Introduction
The morbidity of endometrial cancer (EC) has been
increasing globally, and it is the second most prevalent
gynecological cancer in women after cervical cancer (1,2). In
2018, 382,069 new cases and 89,929 deaths from endometrial cancer
were reported worldwide (3).
Advanced EC often recurs and has a poor prognosis (4,5),
whereas early-stage EC is often completely cured (6).
There are several histological types of EC, of which
endometrioid carcinoma is the most common, while other histological
types include serous cancer, clear cell carcinoma, and mixed
carcinoma. In general, low-grade carcinoma is hormone-sensitive and
includes endometrial carcinoma grades 1 and 2, and has a good
prognosis. In contrast, high-grade carcinoma includes endometrial
carcinoma grade 3, serous carcinoma, and clear cell carcinoma and
has a poor prognosis (7). Several
tumor markers have been used in patients with EC. Cancer antigen
125 (CA 125), cancer antigen 19-9 (CA 19-9), human epididymis
protein 4 (HE4), and carcinoembryonic antigen (CEA) are often used
as serodiagnostic markers for EC (8–13).
However, there is no consensus on the usefulness of these
serodiagnostic and prognostic markers for EC, thus, necessitating a
need for identifying new biomarkers for EC.
Proteomics-based secretome analysis identified
tissue factor pathway inhibitor-2 (TFPI2) as a biomarker for
ovarian clear cell carcinoma (OCCC) in 2013 (14). TFPI2 is a placental glycoprotein and
Kunitz-type serine protease inhibitor with extracellular
matrix-related protease inhibitory activity (15). A retrospective study carried out by
Arakawa et al demonstrated that serum TFPI2 is a useful
diagnostic marker for ovarian cancer (OC), especially OCCC
(16). TFPI2 has also been reported
to be immunohistologically positive in laryngeal, breast, gastric,
pancreatic, renal, and colorectal cancers; however, the staining
intensity is not uniform (17).
Based on the immunohistochemical expression of TFPI2 in ovarian
clear cell carcinoma, we postulated that TFPI2 is also expressed in
endometrial clear cell carcinoma. We then performed immunostaining
for TFPI2 in endometrial carcinoma tissues and found that TFPI2 was
localized in the cytoplasm and nucleus of endometrial carcinomas,
with a staining rate of 63.6%, notably reaching 100% in endometrial
clear cell carcinomas (18).
Therefore, we hypothesized that TFPI2 flows into blood vessels from
endometrial cancer tissue and its serum level increases. However,
there have been no reports to date regarding serum TFPI2 levels.
Therefore, in this study, we aimed to investigate the TFPI2 levels
in the preoperative serum of patients with EC and its utilization
as a prospective prognostic factor.
Materials and methods
Patients
Patients (n=207) who visited the Nara Medical
University for treatment of EC between January 2011 and December
2017 were enrolled in this retrospective study. The patient
inclusion criteria for the study were as follows-i) confirmed
pathological diagnosis of EC, ii) had undergone surgery as an
initial treatment and iii) had not received neoadjuvant
chemotherapy or radiotherapy. The grading of EC was performed as
per the International Federation of Gynecology and Obstetrics
(FIGO) classification, 2009. Patients diagnosed with EC via
histopathology underwent pelvic magnetic resonance imaging (MRI)
and computed tomography (CT) of the chest and abdomen and their CA
125, CA 19-9 and TFPI2 serum levels were measured preoperatively.
Anti-TFPI2 monoclonal antibodies were used to measure serum TFPI2
concentrations via E-test Tosoh II (AIA-PACK TFPI2) and an
automated immunoanalyzer AIA-2000 (Tosoh Corporation, Yokohama,
Japan). Clinical and pathological data of the patients were
obtained retrospectively from the medical records; they included
age, body mass index (BMI), parity, menopausal status, smoking
status, hormone replacement therapy, hyperlipidemia, hypertension,
diabetes, histological type, FIGO stage, myometrial invasion,
lymphovascular invasion, ascites cytology, lymph node metastasis,
and distant metastasis. The acceptable baseline reference values
for CA 125 and CA 19-9 at our institute are 36 U/ml and 38 U/ml
respectively.
Treatment
The patients diagnosed with EC underwent an open or
laparoscopic total hysterectomy and bilateral
salpingo-oophorectomy. Abdominal radical hysterectomy was performed
in patients with EC invading the cervical stroma. Pelvic lymph node
dissection was performed if preoperative pelvic MRI showed <1/2
myometrial invasion of the uterine corpus. In addition, para-aortic
lymphadenectomy was performed if preoperative pelvic MRI showed
≥1/2 myometrial invasion, or if the histopathologic type was
endometrial carcinoma grade 3, serous/clear cell carcinoma, or
carcinosarcoma. Lymphadenectomy was omitted if preoperative pelvic
MRI showed no myometrial invasion of the corpus of the uterus and
the histopathologic type was endometrial carcinoma grade 1 or
2.
