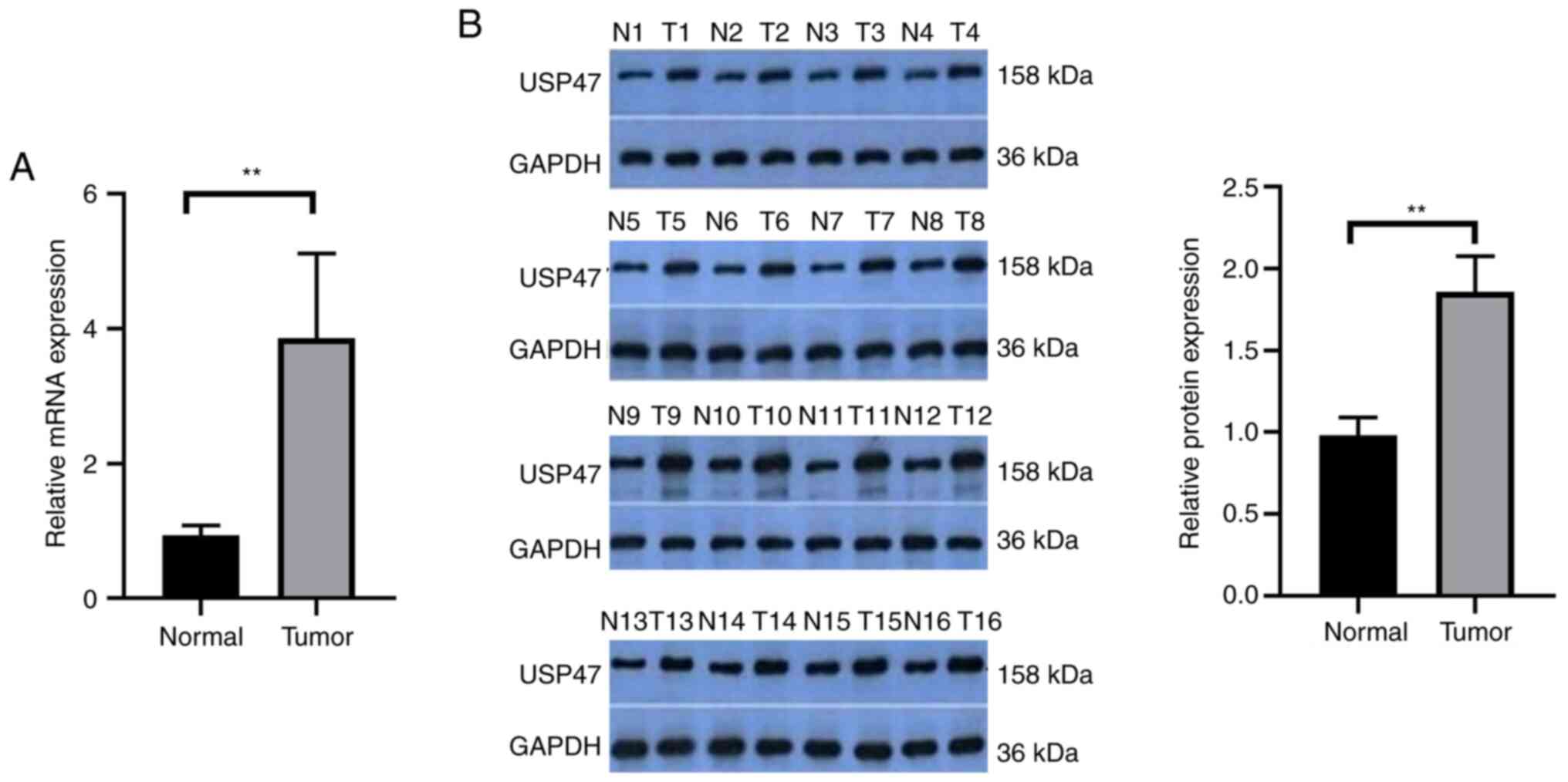
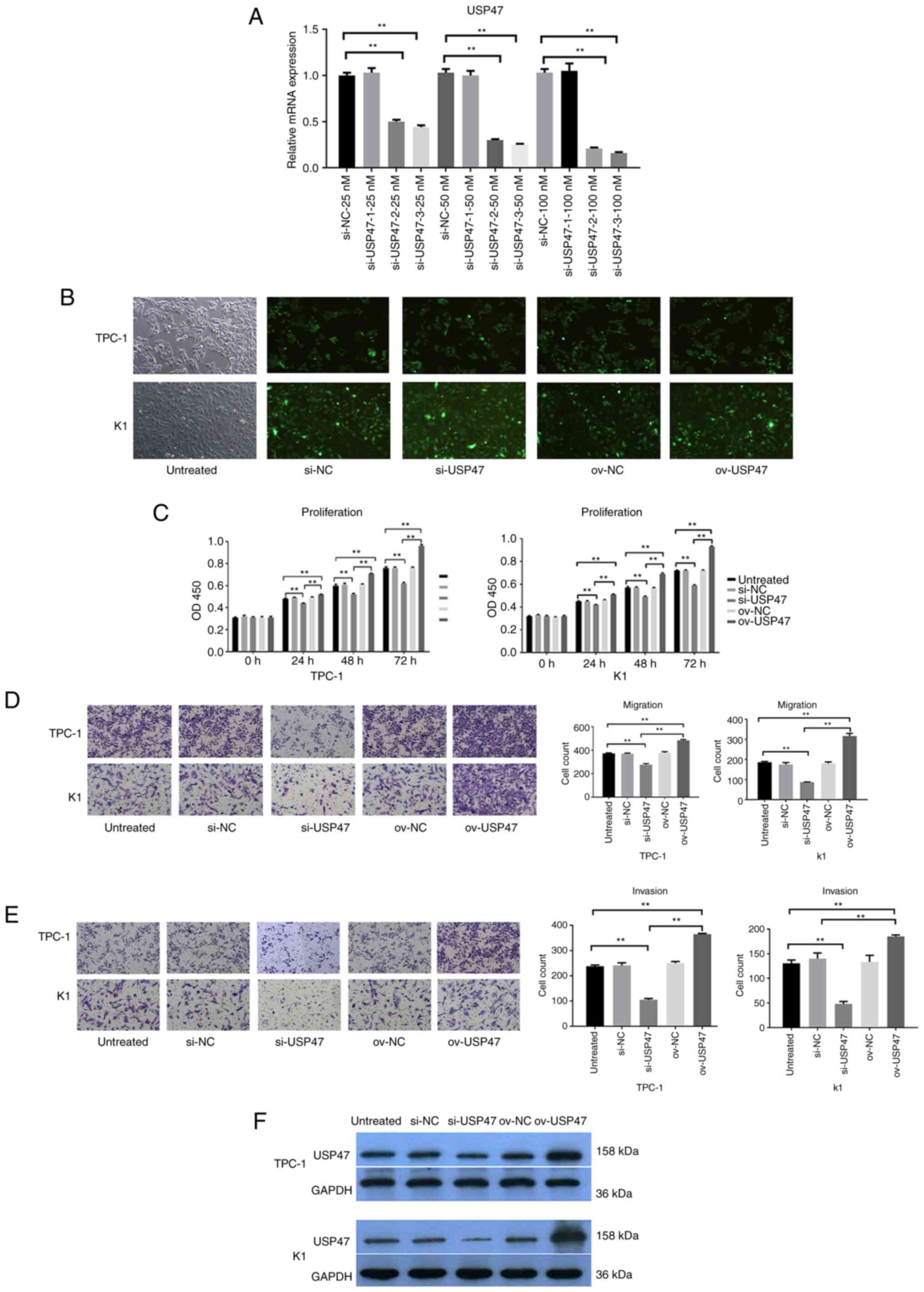
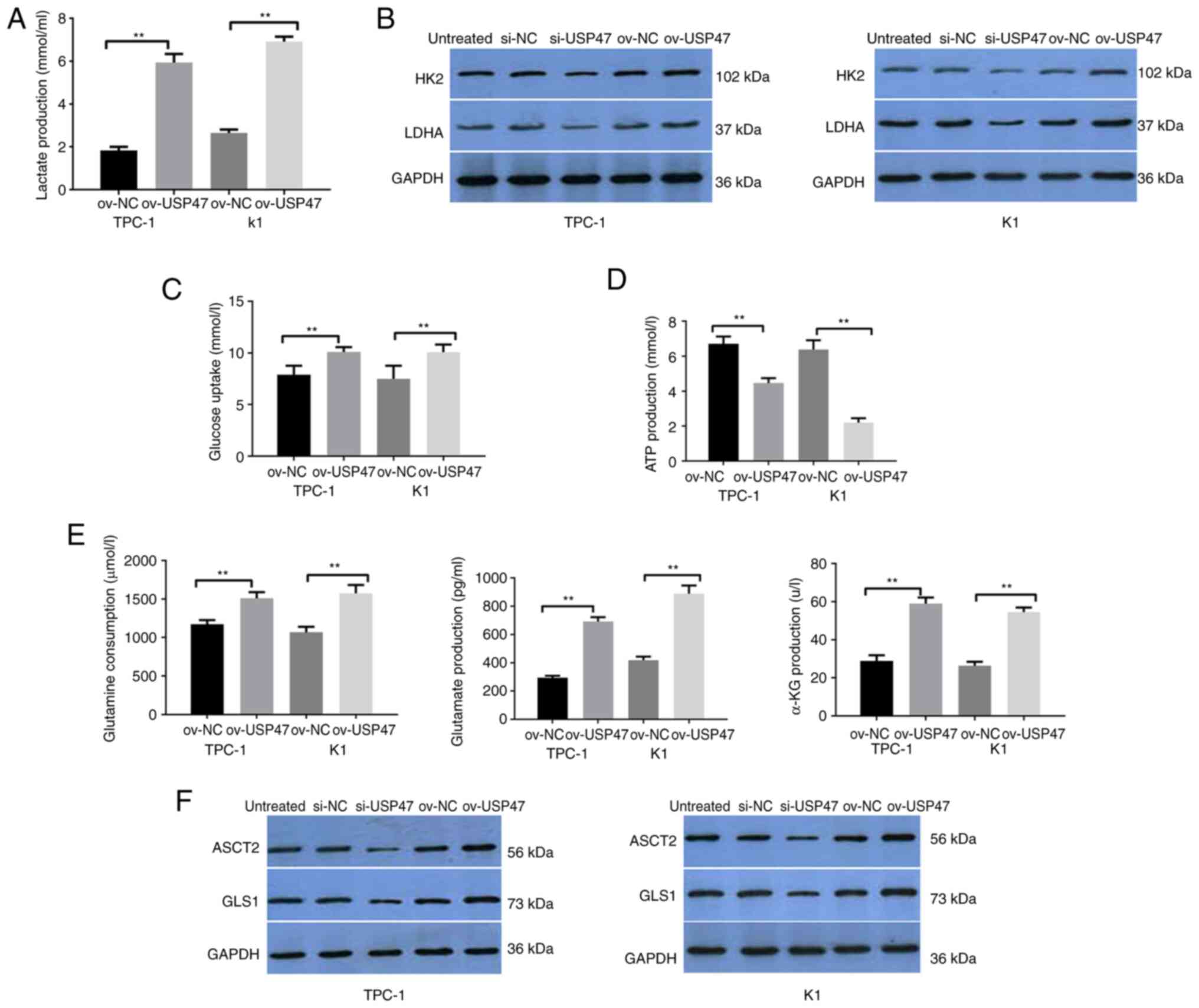
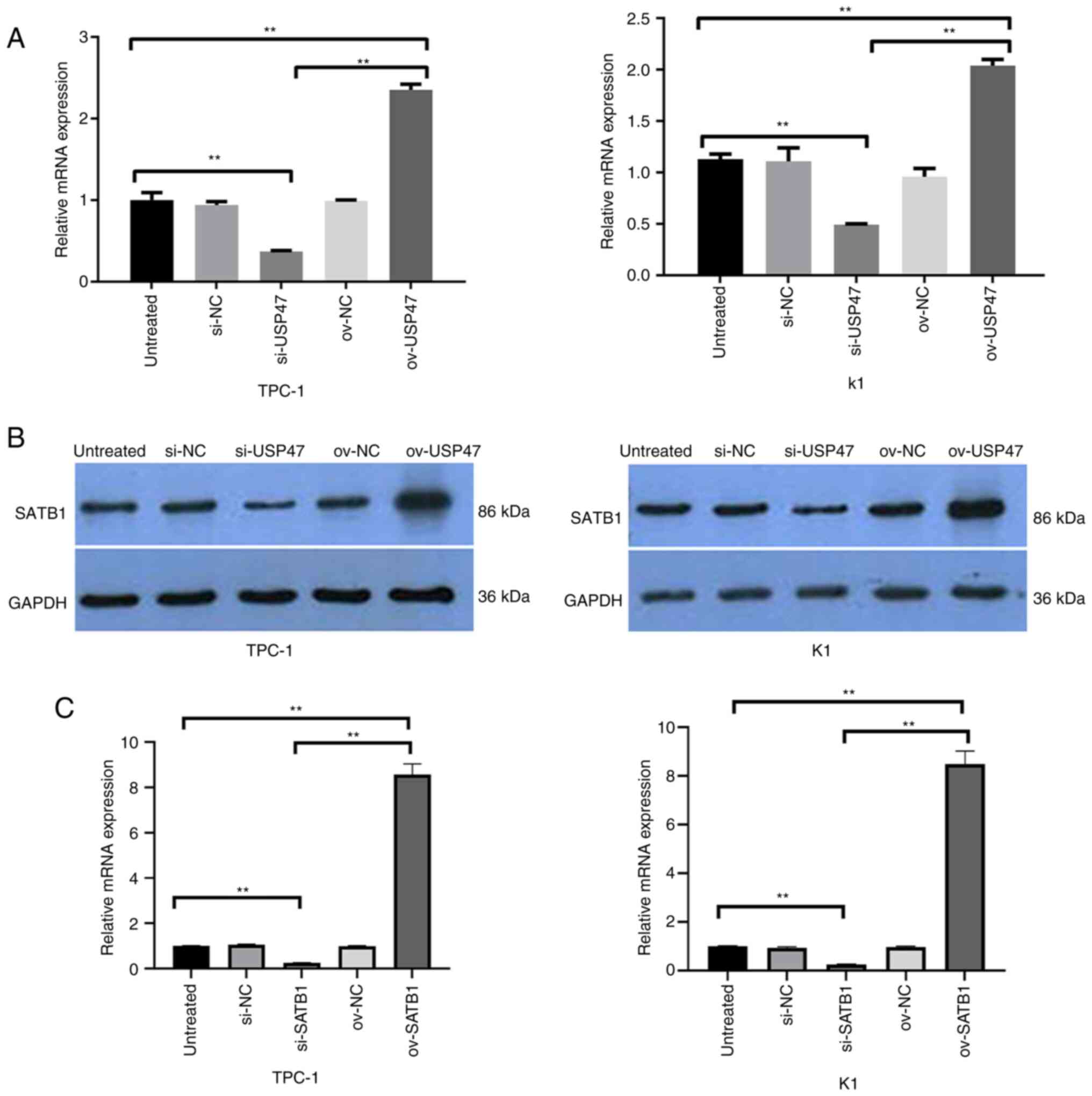
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitahara CM, Schneider AB and Brenner AV:
Thyroid cancer. Thun M, Linet MS, Cerhan JR, Haiman CA and
Schottenfeld D: Cancer Epidemiology and Prevention. 4th edition.
Oxford University Press; pp. 839–860. 2018
|
|
4
|
Carling T and Udelsman R: Thyroid cancer.
Annu Rev Med. 65:125–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chou R, Dana T, Haymart M, Leung AM,
Tufano RP, Sosa JA and Ringel MD: Active surveillance versus
thyroid surgery for differentiated thyroid cancer: A systematic
