Introduction
Meningioma is one of the most common and frequent
primary tumors of the central nervous system, and it originates
from either arachnoid or meningothelial cells in the central
nervous system (1). Recurrence and
extracranial metastases of meningioma are rare, and these have
previously been considered to only be associated with malignant
meningiomas, which are aggressive and associated with a poor
prognosis (1,2). Due to the increased understanding of
benign meningioma obtained from previous studies, it is now known
that benign meningioma also has malignant potential (3–6).
Nakasu et al (7) calculated
that the incidence rate of malignant transformation following
surgery for benign meningioma was 2.98/1,000 person-years by
meta-analysis; 10-year survival after malignant transformation was
50.1%; extent of removal, location and gender were the potential
risk factors associated with benign meningioma malignant
transformation (7). Some
researchers suggest that asymptomatic small-sized tumors can be
followed up with close observation, symptomatic lesions and those
with accelerated growth are primarily treated with maximum gross
total surgical resection, external beam radiotherapy, brachytherapy
or stereotactic radiosurgery (SRS) after surgical resection can be
used in grade 2 and 3 meningiomas and adjuvant therapies might be
required to reduce the recurrence rate in incompletely removed
meningiomas and atypical or malignant meningiomas (8,9).
However, available data on the malignant transformation of
transitional meningioma, a subtype of benign meningioma, are
limited. Ma et al (10)
retrospectively assessed the surgical outcomes of 298 patients who
was diagnosed as transitional meningioma, after a median follow-up
of 61.8 months, 23 patients (8.6%) had developed recurrence and two
patients (0.8%) had died; TM represent an unexpectedly high
recurrence rate (10).
The present report describes the case of a patient
with transitional meningioma, for which total resection was
performed 8 years prior, and transformation to a higher grade and
metastases to the ribs occurred. After a 1-year follow-up, the
recurrent meningioma was slightly larger (1.3 cm mass) than before
(a 1 cm mass). Gamma knife radiosurgery could be an option for the
patient; however, the patient did not consent to radiotherapy, she
still considered conservative treatment.
Case report
A 57-year-old female patient was hospitalized at
Weihai Central Hospital (Weihai, China) in September 2021 due to
right chest pain. Following admission, a computed tomography (CT)
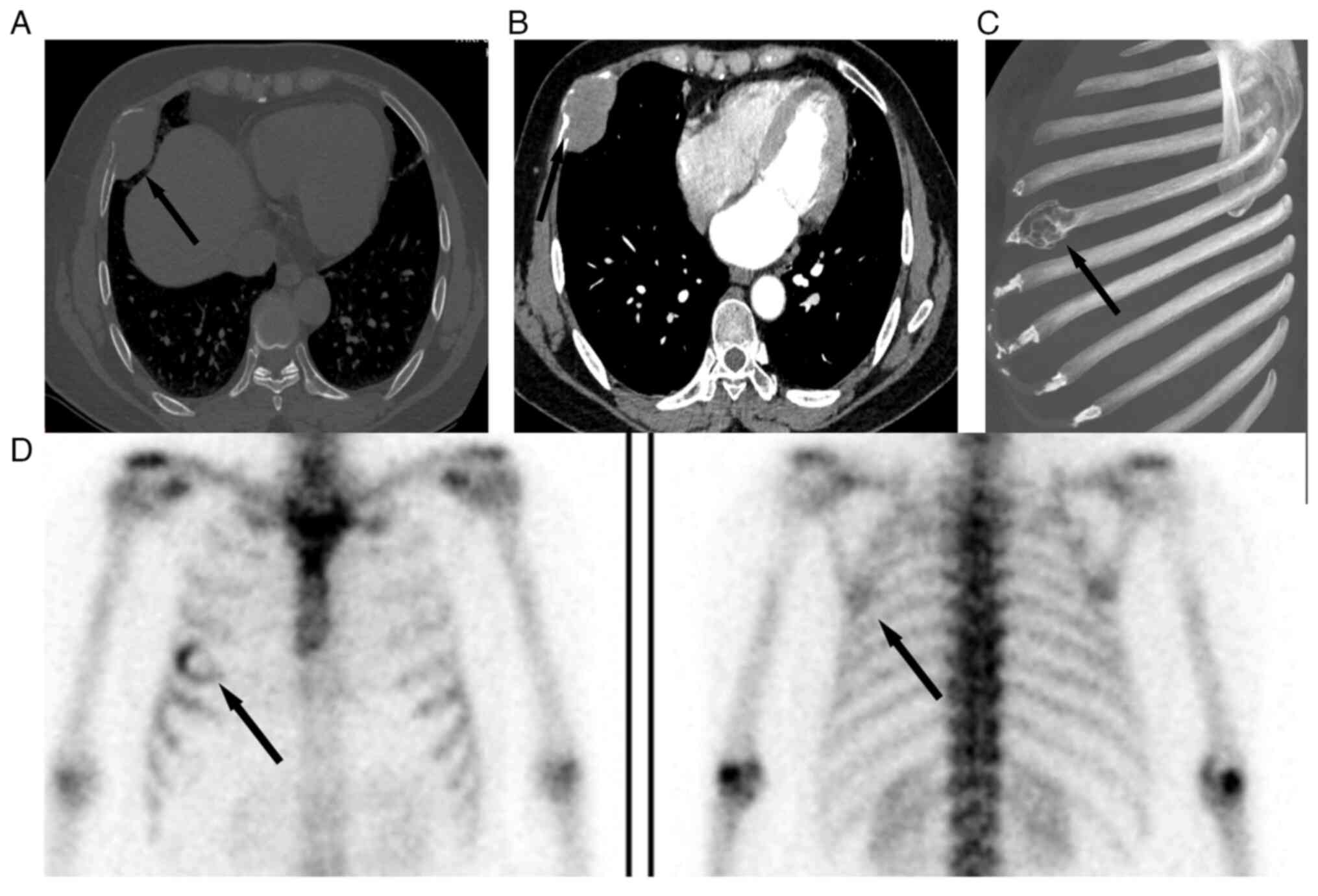
scan of the chest in September 2021 revealed bone destruction of
