Introduction
Adult acute lymphoblastic leukemia (ALL) is one of
the most common types of acute leukemia in adults, accounting for
20–30% of adult acute leukemia cases worldwide (1). A previous study indicated a higher
morbidity in male patients compared with that in female patients
(1.4:1), and a lower morbidity in adults compared with in children
(1:3) (1). The rates of complete
remission (CR) range between 70 and 90%, with long-term
disease-free survival rates ranging between 40 and 50% in ALL cases
positive for the Philadelphia chromosome/BCR-ABL fusion gene
(2). Cardiac involvement is an
uncommon occurrence in patients diagnosed with Philadelphia
chromosome-ALL (Ph+ALL) (3).
Following cardiac invasion, patients often experience recurrent
wheezing symptoms and potential cardiac function impairment,
leading to a decreased survival rate. The present report describes
the case of a patient admitted with cardiac involvement in
Ph+ALL.
Case report
A 48-year-old female patient was admitted to the
Beijing LuHe Hospital (Beijing, China) in September 2020 primarily
due to a 4-year history of ALL. The patient presented with chest
tightness and dyspnea that had occurred for the past 3 days. The
patient was initially diagnosed with Ph+ALL in November 2016, and
achieved CR following regular chemotherapy [cyclophosphamide (0.8 g
day 1), vincristine (4 mg day 2), doxorubicin (80 mg days 3–4),
dexamethasone (70 mg days 1–7) and imatinib (600 mg every day),
