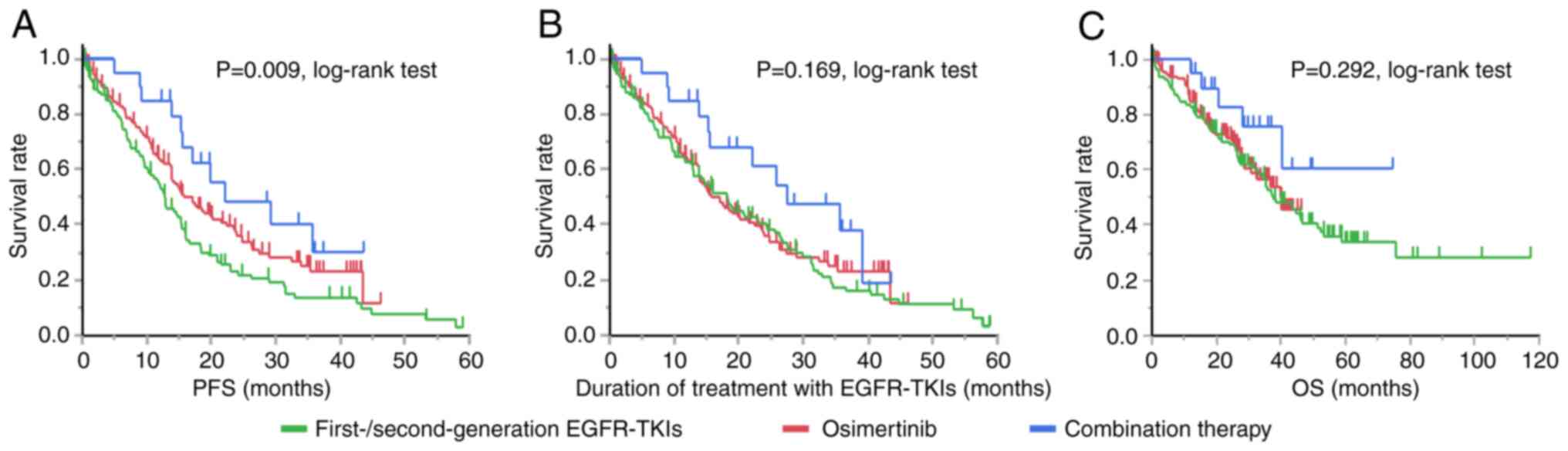
Fig. 1 shows the
Kaplan-Meier curves for PFS, duration of treatment with EGFR-TKIs,
and OS according to the EGFR-TKI received. Among the three
treatments, combined therapy was associated with the longest PFS
(P=0.009, log-rank test), duration of treatment with EGFR-TKIs
(P=0.169, log-rank test) and OS (P=0.292, log-rank test).
Osimertinib monotherapy was associated with a longer PFS than
first- or second-generation EGFR-TKI monotherapy but with a similar
duration of treatment with EGFR-TKIs and OS to those with first- or
second-generation EGFR-TKI monotherapy.
Table II shows an
analysis of the PFS conducted using the Cox proportional hazards
model. Combined therapy was associated with a significant reduction
in the risk of disease progression compared with first- and
second-generation EGFR-TKI monotherapy, suggesting a PFS benefit.
Table III shows an analysis of
the OS conducted using the Cox proportional hazards model. Neither
osimertinib monotherapy nor combined therapy was associated with
any significant reduction in the risk of death compared with first-
and second-generation EGFR-TKI monotherapy. In contrast, the EGFR
mutation status was associated with both PFS and OS.
 | Table II.Analysis using the Cox proportional
hazards model for PFS after the initiation of EGFR-TKI therapy. |
Table II.