the right fifth rib, indicating a soft tissue mass (size, 5×3×2 cm)
(Fig. 1A), with a clear margin and
expansion of the bone cortex. A contrast-enhanced CT scan of the
chest in September 2021 revealed that the soft tissue mass of the
right rib was evenly enhanced and clearly demarcated from the lung
tissue (Fig. 1B). Rib
reconstruction shows bone destruction of the right fifth rib
(Fig. 1C). The CT scan of the chest
suggested a metastatic tumor in the rib. Subsequently, emission CT
also revealed that the right rib exhibited high levels of the
radioactive tracer Technetium-99m (Fig.
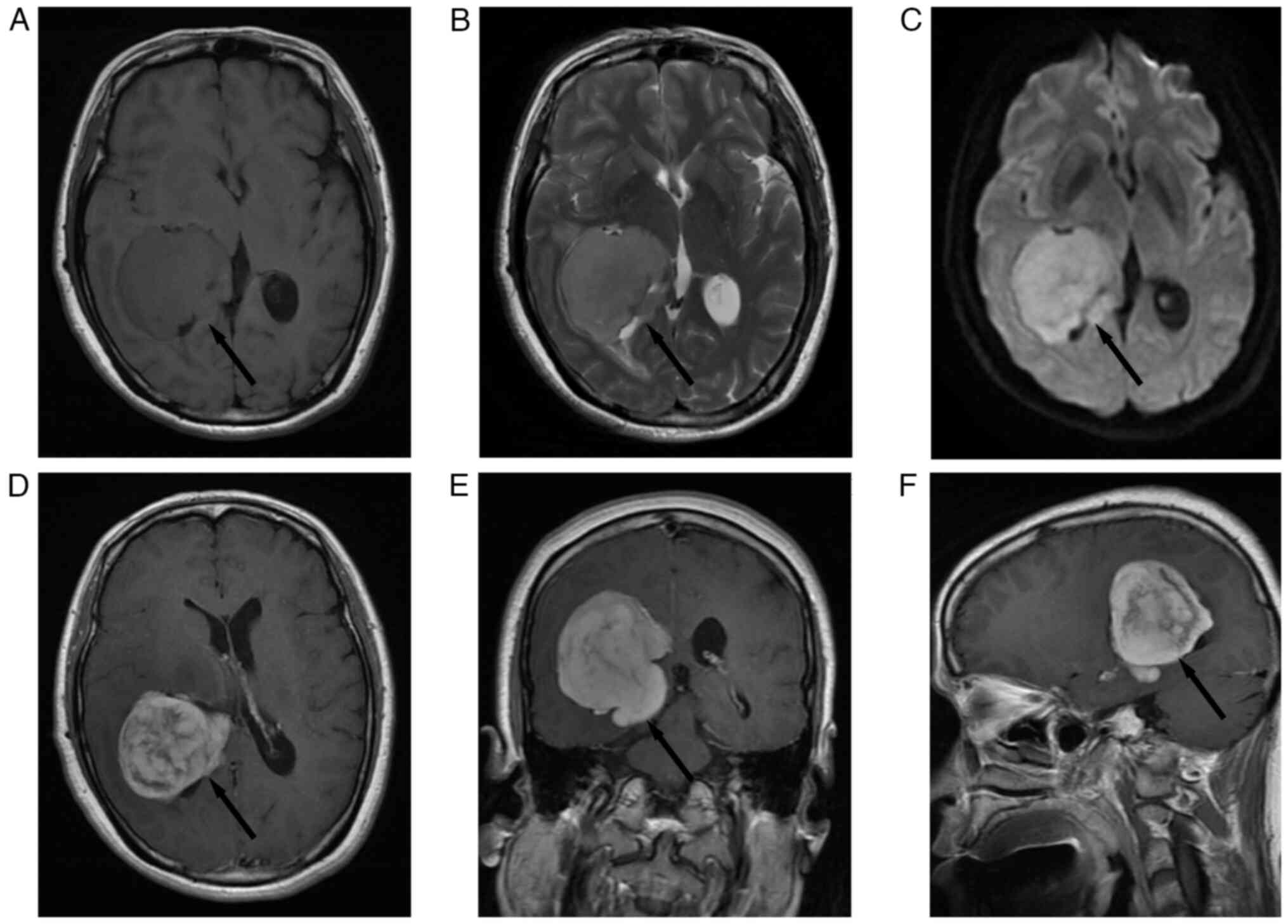
1D). The pre-operative magnetic resonance imaging (MRI) scan of
the patient in September 2013 revealed a ‘mushroom’-like appearance
mass of 5×4×6 cm in size which in the right lateral ventricle,
characterized by an isointense signal on Axial T1-weighted image
(T1WI) (Fig. 2A), a hyperintense
signal with minimal peritumoral edema on T2-weighted image (T2-WI)
(Fig. 2B), diffusion-weighted
imaging (DWI) illustrating a hyperintense signal (Fig. 2C). Axial (Fig. 2D), coronal (Fig. 2E) and sagittal (Fig. 2F) T1-WI gadolinium-enhanced MRI
illustrating heterogeneous contrast enhancement without a dural
tail sign and no metastatic lesions. Complete resection of the
tumor was performed in September 2013. During surgery, it found the
