The cells in the body keep updating themselves
throughout life. Alterations in cell niches caused by physical
wounds, chemical stimulation and genetic hallmarks cause mutations,
and these mutations lead to cancer initiation (1). Cancer has a high mortality rate
worldwide (2), resulting in the
loss of 8.97 million lives, even with advances in medical
technology (3). Cancer treatments,
including surgery, chemotherapy and radiotherapy, have brought
positive results. However, the generalized application of these
measures is often restricted due to severe adverse side effects and
insufficient therapeutic effects. Immunotherapy for cancer has
become an area of great interest to researchers sue to recent
clinical success (4).
Representative antibodies against cytotoxic T lymphocyte antigen-4
(CTLA-4) and programmed cell death 1 in the removal of multiple
malignant cancer types, including melanoma, small cell lung cancer,
and head and neck squamous cell carcinoma, are making milestone
advances in cancer treatment (5,6).
Chimeric antigen receptor T cells also provide beneficial antitumor
impacts (7). However, such means
still fail to implement the fact that it is possible to treat all
individuals. To date, scientists are still struggling to come up
with new treatment strategies.
Pyroptosis, a type of inflammatory programmed cell
death, induces systematic inflammation by releasing
pro-inflammatory intracellular contents (8). With the advancement of research, the
recognition of pyroptosis in cancer is becoming more apparent. Once
inflammasomes activate pyroptosis, it typically provokes a cascade
of reactions, including swelling of tumor cells, plasma membrane
lysis, chromatin fragmentation and exposure of proinflammatory
contents under the influence of caspase-1/4/5/11 (9). This pyroptosis mediated by gasdermin
facilitates immune cell activation and infiltration and results in
a robust inflammatory response and marked tumor regression
(10). Tumors are often called cold
tumors due to resistance to immune checkpoint inhibitors (ICIs) and
small amounts of T-cell infiltration (11). From the perspective of tumor
treatment, induction of pyroptosis may be a potential choice for
the recruitment actions of immune cells. These immune effects
directly cause ‘cold’ tumors that become ‘hot’ tumors with high
levels of T cell infiltration, with the aim to regulate the tumor
microenvironment (12).
However, methods to regulate pyroptosis still need
to be investigated by scientists. Researchers focus on various
multifunctional controlled drug distribution systems comprising of
different polymers to trigger pyroptosis (13,14).
Also, these efforts drive the rapid development of bioengineering
in cancer treatment (15). The
present review discusses the features and mechanisms of pyroptosis,
and evaluates the clinical value in the oncology of pyroptosis.
Furthermore, the present review shares the recent accomplishments
in biological engineering associated with pyroptosis in cancer
research and provides a future outlook.
In the 1990s, researchers observed cell suicide
releases a burst of inflammatory cytokines in macrophages exposed
to salmonella (16,17). However, this subversive form of
programmed cell death (PCD) was termed pyroptosis until 2002
(18). As another conserved type of
PCD, apoptosis, which is mainly initiated by caspase-3 and the
microenvironment, has been the subject of intensive research for
the past 3 decades (19). These
efforts enhanced the comprehensive understanding of features in
apoptosis, including nuclear condensation, membrane blebbing,
caspase-dependent and DNA fragmentation. It is interesting to note
that pyroptosis also contains these characteristics and was
initially regarded as apoptosis for this reason. To some extent,
this overlap delayed the research process. Moreover, the overlap
was not resolved until a clear definition of pyroptosis was given
in 2007 (20). With subsequent
research, the definite difference in morphological and biochemical
characteristics between the apoptosis and pyroptosis has been
demonstrated (Table I) (21).
Regarding morphology, pyroptosis presents
discriminative alternations, such as pore formation, cell swelling
and osmotic lysis with cytosolic contents leakages (22). External challenges promote the
activation of caspases and the release of granzymes in cells.
Finally, gasdermin D (GSDMD) was cleaved to form a transmembrane
pore (23), which results in an
unbalance of transmembrane ion fluxes (24). Following that, incoming water
molecules trigger the plasma membrane break event. Cytoplasmic
swelling induces osmotic cell lysis by releasing pro-inflammatory
cytokines (IL-1β and IL-18), leading to the recruitment of immune
cells (25). From a biochemical
point of view, caspase-1, 4, 5 and 11 act as initiators and
effectors in pyroptosis, which differs from apoptotic caspases
(26,27). Collectively, these characteristics
distinguish pyroptosis and apoptosis, and accelerate our
understanding of them.
