Introduction
According to Global Cancer Statistics, it is
estimated that there were more than a half million new thyroid
cancer cases and >40,000 cancer-related deaths due to thyroid
cancer in 2020 globally; thyroid cancer is the ninth most commonly
diagnosed cancer and ranks 25th in terms of cancer deaths globally
(1). The worldwide incidence rate
is three times higher in females than in males, accounting for 10.1
per 100,000 population in female patients and the incidence in
males is 0.33 per 100,000 population (1). The China Cancer Registry data show
that the incidence of thyroid cancer is rising annually and
threatens human health in Chinese populations (2). However, to date, the precise etiology
of thyroid cancer development remains to be defined. Radiation
exposure is the only validated thyroid cancer risk factor while
other factors, such as being overweight hormonal exposure and
certain environmental pollutants may be associated with thyroid
cancer development (1,3). Histologically, thyroid cancer is
divided into papillary, follicular, medullary, anaplastic or other
rare types; of these, papillary thyroid carcinoma (PTC) comprises
75–85% of diagnosed thyroid cancer cases (4,5). To
date, the incidence of diagnosed PTC has been increasing because of
the use of ultrasonography and other diagnostic imaging equipment
(1,6). PTC prognosis is usually good, with a
10-year survival rate of 90% following standardized therapy
options, such as surgery alone or combined with inhibition of
thyroid-stimulating hormone and radioactive iodine agents (7). Local recurrence and distant metastasis
are primary causes of advanced PTC and death (8). Nevertheless, further understanding of
the pathogenesis and underlying molecular mechanisms of PTC may
help to control PTC progression effectively, as well as to detect
PTC earlier.
The Warburg effect describes a phenomenon
characterized by tumor cell metabolic reprogramming of predominant
energy production by using less efficient aerobic glycolysis in the
cytosol instead of the typical mitochondrial citric acid cycle and
oxidative phosphorylation in normal cells (9). Such a fermentation process only
produces low levels of ATP compared with that of the citric acid
cycle and oxidative phosphorylation but it possesses an advantage
by allowing proliferating tumor cells to use the resulting
nutrients, such as glucose and glutamine, for cell proliferation,
leading to fermentation favoring tumor cell proliferation (10,11).
Previous studies have shown that an increase in aerobic glycolysis
significantly induces myofibroblast activation; meanwhile,
neutrophils and 2-deoxyglucose inhibitors have been shown to
attenuate unilateral ureter obstruction surgery-associated renal
fibrosis, as well as TGF-β1)-induced renal interstitial fibroblasts
(12,13). In addition, histone H2B
monoubiquitination upregulates expression of multiple human
mitochondrial respiratory genes (such as nicotinamide adenine
dinucleotide dehydrogenase ubiquinone) 1 alpha subcomplex subunit
9, Nicotinamide adenine dinucleotide phosphate transhydrogenase
(NNT), and Nicotinamide adenine dinucleotide-hydrogen dehydrogenase
[ubiquinone] 1 beta subcomplex subunit 6 (NDUFB6) proteins in lung
cancer cells, which suppresses the Warburg effect and tumorigenesis
(14); while isocitrate
dehydrogenase 2 has been revealed to stimulate the
hypoxia-inducible factor-1α pathway, which promotes the Warburg
effect and PTC growth (15).
Glutamine metabolism is a type of metabolic
reprogramming in tumor cells (16)
and glutamine demand, a non-essential amino acid, is increased in
cancer cells (17). For example,
glutamine has been reported to be a key amino acid in breast cancer
cell growth (18). Moreover,
prostate cancer cells can be radio sensitized and treated by
targeting glutamine metabolism and autophagy (19), while melanoma cell growth is reduced
by interfering with the glutamine transporter (20). Furthermore, deubiquitinases, a type
of protease that regulates the activity of the ubiquitin/proteasome
system, can regulate cancer development and progression (21). Ubiquitin carboxy-terminal hydrolase
47 (USP47) belongs to the deubiquitination enzyme family of
proteins and counteracts the effects of the E3 ubiquitin ligase in
lung cancer cell proliferation and differentiation (9). Additionally, USP47 deubiquitination
stabilizes YAP-associated protein in colorectal cancer progression
(22), while miR-204-5p inhibit
ovarian cancer cell proliferation via downregulated USP47
expression (23). By contrast, long
non-coding RNA Down syndrome cell adhesion molecule antisense
RNA1-upregulated USP47 expression promotes osteosarcoma progression
(24), while USP47 has been shown
to promote TGF-β2-induced breast cancer cell epithelial-mesenchymal
transition (25).
The present study first detected USP47 expression in
PTC tissues vs. normal tissue and its association with
clinicopathological parameters and follow-up data from patients. In
addition the effects of the expression or knockdown of USP47
protein on biological behaviors, such cell proliferation, the
Warburg effect and glutamine metabolism, in the PTC cell lines
TPC-1 and K1 were investigated. The present study aimed to
determine whether targeting USP47 might serve as a novel strategy
for control of PTC.
Materials and methods
PTC specimens and cell culture
A total of 30 pairs of PTC and distant non-tumorous
tissue (2 cm away from the cancerous lesions; there were no tumor
cells confirmed in these tissue specimens) were collected from
patients treated at the Department of Thyroid and Breast Surgery,
The Second Affiliated Clinical School of Medicine, Fujian Medical
University (Quanzhou, China). From October 2020 to October 2022.
The patients aged 19–68 years old (median: 43 years old), included
22 females and 8 males andwere pathologically confirmed with PTC
and staged according to the 2022 World Health Organization
Classification of Thyroid Neoplasms criteria (26); none of the patients had received
presurgical treatments, such as chemotherapy or radiation therapy.
The present study was approved by the Ethics Review Committees of
The Second Affiliated Hospital of Fujian Medical University (2021;
approval no. 149) and all participants provided signed informed
consent. Fresh tissue specimens were collected from the Surgical
Room, quickly frozen in liquid nitrogen and kept at −80°C. The
clinicopathological and follow-up data were obtained from the
patients' medical records.
The human PTC cell lines TPC-1 and K1 were purchased
from Guangzhou Ryder Liankang Biotechnology Co., Ltd. and the K1
cell line authenticated using short tandem repeat profile analysis.
Cells were cultured in RPMI-1640 supplemented with 5% fetal bovine
serum (FBS; cat. no. SH30087.01) and 1% penicillin-streptomycin
(cat. no. SH30010; both Hyclone; Cytiva) in a humidified incubator
with 5% CO2 and 95% air at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total cellular RNA was isolated from cells with
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse-transcribed into cDNA with an RNase-free DNase І kit
(Promega Corporation), according to the manufacturer's
instructions. cDNA samples were subjected to qPCR using 2X SYBR
Green qPCR SuperMix (Invitrogen; Thermo Fisher Scientific, Inc.) in
a BioPhotometer plus Ebend nucleic acid protein analyzer machine
(Eppendorf). The thermocycling conditions were as follows: 50°C for
2 min, 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec and
60°C for 32 sec. The GAPDH mRNA was used as a control and the
relative USP47 mRNA expression was quantified by the
2−ΔΔCq method (27). The
primers sequences were as follows: USP47 forward,
5′-TATGCAGAGTCATGTTTGAT-3′ and reverse, 5′-ATCAAGATATGTGTCGATTC-3′;
SATB1 forward, 5′-GCTCTGCTGCCCAGGCCAAA-3′ and reverse,
5′-CTGTGTAACTGAATTTTCAA-3′ and GAPDH forward,
5′-GCTCATTTGCAGGGGGGAG-3′ and reverse,
5′-GTTGGTGGTGCAGGAGGCA-3′.
