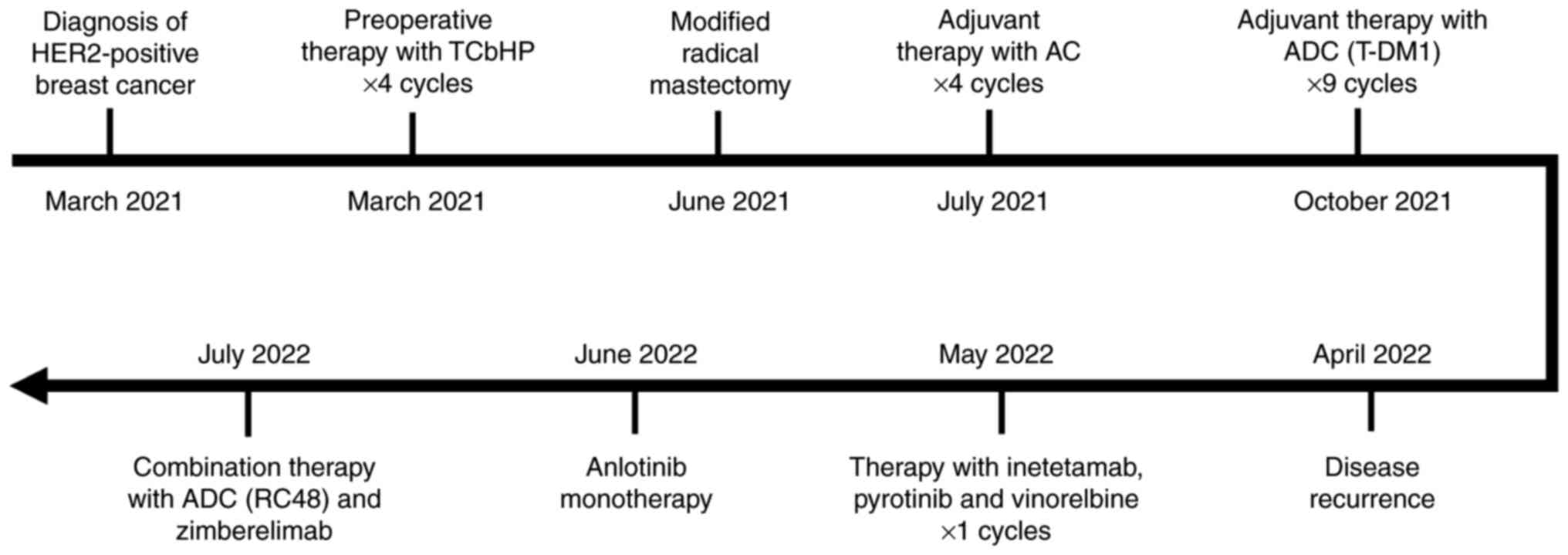
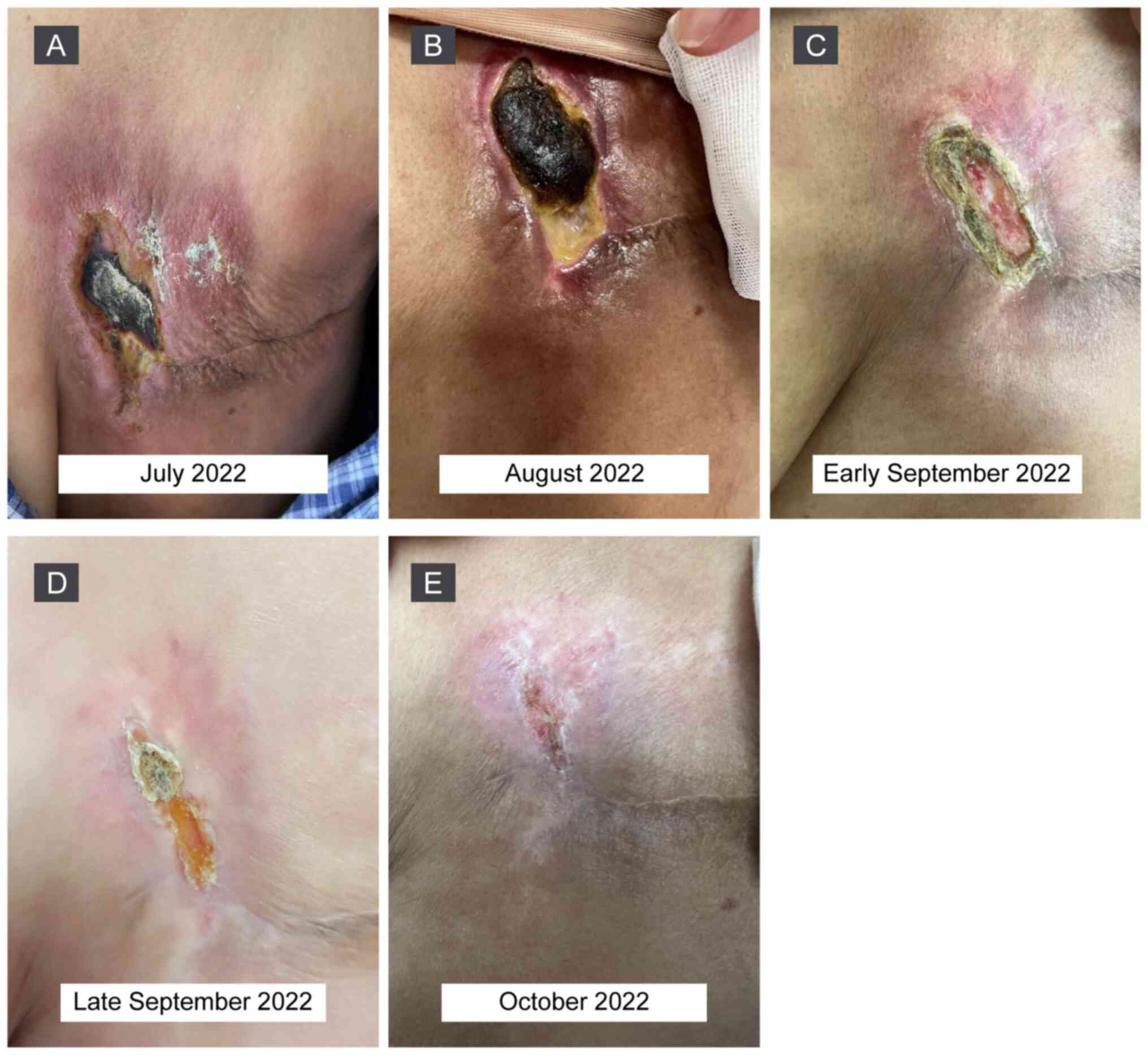
|
1
|
Siddiqui T, Rani P, Ashraf T and Ellahi A:
Enhertu (Fam-trastuzumab-deruxtecan-nxki)-Revolutionizing treatment
paradigm for HER2-low breast cancer. Ann Med Surg (Lond).