Adjuvant chemotherapy was administered in the
following cases: medium risk of recurrence after surgery
(endometrial carcinoma grade 1/2 + ≥1/2 myometrial invasion, and
endometrial carcinoma grade 3 + <1/2 myometrial invasion) and
high risk of recurrence (endometrial carcinoma grade 3 + ≥1/2
myometrial invasion, serous carcinoma/clear cell carcinoma, lymph
node metastasis and distant metastasis). Adjuvant chemotherapy was
omitted in cases with a low risk of recurrence (endometrial
carcinoma grade 1/2 + <1/2 myometrial invasion). The adjuvant
chemotherapy regimen consisted of TC therapy (paclitaxel 175
mg/m2 + carboplatin AUC 5) every 3 weeks for six
courses. In case TC therapy was not administered owing to adverse
events, AP therapy (doxorubicin 60 mg/m2 + cisplatin 50
mg/m2) was administered every 4 weeks for six
courses.
Statistical analysis
Kaplan-Meier life table analysis and log-rank tests
were used to assess survival rates and differences based on
prognostic factors. Disease-free survival (DFS) is defined as the
interval of time after the end of primary cancer treatment where
the patient survives without any signs or symptoms of that cancer.
Overall survival (OS) is defined as the time span from the start of
treatment to death or the last follow-up examination. Multivariate
analysis of prognostic factors for DFS and OS was performed using
the Cox proportional hazards regression model. Receiver operating
characteristic (ROC) curves were used to determine the best cut-off
points for serum TFPI2 levels to predict the OS. The outcome in the
ROC curve was defined as survival or death. Baseline
characteristics, surgical procedure, risk of recurrence and
adjuvant chemotherapy in 207 patients with endometrial cancer
stratified by the cut-off value of TFPI2 were analyzed using the
chi-square test and Fisher's exact test. All statistical analyses
were performed using SPSS software version 28.0 for Windows (IBM
Corp., Armonk, NY, USA). Statistical significance was set at
P<.05.
Results
Patients' clinical
characteristics
All 207 patients with EC had a mean age of 60.3±11.4
(mean ± standard deviation) years (range, 32.0 to 92.0), mean BMI
of 24.4±5.6 kg/m2 (range, 15.1 to 47.5), and median
follow-up of 68.3 months (range, 5.8 to 158.8). The mean
preoperative serum TFPI2, CA 125, and CA 19-9 values were
211.6±27.4 pg/ml (range, 52.0 to 5630.0), 98.8±26.1 U/ml (range,
6.0 to 4019.0), and 86.0±33.1 U/ml (range, 1.0. to 6106.0),
respectively. The cut-off value predicting OS for TFPI2 was
determined from the ROC curve and was 177 pg/ml (Fig. 1). A TFPI2 value of <177 was
defined as negative and a value of >177 as positive. Table I shows the baseline characteristics
of the 207 EC patients stratified according to the cut-off value of
TFPI2. A TFPI2 value of ≥177 pg/ml was significantly correlated
with age ≥65 years (P<0.001), diabetes (P=0.035), FIGO stage
(P<0.001), myometrial invasion (P<0.001), lymphovascular
invasion (P=0.004), lymph node metastasis (P=0.010), distant
metastasis (P=0.002), CA 125 ≥36 U/ml (P<0.001) and CA 19-9 ≥38
U/ml (P<0.001).
 | Table I.Baseline characteristics of 207
patients stratified by the cut-off value of TFPI2. |
Table I.
Baseline characteristics of 207
patients stratified by the cut-off value of TFPI2.
| Variable | Total, n (%) | TFPI2-negative
(<177 pg/ml), n (%) | TFPI2-positive (≥177
pg/ml), n (%) | P-value |
|---|
| Age (years) |
|
|
|
|
|
<65 | 133 (64.3) | 97 (73.5) | 36 (48.0) | <0.001 |
| ≥65 | 74 (35.5) | 35 (26.5) | 39 (52.0) |
|
| BMI
(kg/m2) |
|
|
|
|
|
<25 | 158 (76.3) | 82 (62.1) | 45 (60.0) | 0.438 |
| ≥25 | 49 (23.7) | 50 (37.9) | 30 (40.0) |
|
| Parity |
|
|
|
|
| ≥1 | 149 (72.0) | 95 (72.0) | 54 (72.0) | 0.996 |
| 0 | 58 (28.0) | 37 (28.0) | 21 (28.0) |
|
| Menopausal
status |
|
|
|
|
|