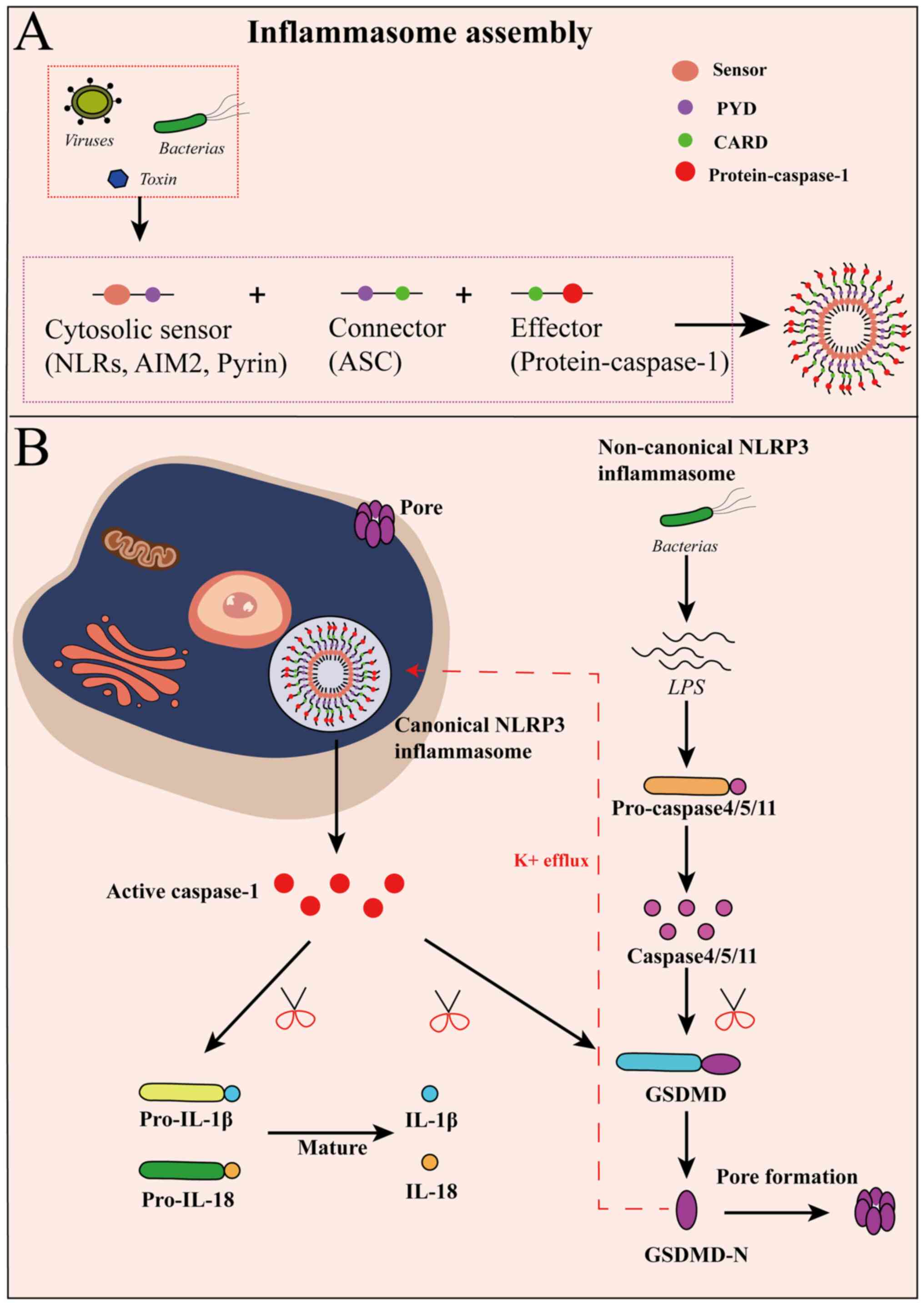
The canonical inflammasome complex is usually
composed of a cytosolic sensor called pattern recognition receptors
(PRRs), an adapter protein named apoptosis-associated speck-like
protein (ASC) containing a C-terminal caspase activation and
recruitment domain (CARD), an N-terminal pyrin domain and
inflammatory caspases (Fig. 1A)
(28,29). PRRs [a nucleotide-binding domain or
leucine-rich repeat receptors (NLRs) or absent in melanoma 2
(AIM2)-like receptors] are capable of recognizing
pathogen-associated molecular patterns and danger-associated
molecular patterns (DAMPs) (30,31).
Then, ASC acts as a connector between sensors and the effector
protein-caspase-1 (32). Finally,
inflammasome assembly activates caspase-1-dependent canonical
pyroptosis by GSDMD-mediated pore formation (33). Meanwhile, the cleavage of caspase-1
results in the maturation of IL-1β and IL-18 (34). Notably, caspase-1 plays an essential
role in the canonical pyroptosis pathway. Among these inflammasome
subtypes, NLRP3 inflammasome containing NLR-protein-3 is considered
to be associated with pyroptosis to sense a wide range of stimuli
(35,36). Some evidence hints that therapies
targeting the NLRP-3 inflammasome might hold potential for
treatment of various types of cancer, including non-small cell lung
cancer, breast cancer and colorectal cancer (37–39).
The non-canonical pyroptosis pathway differs from
the canonical route because of an activation without the
requirement for inflammasomes (40). Lipopolysaccharides (LPS) secreted by
the majority of gram-negative bacteria are directly bound to the
N-terminal CARD of caspase, which activates caspase-4/5 in humans
or caspase-11 in mice (41,42). The activated caspases cleave GSDMD
into N-GSDMD to perforate the cell membrane and drive pyroptosis
(43). Additionally, N-GSDMD
transfers positive feedback to NLRP3 or AIM2 inflammasomes via the
efflux of K+ (44). This
signal induces NLRP3/caspase-1 activation, also leading to the
maturation of IL-1β and IL-18 (45).
The pore-forming family is made up of five members,
including GSDMA, GSDMB, GSDMC, GSDME and GSDMF (46), that can exert their function by the
release of the N-terminus, such as in group A
Streptococcus-driven GSDMA cleavage, lymphocyte-derived
granzyme A-mediated GSDMB cleavage, caspase-8-mediated GSDMC
cleavage, caspase-1/4/5/11-mediated GSDMD cleavage and
caspase-3-mediated GSDME cleavage (47–52).
Among them, GSDMD is considered to have a classic role because it
is a generic substrate for inflammatory cases and a downstream
effector of multiple inflammasomes (53–55).
The cleaved GSDMD-N assembles into ring-shaped oligomers,
permeabilizing the membrane (56).
Both canonical and non-canonical pathways are finally subject to
GSDMD, triggering pyroptosis (57).
Cell death is mediated by accidental cell death
(ACD) or regulated cell death (RCD) (58). RCD is mainly focused on by
researchers due to uncontrollable ACD. An increasing number of
complex molecular controls, including ferroptosis, necroptosis and
pyroptosis, have been identified (59,60).
Intrinsic signal-mediated death processes are involved in the
transformation, growth, invasion and metastases of malignant cells,
which play a guiding role in the treatment of human cancers
(61). As a unique inflammatory
death, pyroptosis is renowned for its particular mechanism and
destructive lethality (57).
