Introduction
Esophageal cancer is an aggressive malignancy of the
digestive system with incidence rates of >450,000 cases
worldwide, and 5-year-survival rates ranging from 15–25% (1). Data from the Chinese Cancer Center in
2022 demonstrated that esophageal cancer was the sixth most common
malignant tumor and the fifth leading cause of cancer-related death
(2). There are two major
histological subtypes of esophageal cancer; namely, esophageal
squamous cell carcinoma (ESCC) and adenocarcinoma (3). ESCC is the predominant histological
classification of esophageal cancer in China with a 10-year
survival rate of 14%, despite recent advances in therapeutics
(4–6). Thus, further investigations into
potential biomarkers for diagnosis and treatment, including
oncogenes and tumor suppressor genes, are required.
MicroRNAs (miRNAs/miRs) are small RNAs that regulate
the expression of complementary mRNAs (7). Numerous roles of miRNA have been
identified in complex biological processes, such as metabolism
(8), cancer (9), programmed cell death (10), cell proliferation, and
differentiation (11). The results
of a previous study demonstrated that there are a higher number of
miRNAs in animals, and their regulatory impact is more pervasive
than was previously suspected. Additionally, the same study showed
that miRNAs can be used as diagnostic and prognostic markers for
cancer (12). For example, miR-195
expression in ESCC is associated with a low survival rate (13). In colorectal cancer, the expression
levels of miR-135b and miR-590-5p are associated with clinical
stage and survival (7,14). miR-31-3p, −3676, −125a-5p, −100-5p,
−125b-5p, −200a-5p, and miR-342 were significantly associated with
the development of chemo- and radio-resistance in patients with
locally advanced cervical cancer (15,16).
miR-378a is an intronic miRNA located in the
peroxisome promoter-activated receptor γ activator 1-β gene and has
been the focus of numerous studies (12,17–20).
miR-378a-3p is the guide strand of miR-378a, and it is extensively
associated with metabolism, while miR-378a-5p is regarded as the
passenger strand (12). Notably,
miR-378a-3p is the target gene of the oncogene Myc, which promotes
tumor formation and angiogenesis in A549 adenocarcinoma cells
(17). The results of further
studies demonstrated that the expression of miR-378a-3p was reduced
in ESCC tissues, which may lead to the overexpression of
GLUT-1/SLC2A1 (21,22). Notably, the downregulation of
miR-378a-3p induced decidual cell apoptosis, which contributed to
early pregnancy loss (23).
Therefore, the biological roles of miR-378a-3p in tumor cells
remain contested. In addition, the association between functions,
energy metabolism, and cell survival mechanisms in ESCC cells
remains to be fully elucidated.
The energy metabolism of cancer cells, which exhibit
increased metabolic requirements, is markedly different from
healthy cells. In addition, cancer cells consume high quantities of
glucose and produce lactic acid rather than catabolizing glucose
through the tricarboxylic acid cycle. During this process, cancer
cells generate higher levels of energy to further support their
rapid proliferation (24). Genes
associated with glycolysis, such as Aldolase A (ALDOA), Enolase 1
(ENO1), lactate dehydrogenase A (LDHA), phosphofructokinase L
(PFKL), phosphoglycerate kinase 1 (PGK1), hexokinase-2 (HK2),
2,3-phosphoglyceraldehyde-3-phosphate dehydrogenation (GAPDH), and
pyruvate kinase M2 (PKM2) participate in the energy metabolism of
nucleosides, amino acids, and glucose, affect cell survival and
apoptosis, and play a role in drug resistance and other biological
mechanisms, to maintain the stability of tissue and the cellular
environment (25,26).
The coding gene of Survivin, BIRC5, is located on
human chromosome 17q25 and consists of three introns and four exons
(27). Survivin is the smallest
member of the inhibitor of apoptosis protein family. Wild-type
Survivin is composed of 142 amino acids and often exists as a
homodimer in the cytoplasm. Numerous previous studies have
demonstrated that Survivin interacts with a variety of endogenous
and exogenous apoptotic regulatory factors, such as Bad and Bcl-2,
and significantly inhibits apoptosis via directly inhibiting the
activities of Caspase-3 and Caspase-7, to block induced apoptosis
(28–31).
In the present study, miR-378a-3p mimic and negative
control were transfected into ECA-109 cells to detect and analyze
the molecular regulatory process of miR-378a-3p. This interfered
with its target genes, energy metabolism and the apoptosis of ESCC
cells. The study will help us gain a deeper understanding of the
regulatory mechanism of miR-378a-3p on tumor cells at the molecular
biology level, and help us to understand the process of its
intervention in tumor cells; it may also provide a novel
theoretical basis for targeting miR-378a-3p as an interventional
measure in the future.
Materials and methods
Cell culture
ESCC cell line ECA-109 was purchased from The BeNa
Culture Collection (BNCC). The BNCC data showed that ECA-109
esophageal cancer cell line, established in 1973, originated from
human esophageal middle squamous cell carcinoma tissue (ESCC).
ECA-109 cells are considered one of the most representative models
of ESCC (32–34). STR profiling was performed to
confirm the identity of the cells. The cells were cultured in RPMI
1640 medium containing 10% FBS and 1% penicillin-streptomycin
solution (penicillin 100 U/ml + streptomycin 100 mg/ml; all from
Biological Industries). Cells were maintained in a humidified
incubator at 37°C supplied with 5% CO2.
Determination of miR-378a-3p
targets
First, the downstream target genes of miR-378a-3p in
miRDB (https://mirdb.org) were screened. In view of its
known interference in some energy metabolism related proteins, the
3′ non-coding regions of all glucose metabolism-related gene mRNAs
were compared. Due to the phenomenon of incomplete matching of
miRNA sequences that interfere with the target protein translation
process (8,9), if the 6–8 nt at the 5′end of miRNA was
complementary to the target gene, and the basic principle of ‘A’ at
the first nucleotide of the miRNA corresponding to the target gene
(8,9), the gene was considered a target gene
(Fig. 1A).