Pre-menopausal | 61 (29.5) | 45 (34.1) | 16 (21.3) | 0.053 |
|
Post-menopausal | 146 (70.5) | 87 (65.9) | 59 (78.7) |
|
| Smoking |
|
|
|
|
| Yes | 184 (88.9) | 14 (10.7) | 8 (10.7) | 0.996 |
| No | 23 (11.1) | 117 (89.3) | 67 (89.3) |
|
| HRT |
|
|
|
|
|
Yes | 8 (3.9) | 7 (5.3) | 1 (1.3) | 0.263 |
| No | 199 (96.1) | 125 (94.7) | 74 (98.7) |
|
| Hyperlipidemia |
|
|
|
|
|
Yes | 33 (15.9) | 23 (17.4) | 10 (13.3) | 0.44 |
| No | 174 (84.1) | 109 (82.6) | 65 (86.7) |
|
| Hypertension |
|
|
|
|
|
Yes | 51 (24.6) | 29 (22.0) | 21 (28.4) | 0.303 |
| No | 156 (75.4) | 103 (78.0) | 53 (71.6) |
|
| Diabetes |
|
|
|
|
|
Yes | 30 (14.5) | 14 (10.6) | 16 (21.3) | 0.035 |
| No | 177 (85.5) | 118 (89.3) | 59 (78.7) |
|
| Histological
type |
|
|
|
|
|
Low-grade carcinoma | 129 (62.3) | 86 (65.2) | 43 (57.3) | 0.177 |
|
Endometrioid carcinoma G1 | 90 (43.5) | 67 (50.8) | 23 (30.7) |
|
|
Endometrioid carcinoma G2 | 39 (18.8) | 19 (14.4) | 20 (26.6) |
|
|
High-grade carcinoma | 78 (37.7) | 46 (34.8) | 32 (42.7) |
|
|
Endometrioid carcinoma G3 | 29 (14.0) | 18 (13.6) | 11 (14.7) |
|
| Clear
cell carcinoma | 11 (5.3) | 3 (2.3) | 8 (10.7) |
|
| Serous
carcinoma | 19 (9.2) | 12 (9.1) | 7 (9.3) |
|
|
Carcinosarcoma | 13 (6.3) | 10 (7.5) | 3 (4.0) |
|
|
Others | 6 (2.9) | 3 (2.3) | 3 (4.0) |
|
| FIGO stage, n
(%) |
|
|
|
|
|
I/II | 158 (76.3) | 113 (85.6) | 45 (60.0) | <0.001 |
|
III/IV | 49 (23.7) | 19 (14.4) | 30 (40.0) |
|
| Myometrial
invasion |
|
|
|
|
|
<1/2 | 142 (68.6) | 106 (80.3) | 36 (48.0) | <0.001 |
|
≥1/2 | 65 (31.4) | 26 (19.7) | 39 (52.0) |
|
| Lymphovascular
invasion |
|
|
|
|
|
Positive | 128 (61.8) | 91 (68.9) | 37 (49.3) | 0.004 |
|
Negative | 79 (38.2) | 41 (31.1) | 38 (50.7) |
|
| Ascites
cytology |
|
|
|
|
|
Positive | 49 (23.7) | 26 (19.7) | 22 (29.7) | 0.102 |
|
Negative | 158 (76.3) | 106 (80.3) | 52 (70.3) |
|
| Lymph node
metastasis |
|
|
|
|
|
Positive | 32 (15.5) | 14 (10.6) | 18 (24.0) | 0.010 |
|
Negative | 175 (84.5) | 118 (89.4) | 57 (76.0) |
|
| Distant
metastasis |
|
|
|
|
|
Positive | 18 (8.7) | 5 (3.8) | 13 (17.3) | 0.002 |
|
Negative | 189 (91.3) | 127 (96.2) | 62 (82.7) |
|
| CA 125 (U/ml) |
|
|
|
|
| <36
U/ml | 136 (70.1) | 98 (79.0) | 38 (54.2) | <0.001 |
| ≥36
U/ml | 58 (29.9) | 26 (21.0) | 32 (45.7) |
|
| CA 19-9 (U/ml) |
|
|
|
|
| <38
U/ml | 120 (62.2) | 85 (69.1) | 35 (50.0) | 0.009 |
| ≥38
U/ml | 73 (37.8) | 38 (30.9) | 35 (50.0) |
|
Treatment and risk of recurrence for
207 endometrial cancer patients
Table II shows the
surgical procedures performed on 207 patients, hysterectomy
(including total abdominal and laparoscopic hysterectomy) and
bilateral salpingo-oophorectomy in 53 patients (25.3%).
Hysterectomy, bilateral salpingo-oophorectomy, and pelvic
lymphadenectomy were performed in 75 patients (35.9%).
Hysterectomy, bilateral salpingo-oophorectomy, pelvic
lymphadenectomy, and para-aortic lymphadenectomy were performed in
79 patients (37.8%, including 4 abdominal radical hysterectomies).
The risk of recurrence was assessed from postoperative pathology
and patients were stratified as low risk (n=84, 40.6%), medium risk
(n=72, 34.8%), and high risk (n=51, 24.6%). Medium and high risk of
recurrence were more common in the group with TFPI2 levels ≥177
pg/ml (P<0.001) (Table II).
Patients with medium risk and high risk of recurrence received
postoperative adjuvant chemotherapy. A total of 123 patients
(59.4%) received adjuvant chemotherapy after surgery due to medium
or high risk of recurrence (Table
II); 111 patients (90.2%) received TC therapy, and 12 patients
(9.8%) received AP therapy.
 | Table II.Surgical procedure, risk of
recurrence and adjuvant chemotherapy performed on a patient with
endometrial cancer. |
Table II.
Surgical procedure, risk of
recurrence and adjuvant chemotherapy performed on a patient with
endometrial cancer.