Advances involving targets and products of pyroptotic pathways
including the GSDM family over the past few years have
revolutionized the status of pyroptosis in treating different types
of cancer (62). A number of cancer
research attempts by scientists focusing on GSDMD have also yielded
positive results. Yan et al (63) enhanced the comprehensive
understanding of cisplatin in patients with triple-negative breast
cancer, demonstrating that treatments are mediated by the GSDMD.
Yuan et al (64)
demonstrated that cucurbitacin B can inhibit non-small cell lung
cancer with GSDMD-independent pyroptosis. These findings highlight
that pore formation induced by GSDMD has a destructive effect on
tumor cells. Furthermore, one study revealed that immune therapy
targeting GSDME in colon cancer has a good effect (65). It has also been indicated that the
presence of GSDME in tumors increases the recruitment of immune
cells (66). Thus, inducting and
activating GSDME may potentially be of clinical value.
Researchers have demonstrated that GSDME-based
measures can convert the microenvironment of tumors with elevated
levels of infiltrating immune cells, assisting in improving the
response to immunotherapy (67–69).
From a therapeutic perspective, pyroptosis is a notable choice for
cancer treatment. However, pyroptosis is restricted in biomedical
applications for the severe side effects caused by the
chemotherapeutic drugs. Finding a safe and effective method in
combination with immunotherapy is essential.
Immune checkpoints (IC) are cell-surface proteins
controlling the initiation, duration and magnitude of immune
responses (70). Tumor development
is generally caused by IC-related immune evasion. Thus, patients
with cancer benefit to a large extent from the application of ICI.
Pyroptosis is positively associated with immune infiltration and
immune characteristics in 30 types of cancer and directly modulates
the expression of immune checkpoint molecules (71). This is consistent with current
cancer treatment strategies to convert cold tumors into a hot ones.
Clinical evidence has also revealed the potential value of
pyroptosis in predicting immunotherapy responses and a theoretical
rationale for combining pyroptosis inducers and immunotherapy in
cancer treatment (72). Pyroptosis
works in the same way as ICIs to strengthen tumor immunity,
exerting a powerful potential in the treatment of cancer (73).
Immunogenic cell death (ICD) is a unique form of
stress-driven cell death that is typically mediated by DAMPs
(74). Applying stressors,
including pathogens, viruses and chemotherapeutic drugs, may be a
novel treatment method to initiate adaptive immunity (75). Notably, pyroptosis seem to act as a
potent stressor to trigger ICD due to exposure to a large amount of
cell debris. From the perspective of immune regulation, cellular
debris provides dendritic cells with antigens and inflammatory
stimuli, and then activates CD8+ T cells to trigger an
immune response called antigen cross-priming (76). Meanwhile, the process by which the
contents of tumor cells are released with pro-inflammatory signals
leads to the efficient immune destruction of cancer cells (77). This type of cascade could reprogram
the tumor immune microenvironment into an immune stimulation state
through the activation of DAMPs following osmotic lysis, ultimately
inhibiting the spread and expansion of the tumor cells (78).
Application of chemical drugs is a good choice for
patients with cancer. Although a number of clinical drugs,
including cisplatin, doxorubicin and dihydroartemisinin, have been
approved by the Food and Drug Administration, the pace of research
has not slowed down (79–81). More efforts are focused on
determining the mechanism of cancer treatment and solving the
problem of drug-resistant tumors. Therapies inducing pyroptosis in
tumor cells are a current solution. For example, doxorubicin, a
common chemotherapy drug, is strongly associated with
caspase-3-mediated GSDME activation and JNK phosphorylation-based
activation (82). Zhang et
al (82) revealed that the
GSDME was cleaved under doxorubicin treatment, resulting in the
death of breast cancer cells. Abnormal activation of the
pyroptosis-related protein caspase3 increases our understanding of
doxorubicin. Cisplatin is another potential drug widely used for
the treatment of various solid cancers, such as testicular,
ovarian, head and neck, bladder, lung, cervical cancer, melanoma
and lymphoma, among others (83).
However, the discovery of treatment routes such as apoptosis and
ferroptosis does not completely explain the excellent effects of
cisplatin in cancer treatment. Notably, pyroptosis sheds new light
on the therapeutic effects of cisplatin. With the development of
research, Yan et al (63)
revealed that cisplatin can activate the NLRP3/caspase-1/GSDMD
pyroptosis pathway by upregulating maternally expressed gene 3
(MEG3). Overall, these clues point to novel targets for treatment
of tumors in the future. However, pyroptosis is restricted in
biomedical applications due to the severe side effects caused by
the chemotherapeutic drugs used. Therefore, finding a safe and
effective method in combination with immunotherapy is
essential.
Recently, the potential of NPs to modulate
biological pyroptosis has been recognized gradually by researchers
(15). Ploetz et al reported
that hybrid metal-organic framework NPs consisting of iron
(Fe3+) and trimesic acids provide an external trigger
for the induction of pyroptosis, according to the extracellular pH
(86). Such particular NPs creating
a controllable platform for iron delivery turned out to be a
success. This success of the tumor treatment model is attributed to
the physical induction of pyroptosis, which activates the tumor
destruction mechanism. Particle effects triggered by physical
stimuli such as sound, light and electricity combined with
pyroptosis will help to promote the progress of tumor treatment
(87). Except for the physical
excitation mode in cancer, the creation of chemically induced cell
pores is also relatively common (88). For instance, some ultrasmall NPs
with diameters <10 nm act as Trojan horses and are successfully
introduced into the pyroptotic cells, releasing extracellular LPS
into cells through endocytosis and, in turn, inducing
GSDMD-N-terminal membrane pores (89). Such chemically-caused cell
inflammatory death is also a success. Notably, NPs-induced
pyroptosis with this chemical form can maintain the continuous
pyroptosis process and control the severity of pyroptosis (15). Consequently, the distribution system
of NPs combined with drugs may be particularly important in the
induction of pyroptosis. A previous study attempted to construct a
nanoparticle carrier loading with indocyanine green and decitabine
to induce activation of pyroptosis for photo-activated cancer cell
pyroptosis and solid tumor immunotherapy (90). This induction method is more
comprehensive and more effective in combining physical and chemical
control. This study detected that selective accumulation of NPs in
tumor activate a sharp increase of cytoplasm Ca2+
concentration after low-dose NIR photo-activation. Subsequently,
the activation of caspase-3 reinforces cleavage with GSDME,
triggering a systemic antitumor immunity by pyroptosis. This type
of comprehensive understanding of NPs could be used as an effective
tool to cause pyroptosis. From the standpoint of a trigger,
nanoparticles play a function in the cell by endocytosis, then
mediate the pyroptosis mode by physical and chemical methods
(15). This method of mediating the
formation of pores inside the cell seems to be more direct, faster
and more efficient compared with chemical drugs.