Western blotting
Total cellular protein was extracted with
radioimmunoprecipitation assay buffer (cat. no. T1070; Beijing
Solarbio Science & Technology Co., Ltd.) and the concentration
of the samples was assayed with a bicinchoninic acid protein assay
kit (Nanjing KGI Biological Development Co., Ltd.), according to
the manufacturer's protocol. The protein samples (20 µg) were
separated by 10% SDS-PAGE [Roche Diagnostics (Shanghai) Co., Ltd.]
and transferred onto polyvinylidene fluoride membranes (Millipore
Sigma). The membranes were blocked at room temperature for 1 h in
5% non-fat milk and incubated in a solution of phosphate-buffered
saline (PBS) containing primary antibody at 4°C overnight. The
primary antibodies were anti-USP47 (cat. no. ab72143; Abcam;
1:2,000) and anti-special AT-rich sequence-binding protein-1
(SATB1; cat. no. ab6688; Abcam). Afterwards, membranes were washed
three times with Tris hydrochloride + 0.2%Tween-20 and incubated
with a horseradish peroxidase-labeled goat anti-rabbit IgG (H+L)
(cat. no. ab6721; Abcam; dilution of 1:20,000) at the room
temperature for 1 h. Next, membranes were briefly washed three
times with Tris hydrochloride + Tween and the positive protein
signaling was visualized with a Western Blotting Detection kit
(Human IgG; Beijing Solarbio). ImageJ software, version 1.8.0.345
(National Institutes of Health), was used to quantitate the band
density. The GAPDH antibody (1:1,000) (cat. no. ab9485; Abcam) was
used as a control. Western blotting was used to detect the protein
expression levels of Lactate dehydrogenase (LDH), KH domain 2
(KH2), Amino acid transport vehicle 2 (ASCT2), and Glutaminase 1
(GLS1) in USP47 overexpressing cells and empty vector control
cells. LDHA rabbit monoclonal antibody (mAb; cat. no. 3582; Cell
Signaling Technology, Inc; 1:1,000); recombinant anti-HK II
antibody (cat. no. ab209847; Abcam; 1:1,000); recombinant
anti-ASCT2 antibody (cat. no. ab237704; 1:1,000); anti-GLS2
antibody (cat. no. ab150474; Abcam; 1:500).
Plasmids and cell infection
The concentration of nucleic acid was 10 nM and the
generation system used was 2nd generation. The ratio of lentivirus
and packaging and envelope plasmids was 10:8:6. Overexpression
plasmids carrying USP47 and SATB1 cDNA contain the plasmid backbone
of the plvx_zsgreen_pgk_puro (LMAI Bio). SATB1 small interfering
(si)RNA were from Sigma Biotech. siRNA sequences were as follows:
SATB1-siRNA-1, 5′-AUUUCUGAAUGUUCUUUCCCCdTdT-3′; SATB1-siRNA-2,
5′-UAGACAUUUCUGAAUGUUCUUdTdT-3′; SATB1-siRNA-3,
5′-UGUUAGACAUUUCUGAAUGUUdTdT-3′ and NC-siRNA,
5′-GAACUGGGGUGCGUGUGAUdTdT-3′. USP47 (si)RNA and NC) targeting cDNA
sequences were synthesized by Guangzhou Ribobio Co., Ltd. as
follows: USP47 siRNA-1, 5′-UACAACCAUGCAUAGGAUUAdTdT-3′; USP47
siRNA-2, 5′-AACGCUGCUACAAUGAUUUGdTdT-3′; USP47 siRNA-3,
5′-AGCAGUCGACUCCAGAAGACdTdT-3′ and USP47 siRNA-NC,
5′-UUCUCCGAACGUGUCACGUUUdTdT-3′. These DNA sequences were cloned
into pLKO.1 lentiviral vector (Addgene, Inc.). Lentiviruses were
packaged and produced in 293(T) cells (Guangzhou Landliangkang
Biotechnology Co., Ltd.) following vector transfection using
Lipofectamine 2000 reagent (cat. no. 11668019; Invitrogen; Thermo
Fisher Scientific, Inc.) for 24 h at 37°C and supernatant was
collected. In brief, TPC-1 or K1 cells were plated into 24-well
cell culture plates (5×104 cells/well), grown at 37°C
overnight, then infected with viral supernatant or control viral
particles at MOI of 10 for 48 h and efficiency of these si-RNAs was
analyzed; the most effective siRNA was used for subsequent
experiments. Stably transduced cells were selected using
puromycin-containing RPMI-1640. The screening concentration was 12
µg/ml and the maintenance concentration was 4 µg/ml).
ELISA
Levels of lactate, ATP, glucose consumption,
glutamate, α-ketoglutarate and glutamine following knockdown of
USP47 expression or SATB1 expression were assessed using ELISA.
kits for lactate (cat. no. WK-SU62; Shanghai Valan Biotechnology
Co., Ltd.), ATP content (cat. no. LE-H3380; Lyell Biological Co.),
glucose consumption (cat. no. Keshun-0017; Shanghai Keshun
Biotechnology Co.), glutamate (cat. no. 140739; Nanjing Senberga
Biotechnology Co.), α-ketoglutarate (cat. no. XGE64578; Shanghai
Sig Biotechnology Co.) and glutamine (cat. no. KL-Gln-Hu; Kanglang
Biotechnology Co.) according to the manufacturer's protocols.
Experiments were performed in duplicate and repeated at least
twice.
Cell proliferation assay
Cells with stable USP47 siRNA infection were plated
into 96-well plates with 1×104 cells/well and grown for
0, 24, 48 and 72 h at 37°C. The cellTiter96AQ single solution (cat.
no. G3582; Promega Corporation) was added to cell culture medium at
a ratio of 1:10 and the cells were further cultured at 37°C for 4
h. The optical density of the cell cultures was measured with a
microplate reader (Multiscan MK3; Thermo Fisher Scientific, Inc.)
at 490 nm and the data were quantified as the percentage of the
control. The experiment was performed in triplicate and repeated
three times.
Transwell assay
Transwell chambers were obtained from Corning, Inc.
to assess PTC cell mobility. In brief, PTC cells were resuspended
in 100 µl serum-free RPMI-1640 and plated into the upper chambers
(1×104 cells per chamber). The filter was precoated with
Matrigel (Corning, Inc.) for 48 h at 37°C for the tumor cell
invasion assay or without Matrigel for the migration assay. A total
of 100 µl RPMI-1640 containing 20% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.) was added to the bottom chambers and the cells
were cultured at 37°C for 12–48 h. Cells remaining on the surface
of the filter were removed using a cotton swab, whereas cells that
had migrated or invaded to the other side of the filters were fixed
in 4% paraformaldehyde at 4°C for 30 min, washed with distilled
water (twice; 2 min), stained with 0.1% crystal violet for 10 min
at room temperature and manually counted under an inverted
fluorescence microscope (Olympus CKX41 and U-CTR30-2). A total of
five microscopic fields (400× magnification) were randomly selected
for each chamber. The assay was performed in duplicate and repeated
three times.