Q4W]. However, in January 2018, relapse was observed in bone marrow
morphology, accompanied by pleural invasion, leading to the
continuation of chemotherapy (Table
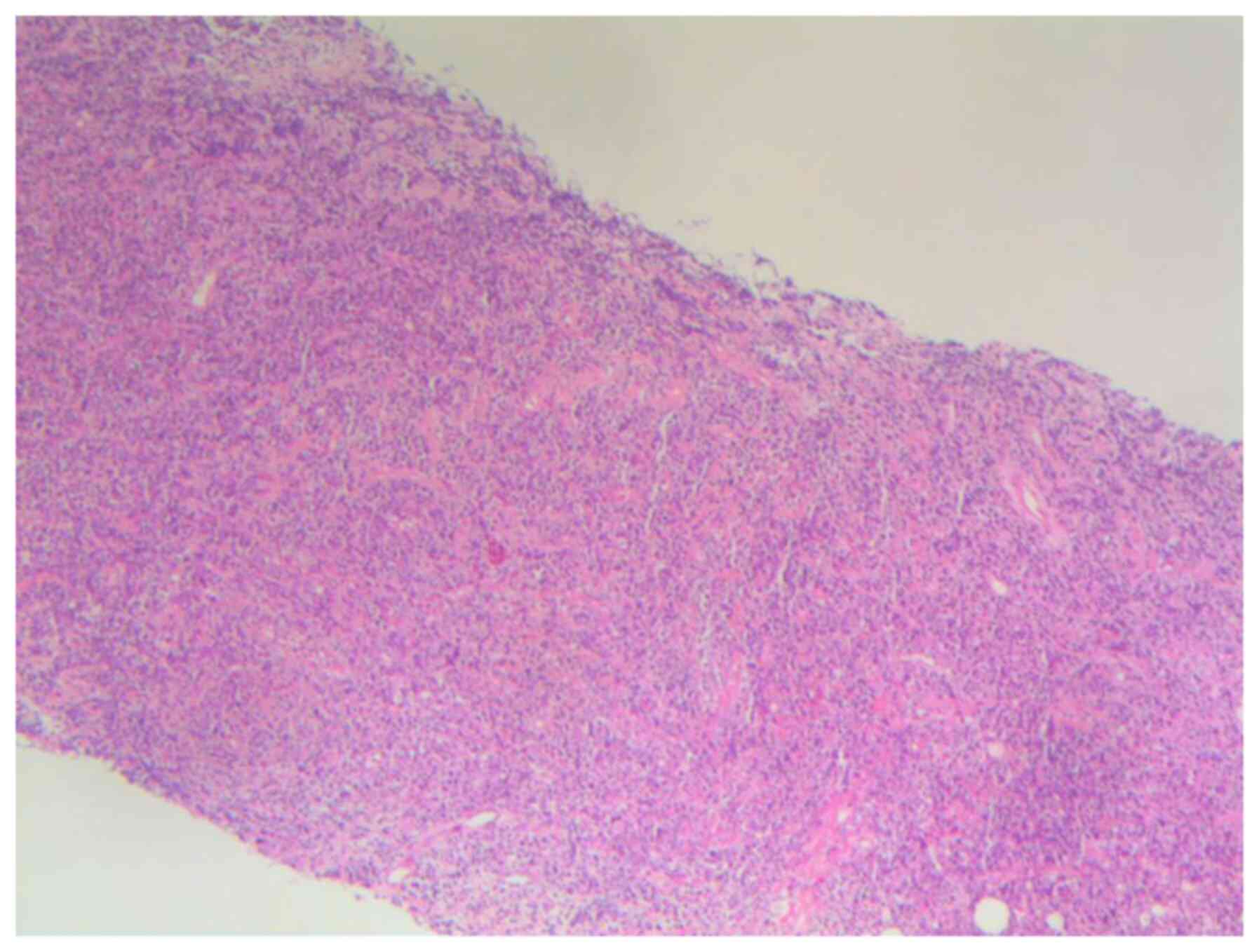
I). In March 2018, a left breast mass was detected, and
subsequent pathological examination confirmed leukemic infiltration
(Fig. 1). Due to the unavailability
of total body irradiation (TBI) treatment in the radiation oncology
department, and considering the severe bone marrow suppression and
rapid disease progression, it was not feasible to refer the patient
to an external facility for radiation therapy. Thus, the patient
was not selected to undergo TBI despite the presence of
extramedullary recurrence. In April 2018, an intensified
conditioning regimen (modified BuCY + IDA + VP16; idarubicin 20 mg
on days 10 and 9, cytarabine 2 g/m2 on day 9,
daunorubicin 0.8 mg/kg every 6 h on days 8, 7 and 6,
cyclophosphamide 1.8 g/m2 on days 5 and 4, somatostatin
250 mg on day 3, etoposide 60 mg/m2 on days 4, 3 and 2)
was administered, followed by salvage sibling-matched allogeneic
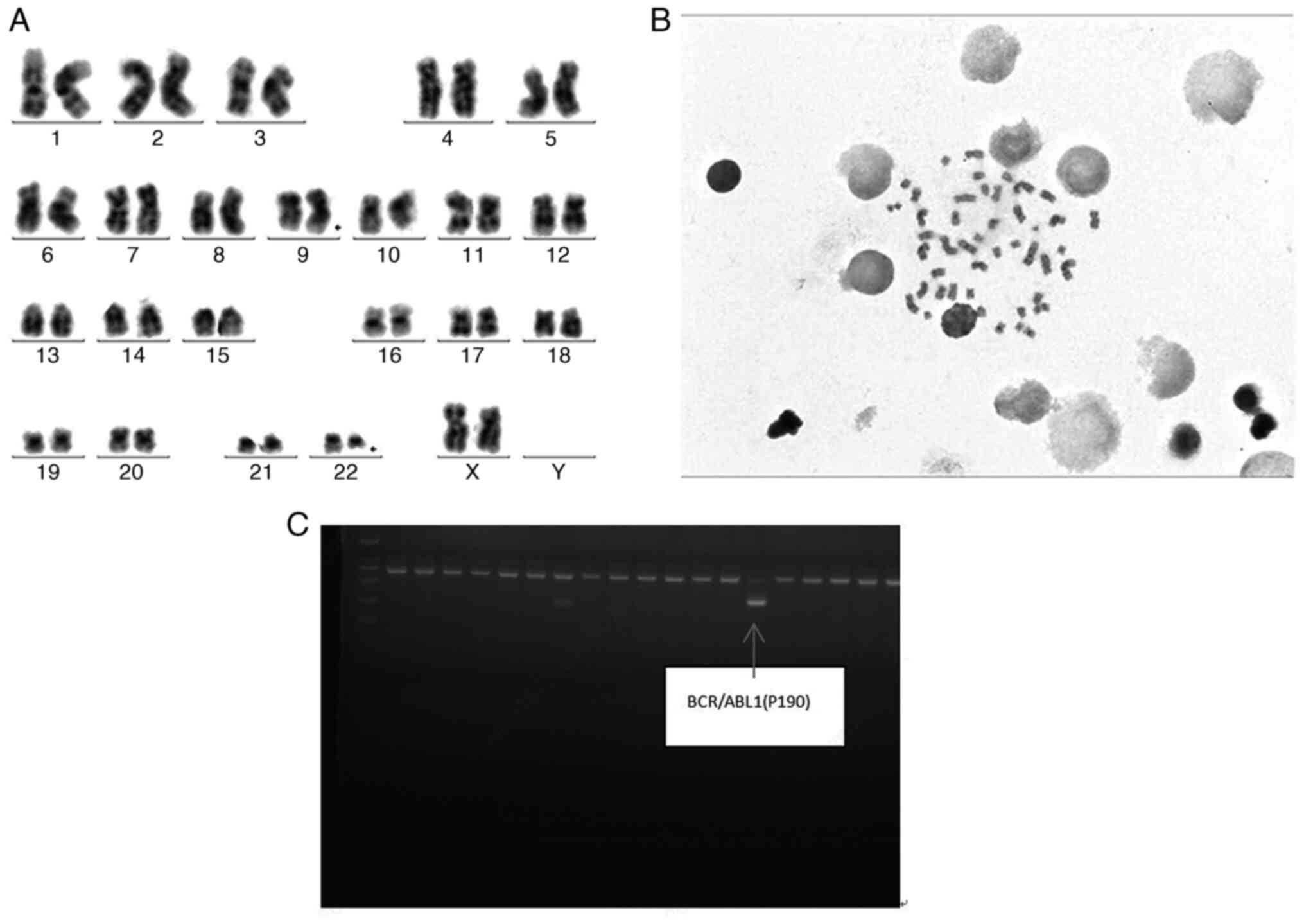
hematopoietic stem cell transplantation. A review conducted by PCR
(Fig. 2) in July 2018 revealed the
presence of the bone marrow BCR/ABL1 fusion gene (Philadelphia
chromosome) and a BCR-ABL1/ABL1 ratio of 11.58%. The patient was
prescribed dasatinib (50 mg; twice daily) and interferon therapy.