meningioma originated from the choroid plexus tissue. In addition,
the texture of the tumor observed was uneven, surrounded by
nodules. The tumor was surgically removed and electrocoagulation of
the choroid plexus was performed. Subsequent to this
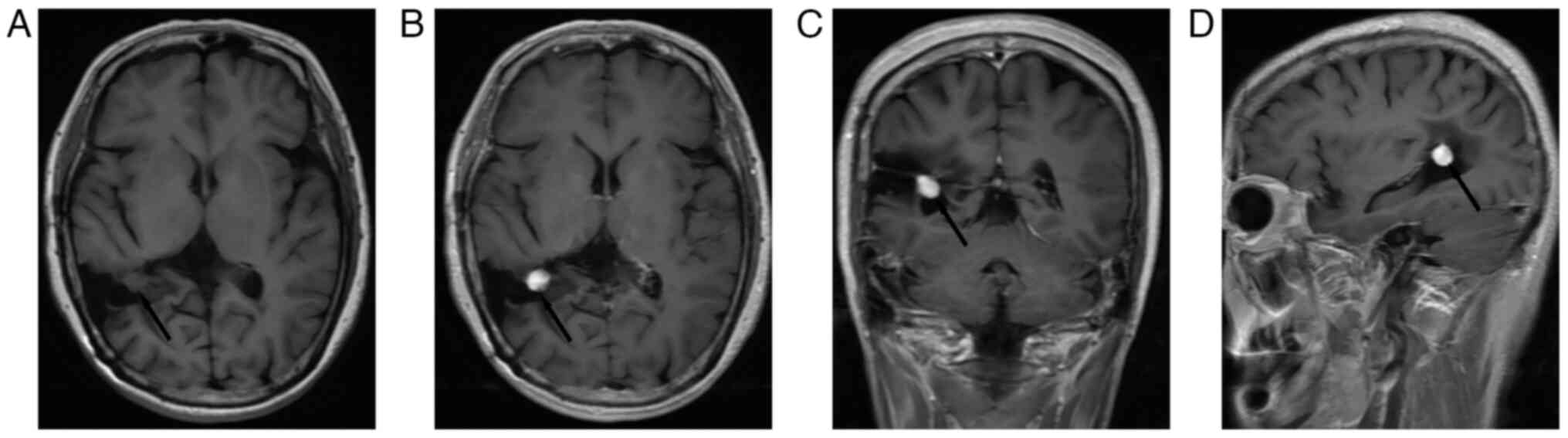
hospitalization, a brain MRI scan in September 2021 revealed a
well-circumscribed mass (diameter, 1 cm) in the area of previous
surgical resection, characterized by an isointense signal on Axial
T1-WI (Fig. 3A), a homogenous
contrast enhancement signal on Axial (Fig. 3B), coronal (Fig. 3C) and sagittal T1-WI (Fig. 3D). We carry out the
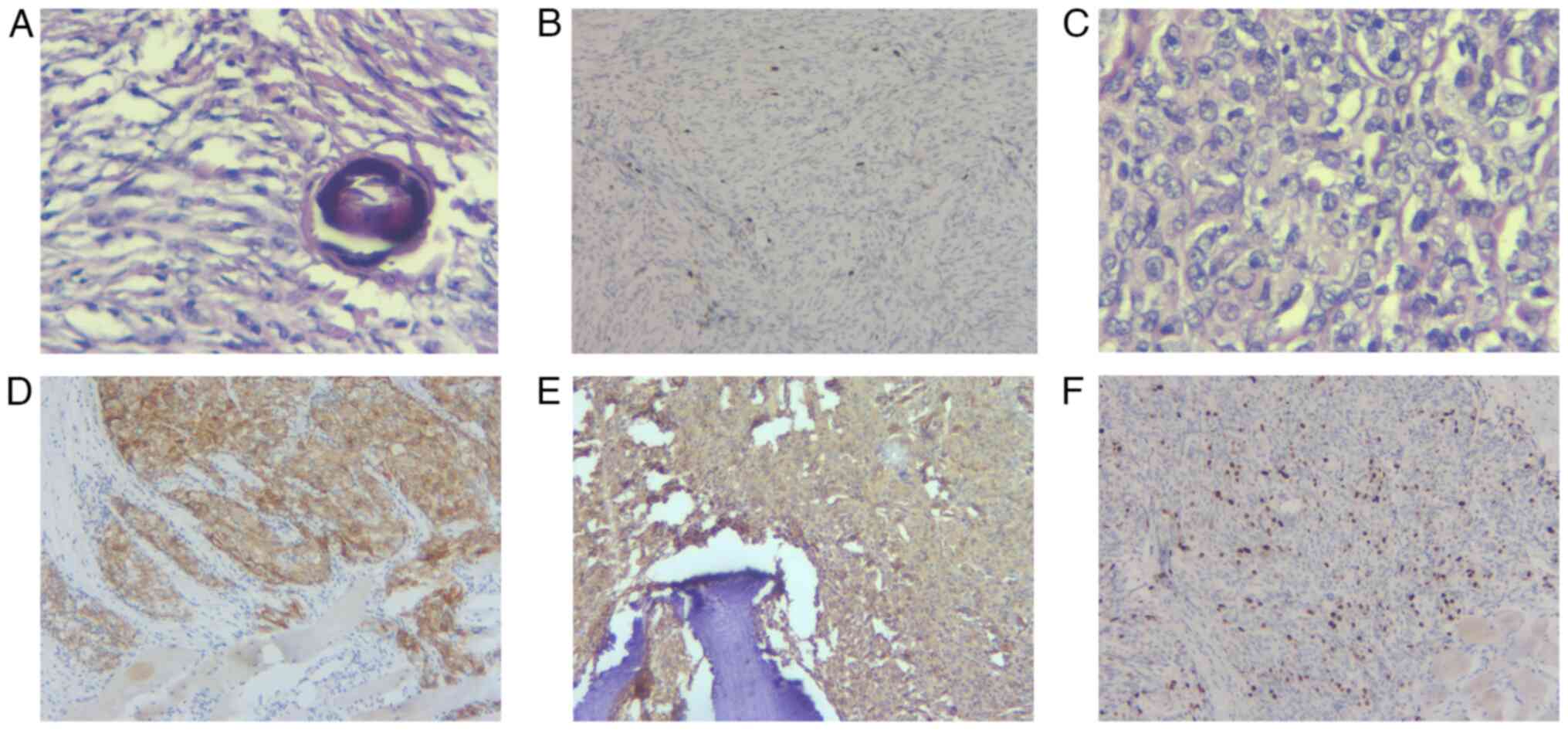
immunohistochemical analysis of the meningioma pathological section
which was resected in September 2013, revealed meningothelial and
fibroblastic features, meanwhile, contained a large amount of
psammoma (Fig. 4A), the patient was
diagnosed with transitional meningioma of World Health Organization
(WHO) grade 1 (11), and the Ki-67
index was ~3% (Fig. 4B). The
patient had no neurological symptoms apart from right chest pain.
Therefore, it was decided to use a minimally invasive surgical
approach to remove the metastatic tumor of the rib. During the
surgery, it was found that the tumor did not invade the lung. The
whole procedure was completed successfully with no complications.