Statistical analysis
IBM SPSS Statistics 27 software was used for
statistical analysis. The paired t test was used to compare paired
samples, while the independent sample t test was used to compare
the mean difference between the two groups. One-way ANOVA) was used
to compare the mean differences between three or more groups. A
total of three independent experiments was performed. Data are
presented as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
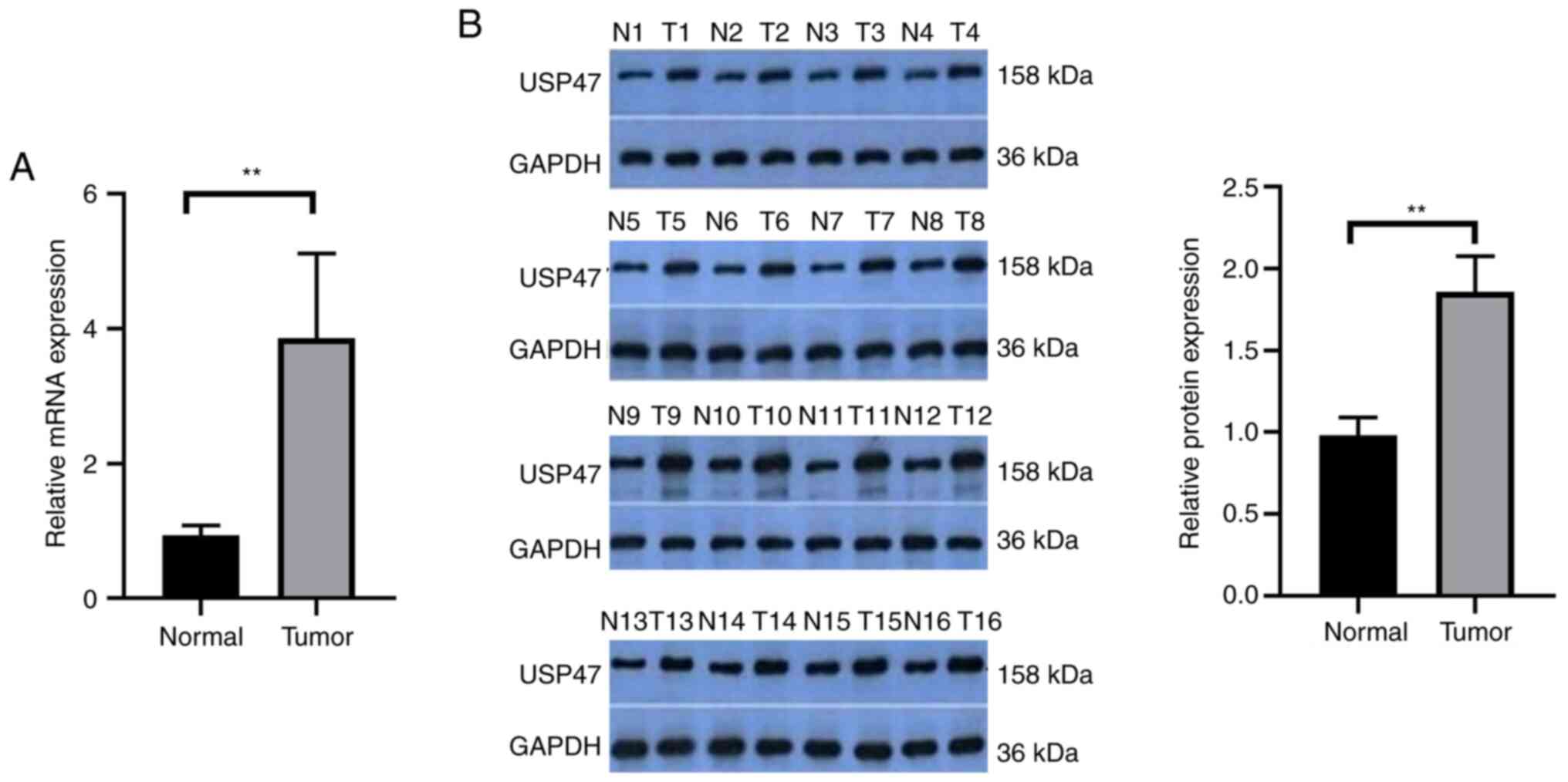
USP47 is upregulated in PTC
tissue
The present study detected the levels of USP47 mRNA
and protein in 30 paired PTC and distant normal tissue samples.
USP47 mRNA and protein were significantly upregulated in PTC tissue
(Fig. 1A and B). Analysis of USP47
mRNA and clinicopathological data from the patients demonstrated
that the expression of USP47 mRNA was associated with tumor size
but not the number of lesions, lymph node metastasis or
extrathyroidal extension of PTC (Table
I).
 | Table I.Association of USP47 mRNA expression
with clinicopathological characteristics of patients with PTC. |
Table I.
Association of USP47 mRNA expression
with clinicopathological characteristics of patients with PTC.
| Characteristic | Number | Relative USP47
expression | T-value | P-value |
|---|
| Sex |
|
|
|
|
|
Female | 22 | 3.89±0.25 | 0.215 | 0.81 |
|
Male | 8 | 3.77±0.52 |
|
|
| Age at diagnosis,
years |
|
|
|
|
|
≤45 | 18 | 4.02±0.30 | 0.901 | 0.38 |
|
>45 | 12 | 3.61±0.33 |
|
|
| Tumor size, cm |
|
|
|
|
|
<2 | 28 | 3.90±0.24 | 2.149 | 0.04 |
| ≥2 | 2 | 3.31±0.12 |
|
|
| N stage (AJCC) |
|
|
|
|
| N0 or
Nx | 12 | 3.93±0.37 | 0.257 | 0.79 |
| N1 | 18 | 3.81±0.29 |
|
|
| Gland outside
invasion |
|
|
|
|
| No | 23 | 4.04±0.25 | 1.507 | 0.14 |
|
Yes | 7 | 3.25±0.46 |
|
|
| Tumor location |
|
|
|
|
| One
lobe | 24 | 9.21±1.32 | 0.928 | 0.36 |
| More
than one lobe | 6 | 9.68±0.82 |
|
|
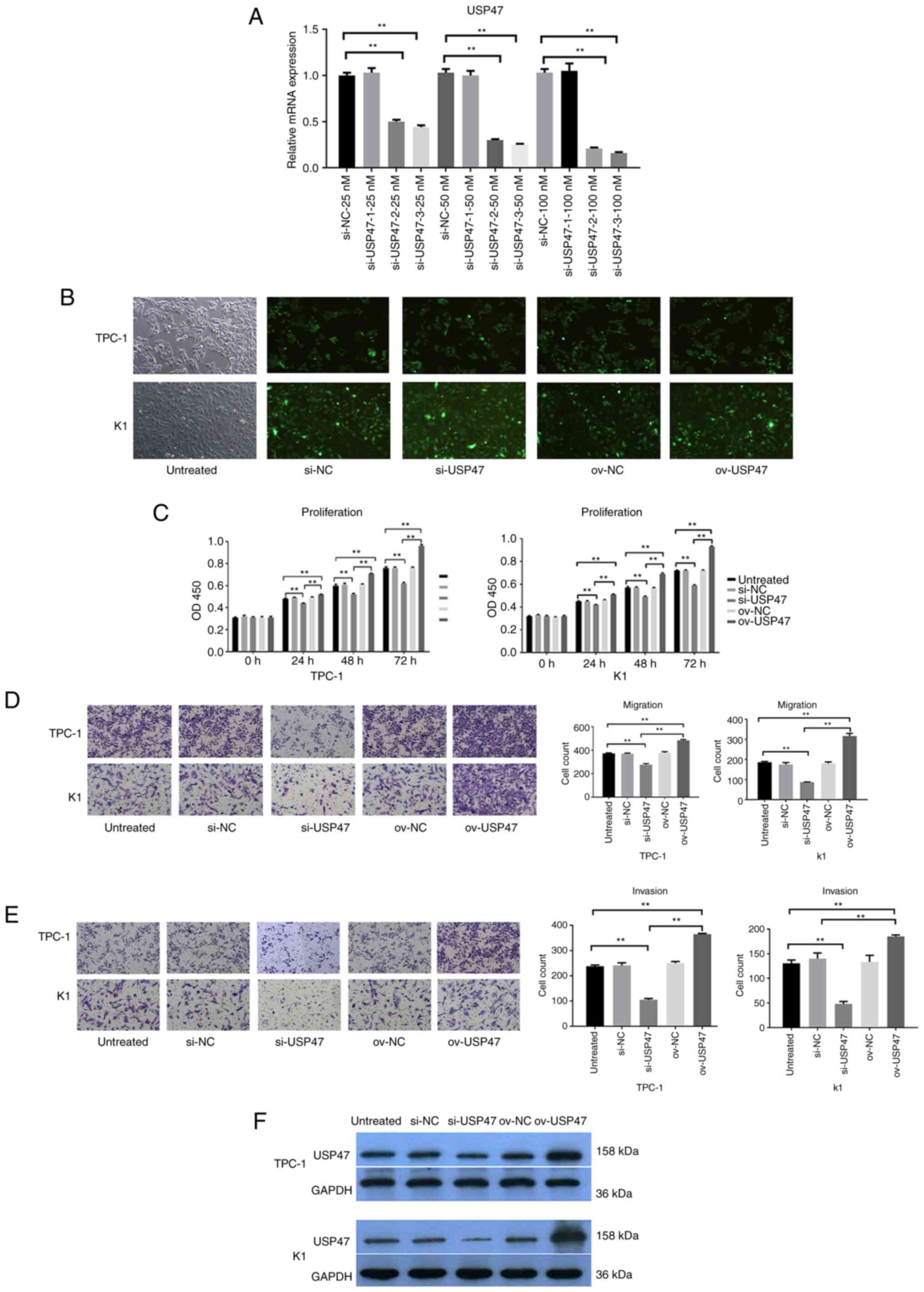
PTC cell proliferation, invasion and
migration is decreased following after knockdown of USP47
expression
To assess the role of USP47 protein in PTC, USP47
expression was knocked down in PTC cell lines with USP47 siRNA.
RT-qPCR data showed that the USP47 mRNA expression was dramatically
reduced after the introduction of siRNA-3 compared with that of
USP47si-NC in both the TPC-1 and K1 cell lines (Fig. 2A) USP47si-3 had the highest
interference efficiency, and it was selected for subsequent
experiments (Fig. 2B). Cell
proliferation assay revealed that knockdown of USP47 expression led