In October 2018, the patient developed chronic skin and oral
graft-versus-host disease, which improved with treatment with
prednisone (1 mg/kg/day) in combination with methotrexate (7.5
mg/week). In January 2019, a left breast mass was observed.
Dasatinib and interferon therapy were continued (Table I), resulting in a reduction of the
breast mass.
 | Table I.Summary of the treatment course of the
patient. |
Table I.
Summary of the treatment course of the
patient.
| Time | Event | Treatment | Outcome |
|---|
| November 2016 | Diagnosis:
Philadelphiachromosome-positive acute lymphoblastic leukemia | VDCP + IM consisting
of cyclophosphamide (0.8 g day 1), vincristine (4 mg day 2),
doxorubicin (80 mg days 3–4), dexamethasone (70 mg days 1–7) and
imatinib (600 mg once every day), Q4W | BM: CR |
| November 2016-January
2018 | ph+ALL | Continuation of
chemotherapy with the previous regimen (VDCP + IM) for 11
cycles | BM: CR |
| January 2018 | BM morphological
relapse and pleural involvement | Cyclophosphamide (1.8
g on days 1 and 2), followed by CAR-T cell infusion (23 ml,
totaling 1.71×108 cells) for two cycles | BM: CR; BCR/ABL1
fusion gene, 0.56% |
| March 2018 | Left breast mass,
pathological diagnosis: Leukemic infiltration. Marrow morphological
relapse | Modified BuCY + IDA +
VP16 once, salvage sibling-matched allogeneic hematopoietic stem
cell transplantation (counts of peripheral blood stem cells,
mononuclear cells: 6.07×108/kg, CD34+:
2.423×106/kg) | BM: CR; BCR/ABL
fusion gene, (−) |
| July 2018 | BCR/ABL fusion gene,
(+); BCR-ABL1/ABL1, 11.58% | Dasatinib (50 mg;
bid); interferon therapy, for 4 months |
|
| October 2018 | Chronic
graft-versus-host disease of the skin and oral cavity | Prednisone (1
mg/kg/day); methotrexate (7.5 mg/week), for 2 months | BM: CR; BCR/ABL
fusion gene, (−) |
| January 2019 | Left breast mass
relapse | Dasatinib; interferon
therapy, for 4 months | Mass was smaller than
before |
| April 2020 | Shortness of breath
on exertion. Echocardiogram revealed moderate pericardial