Analysis using the Cox proportional
hazards model for PFS after the initiation of EGFR-TKI therapy.
| Characteristic | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
Male | 1.16 | 0.86-1.58 | 0.328 |
|
Female | 1.00 |
|
|
| PS |
|
|
|
|
0-1 | 0.68 | 0.42-1.08 | 0.105 |
| ≥2 | 1.00 |
|
|
| EGFR |
|
|
|
|
L858R | 1.39 | 1.03-1.88 | 0.030 |
| del
19 | 1.00 |
|
|
| PD-L1 |
|
|
|
|
1–49% | 0.81 | 0.59-1.12 | 0.206 |
|
≥50% | 1.00 |
|
|
| Brain
metastasis |
|
|
|
| No | 0.79 | 0.57-1.09 | 0.151 |
|
Yes | 1.00 |
|
|
| EGFR-TKI
therapy |
|
|
|
|
First/second generation | 1.00 |
|
|
|
Osimertinib | 0.73 | 0.54-1.00 | 0.053 |
|
Combination therapy | 0.47 | 0.25-0.90 | 0.024 |
 | Table III.Analysis using the Cox proportional
hazards model for OS after the initiation of EGFR-TKI therapy. |
Table III.
Analysis using the Cox proportional
hazards model for OS after the initiation of EGFR-TKI therapy.
| Characteristic | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
Male | 1.31 | 0.89-1.92 | 0.174 |
|
Female | 1.00 |
|
|
| PS |
|
|
|
|
0-1 | 0.53 | 0.30-0.92 | 0.025 |
| ≥2 | 1.00 |
|
|
| EGFR |
|
|
|
|
L858R | 1.88 | 1.28-2.77 | 0.001 |
| del
19 | 1.00 |
|
|
| PD-L1 |
|
|
|
|
1–49% | 1.24 | 0.80-1.90 | 0.334 |
|
≥50% | 1.00 |
|
|
| Brain
metastasis |
|
|
|
| No | 0.86 | 0.58-1.29 | 0.469 |
|
Yes | 1.00 |
|
|
| EGFR-TKI
therapy |
|
|
|
|
First/second generation | 1.00 |
|
|
|
Osimertinib | 0.98 | 0.65-1.48 | 0.925 |
|
Combination therapy | 0.52 | 0.21-1.31 | 0.166 |
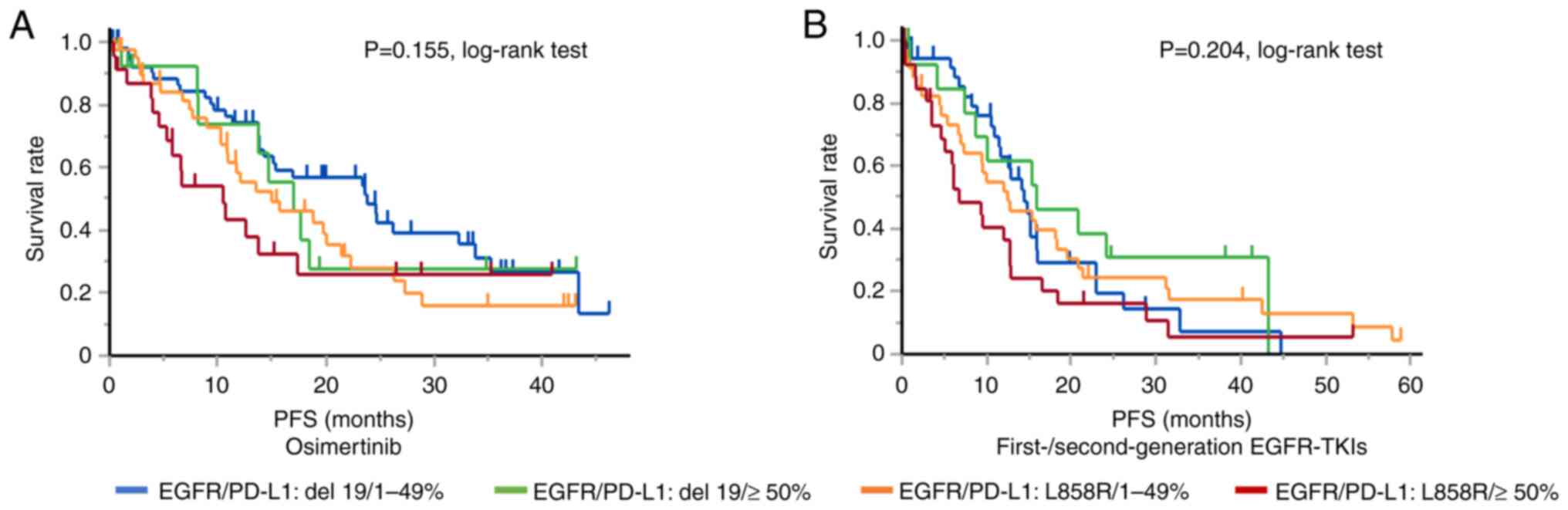
Fig. 2 shows the
Kaplan-Meier curve for PFS according to the tumor PD-L1 expression
level and EGFR mutation status. Although the log-rank test showed
no significant difference in PFS, patients with a PD-L1 TPS of ≥50%
and tumors harboring the exon 21 L858R point mutation showed the
shortest PFS after both osimertinib therapy (10.5 months) and
first- or second-generation EGFR-TKI monotherapy (6.8 months).