Although NPs have the benefits of targeted
administration, there are still some limitations that make them
unsuitable for long-term administration, such as bursting release,
poor biological adhesion and irreversible deformation (91). Recently, hydrogels formed by
cross-linked polymer networks have been widely utilized for cancer
treatment. As a type of degradable multifunctional scaffold,
hydrogels have been instrumental in providing intelligent drug
delivery systems for cancer immunotherapy (92). The polymer, composed mainly of
water, imitates highly hydrophilic biological tissues, a
free-standing viscoelastic mesh, and has mechanical properties in
ranges suitable for living tissues (93). These unique features give hydrogel
the perfect drug delivery capabilities. Some hydrogel-based
investigations have also shown applying hydrogel-mediated
pyroptosis is a good choice in cancer treatment (94–96).
The research using hydrogel as a scaffold for tumor cell pyroptosis
opens up a new perspective. Scientists reported the superiority of
cellulose nanofiber-based hydrogels as a release drug stent
(97,98). Another paper also pointed out the
feasibility of embedding 5-FU on pyroptosis induction in cellulose
nanofiber-based hydrogels (99). It
was discovered that an important pyroptotic phenomenon was present
in breast cancer cells via the caspase 1 cleavage, indicating
pyroptosis-based immunotherapy with a hydrogel carrier is
potential. However, to date, there has not been much research on
hydrogel in the area of pyroptosis-based treatment of cancer.
Since more attention has been focused on treating
diseases with traditional hydrogel in the past, hydrogel-based
therapies in cancer are a compelling trend. Intelligent hydrogels
that react to external stimuli, such as pH, light and temperature,
show a solution-gel transition (100–102). This controlled shapeshift favors
the development of hydrogel in cancer for controlled drug release,
local treatment, fewer side-effects and easy administration. In
recent years, the thermosensitive hydrogel has been applied in
local cancer therapy. Temperature-controlled gels made of natural
polymers such as chitosan, cellulose and hyaluronic acid also
performed well, but these results seem to only meet the ideal
expectations with immune intervention (103,104). Researchers are dedicated to
finding a novel therapy combining the application of the immune
checkpoint and thermosensitive hydrogel. For instance, the strategy
based on thermosensitive hydrogel by releasing the nitric oxide
donor and anti-CTLA-4 micelles achieved good feedback in tumor
immunotherapy. The results demonstrated that hydrogel enhanced drug
retention, and ultimately activated immune modulation within the
tumor injection site (105). This
discovery has expanded our understanding of their influence,
particularly in relation to their role in drug liberation. Drugs
that provoke pyroptosis closely associated with hydrogel will
provide a new perspective.
Pyroptosis is an inflammatory form of cell death
that relies on the formation of pores in the plasma membrane by
proteins of the GSDM family (106). Plasma membranes play an essential
role in the maintenance of homeostasis in mammalian cells. Thus,
disrupting the integrity of the cell membrane will certainly end
the life of the cell (107). A
previous study has revealed that ~20 units of GSDMD-N construct a
large oligomeric ring-shaped pore that directly leads to an
elevation of the intracellular osmotic pressure, cell swelling and,
eventually, bursting of the cell (108,109). These intracellular liberated
substances soon activate a robust immune response, recruit immune
effector cells, and are involved in numerous physiological
pathological processes (110). As
an important strategy for tumor survival and development, immune
evasion of the tumour has emerged as a hot topic in antitumor
research (111,112). Notably, pyroptosis is more
effective at activating immune responses and pushing cancerous
cells to the verge of death (113). The continuous dying of cancerous
cells contributes to an even more violent cascade of inflammation
through exposure to much of the contents of the cell (114). This will inevitably cause the body
to become excessively inflamed. Consequently, how to manage and use
this double-edged sword is especially important. Further
exploration of the control mechanism of pyroptosis and the search
for effective ways to regulate pyroptosis may provide new ideas for
treating related tumors (27).
It must be recognized that numerous medications that
induce pyroptosis do suppress the tumor, but also bring side
effects. There is increasing interest among researchers in drug
delivery systems because of their controlled-release properties.
This opens up new possibilities for the clinical application of
stents based on pyroptosis drugs in the future. Although there are
few studies on the stents combined with pyroptosis, it must be a
new research trend in the future. For instance, injectable
hydrogels are a suitable choice because they are a controlled
release system to control the degree of pyroptosis, and may confine
diseased tissue to a restricted area. With the slow degradation of
the gels, the drugs in the gels are released slowly, which favors
the formation of the GSDMD-mediated pore and the rupture of the
tumor cells. The present study hypothesizes that the initial
exposure of tumor cell contents at the distal end of the gel
accelerates the immune clearance of tumor cells. Meanwhile, the
side effects caused by excessive inflammation are avoided to a
certain extent. It can be categorized as pyroptosis activated by
external transmission signals with a reaction zone. Another
strategy for inducing pyroptosis is inherent in nanomolecular
technology. NPs charging various medicines cause a process of
destruction from within. When the nanoparticles enter the cancer
site, they are ingested by cancer cells, releasing the drug
internally and reacting directly to the activation of the
pyroptotic pathway. This strategy of internally induced pyroptosis
also leads to the destruction of cancer cells and the clearance of
immune responses triggered by the contents of damaged cells.