to a significant decrease in TPC-1 and K1 cell proliferation
(Fig. 2C). Transwell assay revealed
that the migration and invasion abilities of both TPC-1 and K1
cells were significantly inhibited following transfection with
USP47si-3 (Fig. 2D and E). The
aforementioned experiments confirmed that USP47 promoted the
proliferation, invasion, and migration of PTC cells.
USP47 knockdown downregulates the
Warburg effect in PTC in vitro
To understand the effects of USP47 expression and
knockdown in PTC cells, lactate production, lactate dehydrogenase
(LDH) and hexokinase 2 (HK2) activity, ATP content and glucose
consumption were assessed. USP47-overexpressing TPC-1 and K1 cells
demonstrated increased lactate production as well as LDH and HK2
activity compared with vector control-infected cells, whereas
knockdown of USP47 expression in PTC-1 and K1 cells decreased
lactate production, as well as LDH and HK2 activity (Fig. 3A and B). Furthermore, PTC-1 and K1
cells with USP47 overexpression showed a lower ATP content and
glucose consumption than vector control-infected cells, but
knockdown of USP47 expression resulted in higher ATP content and
glucose consumption (Fig. 3C and
D).
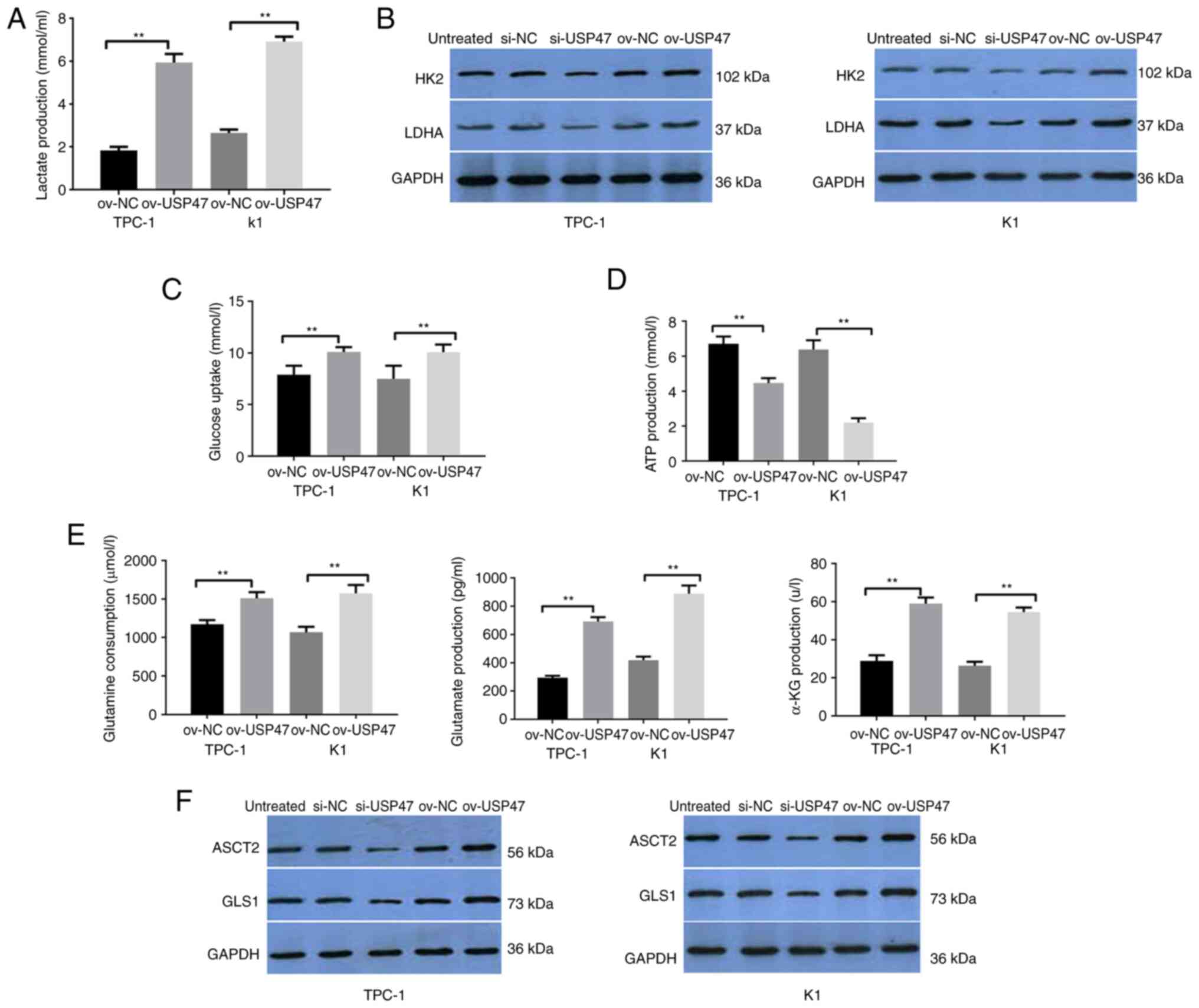 | Figure 3.USP47 promotes the Warburg effect and
glutamine catabolism in PTC cells in vitro. (A) ELISA was
performed to assess (A) lactate production, Western blot to assess
(B) glucose metabolism key enzymes HK2 and LDHA, ELISA to assess
(C) glucose uptake, (D) ATP content and (E) glutamine, glutamate
and α-KG consumption. Western blot analysis (F) of key enzymes
ASCT2 and GLS1 for glutamine metabolism in TPC-1 and K1 cells with
stable USP47 ov. USP47, Ubiquitin carboxyl-terminal hydrolase 47;
PTC, Papillary thyroid carcinoma; LDHA, lactate dehydrogenase; HK2,
Hexokinase 2; ASCT2, Amino acid transport vehicle 2; α-KG,
ketoglutaric acid; GLS1, Glutaminase 1; ov, overexpression; NC,
negative control; si, small interfering. **P<0.01. |
USP47 regulates glutamine
metabolism
Glutamine catabolism is a characteristic metabolic
pattern following cancer cell metabolic reprogramming (28). Thus, the present study assessed
glutamine consumption, glutamate and α-ketoglutarate production and
the enzymatic activity of ASCT2 and GLS1 in TPC-1 and K1 cells with
USP47 overexpression. Compared with the empty vector cells,
glutamine consumption as well as glutamate and α-ketoglutarate
production in PTC-1 and K1 cells overexpressing USP47 were all
increased (Fig. 3E) and levels of
ASCT2 and GLS1 protein were also higher in these cells (Fig. 3F).