Tumor tissue samples were fixed with 10% buffered formalin for 24 h
at room temperature, paraffin-infiltrated overnight. Tissue blocks
were sectioned at 3 µm, dewaxed, and stained with hematoxylin (~5%)
for 5 min, followed by eosin (~1%) staining for 2 min at room.
Hematoxylin and eosin staining were examined using an Olympus BX53
light microscope. The rib metastatic tumor revealed atypical
features with the diffuse arrangement of cells, focal necrosis and
there were mitotic figures up to five figures/10 points (Fig. 4C). The results of
immunohistochemistry (Fixed with 10% buffered formalin for 24 h at
room temperature, paraffin-infiltrated overnight. Tissue blocks
were sectioned at 3 µm. Heated at 70°C for 30 min in an electric
thermostatic drying oven (Shanghai Yiheng Scientific Instrument
Co., Ltd.), dewaxed with xylene at room temperature, rehydrated in
a descending alcohol series at room temperature, and endogenous
peroxidase activity was blocked using 3% H2O2
at room temperature for 10 min, followed by blocking with 10%
normal goat serum (ab7481; Abcam) at 37°C for 30 min. Anti EMA
(E29) (prediluted by the manufacturer; cat. no. Z2048MP; Thermo
Fisher Scientific, Inc.), Ki 67 (SP6) (prediluted by the
manufacturer; cat. no. PIMA514520; Invitrogen™; Thermo
Fisher Scientific, Inc.), Anti Vimentin (v9) (prediluted by the
manufacturer; cat. no. MA1102; Boster Biological Technology).
Incubation Time/Temperature: Anti EMA (E29) 13 min/room
temperature; Ki 67 (SP6) 30–60 min/room temperature and Anti
Vimentin (v9) 5–10 min/room temperature. The secondary antibody was
obtained from the EnVision FLEX/HRP (prediluted; cat. no. K8000;
Agilent Technologies, Inc.) and was used to treat sections at room
temperature for 25 min. Subsequently, a chromogen detection reagent
was applied (EnVision FLEX DAB+ Chromogen; cat. no. K8000, Agilent
Technologies, Inc.). Using an Olympus BX53 light microscope
(Olympus Corporation) revealed the positive expression of
epithelial membrane antigen (EMA; Fig.
4D), vimentin (Fig. 4E) and
progesterone receptor, and the negative expression of S-100
protein, cytokeratin(P), glial fibrillary acidic protein, CD34,
smooth muscle actin and desmin. The Ki-67 positive index was ~15%
(Fig. 4F). Vimentin and EMA
exhibited positive staining, and these are the critical markers
supporting the diagnosis of meningioma (6,12).
Thus, the metastatic tumor in the rib was diagnosed as atypical
meningioma (WHO grade 2). As the patient had no symptoms in
September 2021, the patient selected conservative treatment. After
1-year follow-up, the patient's brain MRI scan in August 2022
revealed the recurrent meningioma was slightly larger (a 1.3 cm
mass) than before (a 1 cm mass). Here, the gamma knife radiosurgery
could be an option for the patient, however, the patient did not
consent to radiotherapy, she still considered conservative
treatment.
Discussion
According to the 2021 WHO tumor classification,
>80% of meningiomas are benign meningiomas (grade 1), 15–20% are
atypical meningiomas (grade 2) and 1–3% are malignant meningiomas
(grade 3), depending on the mitotic rate, brain invasion or
specific histological features, such as rhabdoid and papillary
morphology qualified for CNS WHO grade 3 irrespective of any other
indications for malignancy (11,13).
Transitional meningioma is an uncommon subtype of benign
meningioma, which is characterized by a transitional morphological
appearance between endothelial meningiomas and fibrous meningiomas
(10). The histological tumor grade
of the meningioma is the most crucial predictor for recurrence or
metastasis (11). Thus, it was
previously considered that benign meningiomas rarely metastasize or
recur and can be cured through surgical resection (8). By contrast with benign meningioma,
atypical and anaplastic meningiomas often exhibit a more aggressive
biological potential of malignant tumors, such as an abnormal
proliferative activity and aggressive growth patterns and have a
higher risk of recurrence and poorer progression than benign
meningiomas (8,14). Studies found that the recurrence
rate of WHO grade I meningiomas is 7–23%, WHO grade II meningiomas
is 50–55%, and WHO grade III meningiomas is 72–78% in 5 years after
total resection (8,14). It has previously been considered
rare for atypical and anaplastic meningiomas to exhibit an
increased risk of extracranial metastasis (6,15).