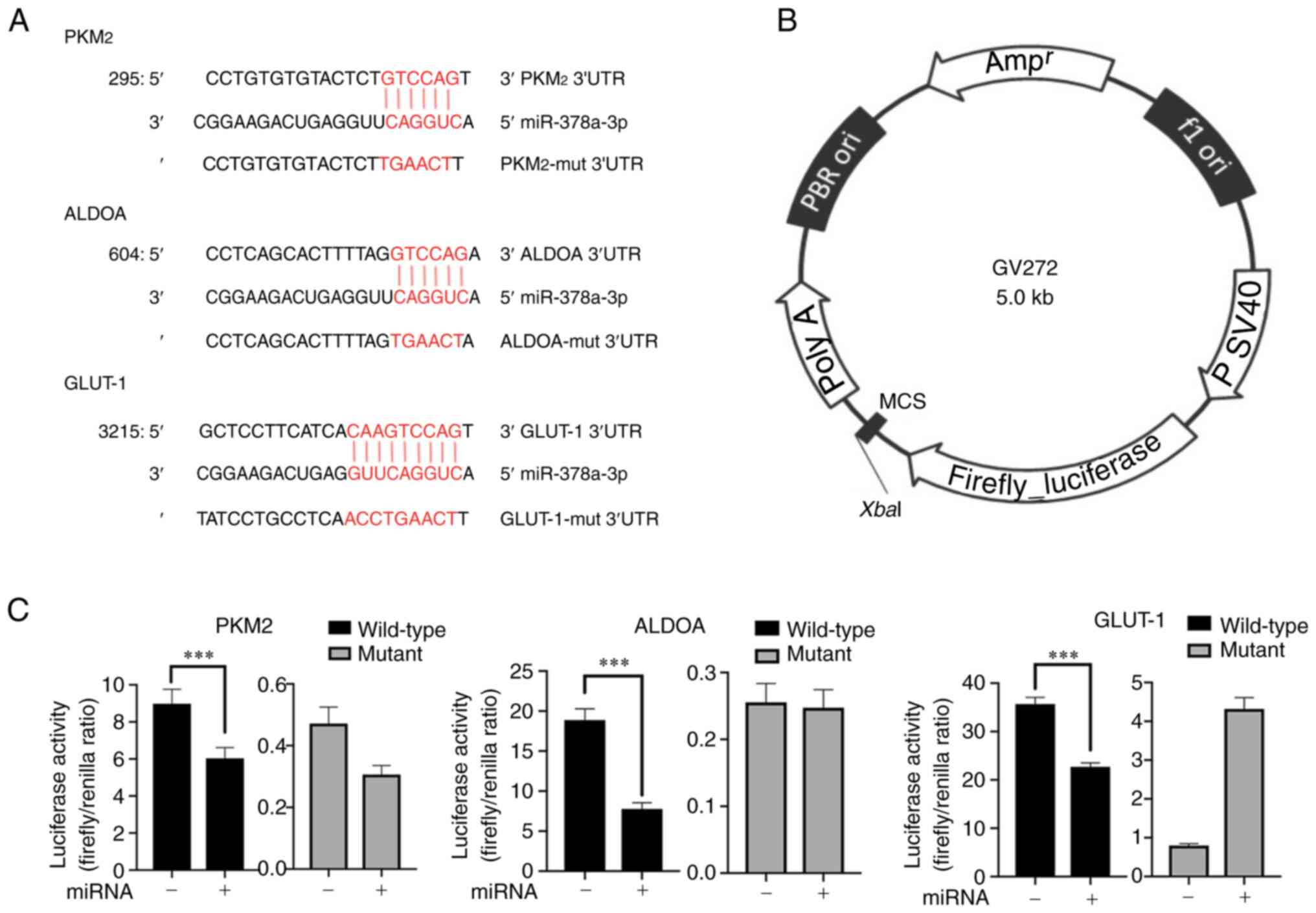 | Figure 1.PKM2, ALDOA, and GLUT-1 are target
genes of miR-378a-3p. (A) The matching sites between miR-378a-3p
and wild-type/mutant genes of PKM2, ALDOA, and GLUT-1. (B)
Construction of the dual-luciferase reporter vector. (C) The
activity of firefly luciferase reporter genes PKM2, ALDOA, and
GLUT-1 was suppressed by miR-378a-3p in ECA-109 cells.
***P<0.001. miR/miRNA, microRNA; mut, mutant; PBR, a man-made
constructed plasmid DNA; ori, the replication origin of plasmid;
ampr, antiampicillin-resistance gene. |
qPCR analysis
Total RNA was extracted from the cells using
TRIzol® reagent (cat. no. 15596026; Thermo Fisher
Scientific, Inc.). The extracted Total RNA was reverse transcribed
into cDNA using the Mir-X miRNA first strand synthesis kit
according to the manufacturer's instructions (cat. no. 638315;
Clontech; Takara Bio USA, Inc.), and qPCR was performed using SYBR
Green I Master (cat. no. 4887352001; Roche Diagnostics), with U6 as
an inner control. A ightCycler 480 II (Roche Diagnostics) was used
for the qPCR. The qPCR conditions were as follows: Pre-denaturation
at 95°C for 15 min, followed by 40 cycles of 10 sec of denaturation
at 95°C, annealing at 60°C for 20 sec and extension at 72°C for 20
sec. The fluorescence signals were collected at 72–95°C after the
reaction for melting curve analysis. The results were analyzed
using a hyperbolic curve and the relative gene expression was
determined. The forward primer sequences of miR-378a-3p were
forward (F), 5′-ACTGGACTTGGAGTCAGAAGGC-3′ and reverse (R), mRQ 3′
primer in Mir-X miRNA First-Strand Synthesis Kit (cat. no. 638315;
Clontech; Takara Bio USA Inc.). Primer sequences for U6 were F,
5′-GGAACGATACAGAGAAGATTAGC-3′ and R, 5′-TGGAACGCTTCACGAATTTGCG-3′.
The comparative Cq method was used to quantify gene levels using
the formula: ΔCq=CqmiRNA-CqU6.
ΔΔCq=ΔCqApoE-ΔCqWT. The relative
quantification for miRNA was calculated as 2−∆∆Cq.
Expression of miRNA was normalized by U6. The specific
primers/sequences for amplifying miRNAs and mRNAs are listed in
Table I.
 | Table I.Basic information on the synthesis
and expression construction for miR-378a-3p. |
Table I.
Basic information on the synthesis
and expression construction for miR-378a-3p.
| miRNAs or gene
clones | Sequence
(5′-3′) | Vector | Supplier |
|---|
|
hsa-miR-378a-3p |
ACUGGACUUGGAGUCAGAAGGC |
| Shanghai GenePharma
Co., Ltd. |
| mimics |
|
|
|
| mimic NC |
UUCUCCGAACGUGUCACGUTT |
| Shanghai GenePharma
Co., Ltd. |
| miR-378a-3p |
GGATCCCCTTCTGACGACAGTCC |
CMV-MCS-polyA-EF1A- | Shanghai Genechem
Co., Ltd. |
| miRNA sponge |
AGTCTTCCCTTCTGACGACAGTC |
zsGreen-sv40-puromycin |
|
|
|
CAGTCTTCCCTTCTGACGACAGT |
|
|
|
|
CCAGTCTTCCCTTCTGACGACAG |
|
|
|
|
TCCAGTCTTCCCTTCTGACGACA |
|
|
|
|
GTCCAGTCTTCCCTTCTGACGAC |
|
|
|
|
AGTCCAGTCTTCACCGGT |
|
|
| miR-378a-3p |
CCGGACTGGACTTGGAGTCAGA | LKD006
pLKD-Ubc-e | OBiO
Technology |
| shRNA-F |
AGGCCTCGAGGCCTTCTGACTC | GFP-U6-shRNA | (Shanghai) Corp.,
Ltd. |
|
|
CAAGTCCAGTTTTTTTG |
|
|
| miR-378a-3p |
AATTCAAAAAAACTGGACTTGG | LKD006
pLKD-Ubc- | OBiO
Technology |
| shRNA-R |
AGTCAGAAGGCCTCGAGGCCTT | eGFP-U6-shRNA | (Shanghai) Corp.,
Ltd. |
|
|
CTGACTCCAAGTCCAGT |
|
|
| miR NC-F | CCGG
TTCTCCGAACGTGTCACG | LKD006
pLKD-Ubc- | OBiO
Technology |
|
|
TTTCAAGAGAACGTGACACGTT |
eGFP-U6-shRNA-(NC) | (Shanghai) Corp.,
Ltd. |
|
| CGGAGAATTTTTTG |
|
|
| miR NC-R |
AATTCAAAAAATTCTCCGAACG | LKD006
pLKD-Ubc- | OBiO
Technology |
|
| TGTCACGT
TCTCTTGAAACGTG | eGFP-U6-(NC) | (Shanghai) Corp.,
Ltd. |
|
| ACACGTTCGGAGAA |
|
|
| ALDOA |
TCTAGATGTCAAGGAAAGTACA | GV272 | Shanghai Genechem
Co., Ltd. |
|
[NM_184041-3utr |
CTCCGAGCGGTCAGGCTGGGG |
|
|
|
(mir378a-3p)-mut] |
CTGCTGCCAGCGAGTCCCTCTT |
|
|
|
|
CGTCTCTAACCACGCCTATTAAG |
|
|
|
|
CGGAGGTGTTCCCAGGCTTAAC |
|
|
|
|
CACACCAGAACGTCAAGTCCCC |
|
|
|
|
CTCCCACTCTTGAAGAGGAGGC |
|
|
|
|
CGCCTCCTCGGGGCTCCAGGCT |
|
|
|
|
GGCTTGCCCGCGCTCTTTCTTCC |
|
|
|
|
CTCGTGACAGTGGTGTGTGGTG |
|
|
|
| TCGTCTCTAGA |
|
|
| ALDOA |
TCTAGAGCTCTGCCCCTTCACCT | GV272 | Shanghai Genechem
Co., Ltd. |
| [NM_184041-3
utr |
AACAGCATAAGATAGGGCTAAC |
|
|
| (mir-378a-3p)] |
AGTTGGGGAGTATGGTTGTAACT |
|
|
|
|
GCTCATGTCTTAGGAGGCTTCAG |
|
|
|
|
CCTCAGCACTTTTAGGTCCAGAA |
|
|
|
|
CTCAAGGGGGGCAGAAGACCCC |
|
|
|
|
TGTGACAAAAACCCACTAACTAG |
|
|
|