effusion | Discontinuation of
dasatinib; treatment with prednisone (0.5 mg/kg/day) and diuretics
(10 mg/day), for 5 months | Outcomes remained
unsatisfactory |
| September 2020 | Increased dyspnea,
echocardiography indicated large pericardial effusion. Diagnosis of
ALL relapse with involvement of the pericardium | PCC | Pathology indicated
primitive cells; flow cytometry revealed 52.37% abnormal primitive
B lymphocytes; IGH, (+); BM: CR |
| September
2020-December 2020 | Recurrent pericardial
effusion | PCC; intrapericardial
injection of dexamethasone (10 mg once); rituximab (375
mg/m2), for 3 months | Repeated pericardial
effusion |
| December 2020 for 2
weeks | Chest tightness and
dyspnea, Echocardiogram showed large pericardial effusion, right
atrial compression, bilateral pleural effusion and an abnormal echo
of the left parietal pleura | PCC; 4D chemotherapy
simulation positioning; radiotherapy (19.8 Gy/1.8 Gy/11 fractions),
subsequent maintenance treatment with dasatinib (50 mg; bid), for 6
months | During a 10-month
follow-up, the patient did not experience radiation pneumonitis,
pericarditis or any impairment in cardiac function |
| After September
2021 | The patient exhibited
spinal cord involvement and bone marrow relapse | The patient opted for
treatment discontinuation |
|
| October 2021 | Patient died of
severe pneumonia |
|
|
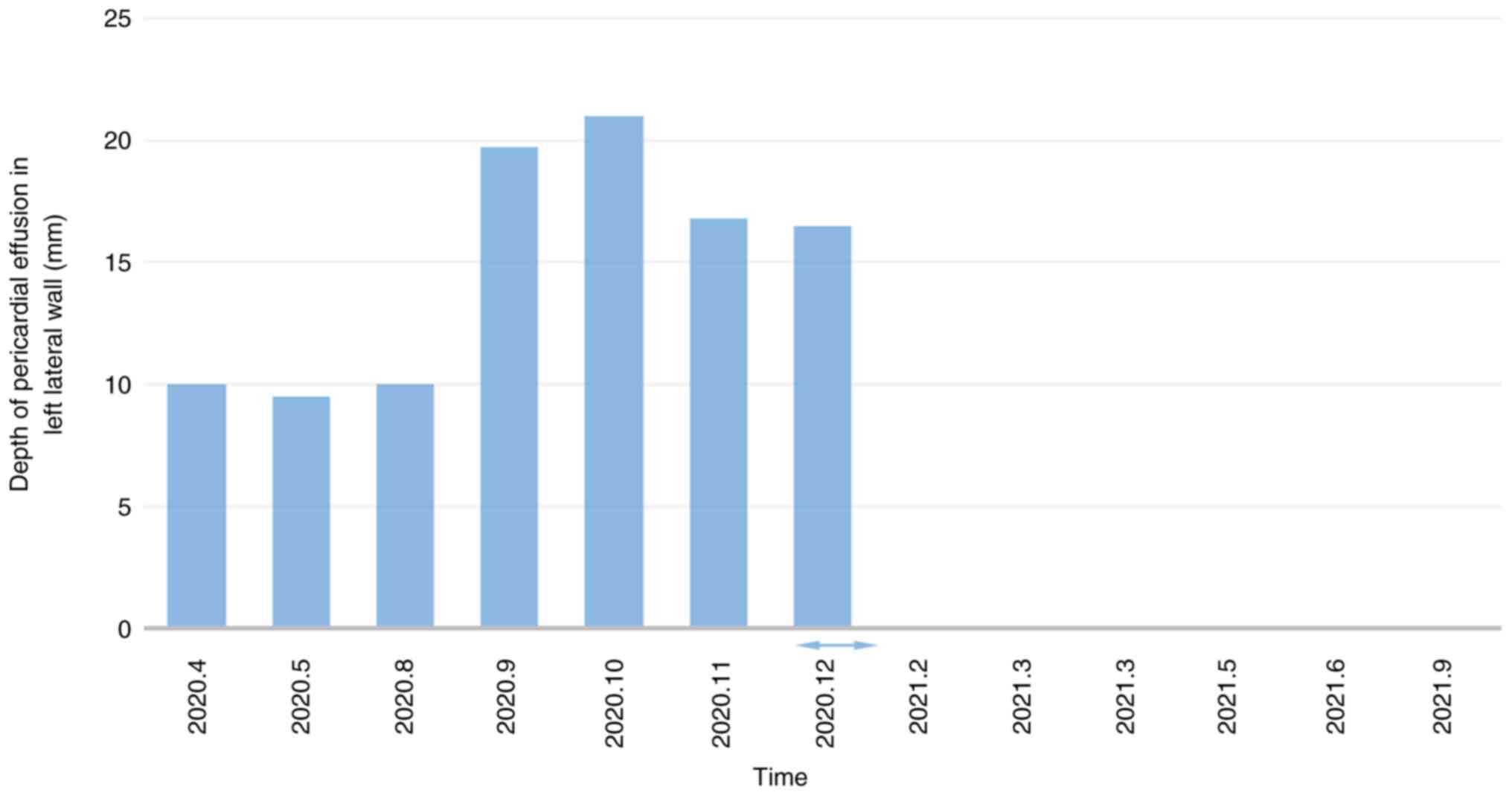
Since April 2020, the patient complained of
intermittent shortness of breath following physical activity.
Echocardiography revealed the presence of moderate pericardial
effusion, which persisted in multiple subsequent examinations.
Considering the possibility of pericardial effusion as a side
effect of dasatinib, the medication was discontinued. Prednisone
(0.5 mg/kg/day) and diuretics (10 mg/day) were administered for 4
months; however, there was no significant improvement in symptoms.
The condition of the patient deteriorated in mid-September 2020,
with echocardiography indicating a large pericardial effusion.