Similarly, reported cases of extracranial metastasis of benign
meningiomas are limited (4,5,7). Some
studies have reported that the lungs are the most common site for
seeding, followed by the liver, bone, skin, lymph nodes and
mediastinum (4,6,16). The
routes of metastasis are blood and the lymphatic vessels, as well
as the cerebrospinal fluid, hematogenous spread is the most common
route of metastasis (4,6,15).
Furthermore, metastasis mostly occurs in postoperative patients,
and the spread of tumor cells following surgery also may lead to
tumor metastasis (14). Based on
aforementioned previous research reports, for the patient described
in the present report, it was hypothesized that rib metastasis
occurred through the vertebral venous plexus, as surgery was
considered to have led to the release of neoplastic cells into torn
veins, allowing tumor cells to access the blood circulation and to
travel from there to the vertebral venous plexus. These vertebral
veins have extensive communication links with the veins of the
intercostal veins of the thoracic-abdominal wall anatomically.
Through these connections, tumor cells can spread from the brain to
the ribs (16). It is considered
reasonable to relate isolated rib metastatic involvement to the
existence of the vertebral venous system.
In the present case, despite the histopathological
confirmation of transitional meningioma (WHO grade 1) during the
initial surgery, the histopathological examination of the rib
metastatic tumor led to a diagnosis of atypical meningioma (WHO
grade 2). Atypical and malignant transformation is a recognized
phenomenon, and it has been reported that 2–10% of benign tumors
exhibit malignant potential (6). A
previous study determined that despite the gross total resection of
benign meningioma, the recurrence rate was ~9.5% (3). Furthermore, it has been demonstrated
that the probability of a recurrent benign meningioma progressing
to an atypical or anaplastic pathology was as high as 28.5%
(15). Recurrent or malignant
transformation usually begins with excessive cell proliferation
(12). Ki-67 is a nuclear antigen
expressed during the active phases of the cell cycle. A higher
Ki-67 proliferative index indicates shorter cell cycle times and
more rapid tumor growth (12). It
has been demonstrated that, in meningioma, the Ki-67 proliferative
index increases with recurrence or malignant progression to grade 3
anaplastic meningioma (11).
Generally, Ki-67 proliferative index of 4% indicates benign
meningioma, while a Ki67 proliferative index of 10–15% is
indicative of aggressive meningioma (6). In the present case, the Ki-67 index
was ~3% in the primary benign meningioma; however, the Ki-67 index
increased to 15% in the recurrent meningioma. The patient exhibited
recurrence of benign meningioma with a worsened tumor grade (WHO
grade 2) based on the immunohistochemical analysis of the
metastatic tumors. Furthermore, the Ki-67 proliferative index was
high in the resected specimen. Thus, Ki-67 may be used as a marker
of tumor cell proliferation to help predict the malignant potential
of meningioma.
In the present case, the meningioma originated from
choroid plexus tissue. During the surgery, the tumor was surgically
removed section by section, and electrocoagulation of the choroid
plexus was performed. Although the tumor was completely resected,
tumor cells may still have been present at the surgical margin,
leading to postoperative tumor recurrence. In addition, the texture
of the tumor observed during surgery was uneven, surrounded by
nodules. The combined MRI revealed a ‘mushroom’-like appearance and
a heterogeneous enhancement of the mass. This may have provided
critical indications of the malignancy or aggressiveness of the
tumor. Although the texture of the tumor was uneven during surgery
and magnetic resonance imaging, indicating a malignancy,
histopathological analysis is considered the gold standard for the
diagnosis of benign meningioma.
The majority of primary meningiomas are diagnosed
and resected prior to the occurrence of distant metastases, which
supports the hypothesis that surgical resection may increase the
risk of iatrogenic metastasis in meningiomas (16). Some cases of metastasis have been
observed even without prior surgery (16). Theoretically, a craniotomy may lead
to the release of tumor cells from a normally cohesive state into
the bloodstream or cerebrospinal fluid. In particular, neoplastic
cells enter the torn vein, and subsequently spread to the heart,
and are released to various organs (16).
With an increasing number of studies reporting the
malignant transformation of meningioma, researchers have found that
chromosomal instability with recurrent cytogenetic alterations is
often associated with the malignant progression of meningioma,
including chromosomal aberrations at 1p, 6q, 9q, 10q, 12q, 14q,15q,
17q, 18q, 20q and 22q (13,14). A previous study reported that a
meningioma with telomerase reverse transcriptase promoter mutations
harbors a higher risk of malignant transformation and a more
aggressive clinical course compared to meningioma without (TERT)
promoter mutations (17).