|
CTCATGAGTGACATGAGCCAGGC |
|
|
|
|
AACATAATGGGTGTTTTATATGAG |
|
|
|
| TAGATGTCTAGA |
|
|
| PKM2 |
TCTAGAGGGCTGAGGACGTGGA | GV272 | Shanghai Genechem
Co., Ltd. |
| [NM_002654 |
CCTCCGGGTGAACTTTGCCATGA |
|
|
|
(mir-378a-3p)-mut] |
ATGTTGGCAAGGCCCGAGGCTTC |
|
|
|
|
TTCAAGAAGGGAGATGTGGTCAT |
|
|
|
|
TGTGCTGACCGGATGTAGCCAGT |
|
|
|
|
GAGAAGGCGTACCCAACACCATG |
|
|
|
|
CGTGTTGTTCCTGTGCCGTGATGG |
|
|
|
|
ACCCCAGAGCCCCTCCTCCAGCC |
|
|
|
|
CCTGTCCCACCCCCTTCCCCCAGC |
|
|
|
|
CCATCCATTAGGCCAGCATCTAGA |
|
|
| PKM2 |
TCTAGAGGGCTGAGGACGTGGAC | GV272 | Shanghai Genechem
Co., Ltd. |
| [NM_002654 |
CTCCGGGTGAACTTTGCCATGAAT |
|
|
| (mir-378a-3p)] |
GTTGGCAAGGCCCGAGGCTTCTTC |
|
|
|
|
AAGAAGGGAGATGTGGTCATTGTG |
|
|
|
|
CTGACCGGATGGCGCCCTGGCTCC |
|
|
|
|
GGCTTCACCAACACCATGCGTGTT |
|
|
|
|
GTTCCTGTGCCGTGATGGACCCCA |
|
|
|
|
GAGCCCCTCCTCCAGCCCCTGTCC |
|
|
|
|
CACCCCCTTCCCCCAGCCCATCCAT |
|
|
|
|
TAGGCCAGCATCTAGA |
|
|
| SLC2A1 |
TCTAGACTCTGGTTCCTCTGTATAC | GV272 | Shanghai Genechem
Co., Ltd. |
|
[NM_006516-3utr |
TACTGCTTCATCTCTAAAGACAGC |
|
|
|
(mir378a-3p)-mut] |
TCATCCTCCTCCTTCACCCCTGAAT |
|
|
|
|
TTCCAGAGCACTTCATCTTATCCTG |
|
|
|
|
CCTCAACCTGAACTTTTTCTGCCA |
|
|
|
|
CTAGTCTGAATTTCATGAGAAGATG |
|
|
|
|
CCGATTTGGTTCCTGTGGGTCCTCA |
|
|
|
|
GCACTATTCAGTACAGTGCTTGATG |
|
|
|
|
CACAGCAGGCACTCATCTAGA |
|
|
| SLC2A1 |
TCTAGACTCTGGTTCCTCTGTATAC | GV272 | Shanghai Genechem
Co., Ltd. |
|
[NM_006516-3utr |
TACTGCTTCATCTCTAAAGACAGC |
|
|
| (mir378a-3p)] |
TCATCCTCCTCCTTCACCCCTGAAT |
|
|
|
|
TTCCAGAGCACTTCATCTGCTCCTT |
|
|
|
|
CATCACAAGTCCAGTTTTCTGCCA |
|
|
|
|
CTAGTCTGAATTTCATGAGAAGATG |
|
|
|
|
CCGATTTGGTTCCTGTGGGTCCTCA |
|
|
|
|
GCACTATTCAGTACAGTGCTTGATG |
|
|
|
|
CACAGCAGGCACTCATCTAGA |
|
|
Overexpression of miR-378a-3p
miRNA mimics and their negative control
counterparts, miRNA-sponge or miRNA-shRNA expression plasmids were
obtained, and the information on their sequences and construction
vectors are shown in Table I.
Transient transfection with miRNA and DNA (expression plasmid) was
performed using Lipofectamine™ 3000 (cat. no. L3000015; Invitrogen;
Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's protocols. Cells (2×105) were seeded into
6-well plates 1 day before transfection with 50 pmol miRNA mimics
and negative control or 2.5 µg plasmid DNA using 5.0 µl
Lipofectamine. After transient transfection (15 h), cells were
washed with PBS (cat. no. C10010500BT; Thermo Fisher Scientific,
Inc.) and used for experiments.
miR-378a-3p-shRNA overexpression and negative
control plasmids (Table I) were
transfected into cells during the logarithmic growth stage using
electroporation (conditions: U, 280 V; C, 900 CF; R, 550 Ω) with
Opti-MEM reduced serum medium (Gibco; Thermo Fisher Scientific,
Inc.) with Gene Pulser XcellTM Electroporation System (Bio-Rad,
USA).
Protein expression and cellular
localization of TF-1 apoptosis-related gene-19 (TFAR19)
Prior to cell inoculation, a cover glass was placed
in each well of a 6-well plate. Cells (1×105) were
inoculated on the cover glass to make cell slides. miR-378a-3p
mimic RNAs were transfected into cells in the logarithmic growth
stage using Lipofectamine 3000. After 15 h of transfection, the
medium was discarded, and the cells were rinsed twice with PBS
buffer. Subsequently, cells were fixed with 4% paraformaldehyde
(cat. no. G0528; GBCBIO Technologies, Inc.) for 30 min at room
temperature (22–25°C), washed twice with PBS and sealed with
blocking buffer for 1 h at room temperature.
Following blocking, cells were incubated overnight
at 4°C with the anti-TFAR19 primary antibody (1:300; cat. no.
K107297P; Beijing Solarbio Science & Technology Co., Ltd.).
Following primary incubation, cells were washed with PBS for 5 min,
and subsequently incubated with FITC-goat anti-rabbit secondary
antibody (1:1,000; cat. no. A0562; Beyotime Institute of
Biotechnology) for 1–2 h at room temperature. Cells were rinsed
with PBS three times for 5 min each time, and stained with DAPI
(Beyotime Institute of Biotechnology) in the dark for 3 min. Cells
were rinsed again using PBS three times for 5 min each time. A drop
of an anti-fluorescence quenching sealing agent was added to the
slide and cells were subsequently observed using an inverted
fluorescence microscope at ×400 magnification (IX83; Olympus
Corporation).