review. Thyroid. 32:351–367. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Udelsman R and Zhang YW: The epidemic of
thyroid cancer in the United States: The role of endocrinologists
and ultrasounds. Thyroid. 24:472–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stenman A, Backman S, Johansson K,
Paulsson JO, Stålberg P, Zedenius J and Juhlin CC: Pan-genomic
characterization of high-risk pediatric papillary thyroid
carcinoma. Endocr Relat Cancer. 28:337–351. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito Y, Miyauchi A, Kihara M, Fukushima M,
Higashiyama T and Miya A: Overall survival of papillary thyroid
carcinoma patients: A single-institution long-term follow-up of
5897 patients. World J Surg. 42:615–622. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng J, Li W, Tan N, Lai X, Jiang W and
Chen G: USP47 stabilizes BACH1 to promote the Warburg effect and
non-small cell lung cancer development via stimulating Hk2 and
Gapdh transcription. Am J Cancer Res. 12:91–107. 2022.PubMed/NCBI
|
|
10
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
López-Lázaro M: The warburg effect: Why
and how do cancer cells activate glycolysis in the presence of
oxygen? Anticancer Agents Med Chem. 8:305–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan
Q, Luo J, Zen K and Yang JW: Inhibiting aerobic glycolysis
suppresses renal interstitial fibroblast activation and renal
fibrosis. Am J Physiol Renal Physiol. 313:F561–F575. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu H, Zhu J, Chang L, Liang C, Li X and
Wang W: 3-Bromopyruvate decreased kidney fibrosis and fibroblast
activation by suppressing aerobic glycolysis in unilateral ureteral
obstruction mice model. Life Sci. 272:1192062021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jing YY, Cai FF, Zhang L, Han J, Yang L,
Tang F, Li YB, Chang JF, Sun F, Yang XM, et al: Epigenetic
regulation of the Warburg effect by H2B monoubiquitination. Cell
Death Differ. 27:1660–1676. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, He Y, Tan Z, Lu J, Li L, Song X, Shi
F, Xie L, You S, Luo X, et al: Wild-type IDH2 promotes the Warburg
effect and tumor growth through HIF1α in lung cancer. Theranostics.
