Introduction
Lung cancer ranks second in the incidence rate of
cancer and is the leading cause of cancer-related deaths worldwide,
with 8.2 million deaths yearly (1).
Non-small cell lung cancer (NSCLC) accounts for ~85% of lung cancer
cases. Immune checkpoint inhibitors (ICIs) target programmed cell
death protein 1 (PD-1) and its ligand (PD-L1) and have
revolutionized the treatment of advanced NSCLC (2,3). PD-1
and PD-L1 trigger T lymphocyte function to kill tumor cells by
blocking the PD-1/PD-L1/2 signaling pathways (4). Several trials have reported the value
of ICIs in treating resectable NSCLC with a significant
pathological response as the primary endpoint (5–7).
However, due to the mechanism of action of ICIs, immune-related
adverse events (irAEs) can occur during treatment. Significant
irAEs can cause surgery delays and/or intraoperative complications.
Tumor progression may occasionally occur due to toxicities or poor
efficacy of ICIs (8,9).
Endocrine adverse events are among the most common
toxicities of ICIs, and the most commonly affected endocrine organ
is the thyroid (10). Most thyroid
dysfunction is asymptomatic or mild. However, patients may present
with symptoms of hypothyroidism, such as fatigue, anorexia,
constipation, bradycardia or weight gain (11,12).
Thyroid dysfunction during PD-1 inhibitor therapy is associated
with a longer progression-free survival time and could be used as a
potential marker to predict an improved response to treatment
(13). However, another study has
refuted this hypothesis (14). In
the present study, a case of thyroid dysfunction and tumor
progression in a patient with stage IIIA NSCLC treated with the
PD-1 inhibitor pembrolizumab is reported.
Case report
A 59-year-old male with a 30 pack-year history of
smoking was transferred to The Peking University People's Hospital