Western blotting
Following 24/48 h transfection, cells were collected
and lysed at 4°C for 30 min using RIPA lysate (Boster Biological
Technology) supplemented with 1% protease inhibitor (Thermo Fisher
Scientific, Inc.) and 1% phosphatase inhibitor (Thermo Fisher
Scientific, Inc.). The supernatant was centrifuged (13,000 × g at
4°C for 15 min) to obtain the total protein. Protein concentration
was determined using a BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). A total of 25 µg protein was loaded per lane on
a 10% SDS-gel, resolved using SDS-PAGE, and transferred to PVDF
membranes (Roche Diagnostics GmbH), which were subsequently blocked
with TBS-Tween-20 (TBS-T) containing 5% skimmed milk at room
temperature for 1 h. Following blocking, membranes were incubated
overnight at 4°C with the following primary antibodies against
GLUT-1 (1:100,000; Abcam, cat. no. ab115730), ALDOA (1:1,000;
Abcam, cat. no. ab252953), PKM2 (1:1,000; Cell Signaling
Technology, Inc., cat. no. 4053T), H2AX (1:1,000; Cell Signaling
Technology, Inc., cat. no. 7631S), p-H2AX (1:1,000; Cell Signaling
Technology, Inc., cat. no. 9718S), Caspase-3 (1:1,000; Cell
Signaling Technology, Inc., cat. no. 9662S), cleaved Caspase-3
(1:1,000; Abcam, cat. no. AB32042), Bad (1:1,000; Cell Signaling
Technology, Inc., cat. no. 9268S), Survivin (1:1,000; Cell
Signaling Technology, Inc., cat. no. 2808S), Bcl-2 (1:1,000; Cell
Signaling Technology, Inc., cat. no. 15071S), β-actin (1:1,000;
Cell Signaling Technology, Inc., cat. no. 3700S), and TFAR19
(1:1,000; Beijing Solarbio Science & Technology Co., Ltd., cat.
no. K107297P). The following secondary antibodies were used:
anti-mouse antibody (Cell Signaling Technology, Inc., cat. no.
7076S, Lot#35, 1:4,000) anti-rabbit antibody (Cell Signaling
Technology, Inc., cat. no. 7074S, Lot#30, 1:3,000). Following
primary incubation, membranes were incubated at room temperature
for 1 h with the secondary antibody, anti-mouse/rabbit
HRP-conjugated antibodies. Following washing with TBS-T, signals
were visualized using an enhanced chemiluminescence detection
reagent (Thermo Fisher Scientific, Inc.). β-actin was used as the
loading control. Image J version 1.53 was used for densitometry
(National Institutes of Health).
Flow cytometry
A FITC Annexin V Apoptosis Detection kit (BD
Biosciences) was used to detect the apoptosis of cells. Transfected
cells in each group were collected, washed twice with pre-cooled
PBS, and suspended in 1X binding buffer. A total of 5 µl FITC and 5
µl PI were added for incubation for 15 min at 25°C. Subsequently,
400 µl 1X binding buffer was added to each tube and analyzed using
flow cytometry (FACSAria II) with BD FACSDiva software (version
8.0.2) (Becton, Dickinson and Company) within 1 h.
ELISA
Cells in the control, NC and miR378-mimics groups
transfected for 24 or 48 h were collected and washed with
pre-cooled PBS three times. A total of 250 µl NP40 lysate was added
to cells for 30 min until cells were fully lysed, and the
supernatant was collected. The ELISA kits (Human ALDOA ELISA Kit,
cat. no. E-EL-H0309-96T, Lot# BRZUPTIBFG; Human GLUT-1 ELISA Kit,
cat. no. E-EL-H1822-96T, Lot# 3MIHWZGF38; Human PKM2 ELISA Kit,
cat. no. E-EL-H1089-96T, Lot# IUNGNXEV9W; Elabscience
Biotechnology, Inc.) were used according to the manufacturer's
instructions. The optical density of each well was measured using a
microplate reader at a wavelength of 450 nm. The concentration of
total protein was considered the level of the enzyme.
Detection of the dual-luciferase
reporter assay
A Dual Luciferase Reporter Gene Assay kit (Beijing
Solarbio Science & Technology Co., Ltd.) was used to assess the
target genes of miR-378a-3p (Table
I). Cells were co-transfected with the wild-type/mutant plasmid
DNA of the respective firefly luciferase target genes +
Renilla luciferase plasmid DNA ± miR-378a-3p plasmid DNA,
and cultured for 24/48 h. Lysate was collected after cells (200 µl
lysate/1×106 cells) were fully lysed at 4°C for 5 min. A
total of 20 µl cell lysate was added to 100 µl 1X
firefly/Renilla luciferase reaction solution, and luciferase
activity was detected. Firefly luciferase activity/Renilla
luciferase activity was measured as luciferase reporter gene
activity.
ATP synthesis inhibition
Oligomycin (concentration, 200 nM) was used in
ECA109 cells in the logarithmic growth phase for 12 or 24 h to
inhibit ATP synthetase and decrease the ATP content in cells.
ATP detection
ATP Detection kit (Beijing Solarbio Science &
Technology Co., Ltd.) was used to determine the ATP content of
cells. Cells were suspended with ATP extract (1 ml/5×106
cells) and lysed using ultrasonication. Chloroform was mixed with
100 µl supernatant or the control and 900 µl ATP detection working
solution, and the absorbance was measured at 10 sec and 3 min 10
sec with a wavelength of 340 nm (recorded as A1 and A2,
respectively), using an ultraviolet spectrophotometer. DA=A2-A1;
DA/(DA standard/C standard) × V extract/5 as ATP content
(µmol/106 cells).
Detection of mitochondrial membrane
potential
An enhanced mitochondrial membrane potential
detection kit (JC-1) (cat. no. C2003S, Beyotime Institute of
Biotechnology) was used to determine the mitochondrial membrane
potential. For each well of a 6-well plate, the media was removed,
cells were washed with PBS once, 1 ml cell culture medium was
added, and 1 ml JC-1 dye working solution was added and mixed
thoroughly. Next cells were incubated for 20 min. After incubation,
the supernatant was aspirated, and cells were washed twice with
JC-1 dye buffer. and 2 ml cell culture medium was added. Cells were
observed using an inverted fluorescence microscope at ×100 and ×400
magnification (IX83; Olympus Corporation). When detecting JC-1
monomers, the excitation light wavelength was set to 490 nm and the
emission light wavelength was set to 530 nm; when observing the
JC-1 polymer, the excitation light wavelength was set to 525 nm and
the emission light wavelength was set to 590 nm.
Statistical analysis
All data were analyzed in GraphPad Prism (version,
8.0.0, GraphPad Software, Inc.]. All quantitative data are
presented as the mean ± standard deviation. A one-way ANOVA
followed by Tukey's post hoc test was used to analyze the
difference between multiple groups. P<0.05 was considered to
indicate a statistically significant difference. All the
experiments were performed at least three times.
Results
Baseline miR-378a-3p expression levels
in ECA-109 cells
A miR-378a-3p-sponge overexpression vector was used
to establish the baseline miR-378-3p expression levels (Table I). The results showed that the
expression of miR-378a-3p was very low in ECA-109 cells (Fig. S1A and B), indicating that its
expression as a tumor suppressor gene was suppressed. At low
levels, miR-378a-3p had limited effect on the normal biological
activity of ECA-109 cells as the miR-378a-3p-sponge had limited
effect on GLUT-1, ALDOA, and PKM2 protein levels in ECA-109 cells
(Fig. S1C and D).