| Parameter | Total, n (%) | TFPI2-negative
(<177 pg/ml), n (%) | TFPI2-positive
(≥177 pg/ml), n (%) | P-value |
|---|
| Surgical
procedure |
|
|
|
|
| TAH +
BSO | 53 (25.6) | 25 (18.9) | 28 (37.3) | 0.012 |
| TAH +
BSO + PLA | 75 (36.2) | 54 (11.4) | 21 (13.3) |
|
| TAH +
BSO + PLA + PALA | 79 (35.9) | 52 (39.4) | 23 (30.7) |
|
| Risk of
recurrence |
|
|
|
|
| Low
risk | 84 (40.6) | 64 (47.7) | 20 (26.7) | <0.001 |
| Median
risk | 72 (34.8) | 47 (35.6) | 25 (33.3) |
|
| High
risk | 51 (24.6) | 21 (15.9) | 30 (40.0) |
|
| Adjuvant
chemotherapy |
|
|
| 0.037 |
| No | 84 (40.6) | 64 (48.5) | 20 (26.7) |
|
|
Yes | 123 (59.4) | 68 (51.5) | 55 (73.3) |
|
Prognostic factors for disease-free
survival
The 5-year DFS rate and OS rates were 73.3 and
83.7%, respectively. The log-rank test was used to assess the risk
factors affecting the DFS and OS in patients with EC. In the
univariate analysis, age ≥65 years (P<0.001), postmenopausal
status (P=0.002), high-grade carcinoma (P<0.001), myometrial
invasion ≥1/2 (P<0.001), lymphovascular invasion (P<0.001),
positive ascites cytology (P<0.001), lymph node metastasis
(P<0.001), distant metastasis (P<0.001), TFPI2 level ≥177
pg/ml (P<0.001), CA 125 level ≥36 U/ml (P<0.001) and CA 19-9
level ≥38 U/ml (P<0.001) were shown to be significantly
associated with DFS (Table III).
When Cox multivariate analysis was applied, age ≥65 years
(P=0.009), postmenopausal status (P=0.017), high-grade carcinoma
(P=0.031), lymphovascular invasion (P=0.023), lymph node metastasis
P=0.004), distant metastasis (P=0.013), TFPI2 level ≥177 pg/ml
(P=0.017) and CA 19-9 level ≥38 U/ml (P=0.045) were found to be
significant independent prognostic factors affecting DFS in
patients with EC (Table III).
 | Table III.Univariate and multivariate analysis
of prognostic factors for disease free survival. |
Table III.
Univariate and multivariate analysis
of prognostic factors for disease free survival.
|
| Univariate
analysis | Cox multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | Patients, n | P-value | HR | 95% CI | P-value |
|---|
| Age (years), n |
|
|
|
|
|
|
<65 | 23 |
|
|
|
|
|
≥65 | 31 | <0.001 | 2.550 | 1.260–5.161 | 0.009 |
| BMI
(kg/m2) |
|
|
|
|
|
|
<25 | 39 |
|
|
|
|
|
≥25 | 15 | 0.058 |
|
|
|
| Parity |
|
|
|
|
|
| ≥1 | 40 |
|
|
|
|
| 0 | 14 | 0.76 |
|
|
|
| Menopausal
status |
|
|
|
|
|
|
Pre-menopausal | 7 |
|
|
|
|
|
Post-menopausal | 47 | 0.002 | 3.645 | 1.263–10.525 | 0.017 |
| Smoking |
|
|
|
|
|
|
Yes | 7 |
|
|
|
|
| No | 47 | 0.577 |