82:1046652022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferraro E, Drago JZ and Modi S:
Implementing antibody-drug conjugates (ADCs) in HER2-positive
breast cancer: State of the art and future directions. Breast
Cancer Res. 23:84–94. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi F, Liu Y, Zhou X, Shen P, Xue R and
Zhang M: Disitamab vedotin: A novel antibody-drug conjugates for
cancer therapy. Drug Deliv. 29:1335–1344. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu B, Wang J, Fang J, Chen X, Han Y, Li Q,
Zhang P, Yuan P, Ma F, Luo Y, et al: Abstract PD4-06: Early
clinical development of RC48-ADC in patients with HER2 positive
metastatic breast cancer. Cancer Res. 80:PD4–06-PD04-06. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Liu Y, Zhang Q, Feng J, Fang J,
Chen X, Han Y, Li Q, Zhang P, Yuan P, et al: RC48-ADC, a
HER2-targeting antibody-drug conjugate, in patients with
HER2-positive and HER2-low expressing advanced or metastatic breast
cancer: A pooled analysis of two studies. J Clin Oncol. 39:1022.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Q, Qian W, Sun X and Jiang S:
Small-molecule inhibitors, immune checkpoint inhibitors, and more:
FDA-approved novel therapeutic drugs for solid tumors from 1991 to
2021. J Hematol Oncol. 15:143–205. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology Breast
Cancer. Version 4. NCCN; Plymouth Meeting, PN: 2023, View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu D, Ma C, Lu P, Gong J, Ye D, Wang S,
Peng P, Bai Y, Song Y, Chen J, et al: Dose escalation and expansion
(phase Ia/Ib) study of GLS-010, a recombinant fully human
antiprogrammed death-1 monoclonal antibody for advanced solid
tumors or lymphoma. Eur J Cancer. 148:1–13. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin N, Zhang M, Bai H, Liu H, Cui J, Ke X,
Zhang H, Liu L, Yan D, Jiang Y, et al: Efficacy and safety of
GLS-010 (zimberelimab) in patients with relapsed or refractory
classical Hodgkin lymphoma: A multicenter, single-arm, phase II
study. Eur J Cancer. 164:117–126. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Markham A: Zimberelimab: First approval.
Drugs. 81:2063–2068. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu X, Xia L, Zhou Q, Zhu J, Wang K, Chen
J, Huang Y, Kurb G, Chang B, Zhao W, et al: GLS-010 (Zimberelimab),
a novel fully human anti-PD-1 mAb in Chinese patients with
recurrent metastatic cervical cancer results from a multicenter,
open-label, single-arm phase II trial. Int J Gynecol Cancer.
30:A1472020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chung HC, Ros W, Delord JP, Perets P,
Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Xu L,
Zeigenfuss S, et al: Efficacy and safety of pembrolizumab in
previously treated advanced cervical cancer results from the phase
II KEYNOTE-158 study. J Clin Oncol. 37:1470–1478. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Health Commission Of The People's
Republic Of China, . Chinese guidelines for diagnosis and treatment
of breast cancer 2018 (English version). Chin J Cancer Res.
31:259–277. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allison KH, Hammond MEH, Dowsett M,
McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR,
Chavez-MacGregor M, Perlmutter J, et al: Estrogen and progesterone
receptor testing in breast cancer: ASCO/CAP guideline update. J
Clin Oncol. 38:1346–1366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, Jessup JM, Brirley JD, Gaspar LE, Schilsky RL, Balch
CM, et al: AJCC Cancer Staging Manual. 8th edition. Springer; New
York, NY: pp. 589–636. 2017, View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ignatov T, Gorbunow F, Eggemann H, Ortmann
O and Ignatov A: Loss of HER2 after HER2-targeted treatment. Breast
Cancer Res Treat. 175:401–408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Branco FP, Machado D, Silva FF, André S,
Catarino A, Madureira R, Pinto JM, Godinho JP, Simões PD, Brito M,
Casa-Nova M, Moreira AR and Passos-Coelho JL: Loss of HER2
amplification and disease prognosis after neoadjuvant treatment of
HER2 amplified breast cancer. Eur J Cancer. 92:S942018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niikura N, Tomotaki A, Miyata H, Iwamoto