Collectively, stent-based efforts to induce pyroptosis may provide
new perspective in future research.
Mrs. Yucheng Qian (Department of Neurology,
Affiliated Changshu Hospital of Nantong University, Suzhou, China)
helped to prepare the figure for the manuscript.
Funding: No funding was received.
Not applicable.
JT wrote the majority of the manuscript. ZZ and YS
edited the manuscript. All authors have read and approved the final
submitted manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Fan J, Feng Z and Chen N: Spermidine as a
target for cancer therapy. Pharmacol Res. 159:1049432020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carioli G, Malvezzi M, Bertuccio P, Hashim
D, Waxman S, Negri E, Boffetta P and La Vecchia C: Cancer mortality
in the elderly in 11 countries worldwide, 1970–2015. Ann Oncol.
30:1344–1355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mattiuzzi C and Lippi G: Current cancer
epidemiology. J Epidemiol Glob Health. 9:217–222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y: Cancer immunotherapy: Harnessing
the immune system to battle cancer. J Clin Invest. 125:3335–3337.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cha JH, Chan LC, Li CW, Hsu JL and Hung
MC: Mechanisms controlling PD-L1 expression in cancer. Mol Cell.
76:359–370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rowshanravan B, Halliday N and Sansom DM:
CTLA-4: A moving target in immunotherapy. Blood. 131:58–67. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang H, Shi Z, Wang P, Wang C, Yang L, Du
G, Zhang H, Shi B, Jia J, Li Q, et al: Claudin18.2-specific
chimeric antigen receptor engineered T cells for the treatment of
gastric Cancer. J Natl Cancer Inst. 111:409–418. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christgen S, Tweedell RE and Kanneganti
TD: Programming inflammatory cell death for therapy. Pharmacol
Ther. 232:1080102022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang
Y, Yu T, Wu X, Shi Y, Ma P and Shu Y: Pyroptosis: A new frontier in
cancer. Biomed Pharmacother. 121:1095952020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loveless R, Bloomquist R and Teng Y:
Pyroptosis at the forefront of anticancer immunity. J Exp Clin
Cancer Res. 40:2642021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan Q, Zhang H, Zheng J and Zhang L:
Turning cold into hot: Firing up the tumor microenvironment. Trends
Cancer. 6:605–618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niu X, Chen L, Li Y, Hu Z and He F:
Ferroptosis, necroptosis, and pyroptosis in the tumor
microenvironment: Perspectives for immunotherapy of SCLC. Semin
Cancer Biol. 86:273–285. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rioja-Blanco E, Arroyo-Solera I, Álamo P,
Casanova I, Gallardo A, Unzueta U, Serna N, Sánchez-García L, Quer
M, Villaverde A, et al: CXCR4-targeted nanotoxins induce
GSDME-dependent pyroptosis in head and neck squamous cell
carcinoma. J Exp Clin Cancer Res. 41:492022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Guo K, Ding M, Zhang B, Xiao N,
Tang Z, Wang Z, Zhang C and Shubhra QTH: Engineered magnetic
polymer nanoparticles can ameliorate breast cancer treatment
inducing pyroptosis-starvation along with chemotherapy. ACS Appl
Mater Interfaces. 14:42541–42557. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu D, Wang S, Yu G and Chen X: Cell death
mediated by the pyroptosis pathway with the aid of nanotechnology:
Prospects for cancer therapy. Angew Chem Int Ed Engl. 60:8018–8034.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gogoi M, Shreenivas MM and Chakravortty D:
Hoodwinking the big-eater to prosper: The salmonella-macrophage
paradigm. J Innate Immun. 11:289–299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zychlinsky A, Prevost MC and Sansonetti
PJ: Shigella flexneri induces apoptosis in infected macrophages.
Nature. 358:167–169. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu X, Chen H, Xu H, Wu Y, Wu C, Jia C, Li
Y, Sheng S, Xu C, Xu H, et al: Role of pyroptosis in traumatic
brain and spinal cord injuries. Int J Biol Sci. 16:2042–2050. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
D'Souza CA and Heitman J: Dismantling the
cryptococcus coat. Trends Microbiol. 9:112–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia X, Wang X, Cheng Z, Qin W, Lei L,
Jiang J and Hu J: The role of pyroptosis in cancer: pro-cancer or
pro-‘host’? Cell Death Dis. 10:6502019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu YJ, Zheng L, Hu YW and Wang Q:
Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta.
476:28–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia S, Zhang Z, Magupalli VG, Pablo JL,
Dong Y, Vora SM, Wang L, Fu TM, Jacobson MP, Greka A, et al:
Gasdermin D pore structure reveals preferential release of mature
interleukin-1. Nature. 593:607–611. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burdette BE, Esparza AN, Zhu H and Wang S:
Gasdermin D in pyroptosis. Acta Pharm Sin B. 11:2768–2782. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Broz P, Pelegrín P and Shao F: The
gasdermins, a protein family executing cell death and inflammation.
Nat Rev Immunol. 20:143–157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fischer FA, Chen KW and Bezbradica JS:
Posttranslational and therapeutic control of gasdermin-mediated
pyroptosis and inflammation. Front Immunol. 12:6611622021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Man SM and Kanneganti TD: Regulation of
inflammasome activation. Immunol Rev. 265:6–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai X, Chen J, Xu H, Liu S, Jiang QX,
Halfmann R and Chen ZJ: Prion-like polymerization underlies signal
transduction in antiviral immune defense and inflammasome
activation. Cell. 156:1207–1222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao S, Chen C, Shi G, Zhou Y, Wei Y, Fan
N, Yang Y, Wu L and Zhang T: Therapeutic potential of the target on
NLRP3 inflammasome in multiple sclerosis. Pharmacol Ther.