ALDOA/GLUT-1/PKM2 are target genes of
miR-378a-3p
To identify the target genes of miR-378a-3p, a
dual-luciferase reporter assay was performed using a GV272 vector
containing the wild-type or mutant miR-378a-3p-binding sequences in
the 3′-untranslated region (UTR) of ALDOA, GLUT-1, PKM2, LDHA, and
GAPDH. miR-378a-3p mimic and the reporter vector were
co-transfected into ECA-109 cells. The results of the present study
indicated that miR-378-3p markedly suppressed the dual-luciferase
activity of key enzymes of glycolysis, including ALDOA, GLUT-1, and
PKM2 (Fig. 1).
Expression levels of
glycolysis-related enzymes are downregulated by miR-378a-3p mimic
transfection
Based on the aforementioned findings that
miR-378a-3p directly targeted glycolytic enzymes, the effects of
miR-378a-3p on the glycolysis pathway were determined. Following
miR-378a-3p transfection, the association between miR-378a-3p and
energy metabolism was assessed. The major enzymes of the glycolysis
pathway were detected using western blotting and ELISA. Protein
expression levels and enzyme activities of PKM2, ALDOA, and GLUT-1
were inhibited between 24 and 48 h following transfection of
miR-378a-3p (Fig. 2).
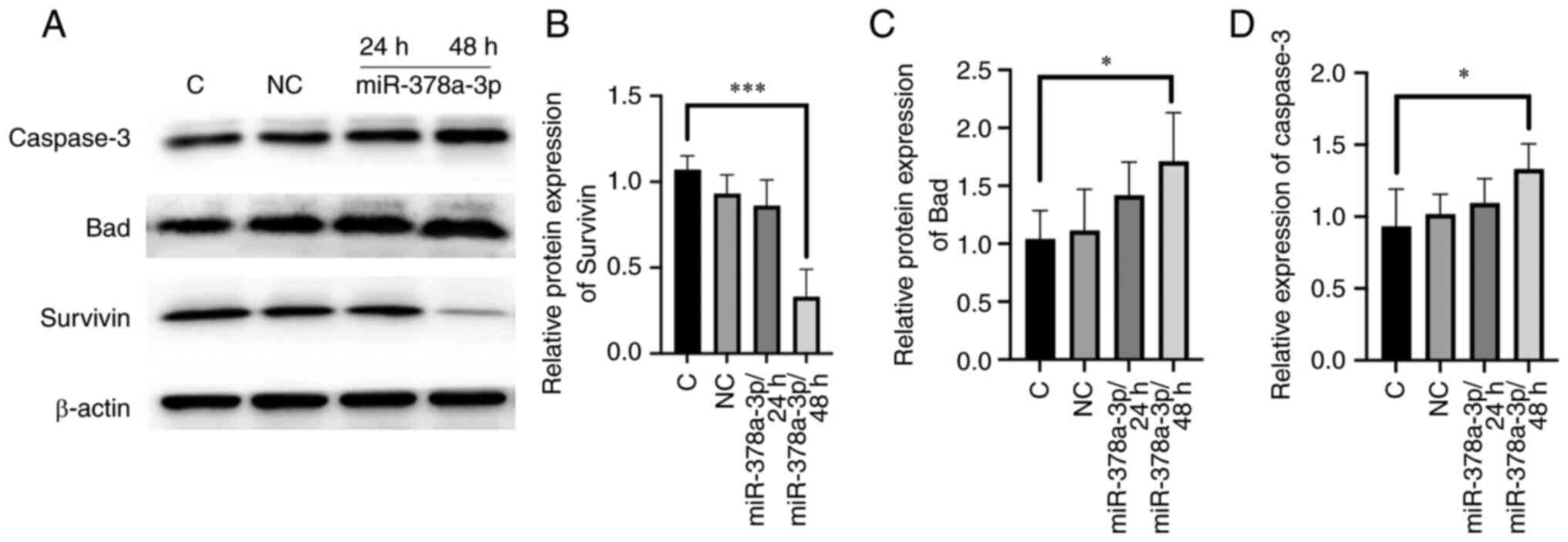
Survivin expression levels are
downregulated, while Bad and Caspase-3 expression levels are
upregulated by miR-378a-3p
To investigate the potential mechanism by which
miR-378a-3p affected the apoptosis of ECA-109 cells at the protein
level, protein expression levels of Bad, Caspase-3, and Survivin
were investigated. The results showed that in the group of
transfected with miR-378a-3p mimics, Survivin expression (an
apoptosis inhibitor protein) was markedly decreased, while
Caspase-3 and Bad expression (apoptosis-related proteins) were
increased (Fig. 3). Cleaved
caspase-3 expression was increased (Fig. S2). To confirm that there was no DNA
damage involved in the early stage of apoptosis, histone variant
H2AX phosphorylation was detected by western blotting, and it was
shown that their protein levels were not increased following
miR-378a-3p overexpression (Fig.
S2).
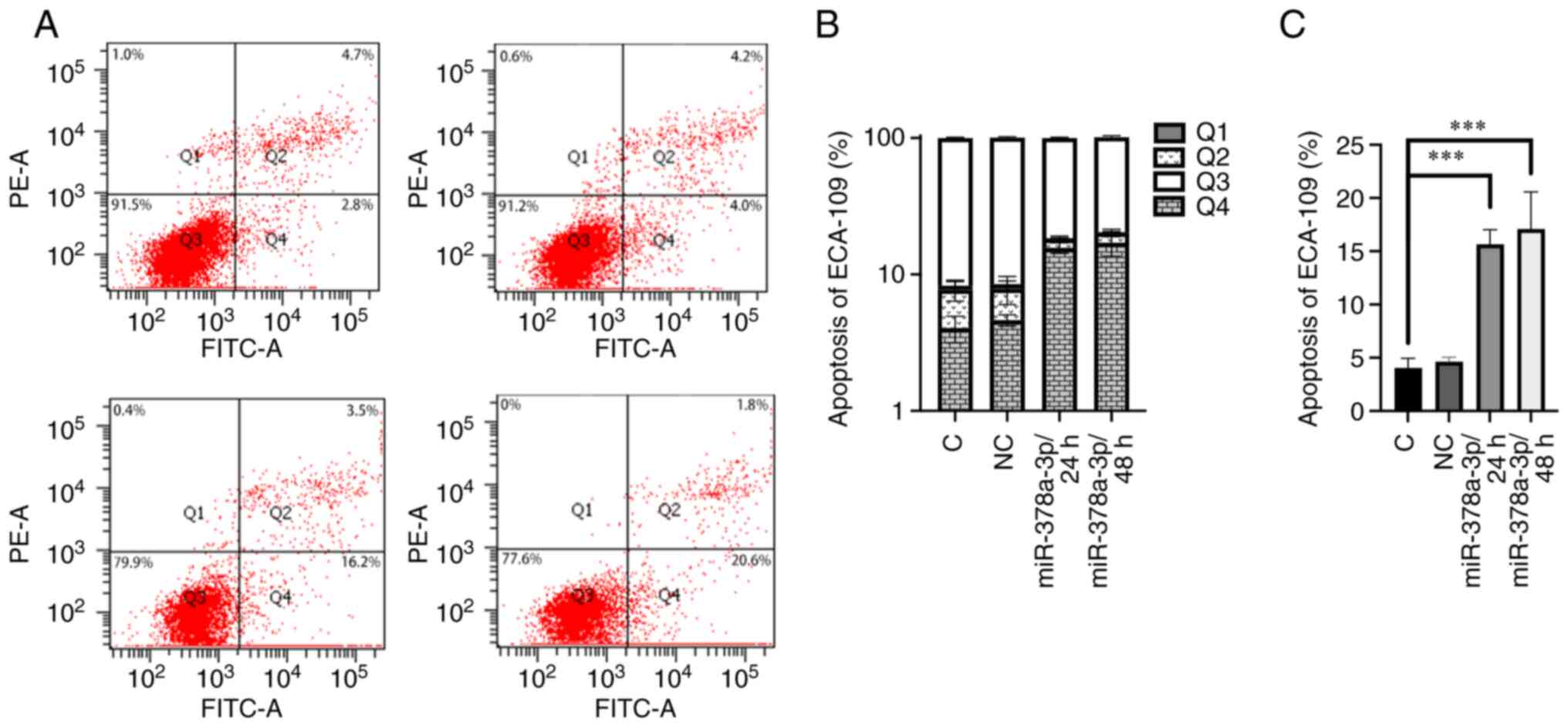
Transfected with miR-378a-3p mimics
promotes cell apoptosis
A cell apoptosis assay was performed to determine
the biological function of miR-378a-3p. ECA-109 cells were
transfected with miR-378a-3p mimics and negative control RNAs, and
the cells were collected 24 and 48 h later. The results showed that
in the miR-378a-3p mimic treatment group, the number of apoptotic
cells in the early stage was significantly higher than that in the
control or miR-NC negative control group (Fig. 4).