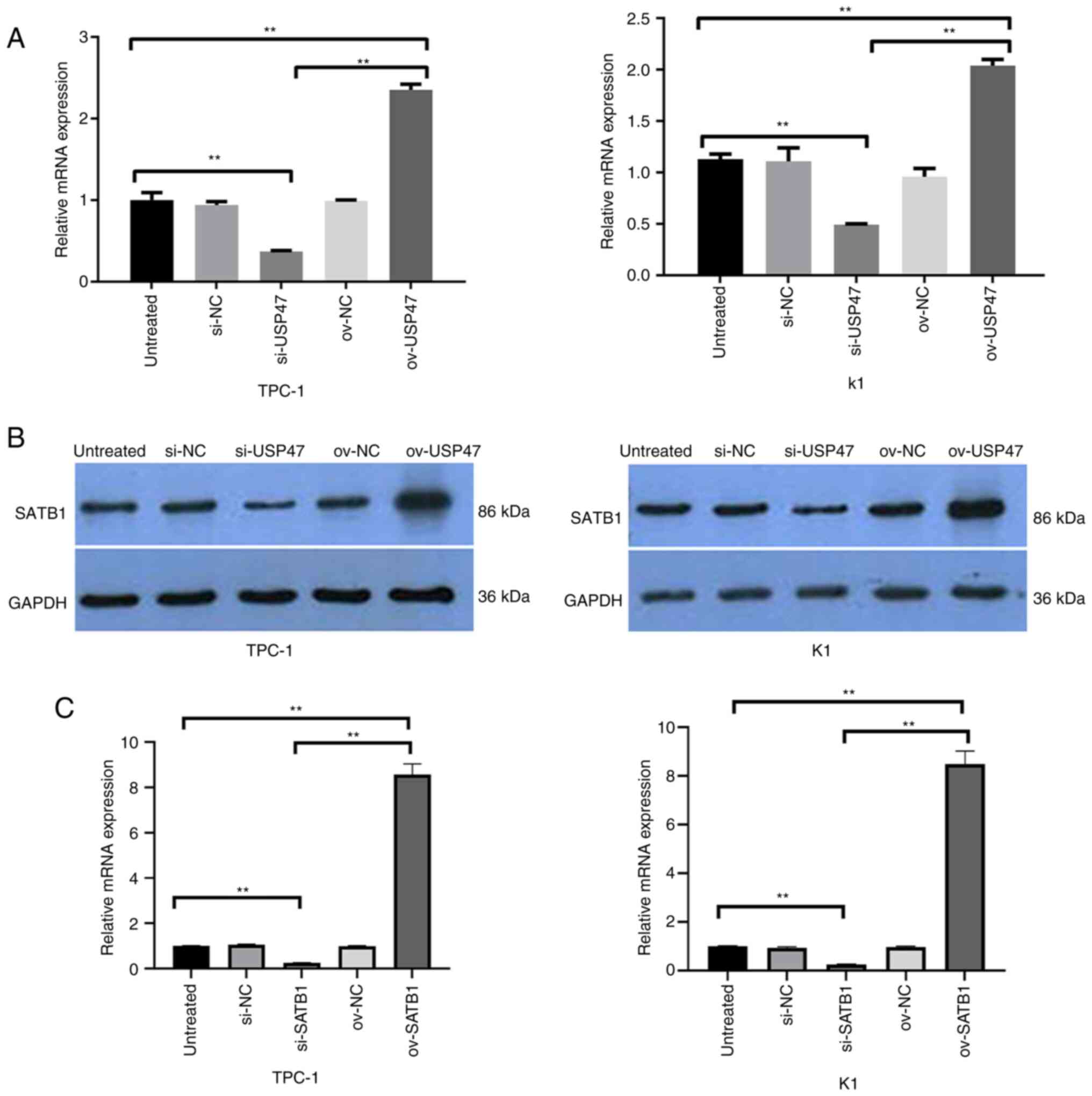
USP47 interacts with SATB1 in PTC
cells in vitro
A previous study has shown that USP47 expression
promotes colon cancer progression via STAB1 deubiquitination
(29), while another study revealed
high SATB1 expression in PTC tissue, which is associated with a
poor PTC prognosis (30).
Therefore, the present study detected the levels of SATB1 mRNA and
protein in TPC-1 and K1 cells following USP47 overexpression and
knockdown. SATB1 mRNA and protein levels were both decreased in
USP47 knockdown TPC-1 and K1 cells, whereas USP47 expression
increased expression of SATB1 mRNA and protein in PTC cells
(Fig. 4A and B). Knockdown of SATB1
was verified by using RT-qPCR in PTC cells (Fig. 4C).
SATB1 promotes glucose and glutamine
metabolism in PTC cells in vitro
SATB1 expression was knocked down in PTC cells,
resulting in decreased lactate production, glucose consumption and
LDH and HK2 activity. ATP content was increased in PTC-1 and K1
cells following knockdown of SATB1 expression, whereas SATB1
overexpression decreased lactate production, glucose consumption
and LDH and HK2 activity) (Fig.
5A-D). Furthermore, compared with the control cells, PTC-1 and
K1 cells infected with USP47 siRNA showed reduced glutamine
consumption, glutamate and α-ketoglutarate production and ASCT2 and
GLS1 activities; overexpression of SATB1 in PTC cells increased
glutamine depletion, production of glutamate and α-ketoglutaric
acid, and ASCT2 and GLS1 activities (Fig. 5E and F).