227:1078802021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lahooti B, Chhibber T, Bagchi S,
Varahachalam SP and Jayant RD: Therapeutic role of inflammasome
inhibitors in neurodegenerative disorders. Brain Behav Immun.
91:771–783. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S, Wang L, Xu Z, Huang Y, Xue R, Yue T,
Xu L, Gong F, Bai S, Wu Q, et al: ASC deglutathionylation is a
checkpoint for NLRP3 inflammasome activation. J Exp Med.
218:e202026372021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang C, Yang T, Xiao J, Xu C, Alippe Y,
Sun K, Kanneganti TD, Monahan JB, Abu-Amer Y, Lieberman J and
Mbalaviele G: NLRP3 inflammasome activation triggers gasdermin
D-independent inflammation. Sci Immunol. 6:eabj38592021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He Y, Hara H and Núñez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng M and Kanneganti TD: The regulation
of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis,
apoptosis, and necroptosis (PANoptosis). Immunol Rev. 297:26–38.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Swanson KV, Deng M and Ting JPY: The NLRP3
inflammasome: Molecular activation and regulation to therapeutics.
Nat Rev Immunol. 19:477–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R,
Qiu WQ, Pan R, Law BYK, Wong VKW, Yu CL, et al: Polyphyllin VI
induces caspase-1-mediated pyroptosis via the induction of
ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer.
Cancers (Basel). 12:1932020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ershaid N, Sharon Y, Doron H, Raz Y, Shani
O, Cohen N, Monteran L, Leider-Trejo L, Ben-Shmuel A, Yassin M, et
al: NLRP3 inflammasome in fibroblasts links tissue damage with
inflammation in breast cancer progression and metastasis. Nat
Commun. 10:43752019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li T, Fu B, Zhang X, Zhou Y, Yang M, Cao
M, Chen Y, Tan Y and Hu R: Overproduction of gastrointestinal 5-HT
promotes colitis-associated colorectal cancer progression via
enhancing NLRP3 inflammasome activation. Cancer Immunol Res.
9:1008–1023. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsu SK, Li CY, Lin IL, Syue WJ, Chen YF,
Cheng KC, Teng YN, Lin YH, Yen CH and Chiu CC: Inflammation-related
pyroptosis, a novel programmed cell death pathway, and its
crosstalk with immune therapy in cancer treatment. Theranostics.
11:8813–8835. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He X, Fan X, Bai B, Lu N, Zhang S and
Zhang L: Pyroptosis is a critical immune-inflammatory response
involved in atherosclerosis. Pharmacol Res. 165:1054472021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zheng X, Chen W, Gong F, Chen Y and Chen
E: The role and mechanism of pyroptosis and potential therapeutic
targets in sepsis: A review. Front Immunol. 12:7119392021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wright SS, Vasudevan SO and Rathinam VA:
Mechanisms and consequences of noncanonical inflammasome-mediated
pyroptosis. J Mol Biol. 434:1672452022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Burzynski LC and Clarke MCH: Death is
coming and the clot thickens, as pyroptosis feeds the fire.
Immunity. 50:1339–1341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kayagaki N, Warming S, Lamkanfi M, Vande
Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al:
Non-canonical inflammasome activation targets caspase-11. Nature.
479:117–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xia S, Hollingsworth LR IV and Wu H:
Mechanism and regulation of gasdermin-mediated cell death. Cold
Spring Harb Perspect Biol. 12:a0364002020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Deng W, Bai Y, Deng F, Pan Y, Mei S, Zheng
Z, Min R, Wu Z, Li W, Miao R, et al: Streptococcal pyrogenic
exotoxin B cleaves GSDMA and triggers pyroptosis. Nature.
602:496–502. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
LaRock DL, Johnson AF, Wilde S, Sands JS,
Monteiro MP and LaRock CN: Group A streptococcus induces
GSDMA-dependent pyroptosis in keratinocytes. Nature. 605:527–531.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y,
Wang Y, Li D, Liu W, Zhang Y, et al: Granzyme A from cytotoxic
lymphocytes cleaves GSDMB to trigger pyroptosis in target cells.
Science. 368:eaaz75482020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang JY, Zhou B, Sun RY, Ai YL, Cheng K,
Li FN, Wang BR, Liu FJ, Jiang ZH, Wang WJ, et al: The metabolite
α-KG induces GSDMC-dependent pyroptosis through death receptor
6-activated caspase-8. Cell Res. 31:980–997. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang K, Sun Q, Zhong X, Zeng M, Zeng H,
Shi X, Li Z, Wang Y, Zhao Q, Shao F and Ding J: Structural
mechanism for GSDMD targeting by autoprocessed caspases in
pyroptosis. Cell. 180:941–955.e20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Barnett KC and Ting JPY: Mitochondrial
GSDMD pores DAMPen pyroptosis. Immunity. 52:424–426. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Z, Gan L, Xu Y, Luo D, Ren Q, Wu S and
Sun C: Melatonin alleviates inflammasome-induced pyroptosis through
inhibiting NF-κB/GSDMD signal in mice adipose tissue. J Pineal Res.
63:2017. View Article : Google Scholar
|
|
55
|
Humphries F, Shmuel-Galia L,
Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, Wilson R, Jiang Z,
Khalighinejad F, Muneeruddin K, et al: Succination inactivates
gasdermin D and blocks pyroptosis. Science. 369:1633–1637. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yi YS: Regulatory roles of the caspase-11
non-canonical inflammasome in inflammatory diseases. Immune Netw.
18:e412018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu P, Zhang X, Liu N, Tang L, Peng C and
Chen X: Pyroptosis: Mechanisms and diseases. Signal Transduct
Target Ther. 6:1282021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tang D, Kang R, Berghe TV, Vandenabeele P
and Kroemer G: The molecular machinery of regulated cell death.