Discussion
Increased tumor expression of PD-L1, evaluated using
the 22C3 antibody, has been associated with poor efficacy of
EGFR-TKI monotherapy, independent of the clinical background,
including the EGFR mutation status (12–20).
The present study showed that combined therapy was significantly
associated with a lower risk of disease progression than first- and
second-generation EGFR-TKI therapy and was associated with the
longest PFS, duration of treatment with EGFR-TKIs, and OS among the
treatments used in patients with PD-L1-positive EGFR-mutant
NSCLC.
A previous phase III clinical trial showed that
osimertinib monotherapy was associated with a longer PFS (8) and OS (9) than first-generation EGFR-TKI
monotherapy (median, 18.9 vs. 10.2 months and 38.6 vs. 31.8 months,
respectively). Regarding second-generation EGFR-TKIs, a network
meta-analysis suggested that osimertinib monotherapy might confer a
longer PFS than second-generation EGFR-TKI monotherapy (afatinib or
dacomitinib), while an observational study using propensity score
analysis showed a similar time to discontinuation of EGFR-TKIs
between osimertinib and afatinib (20.5 vs. 18.6 months,
respectively) (26). It is
difficult to compare the findings of the present study with these
previous reports because we considered patients treated with first-
and second-generation EGFR-TKIs as a single group, but the
multivariate analysis showed no significant reduction in the risk
of disease progression or death, and the Kaplan-Meier curves showed
a similar duration of treatment with EGFR-TKIs and OS between
patients who received osimertinib and those who received first- or
second-generation EGFR-TKI monotherapy.
Previous clinical trials have demonstrated the PFS
benefit of combined therapy regimens, including erlotinib plus VEGF
inhibitors and gefitinib plus cytotoxic agents, compared with
first-generation EGFR-TKI monotherapy. The NEJ026 trial, a phase 3
trial, showed that PFS was significantly longer in combination
therapy with bevacizumab plus erlotinib compared with erlotinib
monotherapy (16.9 months vs. 13.3 months, respectively) (22). Similarly, the Relay trial, another
phase 3 trial, demonstrated that PFS was significantly longer in
the combination therapy with ramucirumab plus erlotinib than that
in the erlotinib monotherapy (19.4 months vs. 12.4 months,
respectively) (23).
Additionally, VEGF plays a major role in
angiogenesis, and VEGF inhibitors exert several functions,
including the inhibition of angiogenesis, improvement of hypoxia in
the tumor microenvironment, normalization of interstitial fluid
pressure, and sensitization of tumors to anticancer therapies
(27). Furthermore, the in
vivo study demonstrated that bevacizumab restored the
sensitivity of EGFR-TKI-resistant tumor cells against erlotinib,
and increasing concentrations of erlotinib in the tumor tissues
were observed (27). This effect
was evident in cell lines with increased VEGF expression. The
present study cannot confirm whether VEGF was upregulated in
PD-L1-positive EGFR-mutant NSCLC. However, it has been reported
that VEGF-neuropilin-2 signaling is associated with PD-L1
expression in prostate cancer (28). Thus, increased tumor VEGF expression
may underlie the efficacy of combined therapy with EGFR-TKIs plus
VEGF inhibitors for PD-L1-positive EGFR-mutant NSCLC.