(Beijing, China) on September 2020. The patient had been
experiencing shortness of breath after activity without apparent
cause for almost 8 months. However, the patient did not complain of
chest pain, cough, expectoration or fever. Chest computed
tomography (CT) performed in another hospital revealed a central
space-occupied lesion in the upper lobe of the left lung with
atelectasis. Positron emission tomography-CT showed enlarged lymph
nodes (zone VI) with no abnormal uptake of
18F-fluoro-2-deoxyglucose. The pathological section diagnosis was
squamous carcinoma. The chest CT scan performed at The Peking
University People's Hospital revealed that the volume of the upper
lobe of the left lung was decreased and consolidated, and the upper
lobe bronchus of the left lung was blocked. The soft tissue density
shadow extended to the left main bronchus and the lower lobe
bronchus of the left lung, resulting in a slight stenosis of the
lower lobe bronchi. The results indicated that the malignant lesion
occurred with multiple partially enlarged lymph nodes in the
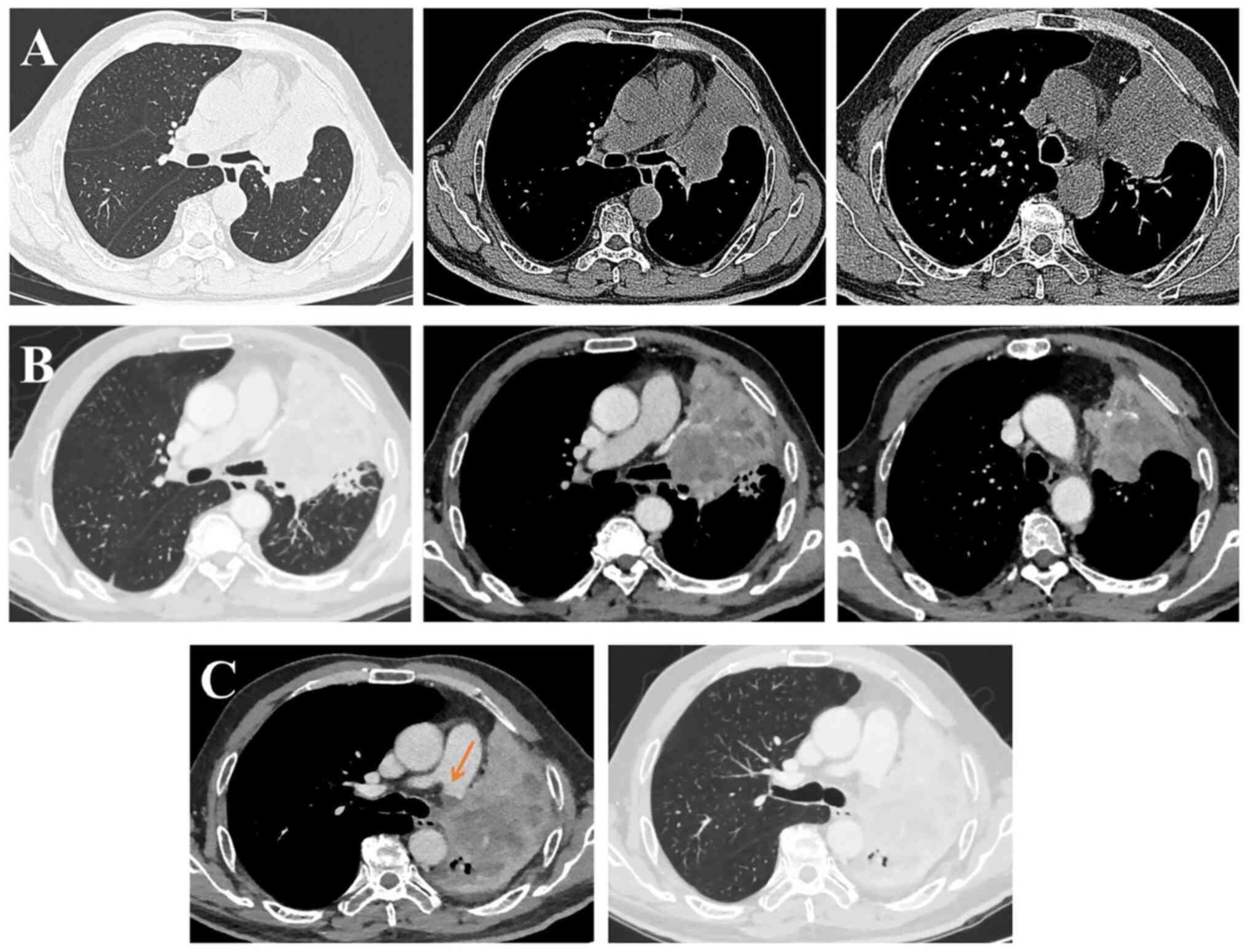
mediastinum (Fig. 1A). The clinical
stage was T4N0M0 (IIIA) according to The Eighth Edition Lung Cancer
Stage Classification (15).
The general condition of the patient was
satisfactory. The Eastern Cooperative Oncology Group Performance
Status (ECOG PS) score (16) was 1.
The thyroid hormone panel was in the normal range: Free
triiodothyronine (FT3), 4.55 pmol/l (normal range, 3.5–6.5 pmol/l);
free thyroxine (FT4), 17.16 pmol/l (normal range, 11.45–23.17
pmol/l); triiodothyronine, 136.05 ng/dl (normal range, 60–180
ng/dl); thyroxine (T4), 10.6 µg/dl (normal range, 3.2–12.6 µg/dl);
thyroid-stimulating hormone (TSH), 1.016 mIU/l (normal range,
0.55–4.78 mIU/l); and thyroglobulin antibodies (TGAb), 21.8 IU/ml
(normal range, <60 IU/ml).