Pericardiocentesis (PCC) was performed to drain the pericardial
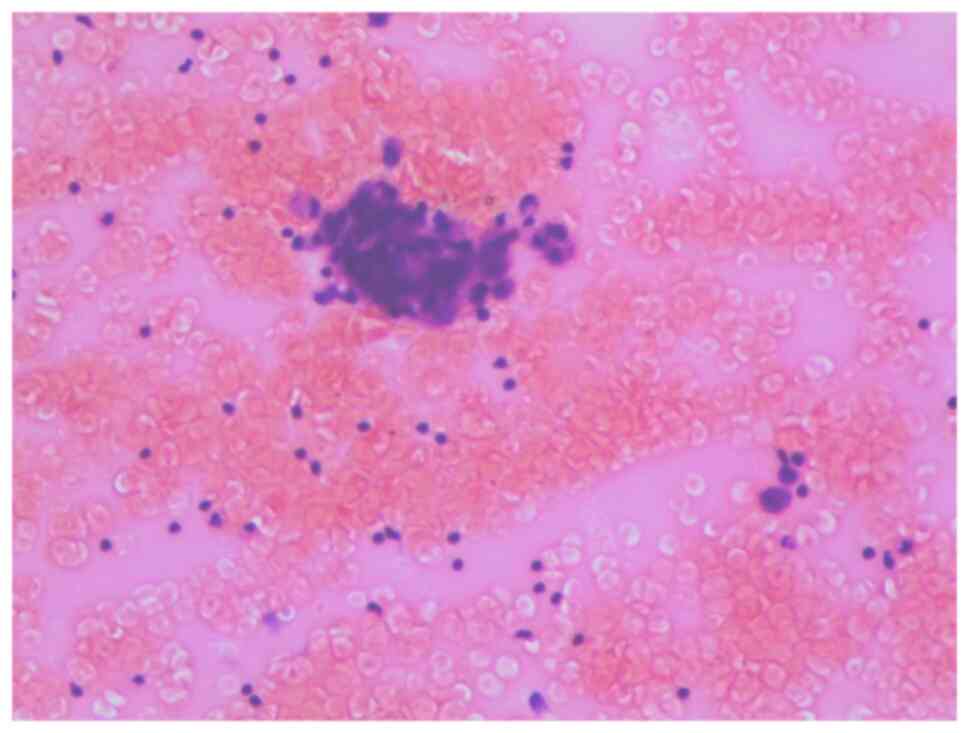
effusion. Pathological analysis of the pericardial effusion
revealed the presence of primitive cells (Fig. 3), and flow cytometry demonstrated
51.28% abnormal primitive B lymphocytes (Fig. 4). It was revealed that abnormal
primitive B cells, under certain circumstances, may express
terminal-deoxynucleotidyl transferase, and are not associated with
graft-versus-leukemia effects. PCR results showed that the
quantitative percentage of the positive BCR-ABL1 fusion gene in the
pericardial effusion was 17.704%, with positive IGH gene
rearrangement (Fig. 2C). Bone
marrow morphology analysis indicated a CR, with the presence of
residual, leukemia immune cells and a neoplasm negative for the
BCR-ABL1 fusion gene (BCR-ABL1 <10-4). Pericardial biopsy was
not performed for this patient due to the following reasons:
Firstly, the Beijing LuHe Hospital does not perform pericardial
biopsies. Secondly, there is a high risk associated with myocardial
biopsies. Additionally, the patient did not undergo a positron
emission tomography (PET)/CT scan because our hospital did not
offer PET imaging at that time, and the patient could not afford
the related expenses. Following the diagnosis of ALL relapse with
involvement of the pericardium, the patient underwent PCC and
received intrapericardial injections of dexamethasone and
rituximab; however, the pericardial effusion recurred. In December
2020, the patient was readmitted to the Beijing LuHe Hospital due
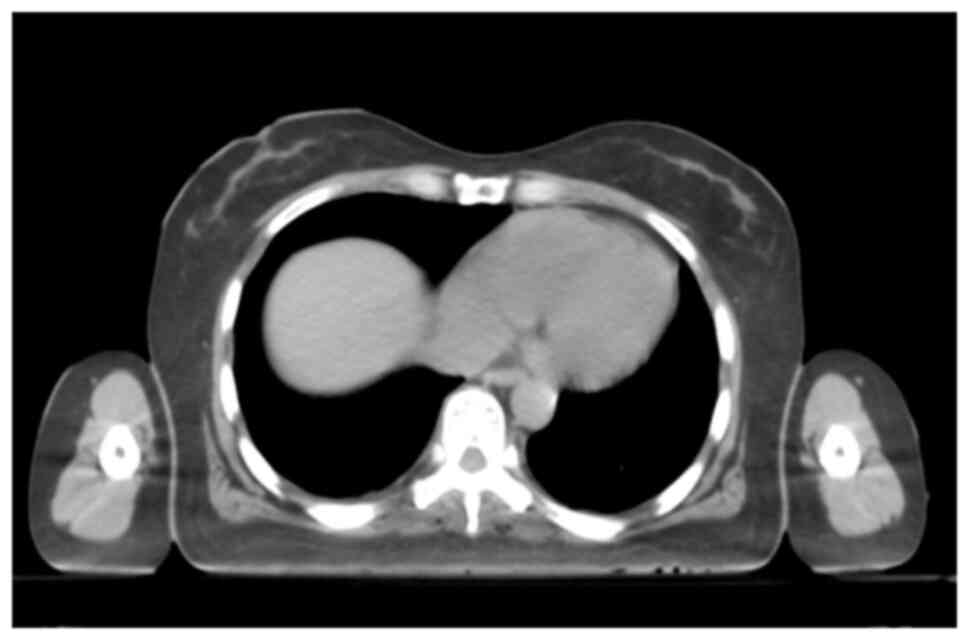
to chest tightness and dyspnea. A transthoracic echocardiogram
revealed a large pericardial effusion, compression of the right
atrium, bilateral pleural effusion and an abnormal echo in the left
parietal pleura (Fig. 5). Following
the diagnosis of leukemia with pericardial and pleural invasion,
the patient underwent pericardial catheter drainage and received
local radiotherapy in the pericardium at a dose of 19.8 Gy/1.8
Gy/11 fractions in December 2020 (Fig.
6). During and after radiotherapy, cardiac function was
monitored using myocardial enzymes, B-type natriuretic peptide and
ejection fraction values, and no cardiac dysfunction was observed
(Fig. 7). Follow-up chest CT scans
revealed the absence of radiation-induced pneumonia in the patient.