The phase 3 NEJ009 trial that evaluated the efficacy
of combination therapy with gefitinib plus cytotoxic agents showed
a longer PFS of 20.9 months (24)
and a longer PFS2 (duration between the randomization until the
progressive disease of the second-line treatment or death) compared
with gefitinib monotherapy (25).
In addition, indirect comparisons have revealed that combined
therapy with gefitinib plus cytotoxic agents was associated with a
significant reduction in the risk of death compared with
monotherapy using first-generation EGFR-TKIs and some
second-generation EGFR-TKIs (29),
suggesting an OS benefit. Although direct comparison is difficult
because of the variations in study designs and patient backgrounds,
the patient survival observed in the present study appears to be
consistent with these prior clinical trials. The present study
suggested that, even in patients with PD-L1-positive EGFR-mutant
NSCLC, combined therapy might confer a longer PFS than first- and
second-generation EGFR-TKI monotherapy.
Notably, sequential osimertinib therapy may yield a
longer OS by prolonging the duration of treatment with EGFR-TKIs
after acquiring the secondary T790M mutation because combined
therapy regimens also contain first-generation EGFR-TKIs (30). Although the difference was not
significant, the Kaplan-Meier curve showed that combined therapy
was associated with the longest duration of treatment with
EGFR-TKIs and OS, and the point estimate of the hazard ratio for OS
was as low as 0.52. However, it is difficult to determine a
definitive conclusion about the superiority or equivalence in terms
of the OS between combined therapy, osimertinib monotherapy, and
first- or second-generation EGFR-TKI monotherapy in patients with
PD-L1-positive EGFR-mutant NSCLC because this study was not a
predesigned clinical trial and may not have had sufficient
statistical power. Additionally, the information on post-TKI
treatment is important for this discussion. However, this
multicenter study did not collect information on post-TKI therapy,
including immune checkpoint inhibitor therapy. This should be
mentioned as a limitation in this study.
Moreover, biomarkers that can predict poor response
to EGFR-TKIs may help in selecting patients who are likely to
benefit from combined therapy (31). PD-L1 expression is upregulated by
oncogenes, including EGFR, ALK, MYC, hypoxia-inducible
factor-1 alpha, phosphatase and tensin homolog loss,
mitogen-activated protein kinase, and KRAS, and PD-L1
expression in EGFR-mutant NSCLC might result from the expression of
multiple oncogenes (14).
Additionally, using next-generation sequencing, the expression of
several oncogenes, including ALK, BCL2, KRAS, and
PIK3CA, has been observed in EGFR-mutant NSCLCs (32). Even in EGFR-mutant NSCLCs, multiple
oncogene signals might contribute to oncogenesis in some cases,
making cell proliferation and survival relatively less dependent on
EGFR signaling.
AXL is a tyrosine kinase receptor that is associated
with resistance and poor outcomes in various cancers. In
EGFR-mutant NSCLC, AXL has also been reported to support resistance
to osimertinib therapy (33). High
expression levels of AXL were detected in 26.1% of tumors in
previously untreated cases of EGFR-mutant NSCLC and were associated
with a shorter PFS (34).
Furthermore, in cell line-based assays, AXL expression was shown to
accelerate PD-L1 expression (34),
and the expression of AXL and PD-L1 was correlated (34,35).
AXL is also a downstream target of Yes-associated protein (YAP)
(36), which is one of the
mechanisms underlying the development of resistance to EGFR-TKIs
(36,37). Based on these reports,
overexpression of AXL or YAP might help explain the poor outcomes
in PD-L1-positive EGFR-mutant NSCLC patients treated with
EGFR-TKIs.