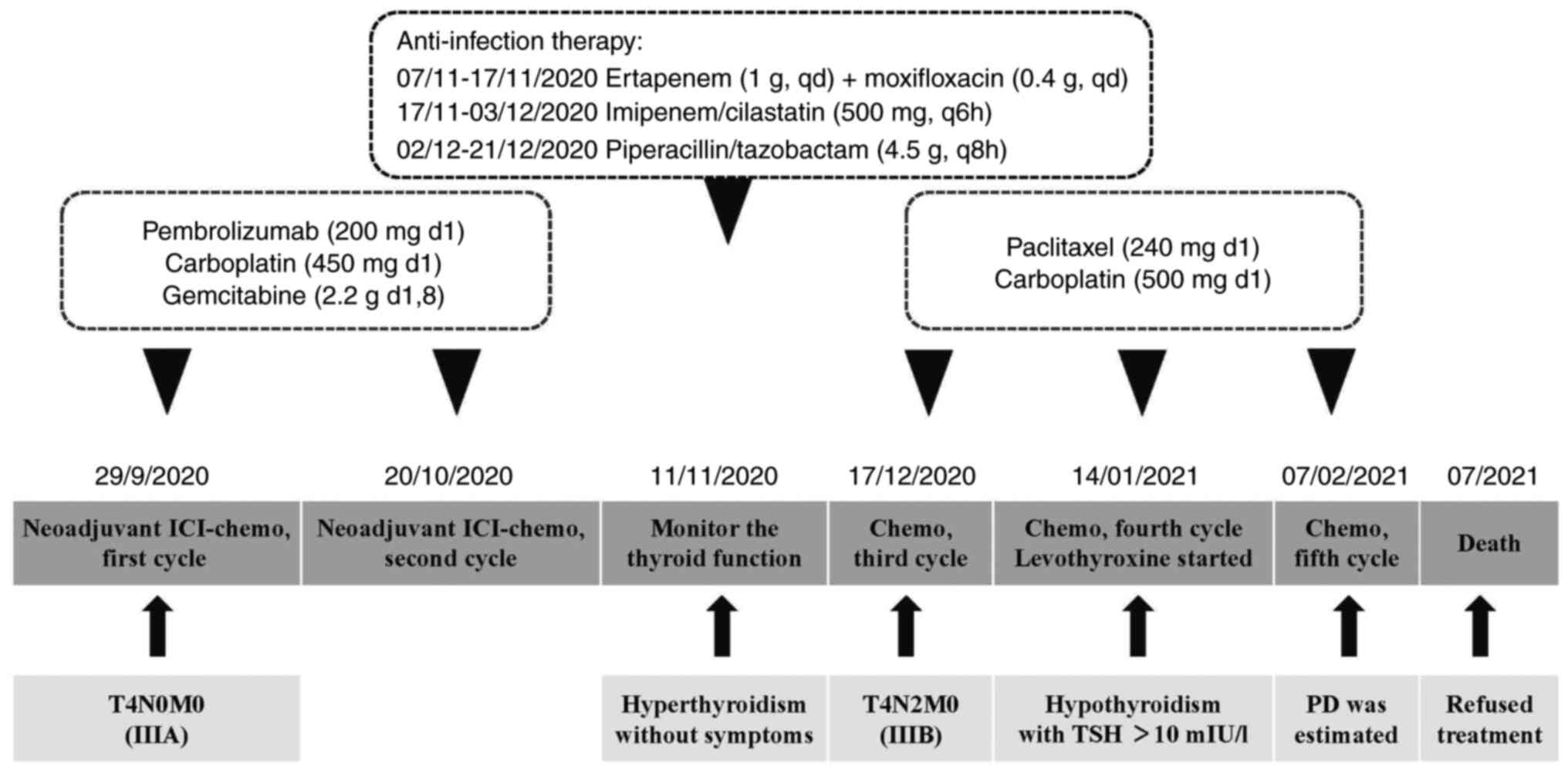
The patient received neoadjuvant therapy before
surgery. For this, two cycles of pembrolizumab (200 mg, day 1) plus
carboplatin (450 mg, day 1) and gemcitabine (2.2 g, days 1 and 8)
were administered from September 29, 2020 to October 20, 2020. On
November 5, 2020, the patient developed an infection of the
thoracic cavity. The maximum body temperature was 39.1°C, with
cough, greenish-yellow sputum and shortness of breath. Ertapenem (1
g, once a day, from November 7 to November 17, 2020), moxifloxacin
(0.4 g, once a day, from November 7 to November 17, 2020),
imipenem/cilastatin (500 mg, every 6 h, from November 17 to
December 3, 2020) and piperacillin/tazobactam (4.5 g, every 8 h,
from December 2 to December 21, 2020) were administered until the
patient's body temperature was normal.