Subsequently, the patient received dasatinib (50 mg; bid) and did
not experience any recurrence of pericardial effusion, radiation
pneumonitis, or pericarditis, nor any impairment in cardiac
function during a 10-month follow-up period until September 2021
(Figs. 8 and 9). The patient exhibited spinal cord
involvement and bone marrow relapse after September 2021. Follow-up
visits were conducted once a month until the patient died in
October 2021 due to severe pneumonia. The course of treatment of
the patient is summarized in Table
I.
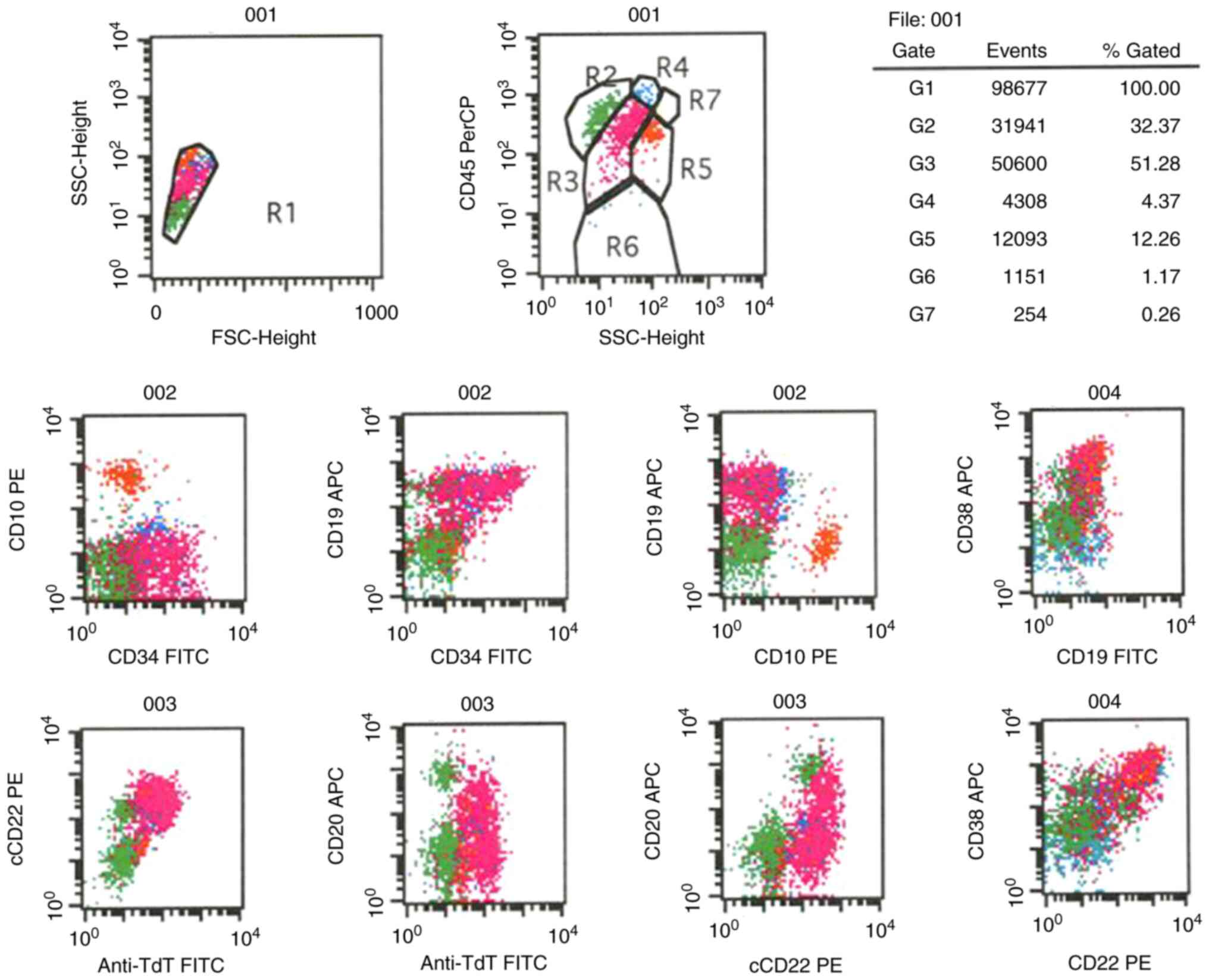 | Figure 4.Flow cytometry plots. R1, all cells;
R2, lymphocytes; R3, abnormal B blasts; R4, monocytes; R5,
granulocytes; R6, nucleated red cells and debris; R7, eosinophils
(G1-7 are the same as R1-7). The results indicated that R3 was an
abnormal B primitive lymphocyte population, accounting for ~51.28%
of cells, and expressing TdT, CD19, cCD22 and CD38, partly
expressing CD34 and CD20, but not expressing CD10. Anti-TdT,
anti-terminal-deoxynucleotidyl transferase; APC, allophycocyanin;
FSC, forward scatter; PE, phycoerythrin; PerCP, peridinin
chlorophyll; SSC, side scatter; cCD22, cytoplasmic CD22. |
Discussion
ALL is a malignant blood disease characterized by
abnormal proliferation, aggregation and infiltration of primary and
juvenile lymphocytes, leading to impaired normal hematopoiesis
(1). While extramedullary organ
involvement is common in ALL, affecting the liver, spleen, lymph
glands, central nervous system and testes, cardiac infiltration is
infrequent in clinical presentations of leukemia (4–6) but
more commonly observed during autopsies (7–9). The
cardiac infiltration rate is ~30% in postmortem examinations of
patients with leukemia and 50% in patients with ALL (9). The diagnosis of leukemia with
extramedullary invasion poses significant difficulties due to the
following reasons: i) Low incidence of cardiac metastasis, ii)
difficulty in obtaining cardiac biopsy for pathological
examination.