Finally, EGFR exon 21 L858R was associated with the
risk of progression and death in the present study. The presence of
compound mutations, defined as multiple mutations in the EGFR
tyrosine kinase domain, has been reported in 15.9 and 24.6% of
EGFR-mutant NSCLCs, depending on the study (32,38),
and is associated with decreased EGFR-TKI sensitivity (38). EGFR compound mutations are more
frequently detected in EGFR-mutant NSCLCs harboring the exon 21
L858R mutation than in those harboring the exon 19 deletion
(38). Moreover, the RBM10 mutation
has been suggested to contribute to decreased EGFR-TKI sensitivity
in NSCLC patients harboring the exon 21 L858R mutation of EGFR.
RBM10 is associated with mRNA alternative splicing of the Bcl-x
gene regulating tumor cell apoptosis, and inactivation of RBM10
diminished apoptosis mediated by EGFR-TKIs. The presence of the
RBM10 mutation might be one of the mechanisms underlying poor
outcomes in NSCLC patients harboring the exon 21 L858R mutation of
EGFR because the RBM10 mutation was more frequently observed in
cases with the exon 21 L858R mutation (39).
There were several limitations of the present study.
Selection bias or an imbalance of patient characteristics may have
affected the results of the analysis, and it is difficult to
completely exclude these because of the retrospective nature of the
study. In addition, the number of patients who received combined
therapy was small, making it difficult to discuss the efficacy of
each combined therapy with EGFR-TKIs plus VEGF inhibitors/cytotoxic
agents.
In conclusion, the results of the present study
showed that combined therapy was associated with a significant
reduction in the risk of disease progression compared with first-
and second-generation EGFR-TKI monotherapy, suggesting that
combined therapy is effective for PD-L1-positive EGFR-mutant NSCLC.
The results of the present study not only imply that tumor PD-L1
expression may help predict the efficacy of EGFR-TKI therapy but
may also lead to the development of precision medicine for
EGFR-mutant NSCLC in the future. However, future studies are needed
to validate the findings of the present study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MI and EM designed the study. YK, RS, DM, HN, MS,
YS, SY, TK, DJ, NY and TH contributed to the data collection and
investigation. MI wrote the original draft of the manuscript. MI
and EM confirm the authenticity of the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted following the
Declaration of Helsinki and Ethical Guidelines for Medical and
Biological Research Involving Human Subjects (Ministry of Health,
Labour and Welfare, Japan) and was approved by the Ethics
Committee, University of Toyama (approval number: R2022070). The
need to obtain informed consent from the study subjects was waived
under the approval of the Ethics Committee, University of Toyama,
and we disclosed information about the study to the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they do not have any
competing interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang JCH, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takano T, Fukui T, Ohe Y, Tsuta K,
Yamamoto S, Nokihara H, Yamamoto N, Sekine I, Kunitoh H, Furuta K
and Tamura T: EGFR mutations predict survival benefit from
gefitinib in patients with advanced lung adenocarcinoma: A
historical comparison of patients treated before and after
gefitinib approval in Japan. J Clin Oncol. 26:5589–5595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kosaka T, Yatabe Y, Endoh H, Yoshida K,
Hida T, Tsuboi M, Tada H, Kuwano H and Mitsudomi T: Analysis of
epidermal growth factor receptor gene mutation in patients with
non-small cell lung cancer and acquired resistance to gefitinib.
Clin Cancer Res. 12:5764–5769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inomata M, Azechi K, Takata N, Hayashi K,
Tokui K, Taka C, Okazawa S, Kambara K, Imanishi S, Miwa T, et al:
Association of tumor PD-L1 expression with the T790M mutation and
progression-free survival in patients with EGFR-mutant non-small
cell lung cancer receiving EGFR-TKI therapy. Diagnostics (Basel).
10:10062020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon BW, Chang B and Lee SH: High PD-L1
expression is associated with unfavorable clinical outcome in
EGFR-mutated lung adenocarcinomas treated with targeted therapy.
Onco Targets Ther. 13:8273–8285. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang CY, Liao WY, Ho CC, Chen KY, Tsai TH,
Hsu CL, Su KY, Chang YL, Wu CT, Hsu CC, et al: Association between
programmed death-ligand 1 expression, immune microenvironments, and
clinical outcomes in epidermal growth factor receptor mutant lung
adenocarcinoma patients treated with tyrosine kinase inhibitors.