8:4050–4061. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cha YJ, Kim ES and Koo JS: Amino acid
transporters and glutamine metabolism in breast cancer. Int J Mol
Sci. 19:9072018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Geldermalsen M, Wang Q, Nagarajah R,
Marshall AD, Thoeng A, Gao D, Ritchie W, Feng Y, Bailey CG, Deng N,
et al: ASCT2/SLC1A5 controls glutamine uptake and tumour growth in
triple-negative basal-like breast cancer. Oncogene. 35:3201–3208.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernhardt S, Bayerlová M, Vetter M,
Wachter A, Mitra D, Hanf V, Lantzsch T, Uleer C, Peschel S, John J,
et al: Proteomic profiling of breast cancer metabolism identifies
SHMT2 and ASCT2 as prognostic factors. Breast Cancer Res.
19:1122017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mukha A, Kahya U and Dubrovska A:
Targeting glutamine metabolism and autophagy: The combination for
prostate cancer radiosensitization. Autophagy. 17:3879–3881. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Q, Beaumont KA, Otte NJ, Font J,
Bailey CG, van Geldermalsen M, Sharp DM, Tiffen JC, Ryan RM,
Jormakka M, et al: Targeting glutamine transport to suppress
melanoma cell growth. Int J Cancer. 135:1060–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fraile JM, Quesada V, Rodriguez D, Freije
JMP and López-Otín C: Deubiquitinases in cancer: New functions and
therapeutic options. Oncogene. 31:2373–2388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan B, Yang Y, Li J, Wang Y, Fang C, Yu FX
and Xu Y: USP47-mediated deubiquitination and stabilization of YAP
contributes to the progression of colorectal cancer. Protein Cell.
11:138–143. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu L, Kolibaba H, Zhang S, Cao M, Niu H,
Mei H, Hao Y, Xu Y and Yin Q: MicroRNA-204-5p inhibits ovarian
cancer cell proliferation by down-regulating USP47. Cell
Transplant. 28 (1 Suppl):51S–58S. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Ding L, Gao F and Fan H: Long
non-coding RNA DSCAM-AS1 upregulates USP47 expression through
sponging miR-101-3p to accelerate osteosarcoma progression. Biochem
Cell Biol. 98:600–611. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silvestrini VC, Thomé CH, Albuquerque D,
de Souza Palma C, Ferreira GA, Lanfredi GP, Masson AP, Delsin LEA,
Ferreira FU, de Souza FC, et al: Proteomics analysis reveals the
role of ubiquitin specific protease (USP47) in epithelial to
mesenchymal transition (EMT) induced by TGFβ2 in breast cells. J
Proteomics. 219:1037342020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cameselle-Teijeiro JM: Changes and
perspectives in the new 2022 WHO classification of thyroid
neoplasms. Rev Esp Patol. 55:145–148. 2022.(In Spanish). PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Moss T, Mangala LS, Marini J, Zhao
H, Wahlig S, Armaiz-Pena G, Jiang D, Achreja A, Win J, et al:
Metabolic shifts toward glutamine regulate tumor growth, invasion
and bioenergetics in ovarian cancer. Mol Syst Biol. 10:7282014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu L, Dong L, Wang Y, Liu L, Long H, Li H,
Li J, Yang X, Liu Z, Duan G, et al: Reversible regulation of SATB1
ubiquitination by USP47 and SMURF2 mediates colon cancer cell
proliferation and tumor progression. Cancer Lett. 448:40–51. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin Q, ZL, Wu W, Lin J, Shi H, Wu X, Yu Y,
Ding M, Huang Z and Qiu J: Role of specific nuclear matrix binding
domain binding protein 1 and microRNA-495-3P in the invasion and
metastasis of papillary thyroid carcinoma. Chin J Exp Surg.