Pericardial invasion in leukemia often presents with
recurrent pericardial effusion, wheezing and reduced cardiac
function. In a specific case, a patient with unexplained heart
failure was ultimately diagnosed with ALL through cardiac MRI and
combined PET/CT imaging. These diagnostic methods revealed
hypermetabolism in the right ventricle, atrium and entire bone
marrow (6). When the patient of
this case first developed pericardial effusion, the possibility of
it being an adverse effect of dasatinib treatment was considered.
At that time, the treatment approach involved discontinuation of
dasatinib and administration of steroids; however, this proved to
be ineffective. Subsequently, the recurrence of pericardial
effusion was confirmed to be due to extramedullary involvement of
ALL based on pathological findings. In the Beijing LuHe Hospital,
such cases were previously uncommon in Ph+ALL but had been
encountered in chronic myelogenous leukemia. However, in these
patients, after discontinuation of therapy and targeted steroid
treatment, resolution of the pericardial effusion was observed.
A definitive diagnosis of pericardial invasion in
ALL was made in this case based on the following reasons: Firstly,
the history of the patient indicated extramedullary invasion of
leukemia. Secondly, recurrent wheezing symptoms were observed.
Thirdly, imaging findings consistently suggested recurrent
pericardial effusion that was unresponsive to medication. Lastly,
cytology, flow cytometry and genetic testing of the pericardial
effusion supported the diagnosis (Figs.
1 and 2). According to the
National Comprehensive Cancer Network (NCCN) guidelines, the
following treatment options are recommended for relapsed/refractory
Ph+ALL: Tyrosine kinase inhibitor (TKI) and multiagent
therapy/corticosteroid/blinatumomab/inotuzumab ozogamicin, or
brexucabtagene autoleucel (following therapy that has included
TKIs) (3). However, at the time,
non-TKI targeted agents were not available in China, and the
patient faced financial constraints. Therefore, following the
relapse of the patient, TKI (dasatinib) therapy was chosen. In this
particular case, despite the administration of pericardial
injections of dexamethasone and rituximab, the patient experienced
recurrent pericardial effusion. Symptom relief could only be
achieved through PCC and drainage, as medical treatment failed to
effectively control the condition. Given the limited efficacy of
medication, local radiotherapy was considered as an alternative
option for symptom control.
In another study, radiotherapy was employed in 38
cases of cardiac and pericardial metastases, with a radiation dose
ranging between 25 and 35 Gy over a period of 3–4 weeks. Among
these cases, six individuals with leukemia and lymphoma received
radiotherapy at doses of 15–20 Gy over 1.5–2 weeks, resulting in
continuous CR lasting for 2–4 months, with a clinical improvement
rate of 60% (6). However, in a
further study, 2 cases of leukemia with cardiac infiltration
experienced recurrent and uncontrollable heart failure within 6
months, leading to death (10,11).
In cases of central nervous system invasion, the
NCCN guidelines recommend lumbar puncture, intrathecal injection or
a combination of these procedures, along with whole-brain and
spinal cord radiotherapy, at doses 18 Gy/1.8–2 Gy. However, the
total doses should be adjusted to 24 Gy/2.0 Gy in testicular
invasion (1). A retrospective study
(12) focusing on 38 cases of
chloroma has highlighted the role of radiotherapy in controlling
local disease and alleviating symptoms with no significant reported
cytotoxicity during extramedullary progression, bone marrow relapse
or when rapid relief was needed. The doses should be at least at 20
Gy, and the usage of 24 Gy/12 times is recommended (12). For ALL cases with cutaneous
involvement, local radiotherapy has demonstrated favorable outcomes
when administered at a recommended dose of 24 Gy/12 fractions
(13). In situations where the
clinical condition does not permit a dose of 24 Gy, a dose range of
6–20 Gy can be considered (14,15).
Furthermore, among 15 cases of leukemia cutis, 50% achieved CR when
treated with medium doses of radiotherapy ranging between 6 and 24
Gy, and the 1-year local control rate was 33%. It is important to
note that patients who experienced a relapse of cutaneous
involvement either had active bone marrow disease during
radiotherapy or experienced marrow recurrence shortly after
treatment. The median survival time after radiotherapy was 5
months, with a range of 0.5–136 months (14).