|
|
|
| HRT |
|
|
|
|
|
|
Yes | 0 |
|
|
|
|
| No | 54 | 0.116 |
|
|
|
| Hyperlipidemia |
|
|
|
|
|
|
Yes | 8 |
|
|
|
|
| No | 46 | 0.746 |
|
|
|
| Hypertension |
|
|
|
|
|
|
Yes | 16 |
|
|
|
|
| No | 38 | 0.335 |
|
|
|
| Diabetes |
|
|
|
|
|
|
Yes | 8 |
|
|
|
|
| No | 46 | 0.881 |
|
|
|
| Histological
type |
|
|
|
|
|
|
Low-grade carcinoma | 20 |
|
|
|
|
|
High-grade carcinoma | 34 | <0.001 | 2.188 | 1.074–4.460 | 0.031 |
| Myometrial
invasion |
|
|
|
|
|
|
<1/2 | 21 |
|
|
|
|
|
≥1/2 | 33 | <0.001 | 0.762 | 0.347–1.675 | 0.499 |
| Lymphovascular
invasion |
|
|
|
|
|
|
Negative | 18 |
|
|
|
|
|
Positive | 35 | <0.001 | 4.628 | 1.118–4.628 | 0.023 |
| Ascites
cytology |
|
|
|
|
|
|
Negative | 30 |
|
|
|
|
|
Positive | 24 | <0.001 | 1.857 | 0.904–3.813 | 0.092 |
| Lymph node
metastasis |
|
|
|
|
|
|
Negative | 35 |
|
|
|
|
|
Positive | 19 | <0.001 | 3.075 | 1.434–6.594 | 0.004 |
| Distant
metastasis |
|
|
|
|
|
|
Negative | 40 |
|
|
|
|
|
Positive | 14 | <0.001 | 2.88 | 1.255–6.605 | 0.013 |
| TFPI2 |
|
|
|
|
|
| <177
pg/ml | 23 |
|
|
|
|
| ≥177
pg/ml | 31 | <0.001 | 2.328 | 1.165–4.650 | 0.017 |
| CA 125 |
|
|
|
|
|
| <36
U/ml | 22 |
|
|
|
|
| ≥36
U/ml | 28 | <0.001 | 1.465 | 0.661–3.244 | 0.347 |
| CA 19-9 |
|
|
|
|
|
| <38
U/ml | 26 |
|
|
|
|
| ≥38
U/ml | 24 | <0.001 | 2.195 | 1.019–4.725 | 0.045 |
Prognostic factors for overall
survival
As per univariate analysis, age ≥65 years
(P<0.001), BMI ≥25 kg/m2 (P=0.019), postmenopausal
status (P=0.009), high-grade carcinoma (P<0.001), myometrial
invasion ≥1/2 (P<0.001), lymphovascular invasion (P<0.001),
positive ascites cytology (P<0.001), lymph node metastasis
(P<0.001), distant metastasis (P<0.001), TFPI2 level ≥177
pg/ml (P<0.001), CA 125 level ≥36 U/ml (P<0.001) and CA 19-9
level ≥38 U/ml (P<0.001) were found to have significant effects
on OS (Table III). However, as
per multivariate analysis, high-grade carcinoma (P=0.041), lymph
node metastasis (P=0.038), distant metastasis (P=0.009) and TFPI2
level ≥177 pg/ml (P=0.043) were found to be significant prognostic
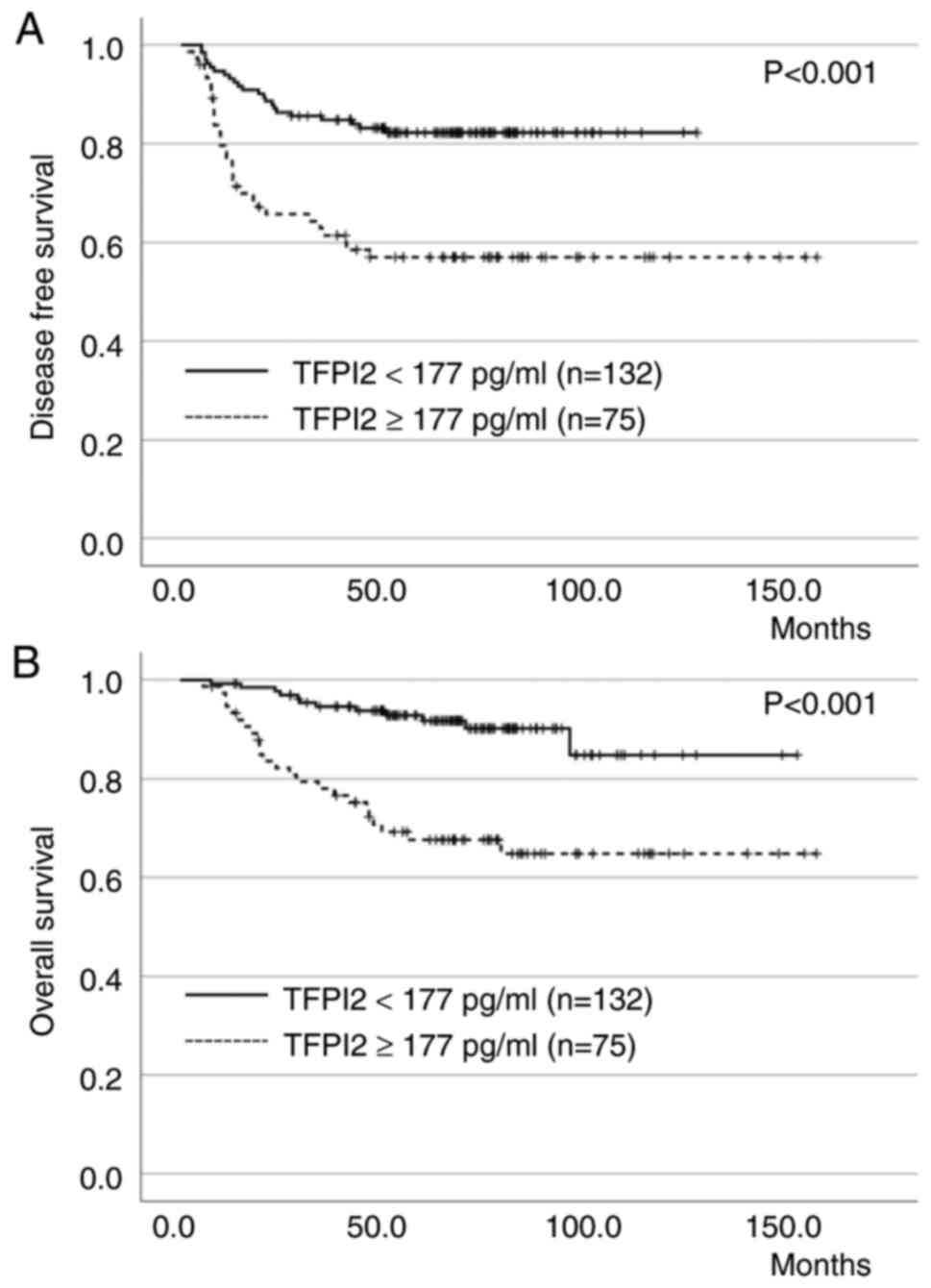
factors affecting OS in patients with EC (Table IV). The DFS and OS curves for
patients with EC according to preoperative TFPI2 levels are shown
in the Fig. 2A and B),
respectively. Patients with positive TFPI2 levels had significantly
worse DFS and OS than those with negative TFPI2 levels
(P<0.001).
 | Table IV.Univariate and multivariate analysis
of prognostic factors for overall survival. |
Table IV.