Furthermore, another study demonstrated that a neurofibromatosis
type 2 mutation activated the Hippo, Notch, PI3K/AKT, mTOR and
RAS/MAPK signaling pathways, with an ensuing increase in cell
proliferation (18). In addition,
some studies have found that stereotactic radiosurgery (19), surgical stress (16) and viral infection (4) can induce the malignant transformation
of intracranial meningioma. These findings provide theoretical
support for the recurrence of benign meningioma and the malignant
progression of transformation into atypical or anaplastic
meningioma.
Notably, intracranial Ewing's sarcoma (ES)/primitive
neuroectodermal tumor, also known as a ‘small round blue cell
tumor’, is a rare entity arising from bone and soft tissue, which
is frequently observed among children and adolescents (20). Intracranial ES may be intra- or
extra-axial with or without bone involvement, and mostly occurs as
a solitary lesion associated with the dura, mimicking a meningioma
in a radiological examination. Intracranial ES mostly exhibits
mixed isointense-to-hypointense signals on T1WI, and
isointense-to-hyperintense signals on T2WI, which is frequently
accompanied by intratumoral hemorrhage or necrosis. In a
post-contrast MRI, it presents as a heterogeneous enhancement.
Therefore, it has been defined as a markedly enhanced solid mass
accompanied by hemorrhagic and cystic components. Notably,
hemorrhaging and necrosis are uncommon in meningioma, mostly
presenting as isointense or hypointense signals on T1WI, and
isointense or hyperintense signals on T2WI, exhibiting a homogenous
contrast enhancement, which is common among in adults than in
children. Furthermore, meningioma usually causes pressure onto the
adjacent parenchyma, whereas parenchyma invasion is observed in ES.
However, imaging may not be helpful in these situations, as both
tumors may present as well-defined dural masses with contrast
enhancement and exhibit bony erosion.
In the present patient, MRI of the primary
meningioma revealed heterogeneous enhancement resembling ES, with
isointense signals on T1WI and hyperintense signals on T2WI.
However, according to the intraoperative findings, the tumor was
located in the lateral ventricles and the tumor originated from the
choroid, therefore, ES was not considered when the diagnosis of the
meningioma was determined using MRI.
To the best of our knowledge, there is no clear
guidance for the management of the malignant transformation and
recurrence of benign meningioma following surgery. Some researchers
have found that gamma knife radiosurgery may be an effective
therapy for the first recurrence of transitional meningioma
(10). Observation is another
option, generally reserved for small, asymptomatic tumors and for
patients that are deemed poor candidates for other therapeutic
options (18). The management of
these patients requires observation and serial monitoring with MRI
scans. Notably, when tumor growth or symptom progression indicate
that observation has failed, additional treatments are then
warranted. As the patient in the present study has no symptoms in
September 2021, further surgery is no longer considered to be in
the best interests of the patient. The patient did not consent to
radiotherapy, ultimately selecting conservative treatment. After a
1-year follow-up, the patient's brain MRI scan in August 2022
revealed the recurrent meningioma was slightly larger (a 1.3 cm
mass) than before (a 1 cm mass). Here, the gamma knife radiosurgery
could be an option for the patient, however, the patient did not
consent to radiotherapy, she still considered conservative
treatment.
In conclusion, although the majority of benign
meningiomas are slow-growing and associated with a good prognosis,
they can also exhibit an aggressive growth and recurrence, and can
present different grades of dedifferentiation from grade 1–3,
associated with different outcomes (4,6,15). Rib
metastasis of meningiomas is uncommon, and meningiomas located
exclusively within the ventricles are also rare (16). The present study describes the case
of a patient with recurrence of transitional meningioma for which
total resection had been performed 8 years prior. This case
highlights the aggressive potential of transitional meningiomas and
emphasizes the need to consider the metastasis of meningioma in the
case of discovered a rib mass in patients with a history of
transitional meningioma. Given this finding, it may be clinically
useful to evaluate annually patients with asymptomatic or small
transitional meningioma by radiological imaging, after 5 years,
this follow up interval can be doubled. The present study also
reviewed previous research that chromosomal instability with
recurrent cytogenetic alterations is often associated with
recurrent and progressive meningiomas. A further understanding of
the mechanisms underlying the recurrence or malignant progression
of transitional meningioma may help to predict the clinical
behavior of meningiomas, which may prove to be beneficial for the
early recognition of high-risk meningiomas or progressive
meningiomas and may lead to the timely adoption of effective
treatment strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ and SY designed and guided the study. SY, JW, BH
and JS analyzed the data and wrote the initial manuscript. WZ, BH
and JS revising it critically for important intellectual content.
SY, JW, JS and BH confirm the authenticity of all the raw data and
analysis and interpretation of data. BH and JS obtained the images
and drafted the figures. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was granted an exemption from
requiring ethics approval from the Ethics Committee of Weihai
Central Hospital (Weihai, China).