Leukemia arises from abnormal proliferation of
immature and undifferentiated progenitor cells, which are known to
be responsive to radiotherapy. The treatment approach in this
particular case was determined by considering the radiation doses
employed in other cases of leukemia with extramedullary invasion
(3,12–15).
Specifically, the patient underwent continuous pericardial drainage
followed by radiotherapy targeting the entire pericardium, left
pleura and pleural effusion. The radiation was administered using 6
MV X-ray irradiation at a dose of 19.8 Gy delivered over 1.8 Gy per
fraction for a total of 11 treatments. During the 10-month
follow-up period, the patient exhibited well-controlled pericardial
effusion without any apparent cardiac function impairment. This
treatment approach demonstrated superior results compared with
previously documented methods (14).
In conclusion, in general, radiotherapy represents a
potential treatment modality for patients experiencing refractory
leukemic pericardial effusion. Radiotherapy can be considered for
cases involving extramedullary progressive solitary lesions in
leukemia, poor response to chemotherapy or isolated relapse
following hematopoietic stem cell transplantation, or for
symptomatic relief. In such situations, a low-dose radiation
therapy regimen (ranging between 6 and 24 Gy) can be explored as a
viable option.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JY was responsible for collecting clinical, imaging
and pathological data of the patient and was responsible for the
conception, design, content and writing of the manuscript. YZ
contributed to collection of clinical and imaging data. YG analyzed
and interpreted data related to radiotherapy and helped revise the
manuscript. HZ analyzed and interpreted data related to
chemotherapy. SW and QL analyzed and interpreted imaging data. JY
and YZ confirm the authenticity of all the raw data. All authors
agreed to be accountable for all aspects of the work. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written consent for publication has been obtained
from the son of the patient. All identifying information has been
removed or anonymized to ensure confidentiality.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hematology Branch of Chinese Medical
Association, . Hematological Tumor Professional Committee of
Chinese Anti-cancer Association. Chinese J Hematol. 33:789–792.
2012.(In Chinese).
|
|
2
|
Hematology Oncology Committee, Chinese
Anti-Cancer Association, Leukemia & Lymphoma Group, Chinese
Society of Hematology, . Chinese Medical Association: Chinese
guidelines for diagnosis and treatment of adult acute lymphoblastic
leukemia (2021). Zhonghua Xue Ye Xue Za Zhi. 42:705–716. 2021.(In
Chinese). PubMed/NCBI
|
|
3
|
National Comprehensive Cancer Network
(NCCN), . NCCN Guidelines for Acute Lymphoblastic Leukemia
V.1.2023. NCCN; Plymouth Meeting, PA: 2023
|
|
4
|
Barbaric D, Holley D, Lau KC and McCowage
G: It is ALL in the heart: A patient with acute lymphoblastic
leukemia and cardiac infiltration at time of diagnosis. Leuk
Lymphoma. 43:2417–2419. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He W, Huang Y and Zhang Y: Heart
infiltration for the starting performance of 1 case with acute
lymphocytic leukemia. J Leukemia Lymphoma. 10:21–22. 2001.
|
|
6
|
Werner RA, Rudelius M, Thurner A, Higuchi
T and Lapa C: Cardiac manifestation of acute lymphoblastic
leukemia. Clin Nucl Med. 41:570–571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sumners JE, Johnson WW and Ainger LE:
Childhood leukemic heart disease. A study of 116 hearts of children
dying of leukemia. Circulation. 40:575–581. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roberts WC, Bodey GP and Wertlake PT: The
heart in acute leukemia. A study of 420 autopsy cases. Am J
Cardiol. 21:388–412. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Javier BV, Yount WJ, Crosby DJ and Hall
TC: Cardiac metastasis in lymphoma and leukemia. Dis Chest.
52:481–484. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ge X, et al: Extramedullary invasion of
leukemia. J Clin Med. 3.5:290–291. 1987.
|
|
11
|
Sheikh IN, Ragoonanan D, Franklin A,
Srinivasan C, Zhao B, Petropoulos D, Mahadeo KM, Tewari P and
Khazal SJ: Cardiac relapse of acute lymphoblastic leukemia
following hematopoietic stem cell transplantation: A case report
and review of literature. Cancers (Basel). 13:58142021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bakst R, Wolden S and Yahalom J: Radiation
therapy for chloroma (granulocytic sarcoma). Int J Radiat Oncol
Biol Phys. 82:1816–1822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bakst RL, Dabaja BS, Specht LK and Yahalom
J: Use of radiation in extramedullary leukemia/chloroma: Guidelines
from the international lymphoma radiation oncology group. Int J
Radiat Oncol Biol Phys. 102:314–319. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bakst R and Yahalom J: Radiation therapy
for leukemia cutis. Pract Radiat Oncol. 1:182–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elsayad K, Oertel M, Haverkamp U and Eich
HT: The effectiveness of radiotherapy for leukemia cutis. J Cancer
Res Clin Oncol. 143:851–859. 2017. View Article : Google Scholar : PubMed/NCBI
|