Eur J Cancer. 124:110–122. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lan B, Wang Y, Wu J, Wang K and Wang P:
The predictive and prognostic effects of PD-L1 expression on TKI
treatment and survival of EGFR-mutant NSCLC: A meta-analysis.
Medicine (Baltimore). 100:e270382021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng Z, Lin H, Zhou K, Deng S and Mei J:
Predictive value of pretreatment PD-L1 expression in EGFR-mutant
non-small cell lung cancer: A meta-analysis. World J Surg Oncol.
19:1452021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshimura A, Yamada T, Okuma Y, Fukuda A,
Watanabe S, Nishioka N, Takeda T, Chihara Y, Takemoto S, Harada T,
et al: Impact of tumor programmed death ligand-1 expression on
osimertinib efficacy in untreated EGFR-mutated advanced non-small
cell lung cancer: A prospective observational study. Transl Lung
Cancer Res. 10:3582–3593. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakata Y, Sakata S, Oya Y, Tamiya M,
Suzuki H, Shibaki R, Okada A, Kobe H, Matsumoto H, Yokoi T, et al:
Osimertinib as first-line treatment for advanced epidermal growth
factor receptor mutation-positive non-small-cell lung cancer in a
real-world setting (OSI-FACT). Eur J Cancer. 159:144–153. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shiozawa T, Numata T, Tamura T, Endo T,
Kaburagi T, Yamamoto Y, Yamada H, Kikuchi N, Saito K, Inagaki M, et
al: Prognostic implication of PD-L1 expression on osimertinib
treatment for EGFR-mutated non-small cell lung cancer. Anticancer
Res. 42:2583–2590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu KH, Tseng JS, Yang TY, Chen KC, Su KY,
Yu SL, Chen JJW, Huang YH and Chang GC: PD-L1 strong expressions
affect the clinical outcomes of osimertinib in treatment naïve
advanced EGFR-mutant non-small cell lung cancer patients. Sci Rep.
12:97532022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inomata M, Matsumoto M, Mizushima I, Seto
Z, Hayashi K, Tokui K, Taka C, Okazawa S, Kambara K, Imanishi S, et
al: Association of tumor PD-L1 expression with time on treatment
using EGFR-TKIs in patients with EGFR-mutant non-small cell lung
cancer. Cancer Diagn Progn. 2:324–329. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito H, Fukuhara T, Furuya N, Watanabe K,
Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori
K, et al: Erlotinib plus bevacizumab versus erlotinib alone in
patients with EGFR-positive advanced non-squamous non-small-cell
lung cancer (NEJ026): Interim analysis of an open-label,
randomised, multicentre, phase 3 trial. Lancet Oncol. 20:625–635.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakagawa K, Garon EB, Seto T, Nishio M,
Ponce Aix S, Paz-Ares L, Chiu CH, Park K, Novello S, Nadal E, et
al: Ramucirumab plus erlotinib in patients with untreated,
EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 20:1655–1669. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hosomi Y, Morita S, Sugawara S, Kato T,
Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, Minato K, et
al: Gefitinib alone versus gefitinib plus chemotherapy for
non-small-cell lung cancer with mutated epidermal growth factor
receptor: NEJ009 study. J Clin Oncol. 38:115–123. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyauchi E, Morita S, Nakamura A, Hosomi
Y, Watanabe K, Ikeda S, Seike M, Fujita Y, Minato K, Ko R, et al:
Updated analysis of NEJ009: Gefitinib-Alone versus gefitinib plus
chemotherapy for non-small-cell lung cancer with mutated EGFR. J
Clin Oncol. 40:3587–3592. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ito K, Morise M, Wakuda K, Hataji O,
Shimokawaji T, Takahashi K, Furuya N, Takeyama Y, Goto Y, Abe T, et
al: A multicenter cohort study of osimertinib compared with
afatinib as first-line treatment for EGFR-mutated non-small-cell
lung cancer from practical dataset: CJLSG1903. ESMO Open.