Patient consent for publication
The patient provided written informed consented to
the data and images being obtained for research purposes and
consented to their publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Modha A and Gutin PH: Diagnosis and
treatment of atypical and anaplastic meningiomas: A review.
Neurosurgery. 57:538–550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Champeaux C, Jecko V, Houston D, Thorne L,
Dunn L, Fersht N, Khan AA and Resche-Rigon M: Malignant meningioma:
An international multicentre retrospective study. Neurosurgery.
85:E461–E469. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scheck AC and Hendricks W: Molecular
biological determinants of meningioma progression and aggressive
behavior. Front Biosci (Elite Ed). 1:390–414. 2009.PubMed/NCBI
|
|
4
|
Ito K, Imagama S, Ando K, Kobayashi K,
Shido Y, Go Y, Arima H, Kanbara S, Hirose T, Matsuyama Y, et al:
Intraspinal meningioma with malignant transformation and distant
metastasis. Nagoya J Med Sci. 79:97–102. 2017.PubMed/NCBI
|
|
5
|
Pereira BJA, de Almeida AN, Paiva WS, de
Aguiar PHP, Teixeira MJ and Marie SKN: Natural history of
intraventricular meningiomas: Systematic review. Neurosurg Rev.
43:513–523. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sheng B, Liu Y and Liu C: Liver metastasis
from typical meningioma. World Neurosurg. 145:334–337. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakasu S, Notsu A, Na K and Nakasu Y:
Malignant transformation of WHO grade I meningiomas after surgery
or radiosurgery: Systematic review and meta-analysis of
observational studies. Neurooncol Adv. 2:vdaa1292020.PubMed/NCBI
|
|
8
|
Zhao L, Zhao W, Hou Y, Wen C, Wang J, Wu P
and Guo Z: An overview of managements in meningiomas. Front Oncol.
10:15232020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alruwaili AA and De Jesus O: Meningioma.
StatPearls [Internet]. StatPearls Publishing LLC; 2023
|
|
10
|
Ma XJ, Zhang GJ, Wang W, Li D, Wu Z and
Zhang JT: Proposed treatment for intracranial transitional
meningioma: A single-center series of 298 cases. World Neurosurg.
127:e280–e287. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldbrunner R, Stavrinou P, Jenkinson MD,
Sahm F, Mawrin C, Weber DC, Preusser M, Minniti G, Lund-Johansen M,
Lefranc F, et al: EANO guideline on the diagnosis and management of
meningiomas. Neuro Oncol. 23:1821–1834. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotecha RS, Junckerstorff RC, Lee S, Cole
CH and Gottardo NG: Pediatric meningioma: Current approaches and
future direction. J Neurooncol. 104:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng W, Wu P, Yuan M, Yuan B, Zhu L, Zhou
J and Li Q: Potential molecular mechanisms of recurrent and
progressive meningiomas: A review of the latest literature. Front
Oncol. 12:8504632022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen HCB, Mady LJ, Panara K, Andrianus
S, Cooper K, Chen IH, Chalian AA and Brody RM: Metastatic
meningioma of the neck: A case report and systematic review. ORL J
Otorhinolaryngol Relat Spec. 84:361–369. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomas RZ and Dalal I: Extracranial
metastases of anaplastic meningioma. BJR Case Rep.
3:201500922017.PubMed/NCBI
|
|
16
|
Surov A, Gottschling S, Bolz J, Kornhuber
M, Alfieri A, Holzhausen HJ, Abbas J and Kösling S: Distant
metastases in meningioma: An underestimated problem. J Neurooncol.
112:323–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spiegl-Kreinecker S, Lötsch D, Neumayer K,
Kastler L, Gojo J, Pirker C, Pichler J, Weis S, Kumar R, Webersinke
G, et al: TERT promoter mutations are associated with poor
prognosis and cell immortalization in meningioma. Neuro Oncol.
20:1584–1593. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patel B, Desai R, Pugazenthi S, Butt OH,
Huang J and Kim AH: Identification and management of aggressive
meningiomas. Front Oncol. 12:8517582022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Basalamah A, Al-Bolbol M, Ahmed O, Ali N
and Al-Rashed S: Stereotactic radiosurgery (SRS) induced
higher-grade transformation of a benign meningioma into atypical
meningioma. Case Rep Surg. 2022:44785612022.PubMed/NCBI
|
|
20
|
Huang J, Ghent F, Levingston R and
Scholsem M: Intracranial ewing sarcoma-a case report. Surg Neurol
Int. 11:1342020. View Article : Google Scholar : PubMed/NCBI
|