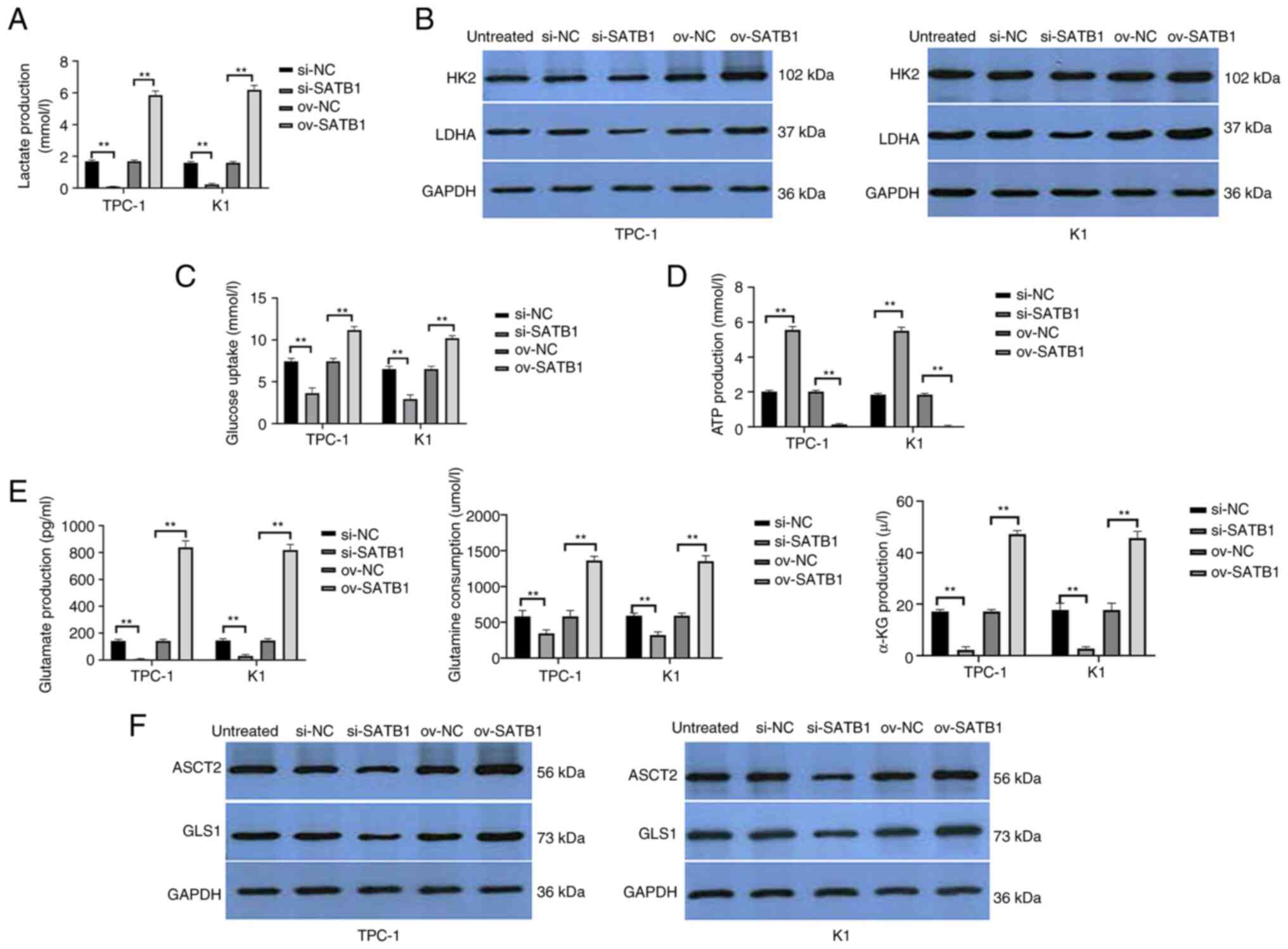 | Figure 5.SATB1 regulates the Warburg effect
and glutamine catabolism in vitro in papillary thyroid
carcinoma cells. (A) ELISA was performed with stable
SATB1-overexpressing or -knockdown TPC-1 and K1 cells for analysis
of (A) lactate, Western blot to assess (B) glucose metabolism key
enzymes HK2 and LDHA, ELISA to assess (C) glucose uptake, (D) ATP
content and (E) glutamine, glutamate and α-KG consumption. Western
blot analysis (F) of key enzymes ASCT2 and GLS1 for glutamine
metabolism. SATB1, special AT-rich sequence-binding protein-1; LDH,
Lactate dehydrogenase; HK2, Hexokinase 2; ASCT2, amino acid
transport vehicle 2; GLS1, glutaminase 1; si, small interfering;
NC, negative control; ov, overexpression. **P<0.01. |
Discussion
The present study analyzed the expression of USP47
mRNA and protein in 30 paired PTC and normal tissue samples. The
expression of USP47 mRNA and protein was upregulated in PTC
compared with normal tissues and that upregulated USP47 mRNA
expression was associated with the PTC tumor size. Effects of USP47
and SATB1 overexpression and knockdown on the malignant behaviors
of tumor cells were assessed. USP47 expression induced PTC cell
proliferation, migration and invasion, whereas knockdown of USP47
expression caused the opposite effects in PTC cells in
vitro. Compared with vector control-infected cells,
USP47-overexpressing TPC-1 and K1 cells showed increased lactate
production as well as LDH and HK2 levels, whereas knockdown of
USP47 expression demonstrated decreased lactate production as well
as LDH and HK2 activity in PTC-1 and K1 cells. However,
USP47-overexpressing PTC-1 and K1 cells exhibited lower ATP content
and glucose consumption than vector control-infected cells, whereas
knockdown of USP47 expression had the opposite effects. In
addition, USP47 interacted with and stabilized SATB1 protein in PTC
cells in vitro and knockdown of SATB1 expression in PTC
cells decreased the levels of lactate production and glucose
consumption as well as LDH and HK2 activity but increased the ATP
content; SATB1 overexpression had the opposite effects. PTC-1 and
K1 cells infected with USP47 siRNA showed decreased glutamine
consumption, glutamate and α-ketoglutarate production and ASCT2 and
GLS1 activities; overexpression of SATB1 in PTC cells had the
opposite effects. In summary, the present study demonstrated that
USP47 expression induced the Warburg effect and glutamine
metabolism in PTC cells by stabilizing SATB1 expression during the
progression of PTC. Future studies should investigate knockdown of
USP47 and/or SATB1 expression as a therapeutic strategy to control
PTC progression clinically.
The recombinant human USP47 protein has been
characterized in a previous study (31). USP47 is a monomeric protein ~146 kDa
in size. Substitution of Cys109 with Ser significantly abrogates
enzymatic activity of USP47 and that N-ethylmaleimide, Zn ion and
ubiquitin aldehyde can also dose-dependently inhibit the activity
of USP47. In addition, a recent proteomics analysis demonstrated
that USP47 plays a role in regulating breast cancer cell
epithelial-mesenchymal transition following TGF-β2 treatment
(25). Moreover, USP47 has been
shown to mediate deubiquitination and stabilization of
yes-associated protein during colorectal cancer progression
(22). USP47 enhances tumorigenesis
via downregulation of p53 by deubiquitination of ribosomal protein
S2 (32). Furthermore, loss of
USP47 expression decreases the transcription of SATB1-targeting
genes and suppresses proliferation, migration and tumorigenesis in
a mouse colon cancer model (29).
USP47 interacts with β-transducin repeat-containing protein, which
promotes cell survival (33).