Univariate and multivariate analysis
of prognostic factors for overall survival.
|
| Univariate
analysis | Cox multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | Patients, n | P-value | HR | 95% CI | P-value |
|---|
| Age (year) |
|
|
|
|
|
|
<65 | 21 |
|
|
|
|
|
≥65 | 15 | <0.001 | 1.575 | 0.653–3.799 | 0.312 |
| BMI
(kg/m2) |
|
|
|
|
|
|
<25 | 28 |
|
|
|
|
|
≥25 | 8 | 0.019 | 0.555 | 0.229–1.345 | 0.192 |
| Parity |
|
|
|
|
|
| ≥1 | 29 |
|
|
|
|
| 0 | 7 | 0.203 |
|
|
|
| Menopausal
status |
|
|
|
|
|
|
Pre-menopausal | 4 |
|
|
|
|
|
Post-menopausal | 32 | 0.009 | 2.981 | 0.802–11.079 | 0.103 |
| Smoking |
|
|
|
|
|
|
Yes | 5 |
|
|
|
|
| No | 31 | 0.557 |
|
|
|
| HRT |
|
|
|
|
|
|
Yes | 0 |
|
|
|
|
| No | 36 | 0.256 |
|
|
|
| Hyperlipidemia |
|
|
|
|
|
|
Yes | 11 |
|
|
|
|
| No | 25 | 0.463 |
|
|
|
| Hypertension |
|
|
|
|
|
|
Yes | 7 |
|
|
|
|
| No | 29 | 0.48 |
|
|
|
| Diabetes |
|
|
|
|
|
|
Yes | 5 |
|
|
|
|
| No | 31 | 0.935 |
|
|
|
| Histological
type |
|
|
|
|
|
|
Low-grade carcinoma | 11 |
|
|
|
|
|
High-grade carcinoma | 25 | <0.001 | 2.439 | 1.038–5.714 | 0.041 |
| Myometrial
invasion |
|
|
|
|
|
|
<1/2 | 11 |
|
|
|
|
|
≥1/2 | 25 | <0.001 | 1.226 | 0.440–3.643 | 0.662 |
| Lymphovascular
invasion |
|
|
|
|
|
|
Negative | 12 |
|
|
|
|
|
Positive | 23 | <0.001 | 1.46 | 0.579–3.684 | 0.423 |
| Ascites
cytology |
|
|
|
|
|
|
Negative | 18 |
|
|
|
|
|
Positive | 18 | <0.001 | 1.173 | 0.469–2.935 | 0.733 |
| Lymph node
metastasis |
|
|
|
|
|
|
Negative | 22 |
|
|
|
|
|
Positive | 14 | <0.001 | 2.116 | 1.049–5.702 | 0.038 |
| Distant
metastasis |
|
|
|
|
|
|
Negative | 24 |
|
|
|
|
|
Positive | 12 | <0.001 | 3.604 | 1.376–9.439 | 0.009 |
| TFPI2 |
|
|
|
|
|
| <177
pg/ml | 12 |
|
|
|
|
| ≥177
pg/ml | 24 | <0.001 | 2.42 | 1.021–5.928 | 0.043 |
| CA 125 |
|
|
|
|
|
| <36
U/ml | 15 |
|
|
|
|
| ≥36
U/ml | 19 | <0.001 | 1.044 | 0.385–2.829 | 0.933 |
| CA19-9 |
|
|
|
|
|
| <38
U/ml | 16 |
|
|
|
|
| ≥38
U/ml | 18 | <0.001 | 2.193 | 0.848–5.668 | 0.105 |
Discussion
Several studies have been carried out on tumor
markers in patients with EC, and CA 125, CA 19-9, human epididymis
protein 4 (HE4), and carcinoembryonic antigen (CEA) are the often
used serodiagnostic markers for EC (8–13). CA
125 is the most widely used tumor marker for EC, and there have
been several reports stating that preoperative CA 125 can be a
prognostic factor for the OS of EC patients (19–22).
Chao et al reported that a preoperative CA 125 level of 105
U/ml in patients under 49 years of age and 35 U/ml in patients over
50 years of age are prognostic factors for EC (19). Furthermore, Pinar et al
reported that a preoperative CA 125 level ≥35 U/ml is a poor
prognostic factor for EC (20).
Yilmaz et al also reported that a CA 125 level of 35 U/ml
was the cut-off value for the OS (21) and in other studies, the reported
cut-off value for CA 125 was 20 U/ml for EC (23,24).
Previous studies have shown that CEA (12,22)
and CA 19-9 (22) are potential
prognostic factors for OS in patients with EC. In addition, several
recent reports have suggested HE4 as a useful biomarker for EC as
well as OC (25,26). Moreover, serum HE4 is an independent
risk factor for decreased DFS and OS in patients with EC and has
been reported to be a more useful biomarker than CA 125 (25).
TFPI2 is a 32-kDa protein that was reported for the
first time by Miyagi et al as a protease inhibitor that is
exclusively expressed in the placenta of pregnant women (15). However, further studies showed that
TFPI2 is produced in vascular endothelial cells, platelets, and
macrophages (27). Moreover,
immunohistochemistry studies have revealed that TFPI2 is localized
in normal muscular, skeletal, breast, liver, kidney, pancreas,
stomach, and colon tissues (17).
In normal human tissues, TFPI2 is thought to be involved in the
processes of coagulation, angiogenesis, inflammation, and apoptosis
(28). Using proteomic analysis
technology, Arakawa et al analyzed the culture medium of OC
cells and found that OCCC specifically produces TFPI2 (14). Previously, we have reported the
diagnostic accuracy and usefulness of TFPI2 in differentiating OC
from benign ovarian tumors (29).