Cell Res. 29:347–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi
AA and Lei P: Ferroptosis: Mechanisms and links with diseases.
Signal Transduct Target Ther. 6:492021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao
C, Liu W, Deng H, Li J, Ning P and Wang Z: Pyroptosis in
inflammatory diseases and cancer. Theranostics. 12:4310–4329. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yan H, Luo B, Wu X, Guan F, Yu X, Zhao L,
Ke X, Wu J and Yuan J: Cisplatin induces pyroptosis via activation
of MEG3/NLRP3/caspase-1/GSDMD pathway in triple-negative breast
cancer. Int J Biol Sci. 17:2606–2621. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yuan R, Zhao W, Wang QQ, He J, Han S, Gao
H, Feng Y and Yang S: Cucurbitacin B inhibits non-small cell lung
cancer in vivo and in vitro by triggering
TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol Res.
170:1057482021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J,
Wang K, Sun X and Zheng J: Cleavage of GSDME by caspase-3
determines lobaplatin-induced pyroptosis in colon cancer cells.
Cell Death Dis. 10:1932019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu
X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, et al: Gasdermin E
suppresses tumour growth by activating anti-tumour immunity.
Nature. 579:415–420. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ibrahim J, De Schutter E and Op de Beeck
K: GSDME: A potential ally in cancer detection and treatment.
Trends Cancer. 7:392–394. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jia Y, Wang X, Deng Y, Li S, Xu X, Qin Y
and Peng L: Pyroptosis provides new strategies for the treatment of
cancer. J Cancer. 14:140–151. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jiang M, Qi L, Li L and Li Y: The
caspase-3/GSDME signal pathway as a switch between apoptosis and
pyroptosis in cancer. Cell Death Discov. 6:1122020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Carlino MS, Larkin J and Long GV: Immune
checkpoint inhibitors in melanoma. Lancet. 398:1002–1014. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lou X, Li K, Qian B, Li Y, Zhang D and Cui
W: Pyroptosis correlates with tumor immunity and prognosis. Commun
Biol. 5:9172022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li S, Chen P, Cheng B, Liu Y, Zhang X, Xu
Q, Huang M, Dai X, Huang K, Zhang L, et al: Pyroptosis predicts
immunotherapy outcomes across multiple cancer types. Clin Immunol.
245:1091632022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gao W, Wang X, Zhou Y, Wang X and Yu Y:
Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor
immunotherapy. Signal Transduct Target Ther. 7:1962022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Krysko DV, Garg AD, Kaczmarek A, Krysko O,
Agostinis P and Vandenabeele P: Immunogenic cell death and DAMPs in
cancer therapy. Nat Rev Cancer. 12:860–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Galluzzi L, Vitale I, Warren S, Adjemian
S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch
E, et al: Consensus guidelines for the definition, detection and
interpretation of immunogenic cell death. J Immunother Cancer.
8:e0003372020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yatim N, Jusforgues-Saklani H, Orozco S,
Schulz O, Barreira da Silva R, Reis e Sousa C, Green DR, Oberst A
and Albert ML: RIPK1 and NF-κB signaling in dying cells determines
cross-priming of CD8+ T cells. Science. 350:328–334.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zeng C, Wang R and Tan H: Role of
pyroptosis in cardiovascular diseases and its therapeutic
implications. Int J Biol Sci. 15:1345–1357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Song M, Cui M and Liu K: Therapeutic
strategies to overcome cisplatin resistance in ovarian cancer. Eur
J Med Chem. 232:1142052022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Aloss K and Hamar P: Recent preclinical
and clinical progress in liposomal doxorubicin. Pharmaceutics.
15:8932023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang Z, Zhang H, Li D, Zhou X, Qin Q and
Zhang Q: Caspase-3-mediated GSDME induced pyroptosis in breast
cancer cells through the ROS/JNK signalling pathway. J Cell Mol
Med. 25:8159–8168. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ghosh S: Cisplatin: The first metal based
anticancer drug. Bioorg Chem. 88:1029252019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Stater EP, Sonay AY, Hart C and Grimm J:
The ancillary effects of nanoparticles and their implications for
nanomedicine. Nat Nanotechnol. 16:1180–1194. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kirtane AR, Verma M, Karandikar P, Furin
J, Langer R and Traverso G: Nanotechnology approaches for global
infectious diseases. Nat Nanotechnol. 16:369–384. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ploetz E, Zimpel A, Cauda V, Bauer D, Lamb
DC, Haisch C, Zahler S, Vollmar AM, Wuttke S and Engelke H:
Metal-organic framework nanoparticles induce pyroptosis in cells
controlled by the extracellular pH. Adv Mater. 32:e19072672020.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang Y, Yu W, Chen M, Zhang B, Zhang L
and Li P: The applications of nanozymes in cancer therapy: Based on
regulating pyroptosis, ferroptosis and autophagy of tumor cells.
Nanoscale. Jun 28–2023.(Epub ahead of print). View Article : Google Scholar
|
|
88
|
Yang F, Bettadapura SN, Smeltzer MS, Zhu H
and Wang S: Pyroptosis and pyroptosis-inducing cancer drugs. Acta
Pharmacol Si. 43:2462–2473. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wu D, Zhu X, Ao J, Song E and Song Y:
Delivery of ultrasmall nanoparticles to the cytosolic compartment
of pyroptotic J774A.1 macrophages via GSDMDNterm
membrane pores. ACS Appl Mater Interfaces. 13:50823–50835. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhao P, Wang M, Chen M, Chen Z, Peng X,
Zhou F, Song J and Qu J: Programming cell pyroptosis with
biomimetic nanoparticles for solid tumor immunotherapy.
Biomaterials. 254:1201422020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kuchur OA, Tsymbal SA, Shestovskaya MV,
Serov NS, Dukhinova MS and Shtil AA: Metal-derived nanoparticles in
tumor theranostics: Potential and limitations. J Inorg Biochem.