Knockdown of USP47 expression has been demonstrated to overcome
chronic myelogenous leukemia resistance to a tyrosine kinase
inhibitor and to eliminate leukemia stem/progenitor cells (34) and USP47 can also regulate the
stemness of colorectal cancer cells (35). The aforementioned studies indicate
that USP47 can be an oncogene or exert oncogenic activity in human
malignancies. The present study confirmed that USP47 was highly
expressed in PTC tissue and that increased USP47 mRNA expression
was associated with tumor size in patients with PTC. Moreover,
USP47 expression induced PTC cell growth and mobility, whereas
knockdown of USP47 expression exerted the opposite effects in PTC
cells in vitro. Compared with the vector control-infected
cells, USP47-overexpressing TPC-1 and K1 cells exhibited an
increase in the Warburg effect. The present findings in PTC cells
were consistent with those of previous studies of other human
malignancy (22,25,29,32,33),
although the underlying gene targets may be different.
SATB1 serves as the ‘genome organizer’ to organize
chromatin into spatial loops, which serve as docking sites for
transcription factor binding and chromatin-modifying enzymes
(29,36,37). A
previous study reported that SATB1 expression promotes PTC
progression, whereas inhibition of SATB1 expression reverses the
malignant biological behaviors of TPC-1 cells (30). SATB1 can reprogram chromatin and
tumor cell transcription profiles to enhance tumor cell growth and
mobility and inhibit apoptosis resulting in disease progression
(36,38). SATB1 expression is upregulated in
various human cancers (39–41). For example, knockdown of SATB1
suppresses esophageal cancer cell proliferation and mobility and
SATB1 promotes esophageal cancer development by upregulating
expression of fibronectin and platelet-derived growth factor
receptor β (36). Thus, targeting
SATB1 may effectively control human cancers. Our previous study
showed that the protein and mRNA expression of SATB1 is upregulated
in PTC and associated with USP47 expression in PTC tissue (30). Nevertheless, additional s Studies
are required to reveal how USP47 regulates SATB1 expression
mechanistically.
Ubiquitylation is an enzymatic modification of
translated proteins in cells that is necessary for a variety of
cell functions, such as genomic maintenance, cell cycle, antigen
processing, DNA repair and cell differentiation and development
(42,43); however, deubiquitination is
associated with cell functions such as cell cycle distribution,
proteasome- and lysosome-dependent protein degradation, DNA repair
and microbial pathogenesis (44).
Deubiquitination is the reverse process of ubiquitination, the
primary role of which is to remove ubiquitin from substrates
(45). For example, USP47, a
deubiquitinase, maintains the stemness of colorectal cancer cells
(35) and promotes the
phosphorylation and survival of RelA gastric cancer cells (46). USP47 promotes lung squamous cell
carcinoma cell proliferation (47)
and stabilizes BTB domain and CNC homolog 1 for induction of the
Warburg effect in non-small cell lung cancer (9). By contrast, a recent study
demonstrated that long non-coding RNA zinc finger protein 883
mediated USP47 upregulation suppresses NLRP3 ubiquitination to
induce epilepsy development (48).
The present data revealed that USP47 expression induced PTC cell
proliferation and invasion via upregulation of the Warburg effect
and glutamine metabolism in PTC cells. However, the present data
need to be confirmed in vivo and in the clinic. It is also
necessary to investigate how USP47 regulates SATB1 and its
downstream factors in the control of PTC progression.
Nevertheless, the present study had limitations. For
example, the present study demonstrated that USP47 promoted
malignant biological behavior and metabolism of PTC but did not
determine the mechanism of USP47 action. Second, the present
results do not prove that STAB1 is stabilized by USP47; further
studies are required to determine the underlying regulatory
mechanism.
To the best of our knowledge, the present study is
the first to demonstrate upregulated USP47 expression in PTC
tissues, which was associated with the PTC tumor size. Moreover,
USP47 overexpression induced PTC cell viability and mobility by
enhancing the Warburg effect and glutamine metabolism. At the gene
level, USP47 interacted with SATB1 protein to stabilize it, thereby
enhancing glucose and glutamine metabolism in PTC cells. The
present study suggested that targeting the USP47/SATB1 axis may be
a potentially novel approach for controlling PTC progression in the
clinic.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant from the
Fujian Provincial Natural Science Foundation (grant no.
2021J01251).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL, WW and LihZ performed experiments. JL, XW and HS
analyzed the results. GL wrote and revised the manuscript. LitZ, YY
and WQ designed the study. YY and WQ revised the manuscript. JC. HD
and XC have made substantial contributions to interpretation of
data. All authors have read and approved the final manuscript. GL
and WW confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no. 149) by
the Ethics Review Committees of the Second Affiliated Hospital,
Fujian Medical University Fujian, China and followed the principles
of the Declaration of Helsinki. Written informed consent was
obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitahara CM, Schneider AB and Brenner AV:
Thyroid cancer. Thun M, Linet MS, Cerhan JR, Haiman CA and
Schottenfeld D: Cancer Epidemiology and Prevention. 4th edition.
Oxford University Press; pp. 839–860. 2018
|
|
4
|
Carling T and Udelsman R: Thyroid cancer.
Annu Rev Med. 65:125–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chou R, Dana T, Haymart M, Leung AM,
Tufano RP, Sosa JA and Ringel MD: Active surveillance versus
thyroid surgery for differentiated thyroid cancer: A systematic
review. Thyroid. 32:351–367. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Udelsman R and Zhang YW: The epidemic of
thyroid cancer in the United States: The role of endocrinologists
and ultrasounds. Thyroid. 24:472–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stenman A, Backman S, Johansson K,
Paulsson JO, Stålberg P, Zedenius J and Juhlin CC: Pan-genomic
characterization of high-risk pediatric papillary thyroid
carcinoma. Endocr Relat Cancer. 28:337–351. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito Y, Miyauchi A, Kihara M, Fukushima M,
Higashiyama T and Miya A: Overall survival of papillary thyroid
carcinoma patients: A single-institution long-term follow-up of
5897 patients. World J Surg. 42:615–622. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng J, Li W, Tan N, Lai X, Jiang W and
Chen G: USP47 stabilizes BACH1 to promote the Warburg effect and
non-small cell lung cancer development via stimulating Hk2 and
Gapdh transcription. Am J Cancer Res. 12:91–107. 2022.PubMed/NCBI
|
|
10
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
López-Lázaro M: The warburg effect: Why
and how do cancer cells activate glycolysis in the presence of
oxygen? Anticancer Agents Med Chem. 8:305–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan
Q, Luo J, Zen K and Yang JW: Inhibiting aerobic glycolysis
suppresses renal interstitial fibroblast activation and renal
fibrosis. Am J Physiol Renal Physiol. 313:F561–F575. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu H, Zhu J, Chang L, Liang C, Li X and
Wang W: 3-Bromopyruvate decreased kidney fibrosis and fibroblast
activation by suppressing aerobic glycolysis in unilateral ureteral
obstruction mice model. Life Sci. 272:1192062021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jing YY, Cai FF, Zhang L, Han J, Yang L,
Tang F, Li YB, Chang JF, Sun F, Yang XM, et al: Epigenetic
regulation of the Warburg effect by H2B monoubiquitination. Cell
Death Differ. 27:1660–1676. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, He Y, Tan Z, Lu J, Li L, Song X, Shi
F, Xie L, You S, Luo X, et al: Wild-type IDH2 promotes the Warburg
effect and tumor growth through HIF1α in lung cancer. Theranostics.
8:4050–4061. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cha YJ, Kim ES and Koo JS: Amino acid
transporters and glutamine metabolism in breast cancer. Int J Mol
Sci. 19:9072018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Geldermalsen M, Wang Q, Nagarajah R,
Marshall AD, Thoeng A, Gao D, Ritchie W, Feng Y, Bailey CG, Deng N,
et al: ASCT2/SLC1A5 controls glutamine uptake and tumour growth in
triple-negative basal-like breast cancer. Oncogene. 35:3201–3208.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernhardt S, Bayerlová M, Vetter M,
Wachter A, Mitra D, Hanf V, Lantzsch T, Uleer C, Peschel S, John J,
et al: Proteomic profiling of breast cancer metabolism identifies
SHMT2 and ASCT2 as prognostic factors. Breast Cancer Res.