TFAR19 nuclear translocation is
enhanced by miR-378a-3p
TFAR19, also known as programmed cell death 5
(PDCD5), cellular localization analysis was performed to verify the
role of miR-378a-3p in promoting cell apoptosis. There was notable
TFAR19 nuclear translocation in the miR-378a-3p mimics transfected
group (Fig. 5A-D).
 | Figure 5.TFAR19 nuclear translocation is
observed in the group transfected with miR-378a-3p mimics. (A-D)
Compared with the control or miR-negative control, a strong
positive reaction of FITC-TFAR19 staining was observed in the
miR-378a-3p mimics transfection group. Red arrow, TFAR19 levels
were significantly higher in the nucleus than in the cytoplasm,
defined as strongly positive. White arrow, TFAR19 levels were
higher in the nucleus than in the cytoplasm, defined as positive.
In other cells, TFAR19 levels were equivalent in the nucleus and
cytoplasm, or lower in the nucleus than in the cytoplasm, defined
as negative. (B) DAPI staining of each group. (C) FITC-TFAR19 and
DAPI overlay images. (E) ATP content of cells was significantly
decreased in the group transfected with miR-378a-3p mimics.
*P<0.05, **P<0.01, ***P<0.001. NC, negative control; C,
control; miR, microRNA; TF-1 apoptosis-related gene-19. |
Energy metabolism is suppressed by
miR-378a-3p
To further clarify the impact of miR-378a-3p on
cancer cell apoptosis, the ATP content was determined. Following
transfection with miR-378a-3p mimics or negative control miRNA for
24/48 h, it was shown that miR-378a-3p blocked energy metabolism,
resulting in a decrease in ATP in the miR-378a-3p group (Fig. 5E). The mitochondrial membrane
potential was also significantly decreased (Figs. S3 and S4).
Oligomycin leads to ATP loss, reduced
expression of Survivin and Bcl-2, and increased cell apoptosis
To further verify the association between energy
metabolism and apoptosis, oligomycin was used to inhibit ATP
synthase. The results of the present study demonstrated that the
ATP content, and the protein expression levels of Survivin and
Bcl-2 were significantly decreased following treatment with
oligomycin. These results indicated that inhibition of ATP
synthesis may lead to apoptosis via alterations in the expression
of Survivin and Bcl-2 (Fig. 6).
Discussion
miRNAs have been widely studied due to their
anti-oncogenic functions. miRNAs are small non-coding RNA molecules
that bind target mRNAs via complementary sequences in the 3′-UTR to
inhibit their expression. Low expression of miR-378a-3p is closely
associated with an unfavorable prognosis, which has been
demonstrated in numerous different types of cancer cells, such as
pancreatic cancer (35), ovarian
cancer (36), and ESCC tissues
(21). However, the results of
previous studies involving the biological functions of miR-378 in
tumor cells remain contested. Gao et al (37) demonstrated that miR-378 was involved
in the inhibition of apoptosis of tumor cells by impacting the
activity of p53 and Caspase-3 in lung cancer cells (37). In gastric cancer tissues, Yang et
al (38) demonstrated that
miR-378 may be a tumor suppressor gene, promoting cell apoptosis
through BMP2 (38). Cui et
al (39) also showed that
miR-378a-3p/5p inhibited the tumor migration and invasion of oral
squamous carcinoma cells through decreasing KLK4 expression
(39). The miR-378a-3p/GLUT-1
regulatory model may be a novel therapeutic target in the metabolic
remodeling of patients with ESCC (22). Survivin is an important inhibitor of
apoptosis and plays a role in the survival of tumor cells (31). Survivin supports the survival of
tumor cells by inhibiting the endogenous apoptosis pathway and the
activity of apoptosis-related factors (30). In addition, Survivin is associated
with the Akt/mTOR and NF-κB signaling pathways, which have
previously been shown to be active in different cancer cell lines
(40–47). Moreover, the results of a previous
study demonstrated the positive cyclic regulation of Survivin and
NF-κB (48). However, there are no
studies assessing the association between miR-378a-3p and Survivin
to the best of our knowledge. Thus, further investigations into the
regulatory mechanisms of energy metabolism and apoptosis in ESCC
cells may improve the current understanding of its regulation, and
provide a novel experimental basis for determining the biological
role and significance of miR-378a-3p in ESCC cells.
The glycolytic bioenergetics pathway is an important
source of energy in tumor cells. The primary metabolic enzymes in
this pathway include GLUT-1, HK2, PFKL, ALDOA, GAPDH, PGK1, ENO1,
PKM2, and LDHA. Eichner et al (49) determined that miR-378a-3p decreased
HK2 and LDHA expression, which may enhance oxidative
phosphorylation, and suppress cell growth and tumorigenicity. Wang
et al (50) also showed that
miR-378a-3p repressed GLUT-1 expression by directly targeting its
3′UTR, and thus accelerated oral squamous cell carcinoma
metastasis.
In the present study, it was first confirmed that
ALDOA and PKM2 were the direct downstream target genes of
miR-378a-3p. The results of the present study demonstrated that
miR-378a-3p may block energy production in tumor cells by
inhibiting the protein expression levels of GLUT-1, ALDOA, and
PKM2. Following miR-378a-3p overexpression, ATP content, and
mitochondrial membrane potential were reduced and apoptosis was
promoted via Survivin, Bcl-2, Bad, and Caspase-3. Moreover, to
determine the association between cell energy metabolism and
apoptosis, an inhibitor of ATP synthesis, oligomycin, was used to
treat cells. The results of the present study demonstrated that the
levels of apoptosis inhibitory proteins (Survivin and Bcl-2) were
reduced, and apoptosis was increased following treatment; thus,
confirming that miR-378a-3p may induce apoptosis through the
intervention of cell energy metabolism. TFAR19 was initially
identified as a widely expressed apoptosis-accelerating protein.