38:139–143. 2021.(In Chinese).
|
|
31
|
Piao J, Tashiro A, Nishikawa M, Aoki Y,
Moriyoshi E, Hattori A and Kakeya H: Expression, purification and
enzymatic characterization of a recombinant human
ubiquitin-specific protease 47. J Biochem. 158:477–484.
2015.PubMed/NCBI
|
|
32
|
Cho J, Park J, Shin SC, Jang M, Kim JH,
Kim EE and Song EJ: USP47 promotes tumorigenesis by negative
regulation of p53 through deubiquitinating ribosomal protein S2.
Cancers (Basel). 12:11372020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peschiaroli A, Skaar JR, Pagano M and
Melino G: The ubiquitin-specific protease USP47 is a novel
beta-TRCP interactor regulating cell survival. Oncogene.
29:1384–1393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lei H, Xu HZ, Shan HZ, Liu M, Lu Y, Fang
ZX, Jin J, Jing B, Xiao XH, Gao SM, et al: Targeting USP47
overcomes tyrosine kinase inhibitor resistance and eradicates
leukemia stem/progenitor cells in chronic myelogenous leukemia. Nat
Commun. 12:512021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang S, Ju X, Yang Q, Zhu Y, Fan D, Su G,
Kong L and Li Y: USP47 maintains the stemness of colorectal cancer
cells and is inhibited by parthenolide. Biochem Biophys Res Commun.
562:21–28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glatzel-Plucińska N, Piotrowska A,
Dzięgiel P and Podhorska-Okołów M: The role of SATB1 in tumour
progression and metastasis. Int J Mol Sci. 20:41562019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kohwi-Shigematsu T, Kohwi Y, Takahashi K,
Richards HW, Ayers SD, Han HJ and Cai S: SATB1-mediated functional
packaging of chromatin into loops. Methods. 58:243–254. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding M, Pan J, Guo Z, Liu Q, Yang C and
Mao L: SATB1 is a novel molecular target for cancer therapy. Cancer
Invest. 36:28–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qi H, Fu X, Li Y, Pang X, Chen S, Zhu X,
Li F and Tan W: SATB1 promotes epithelial-mesenchymal transition
and metastasis in prostate cancer. Oncol Lett. 13:2577–2582. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang S, Tong YX, Xu XS, Lin H and Chao
TF: Prognostic significance of SATB1 in gastrointestinal cancer: A
meta-analysis and literature review. Oncotarget. 8:48410–48423.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou D, Ye C, Pan Z and Deng Y: SATB1
knockdown inhibits proliferation and invasion and decreases
chemoradiation resistance in nasopharyngeal carcinoma cells by
reversing EMT and suppressing MMP-9. Int J Med Sci. 18:42–52. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mukhopadhyay D and Riezman H:
Proteasome-independent functions of ubiquitin in endocytosis and
signaling. Science. 315:201–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peng J, Schwartz D, Elias JE, Thoreen CC,
Cheng D, Marsischky G, Roelofs J, Finley D and Gygi SP: A
proteomics approach to understanding protein ubiquitination. Nat
Biotechnol. 21:921–926. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Reyes-Turcu FE, Ventii KH and Wilkinson
KD: Regulation and cellular roles of ubiquitin-specific
deubiquitinating enzymes. Annu Rev Biochem. 78:363–397. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Heger K, Wickliffe KE, Ndoja A, Zhang J,
Murthy A, Dugger DL, Maltzman A, de Sousa E Melo F, Hung J, Zeng Y,
et al: OTULIN limits cell death and inflammation by
deubiquitinating LUBAC. Nature. 559:120–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Naghavi L, Schwalbe M, Ghanem A and
Naumann M: Deubiquitinylase USP47 promotes RelA phosphorylation and
survival in gastric cancer cells. Biomedicines. 6:622018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu L, Fu J and Shen C: Ubiquitin specific
peptidase 47 promotes proliferation of lung squamous cell
carcinoma. Genes Genomics. 44:721–731. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gong L, Han Y, Chen R, Yang P and Zhang C:
LncRNA ZNF883-mediated NLRP3 inflammasome activation and epilepsy
development involve USP47 upregulation. Mol Neurobiol.
59:5207–5221. 2022. View Article : Google Scholar : PubMed/NCBI
|