19:1122017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mukha A, Kahya U and Dubrovska A:
Targeting glutamine metabolism and autophagy: The combination for
prostate cancer radiosensitization. Autophagy. 17:3879–3881. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Q, Beaumont KA, Otte NJ, Font J,
Bailey CG, van Geldermalsen M, Sharp DM, Tiffen JC, Ryan RM,
Jormakka M, et al: Targeting glutamine transport to suppress
melanoma cell growth. Int J Cancer. 135:1060–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fraile JM, Quesada V, Rodriguez D, Freije
JMP and López-Otín C: Deubiquitinases in cancer: New functions and
therapeutic options. Oncogene. 31:2373–2388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan B, Yang Y, Li J, Wang Y, Fang C, Yu FX
and Xu Y: USP47-mediated deubiquitination and stabilization of YAP
contributes to the progression of colorectal cancer. Protein Cell.
11:138–143. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu L, Kolibaba H, Zhang S, Cao M, Niu H,
Mei H, Hao Y, Xu Y and Yin Q: MicroRNA-204-5p inhibits ovarian
cancer cell proliferation by down-regulating USP47. Cell
Transplant. 28 (1 Suppl):51S–58S. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Ding L, Gao F and Fan H: Long
non-coding RNA DSCAM-AS1 upregulates USP47 expression through
sponging miR-101-3p to accelerate osteosarcoma progression. Biochem
Cell Biol. 98:600–611. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silvestrini VC, Thomé CH, Albuquerque D,
de Souza Palma C, Ferreira GA, Lanfredi GP, Masson AP, Delsin LEA,
Ferreira FU, de Souza FC, et al: Proteomics analysis reveals the
role of ubiquitin specific protease (USP47) in epithelial to
mesenchymal transition (EMT) induced by TGFβ2 in breast cells. J
Proteomics. 219:1037342020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cameselle-Teijeiro JM: Changes and
perspectives in the new 2022 WHO classification of thyroid
neoplasms. Rev Esp Patol. 55:145–148. 2022.(In Spanish). PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Moss T, Mangala LS, Marini J, Zhao
H, Wahlig S, Armaiz-Pena G, Jiang D, Achreja A, Win J, et al:
Metabolic shifts toward glutamine regulate tumor growth, invasion
and bioenergetics in ovarian cancer. Mol Syst Biol. 10:7282014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu L, Dong L, Wang Y, Liu L, Long H, Li H,
Li J, Yang X, Liu Z, Duan G, et al: Reversible regulation of SATB1
ubiquitination by USP47 and SMURF2 mediates colon cancer cell
proliferation and tumor progression. Cancer Lett. 448:40–51. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin Q, ZL, Wu W, Lin J, Shi H, Wu X, Yu Y,
Ding M, Huang Z and Qiu J: Role of specific nuclear matrix binding
domain binding protein 1 and microRNA-495-3P in the invasion and
metastasis of papillary thyroid carcinoma. Chin J Exp Surg.
38:139–143. 2021.(In Chinese).
|
|
31
|
Piao J, Tashiro A, Nishikawa M, Aoki Y,
Moriyoshi E, Hattori A and Kakeya H: Expression, purification and
enzymatic characterization of a recombinant human
ubiquitin-specific protease 47. J Biochem. 158:477–484.
2015.PubMed/NCBI
|
|
32
|
Cho J, Park J, Shin SC, Jang M, Kim JH,
Kim EE and Song EJ: USP47 promotes tumorigenesis by negative
regulation of p53 through deubiquitinating ribosomal protein S2.
Cancers (Basel). 12:11372020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peschiaroli A, Skaar JR, Pagano M and
Melino G: The ubiquitin-specific protease USP47 is a novel
beta-TRCP interactor regulating cell survival. Oncogene.
29:1384–1393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lei H, Xu HZ, Shan HZ, Liu M, Lu Y, Fang
ZX, Jin J, Jing B, Xiao XH, Gao SM, et al: Targeting USP47
overcomes tyrosine kinase inhibitor resistance and eradicates
leukemia stem/progenitor cells in chronic myelogenous leukemia. Nat
Commun. 12:512021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang S, Ju X, Yang Q, Zhu Y, Fan D, Su G,
Kong L and Li Y: USP47 maintains the stemness of colorectal cancer
cells and is inhibited by parthenolide. Biochem Biophys Res Commun.
562:21–28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glatzel-Plucińska N, Piotrowska A,
Dzięgiel P and Podhorska-Okołów M: The role of SATB1 in tumour
progression and metastasis. Int J Mol Sci. 20:41562019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kohwi-Shigematsu T, Kohwi Y, Takahashi K,
Richards HW, Ayers SD, Han HJ and Cai S: SATB1-mediated functional
packaging of chromatin into loops. Methods. 58:243–254. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding M, Pan J, Guo Z, Liu Q, Yang C and
Mao L: SATB1 is a novel molecular target for cancer therapy. Cancer
Invest. 36:28–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qi H, Fu X, Li Y, Pang X, Chen S, Zhu X,
Li F and Tan W: SATB1 promotes epithelial-mesenchymal transition
and metastasis in prostate cancer. Oncol Lett. 13:2577–2582. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang S, Tong YX, Xu XS, Lin H and Chao
TF: Prognostic significance of SATB1 in gastrointestinal cancer: A
meta-analysis and literature review. Oncotarget. 8:48410–48423.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou D, Ye C, Pan Z and Deng Y: SATB1
knockdown inhibits proliferation and invasion and decreases
chemoradiation resistance in nasopharyngeal carcinoma cells by
reversing EMT and suppressing MMP-9. Int J Med Sci. 18:42–52. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mukhopadhyay D and Riezman H:
Proteasome-independent functions of ubiquitin in endocytosis and
signaling. Science. 315:201–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peng J, Schwartz D, Elias JE, Thoreen CC,
Cheng D, Marsischky G, Roelofs J, Finley D and Gygi SP: A
proteomics approach to understanding protein ubiquitination. Nat
Biotechnol. 21:921–926. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Reyes-Turcu FE, Ventii KH and Wilkinson
KD: Regulation and cellular roles of ubiquitin-specific
deubiquitinating enzymes. Annu Rev Biochem. 78:363–397. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Heger K, Wickliffe KE, Ndoja A, Zhang J,
Murthy A, Dugger DL, Maltzman A, de Sousa E Melo F, Hung J, Zeng Y,
et al: OTULIN limits cell death and inflammation by
deubiquitinating LUBAC. Nature. 559:120–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Naghavi L, Schwalbe M, Ghanem A and
Naumann M: Deubiquitinylase USP47 promotes RelA phosphorylation and
survival in gastric cancer cells. Biomedicines. 6:622018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu L, Fu J and Shen C: Ubiquitin specific
peptidase 47 promotes proliferation of lung squamous cell
carcinoma. Genes Genomics. 44:721–731. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gong L, Han Y, Chen R, Yang P and Zhang C:
LncRNA ZNF883-mediated NLRP3 inflammasome activation and epilepsy
development involve USP47 upregulation. Mol Neurobiol.
59:5207–5221. 2022. View Article : Google Scholar : PubMed/NCBI
|