At a cut-off value of 191 pg/ml for TFPI2 levels, the sensitivity,
specificity, and area under the curve (AUC) for discriminating OC
from benign ovarian tumors were 64.7, 91.5%, and 0.893,
respectively (29). The Ministry of
Health, Labor and Welfare in Japan has officially approved the use
of TFPI2 by as a serodiagnostic marker for OC.
TFPI2 was reported to be stained in the nucleus,
cytoplasm, and extracellular matrix in OCCC, whereas TFPI2 was not
expressed in non-ovarian clear cell carcinomas (27). However, serum TFPI2 levels were
elevated in non-ovarian clear cell carcinoma patients, suggesting
that non-tumor cells such as macrophages, platelets, and vascular
endothelial cells produce TFPI2 (17). Moreover, elevated serum TFPI2 levels
in patients with EC are thought to be derived from EC cells
(18). Therefore, more studies need
to be conducted to reveal the potential source of TFPI2 in
different cancers. The usefulness of serum TFPI2 as a tumor marker
in EC has never been reported. In this study, we determined the
serum TFPI2 levels in uterine EC and evaluated whether TFPI2 could
be a prognostic factor. Additionally, we investigated the
association between various parameters and serum TFPI2 levels. In
patients with EC, serum TFPI2 levels were associated with
clinicopathological factors, such as the FIGO stage, myometrial
invasion, lymphovascular invasion, lymph node metastasis, and
distant metastasis. These findings suggest that serum TFPI2 levels
reflect the aggressiveness and progression of EC. Therefore,
patients with high TFPI2 levels are considered to have a poor
prognosis. Although TFPI2 values were also correlated with CA 125
and CA 19-9 values, multivariate analysis revealed that TFPI2 was
superior to CA 125 and CA 19-9 as a prognostic marker for OS in
uterine cancer.
Thus, to the best of our knowledge, this is the
first report of elevated serum TFPI2 levels in patients with EC and
its use as an independent prognostic factor for EC. In this study,
a preoperative serum TFPI2 level of 177 pg/ml was found to be the
cutoff value predicting a worse OS. The prognosis of patients with
EC with high-grade carcinoma, lymph node metastasis, distant
metastasis and preoperative TFPI2 level ≥177 pg/ml was poor in
multivariate analysis. A previous retrospective cohort study of
2,948 patients with EC also reported distant metastasis,
particularly to the brain, as an independent prognostic factor for
OS and multiple distant metastases were associated with a poor
prognosis (30). Our study had few
limitations. Although serum TFPI2 levels are elevated in patients
with EC, the biological properties and role of TFPI2 in EC remain
unclear. Therefore, further studies on TFPI2, including a large
prospective study involving the collection of EC samples from more
institutions, are required.
In summary, in this study we have demonstrated for
the first time that the preoperative serum TFPI2 level, along with
histological type, lymph node metastasis and distant metastasis,
can be used as a potential prognostic factor for OS in patients
with EC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RK conceived and designed the study and drafted the
manuscript. TM, SY, RM, KI and YY collected and analyzed the data
and produced the tables and figures. RK, FK and NK analyzed and
interpreted the data. RK and TM confirm the authenticity of all the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Ethics
Committee of Nara Medical University, Kashihara, Japan (approval
no. 3401). This study was conducted in accordance with the
guidelines of the Declaration of Helsinki. This was a single-center
retrospective study based on medical records and histopathological
findings. All patient information was anonymized; thus, the need
for informed consent was waived, and information regarding the
implementation of the survey was disclosed by the opt-out
method.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao M, Li H, Sun D and Chen W: Cancer
burden of major cancers in China: A need for sustainable actions.
Cancer Commun (Lond). 40:205–210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Colombet M, Soerjomataram I,
Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer
incidence and mortality patterns in Europe: Estimates for 40
countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brooks RA, Fleming GF, Lastra RR, Lee NK,
Moroney JW, Son CH, Tatebe K and Veneris JL: Current
recommendations and recent progress in endometrial cancer. CA
Cancer J Clin. 69:258–279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Travaglino A, Raffone A, Saccone G, De
Luca C, Mollo A, Mascolo M, De Placido G, Insabato L and Zullo F:
Immunohistochemical nuclear expression of β-catenin as a surrogate
of CTNNB1 exon 3 mutation in endometrial cancer. Am J Clin Pathol.
151:529–538. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO consensus conference on endometrial
cancer: Diagnosis, treatment and follow-up. Radiother Oncol.
117:559–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brassard L and Bessette P: Value of
gynecological cytology and CA 125 level for the prediction of
extrauterine malignancy in endometrial cancer. J Obstet Gynaecol
Can. 34:657–663. 2012.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roelofsen T, Mingel M, Hendriks JC, Samlal
RA, Snijders MP, Aalders AL, Bulten J, van Ham MA and Massuger LF:
Preoperative CA-125 predicts extra-uterine disease and survival in
uterine papillary serous carcinoma patients. Int J Biol Markers.
27:e263–e271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yildiz A, Yetimalar H, Kasap B, Aydin C,
Tatar S, Soylu F and Yildiz FS: Preoperative serum CA 125 level in
the prediction of the stage of disease in endometrial carcinoma.