TFAR19 rapidly translocates from the cytoplasm to the nucleus in
the early stage of apoptosis (30,51).
The results of the present study demonstrated that TFAR19 nuclear
translocation was significant in the miR-378a-3p transfection
group. Moreover, the results of the present study demonstrated that
miR-378a-3p promoted apoptosis of tumor cells was mediated by an
endogenous apoptosis mechanism related to the impairment of
mitochondrial membrane potential, particularly in the early stage.
Survivin, Bcl-2, Bad, and Caspase-3 are common proteins in the
apoptotic pathway. Notably, alterations in the expression levels of
Survivin, Bcl-2, Bad, and Caspase-3 in the miR-378a-3p
overexpression group demonstrated that the mode of cell death
induced by miR-378a-3p was apoptosis. These results indicated that
miR-378a-3p promotes the apoptosis of tumor cells by increasing the
expression and the activity of Bad and Caspase-3, and reducing the
expression of Survivin and Bcl-2. The results demonstrating the
energy metabolism of ESCC cells provide a novel experimental basis
to further understand the association between energy metabolism and
apoptosis. Notably, the results of the present study are consistent
with those of previous studies, demonstrating the decline of
metabolism, including the reduction of glycolysis, ATP levels, and
protein production, and the triggering of the apoptosis pathway
(49,50). Compared with previous studies, it is
hypothesized that miR-378a-3p may promote apoptosis through
interfering with cell energy metabolism in ESCC cells (16,30,32).
As energy metabolism is vital for the survival of
tumor cells, further investigations into the specific molecular
mechanism of miR-378a-3p in the regulation of enzymes associated
with energy metabolism are required.
The present study is limited, as the mechanism by
which miR-378a-3p inhibited the enzyme activity of HK2 and LDHA in
ECA-109 cells remains to be fully elucidated. Thus, whether
miR-378a-3p induced cell apoptosis via Survivin and the
Akt/mTOR/NF-κB signaling pathway requires further investigation.
Additionally, the influence of miR-378a-3p on cell migration,
invasion, and proliferation should be determined.
In conclusion, the results of the present study
revealed a novel association between miR-378a-3p, glycolysis, and
apoptosis. Specifically, miR-378a-3p may block energy production
and promote the apoptosis of tumor cells by downregulating
glycolytic enzyme expression in ECA-109 cells. miR-378a-3p inhibits
ATP synthase, increases the expression levels and activity of Bad
and Caspase-3, and decreases the expression levels of Survivin and
Bcl-2. These results provide novel evidence demonstrating that
miR-378a-3p is a tumor suppressor gene. In addition, the results of
the present study demonstrated that Survivin, Bad, and Bcl-2 may be
associated with the miR-378a-3p-induced inhibition of energy
production, and the miR-378a-3p-induced promotion of apoptosis. The
anti-tumor effect of miR-378a-3p may provide a novel treatment
option for the management of ESCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by funding the National Nature Science
Foundation of China (grant nos. 81460359 and 81460419).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and HWL conceived and designed the experiments.
YQ, SX, YJD, and LTH performed the experiments. YQ, YJZ and GPZ
analyzed the data. YQ, YJZ and HWL confirm the authenticity of all
the raw data. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng R, Zhang S, Zeng H, Wang S, Sun K,
Sun R, Li L, Wei W and Wei J: Cancer incidence and mortality in
China, 2016. J National Cancer Center. 2:1–9. 2022. View Article : Google Scholar
|
|
3
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo P, Huang ZL, Yu P and Li K: Trends in
cancer mortality in China: An update. Ann Oncol. 23:2755–2762.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dubecz A, Gall I, Solymosi N, Schweigert
M, Peters JH, Feith M and Stein HJ: Temporal trends in long-term
survival and cure rates in esophageal cancer: A SEER database
analysis. J Thorac Oncol. 7:443–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohashi S, Miyamoto S, Kikuchi O, Goto T,
Amanuma Y and Muto M: Recent advances from basic and clinical
studies of esophageal squamous cell carcinoma. Gastroenterology.
149:1700–1715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rottiers V and Naar AM: MicroRNAs in
metabolism and metabolic disorders. Nat Rev Mol Cell Biol.
13:239–250. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther. 1:150042016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su Z, Yang Z, Xu Y, Chen Y and Yu QJO:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ivey KN and Srivastava D: MicroRNAs as
regulators of differentiation and cell fate decisions. Cell Stem
Cell. 7:36–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Machado IF, Teodoro JS, Palmeira CM and
Rolo AP: miR-378a: A new emerging microRNA in metabolism. Cell Mol
Life Sci. 77:1947–1958. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu B, Qu J, Xu F, Guo Y, Wang Y, Yu H and
Qian B: MiR-195 suppresses non-small cell lung cancer by targeting
CHEK1. Oncotarget. 6:9445–9456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valeri N, Braconi C, Gasparini P, Murgia
C, Lampis A, Paulus-Hock V, Hart JR, Ueno L, Grivennikov SI, Lovat
F, et al: MicroRNA-135b promotes cancer progression by acting as a
downstream effector of oncogenic pathways in colon cancer. Cancer
Cell. 25:469–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pedroza-Torres A, Campos-Parra AD,
Millan-Catalan O, Loissell-Baltazar YA, Zamudio-Meza H, Cantú de
León D, Montalvo-Esquivel G, Isla-Ortiz D, Herrera LA,
Ángeles-Zaragoza Ó, et al: MicroRNA-125 modulates radioresistance
through targeting p21 in cervical cancer. Oncol Rep. 39:1532–1540.
2018.PubMed/NCBI
|
|
16
|
Pedroza-Torres A, Fernández-Retana J,
Peralta-Zaragoza O, Jacobo-Herrera N, Cantú de Leon D, Cerna-Cortés
JF, Lopez-Camarillo C and Pérez-Plasencia C: A microRNA expression
signature for clinical response in locally advanced cervical
cancer. Gynecol Oncol. 142:557–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen LT, Xu SD, Xu H, Zhang JF, Ning JF
and Wang SF: MicroRNA-378 is associated with non-small cell lung
cancer brain metastasis by promoting cell migration, invasion and
tumor angiogenesis. Med Oncol. 29:1673–1680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin Y, Liang R, Lu P, Lai L and Zhu X:
Depicting the Implication of miR-378a in Cancers. Technol Cancer
Res Treat. 21:153303382211343852022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krist B, Florczyk U,
Pietraszek-Gremplewicz K, Józkowicz A and Dulak J: The role of
miR-378a in metabolism, angiogenesis, and muscle biology. Int J
Endocrinol. 2015:2817562015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y and Du J: miR-378a-3p regulates
glioma cell chemosensitivity to cisplatin through IGF1R. Open Life
Sci. 16:1175–1181. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding N, Sun X, Wang T, Huang L, Wen J and
Zhou Y: miR-378a-3p exerts tumor suppressive function on the
tumorigenesis of esophageal squamous cell carcinoma by targeting
Rab10. Int J Mol Med. 42:381–391. 2018.PubMed/NCBI
|
|
22
|
Liu H, Zhang Q, Song Y, Hao Y, Cui Y,
Zhang X, Zhang X, Qin Y, Zhu G, Wang F, et al: Long non-coding RNA
SLC2A1-AS1 induced by GLI3 promotes aerobic glycolysis and
progression in esophageal squamous cell carcinoma by sponging
miR-378a-3p to enhance GLUT-1 expression. J Exp Clin Cancer Res.