T, Kawai M, Anan K, Hayashi N, Aogi K, Ishida T, Masuoka H, et al:
Changes in tumor expression of HER2 and hormone receptors status
after neoadjuvant chemotherapy in 21,755 patients from the Japanese
breast cancer registry. Ann Oncol. 27:480–487. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niikura N, Liu J, Hayashi N, Mittendorf
EA, Gong Y, Palla SL, Tokuda Y, Gonzalez-Angulo AM, Hortobagyi GN
and Ueno NT: Loss of human epidermal growth factor receptor 2
(HER2) expression in metastatic sites of HER2-overexpressing
primary breast tumors. J Clin Oncol. 30:593–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mittendorf EA, Wu Y, Scaltriti M,
Meric-Bernstam F, Hunt KK, Dawood S, Esteva FJ, Buzdar AU, Chen H,
Eksambi S, et al: Loss of HER2 amplification following
trastuzumab-based neoadjuvant systemic therapy and survival
outcomes. Clin Cancer Res. 15:7381–7388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Modi S, Park H, Murthy RK, Iwata H, Tamura
K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, et
al: Antitumor activity and safety of trastuzumab deruxtecan in
patients with HER2-low-expressing advanced breast cancer: Results
from a phase Ib Study. J Clin Oncol. 38:1887–1896. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Modi S, Jacot W, Yamashita T, Sohn J,
Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, et al:
Trastuzumab deruxtecan in previously treated HER2-low advanced
breast cancer. N Engl J Med. 387:9–20. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Z, Li J, Chen J, Liu Y, Wang K, Nie
J, Wang X, Hao C, Yin Y, Wang S, et al: Chinese society of clinical
oncology (CSCO) breast cancer guidelines 2022. Transl Breast Cancer
Res. 3:13–40. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology Breast
Cancer. Version 8. NCCN; Plymouth Meeting, PN: 2021, View Article : Google Scholar : PubMed/NCBI
|
|
26
|
von Minckwitz G, Huang CS, Mano MS, Loibl
S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A,
Redondo A, et al: Trastuzumab Emtansine for residual invasive
HER2-positive breast cancer. N Engl J Med. 380:617–628. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Xu MZ, Wang L, Jiang J, Dong LH,
Chen F, Dong K and Song HF: Conjugating MMAE to a novel anti-HER2
antibody for selective targeted delivery. Eur Rev Med Pharmacol
Sci. 24:12929–12937. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rinnerthaler G, Gampenrieder SP and Greil
R: HER2 directed antibody-drug-conjugates beyond T-DM1 in breast
cancer. Int J Mol Sci. 20:11152019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li L, Zhang D, Liu B, Lv D, Zhai J, Guan
X, Yi Z and Ma F: Antibody-drug conjugates in HER2-positive breast
cancer. Chin Med J (Engl). 135:261–267. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muller P, Kreuzaler M, Khan T, Thommen DS,
Martin K, Glatz K, Savic S, Harbeck N, Nitz U, Gluz O, et al:
Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly
susceptible to CTLA-4/PD-1 blockade. Sci Transl Med.
7:315ra1882015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwata TN, Ishii C, Ishida S, Ogitani Y,
Wada T and Agatsuma T: A HER2-targeting antibody-drug conjugate,
trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a
mouse model. Mol Cancer Ther. 17:1494–1503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang L, Wang R, Xie K, Zhang J, Tao F, Pi
C, Feng Y, Gu H and Fang J: A HER2 target antibody drug conjugate
combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in
hPD-1 transgenic mouse model and contributes immune memory
formation. Breast Cancer Res Treat. 191:51–61. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang T, Kang L, Li D and Song Y:
Immunotherapy for HER-2 positive breast cancer. Front Oncol.
13:10979832023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Emens LA, Esteva FJ, Beresford M, Saura C,
De Laurentiis M, Kim SB, Im SA, Wang Y, Salgado R, Mani A, et al:
Trastuzumab emtansine plus atezolizumab versus trastuzumab
emtansine plus placebo in previously treated, HER2-positive
advanced breast cancer (KATE2): A phase 2, multicentre, randomised,
double-blind trial. Lancet Oncol. 21:1283–1295. 2020. View Article : Google Scholar : PubMed/NCBI
|