Eur J Obstet Gynecol Reprod Biol. 164:191–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baser E, Gungor T, Togrul C, Turkoglu O
and Celen S: Preoperative prediction of poor prognostic parameters
and adjuvant treatment in women with pure endometrioid type
endometrial cancer: What is the significance of tumor markers? Eur
J Gynaecol Oncol. 35:513–518. 2014.PubMed/NCBI
|
|
12
|
Hashiguchi Y, Kasai M, Fukuda T, Ichimura
T, Yasui T and Sumi T: Serum carcinoembryonic antigen as a tumour
marker in patients with endometrial cancer. Curr Oncol.
23:e439–e442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bian J, Sun X, Li B and Ming L: Clinical
significance of serum HE4, CA125, CA724, and CA19-9 in patients
with endometrial cancer. Technol Cancer Res Treat. 16:435–439.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arakawa N, Miyagi E, Nomura A, Morita E,
Ino Y, Ohtake N, Miyagi Y, Hirahara F and Hirano H: Secretome-based
identification of TFPI2, a novel serum biomarker for detection of
ovarian clear cell adenocarcinoma. J Proteome Res. 12:4340–4350.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyagi Y, Koshikawa N, Yasumitsu H, Miyagi
E, Hirahara F, Aoki I, Umeda M and Miyazaki K: cDNA cloning and
mRNA expression of a serine proteinase inhibitor secreted by cancer
cells: Identification as placental protein 5 and tissue factor
pathway inhibitor-2. J Biochem. 116:939–942. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arakawa N, Kobayashi H, Yonemoto N,
Masuishi Y, Ino Y, Shigetomi H, Furukawa N, Ohtake N, Miyagi Y,
Hirahara F, et al: Clinical significance of tissue factor pathway
inhibitor 2, a serum biomarker candidate for ovarian clear cell
carcinoma. PLoS One. 11:e01656092016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wojtukiewicz MZ, Sierko E, Zimnoch L,
Kozlowski L and Kisiel W: Immunohistochemical localization of
tissue factor pathway inhibitor-2 in human tumor tissue. Thromb
Haemost. 90:140–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawaguchi R, Maehana T, Sugimoto S,
Kawahara N, Iwai K, Yamada Y and Kimura F: Immunohistochemical
analysis of the tissue factor pathway inhibitor-2 in endometrial
clear cell carcinoma: A single-center retrospective study. Int J
Gynecol Pathol. May 31–2023.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chao A, Tang YH, Lai CH, Chang CJ, Chang
SC, Wu TI, Hsueh S, Wang CJ, Chou HH and Chang TC: Potential of an
age-stratified CA125 cut-off value to improve the prognostic
classification of patients with endometrial cancer. Gynecol Oncol.
129:500–504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pinar Cilesiz Goksedef B, Gorgen H, Baran
SY, Api M and Cetin A: Preoperative serum CA 125 level as a
predictor for metastasis and survival in endometrioid endometrial
cancer. J Obstet Gynaecol Can. 33:844–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yilmaz Baran Ş, Alemdaroğlu S, Doğan
Durdağ G, Yüksel Şimşek S, Bolat F, Köse F and Çelik H: What is the
predictive value of preoperative CA 125 level on the survival rate
of type 1 endometrial cancer? J Med Sci. 51:335–341.
2021.PubMed/NCBI
|
|
22
|
Lo SS, Cheng DK, Ng TY, Wong LC and Ngan
HY: Prognostic significance of tumour markers in endometrial
cancer. Tumour Biol. 18:241–249. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alagoz T, Buller RE, Berman M, Anderson B,
Manetta A and DiSaia P: What is a normal CA125 level? Gynecol
Oncol. 53:93–97. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurihara T, Mizunuma H, Obara M, Andoh K,
Ibuki Y and Nishimura T: Determination of a normal level of serum
CA125 in postmenopausal women as a tool for preoperative evaluation
and postoperative surveillance of endometrial carcinoma. Gynecol
Oncol. 69:192–196. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brennan DJ, Hackethal A, Metcalf AM,
Coward J, Ferguson K, Oehler MK, Quinn MA, Janda M, Leung Y,
Freemantle M, et al: Serum HE4 as a prognostic marker in
endometrial cancer-a population based study. Gynecol Oncol.
132:159–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abbink K, Zusterzeel PL, Geurts-Moespot
AJ, Herwaarden AEV, Pijnenborg JM, Sweep FC and Massuger LF: HE4 is
superior to CA125 in the detection of recurrent disease in
high-risk endometrial cancer patients. Tumour Biol.
40:10104283187571032018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ota Y, Koizume S, Nakamura Y, Yoshihara M,
Takahashi T, Sato S, Myoba S, Ohtake N, Kato H, Yokose T, et al:
Tissue factor pathway inhibitor-2 is specifically expressed in
ovarian clear cell carcinoma tissues in the nucleus, cytoplasm and
extracellular matrix. Oncol Rep. 45:1023–1032. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chand HS, Foster DC and Kisiel W:
Structure, function and biology of tissue factor pathway
inhibitor-2. Thromb Haemost. 94:1122–1130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kobayashi H, Yamada Y, Kawaguchi R, Ootake
N, Myoba S and Kimura F: Tissue factor pathway inhibitor 2: A
potential diagnostic marker for discriminating benign from
malignant ovarian tumors. J Obstet Gynaecol Res. 48:2442–2451.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Chi S, Zhou X, Zhao R, Xiao C and
Wang H: Prognostic value of distant metastatic sites in stage IV
endometrial cancer: A SEER database study of 2948 women. Int J
Gynaecol Obstet. 149:16–23. 2020. View Article : Google Scholar : PubMed/NCBI
|