209:1111172020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cui R, Wu Q, Wang J, Zheng X, Ou R, Xu Y,
Qu S and Li D: Hydrogel-by-design: Smart delivery system for cancer
immunotherapy. Front Bioeng Biotechnol. 9:7234902021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Guimarães CF, Ahmed R, Marques AP, Reis RL
and Demirci U: Engineering hydrogel-based biomedical photonics:
Design, fabrication, and applications. Adv Mater. 33:e20065822021.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yin Y, Li X, Ma H, Zhang J, Yu D, Zhao R,
Yu S, Nie G and Wang H: In situ transforming RNA nanovaccines from
polyethylenimine functionalized graphene oxide hydrogel for durable
cancer immunotherapy. Nano Lett. 21:2224–2231. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sepantafar M, Maheronnaghsh R, Mohammadi
H, Radmanesh F, Hasani-Sadrabadi MM, Ebrahimi M and Baharvand H:
Engineered hydrogels in cancer therapy and diagnosis. Trends
Biotechnol. 35:1074–1087. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Guedes G, Wang S, Fontana F, Figueiredo P,
Lindén J, Correia A, Pinto RJB, Hietala S, Sousa FL and Santos HA:
Dual-crosslinked dynamic hydrogel incorporating {Mo154}
with pH and NIR responsiveness for chemo-photothermal therapy. Adv
Mater. 33:e20077612021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Guan QF, Yang HB, Han ZM, Ling ZC, Yin CH,
Yang KP, Zhao YX and Yu SH: Sustainable cellulose-nanofiber-based
hydrogels. ACS Nano. 15:7889–7898. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Basti ATK, Jonoobi M, Sepahvand S, Ashori
A, Siracusa V, Rabie D, Mekonnen TH and Naeijian F: Employing
cellulose nanofiber-based hydrogels for burn dressing. Polymers
(Basel). 14:12072022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Balahura LR, Dinescu S, Balaș M, Cernencu
A, Lungu A, Vlăsceanu GM, Iovu H and Costache M: Cellulose
nanofiber-based hydrogels embedding 5-FU promote pyroptosis
activation in breast cancer cells and support human adipose-derived
stem cell proliferation, opening new perspectives for breast tissue
engineering. Pharmaceutics. 13:11892021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Gonsalves A, Tambe P, Le D, Thakore D,
Wadajkar AS, Yang J, Nguyen KT and Menon JU: Synthesis and
characterization of a novel pH-responsive drug-releasing
nanocomposite hydrogel for skin cancer therapy and wound healing. J
Mater Chem B. 9:9533–9546. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang S, Zhang Z, Wei S, He F, Li Z, Wang
HH, Huang Y and Nie Z: Near-infrared light-controllable MXene
hydrogel for tunable on-demand release of therapeutic proteins.
Acta Biomater. 130:138–148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zheng Y, Wang W, Zhao J, Wu C, Ye C, Huang
M and Wang S: Preparation of injectable temperature-sensitive
chitosan-based hydrogel for combined hyperthermia and chemotherapy
of colon cancer. Carbohydr Polym. 222:1150392019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang W, Jin X, Li H, Zhang RR and Wu CW:
Injectable and body temperature sensitive hydrogels based on
chitosan and hyaluronic acid for pH sensitive drug release.
Carbohydr Polym. 186:82–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Phan VHG, Murugesan M, Huong H, Le TT,
Phan TH, Manivasagan P, Mathiyalagan R, Jang ES, Yang DC, Li Y and
Thambi T: Cellulose nanocrystals-incorporated thermosensitive
hydrogel for controlled release, 3D printing, and breast cancer
treatment applications. ACS Appl Mater Interfaces. 14:42812–42826.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kim J, Francis DM, Sestito LF, Archer PA,
Manspeaker MP, O'Melia MJ and Thomas SN: Thermosensitive hydrogel
releasing nitric oxide donor and anti-CTLA-4 micelles for
anti-tumor immunotherapy. Nat Commun. 13:14792022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Robinson N, Ganesan R, Hegedűs C, Kovács
K, Kufer TA and Virág L: Programmed necrotic cell death of
macrophages: Focus on pyroptosis, necroptosis, and parthanatos.
Redox Biol. 26:1012392019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang Y, Chen X, Gueydan C and Han J:
Plasma membrane changes during programmed cell deaths. Cell Res.
28:9–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ding J, Wang K, Liu W, She Y, Sun Q, Shi
J, Sun H, Wang DC and Shao F: Pore-forming activity and structural
autoinhibition of the gasdermin family. Nature. 535:111–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli
VG, Wu H and Lieberman J: Inflammasome-activated gasdermin D causes
pyroptosis by forming membrane pores. Nature. 535:153–158. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T,
Huang J, Wang F, Zhou F and Zhang L: Role of pyroptosis in
inflammation and cancer. Cell Mol Immunol. 19:971–992. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Liu Z, Wang T, She Y, Wu K, Gu S, Li L,
Dong C, Chen C and Zhou Y: N6-methyladenosine-modified
circIGF2BP3 inhibits CD8+ T-cell responses to facilitate
tumor immune evasion by promoting the deubiquitination of PD-L1 in
non-small cell lung cancer. Mol Cancer. 20:1052021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Xia R, Geng G, Yu X, Xu Z, Guo J, Liu H,
Li N, Li Z, Li Y, Dai X, et al: LINC01140 promotes the progression
and tumor immune escape in lung cancer by sponging multiple
microRNAs. J Immunother Cancer. 9:e0027462021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Hou J, Hsu JM and Hung MC: Molecular
mechanisms and functions of pyroptosis in inflammation and
antitumor immunity. Mol Cell. 81:4579–4590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wei Y, Yang L, Pandeya A, Cui J, Zhang Y
and Li Z: Pyroptosis-induced inflammation and tissue damage. J Mol
Biol. 434:1673012022. View Article : Google Scholar : PubMed/NCBI
|