6:1001152021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Takayama K, Wang S, Shiraishi Y,
Gotanda K, Harada T, Furuyama K, Iwama E, Ieiri I, Okamoto I and
Nakanishi Y: Addition of bevacizumab enhances antitumor activity of
erlotinib against non-small cell lung cancer xenografts depending
on VEGF expression. Cancer Chemother Pharmacol. 74:1297–1305. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang M, Wisniewski CA, Xiong C, Chhoy P,
Goel HL, Kumar A, Zhu LJ, Li R, St Louis PA, Ferreira LM, et al:
Therapeutic blocking of VEGF binding to neuropilin-2 diminishes
PD-L1 expression to activate antitumor immunity in prostate cancer.
Sci Transl Med. 15:eade58552023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Liu J, Cai X, Pan Z, Liu J, Yin W,
Chen H, Xie Z, Liang H, Wang W, et al: Efficacy and safety of first
line treatments for patients with advanced epidermal growth factor
receptor mutated, non-small cell lung cancer: Systematic review and
network meta-analysis. BMJ. 367:l54602019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maemondo M: Potential of combination
therapy in EGFR mutated lung cancer. Ann Transl Med. 8:5182020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moore S and Wheatley-Price P: EGFR
combination therapy should become the new standard first-line
treatment in advanced EGFR-mutant NSCLC. J Thorac Oncol.
16:1788–1792. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim EY, Cho EN, Park HS, Hong JY, Lim S,
Youn JP, Hwang SY and Chang YS: Compound EGFR mutation is
frequently detected with co-mutations of actionable genes and
associated with poor clinical outcome in lung adenocarcinoma.
Cancer Biol Ther. 17:237–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taniguchi H, Yamada T, Wang R, Tanimura K,
Adachi Y, Nishiyama A, Tanimoto A, Takeuchi S, Araujo LH, Boroni M,
et al: AXL confers intrinsic resistance to osimertinib and advances
the emergence of tolerant cells. Nat Commun. 10:2592019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshimura A, Yamada T, Serizawa M, Uehara
H, Tanimura K, Okuma Y, Fukuda A, Watanabe S, Nishioka N, Takeda T,
et al: High levels of AXL expression in untreated EGFR-mutated
non-small cell lung cancer negatively impacts the use of
osimertinib. Cancer Sci. 114:606–618. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsukita Y, Fujino N, Miyauchi E, Saito R,
Fujishima F, Itakura K, Kyogoku Y, Okutomo K, Yamada M, Okazaki T,
et al: Axl kinase drives immune checkpoint and chemokine signalling
pathways in lung adenocarcinomas. Mol Cancer. 18:242019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee JE, Park HS, Lee D, Yoo G, Kim T, Jeon
H, Yeo MK, Lee CS, Moon JY, Jung SS, et al: Hippo pathway effector
YAP inhibition restores the sensitivity of EGFR-TKI in lung
adenocarcinoma having primary or acquired EGFR-TKI resistance.
Biochem Biophys Res Commun. 474:154–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McGowan M, Kleinberg L, Halvorsen AR,
Helland Å and Brustugun OT: NSCLC depend upon YAP expression and
nuclear localization after acquiring resistance to EGFR inhibitors.
Genes Cancer. 8:497–504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kohsaka S, Nagano M, Ueno T, Suehara Y,
Hayashi T, Shimada N, Takahashi K, Suzuki K, Takamochi K, Takahashi
F and Mano H: A method of high-throughput functional evaluation of
EGFR gene variants of unknown significance in cancer. Sci Transl
Med. 9:eaan65662017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nanjo S, Wu W, Karachaliou N, Blakely CM,
Suzuki J, Chou YT, Ali SM, Kerr DL, Olivas VR, Shue J, et al:
Deficiency of the splicing factor RBM10 limits EGFR inhibitor
response in EGFR-mutant lung cancer. J Clin Invest.
132:e1450992022. View Article : Google Scholar : PubMed/NCBI
|