40:2872021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong L, Yu T, Xu H, Hou N, Cheng Q, Lai L,
Wang Q, Sheng J and Huang H: Down-regulation of miR-378a-3p induces
decidual cell apoptosis: A possible mechanism for early pregnancy
loss. Hum Reprod. 33:11–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong X, Zhong L, Xie Y, Zheng K, Pang J,
Li Y, Yang Y, Xu X, Mi P, Cao H, et al: Matrine Reverses the
Warburg effect and suppresses colon cancer cell growth via
negatively regulating HIF-1α. Front Pharmacol. 10:14372019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tirpe AA, Gulei D, Ciortea SM, Crivii C
and Berindan-Neagoe I: Hypoxia: Overview on Hypoxia-Mediated
mechanisms with a focus on the role of HIF genes. Int J Mol Sci.
20:61402019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Comelli M, Di Pancrazio F and Mavelli I:
Apoptosis is induced by decline of mitochondrial ATP synthesis in
erythroleukemia cells. Free Radic Biol Med. 34:1190–1199. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marzieh A, Saeed T, Elina K, Omid V,
Mortaza TA, Mahshid T and Amir S: Caspase-3: Structure, function,
and biotechnological aspects. Biotechnol Appl Biochem.
69:1633–1645. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Altieri DC: Survivin-The inconvenient IAP.
Semin Cell Dev Biol. 39:91–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Budhidarmo R and Day CL: IAPs: Modular
regulators of cell signalling. Semin Cell Dev Biol. 39:80–90. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chu SF, Zhang Z, Zhang W, Zhang MJ, Gao Y,
Han N, Zuo W, Huang HY and Chen NH: Upregulating the expression of
Survivin-HBXIP complex contributes to the protective role of
IMM-H004 in transient global cerebral Ischemia/Reperfusion. Mol
Neurobiol. 54:524–540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai L, Li JL, Liang XQ, Li L, Feng Y, Liu
HZ, Wei WE, Ning SF and Zhang LT: Flowers of Camellia nitidissima
cause growth inhibition, cell-cycle dysregulation and apoptosis in
a human esophageal squamous cell carcinoma cell line. Mol Med Rep.
14:1117–1122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Ma Q, Shi Y, Li X, Wang M, Wang
J, Ge J, Chen Z, Wang Z and Jiang H: A novel
5-fluorouracil-resistant human esophageal squamous cell carcinoma
cell line Eca-109/5-FU with significant drug resistance-related
characteristics. Oncol Rep. 37:2942–2954. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao J, Shen X, Li H, Xu J, Shao S, Huang
JX and Lin M: LncRNA-ECM is overexpressed in esophageal squamous
cell carcinoma and promotes tumor metastasis. Oncol Lett.
16:3935–3942. 2018.PubMed/NCBI
|
|
35
|
Liu L, Han S, Xiao X, An X, Gladkich J,
Hinz U, Hillmer S, Hoppe-Tichy T, Xu Y, Schaefer M, et al:
Glucocorticoid-induced microRNA-378 signaling mediates the
progression of pancreatic cancer by enhancing autophagy. Cell Death
Dis. 13:10522022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu ZH, Yao TZ and Liu W: miR-378a-3p
sensitizes ovarian cancer cells to cisplatin through targeting
MAPK1/GRB2. Biomed Pharmacother. 107:1410–1417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao S, Yu Y, Liu L, Meng J and Li G:
Circular RNA hsa_circ_0007059 restrains proliferation and
epithelial-mesenchymal transition in lung cancer cells via
inhibiting microRNA-378. Life Scis. 233:1166922019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang YJ, Luo S and Wang LS: Effects of
microRNA-378 on epithelial-mesenchymal transition, migration,
invasion and prognosis in gastric carcinoma by targeting BMP2. Eur
Rev Med Pharmacol Sci. 23:5176–5186. 2019.PubMed/NCBI
|
|
39
|
Cui Z, Sun S, Liu Q, Zhou X, Gao S, Peng P
and Li Q: MicroRNA-378-3p/5p suppresses the migration and
invasiveness of oral squamous carcinoma cells by inhibiting KLK4
expression. Biochem Cell Biol. 98:154–163. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu JH, Yang S, Nan CJ, Zhou CC, Lu DQ, Li
S and Mu HQ: MiR-182 affects renal cancer cell proliferation,
apoptosis, and invasion by regulating PI3K/AKT/mTOR signaling
pathway. Eur Rev Med Pharmacol Sci. 22:351–357. 2018.PubMed/NCBI
|
|
41
|
Ou DL, Lee BS, Lin LI, Liou JY, Liao SC,
Hsu C and Cheng AL: Vertical blockade of the IGFR-PI3K/Akt/mTOR
pathway for the treatment of hepatocellular carcinoma: The role of
survivin. Mol Cancer. 13:22014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng Y, Lin ZM, Ge N, Zhang DL, Huang J
and Kong F: Ursolic acid induces apoptosis of prostate cancer cells
via the PI3K/Akt/mTOR pathway. Am J Chin Med. 43:1471–1486. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Khwairakpam AD, Monisha J, Roy NK,
Bordoloi D, Padmavathi G, Banik K, Khatoon E and Kunnumakkara AB:
Vietnamese coriander inhibits cell proliferation, survival and
migration via suppression of Akt/mTOR pathway in oral squamous cell
carcinoma. J Basic Clin Physiol Pharmacol. 312019.doi:
10.1515/jbcpp-2019-0162. PubMed/NCBI
|
|
44
|
Guo RH, Wang TS, Shen H, Ge HM, Sun J,
Huang ZH and Shu YQ: Involvement of mTOR and survivin inhibition in
tamoxifen-induced apoptosis in human hepatoblastoma cell line
HepG2. Biomed Pharmacother. 64:249–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li W, Du D and Li Y: Id-1 promotes
reendothelialization in the early phase after vascular injury
through activation of NFkB/survivin signaling pathway. Drug Des
Devel Ther. 13:3799–3811. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fukayama M, Hino R and Uozaki H:
Epstein-Barr virus and gastric carcinoma: Virus-host interactions
leading to carcinoma. Cancer Sci. 99:1726–1733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jeyasuria P, Subedi K, Suresh A and Condon
JC: Elevated levels of uterine anti-apoptotic signaling may
activate NFKB and potentially confer resistance to caspase
3-mediated apoptotic cell death during pregnancy in mice. Biol
Reprod. 85:417–424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zeng W, Li H, Chen Y, Lv H, Liu L, Ran J,
Sun X, Bieerkehazhi S, Liu Y, Li X, et al: Survivin activates NF-κB
p65 via the IKKβ promoter in esophageal squamous cell carcinoma.
Mol Med Rep. 13:1869–1880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eichner LJ, Perry MC, Dufour CR, Bertos N,
Park M, St-Pierre J and Giguère V: miR-378(*) mediates metabolic
shift in breast cancer cells via the PGC-1β/ERRγ transcriptional
pathway. Cell Metab. 12:352–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Zhang X, Wang Z, Hu Q, Wu J, Li Y,
Ren X, Wu T, Tao X, Chen X, et al: LncRNA-p23154 promotes the
invasion-metastasis potential of oral squamous cell carcinoma by
regulating GLUT-1-mediated glycolysis. Cancer Lett. 434:172–183.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Y, Sun R, Han W, Zhang Y, Song Q, Di
C and Ma D: Nuclear translocation of PDCD5 (TFAR19): An early
signal for apoptosis? FEBS Lett. 509:191–196. 2011. View Article : Google Scholar : PubMed/NCBI
|