During the hospital stay, the patient was found to
have thyroid dysfunction and tumor progression. Thyroid function
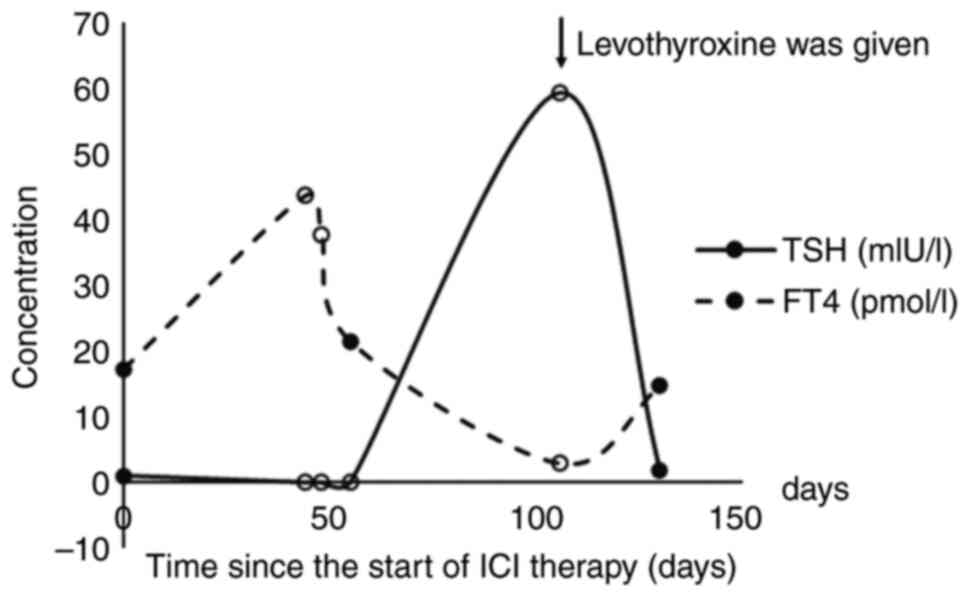
was found to be abnormal on November 11, 2020: FT4 increased to
43.54 pmol/l; FT3 increased to 8.33 pmol/l; T4 increased to 14
µg/dl; TSH decreased to 0.001 mIU/l; TGAb increased to 223.8 IU/ml.
Immune-related thyroid dysfunction was hypothesized to be the
possible cause of this abnormal thyroid function. No treatment was
given for this dysfunction, and thyroid function was regularly
monitored according to clinical guidelines.
An enhanced chest CT was performed on December 8,
2020. It showed tumor metastasis with enlarged ipsilateral
mediastinal lymph nodes (zone VI). The maximum tumor size was 12 cm
(Fig. 1B). The clinical stage was
diagnosed as T4N2M0 (IIIB) on December 14, 2020, according to the
patient's clinical condition and chest CT. Unfortunately, the
patient could not receive surgery due to tumor progression in the
left main bronchus and pulmonary artery, and the R0 resection was
challenging. The ECOG PS score increased to 2. However, the patient
refused immunotherapy due to concerns about irAEs.
The patient received a third cycle of chemotherapy
with liposomal paclitaxel (240 mg, day 1) and carboplatin (500 mg,
day 1) as a second-line treatment on December 17, 2020. On January
14, 2021, a fourth cycle of chemotherapy (240 mg liposomal
paclitaxel on day 1; 500 mg carboplatin on day 1) was administered.
Meanwhile, laboratory examination demonstrated that the patient had
developed hypothyroidism, with FT3 decreasing to 1.04 pmol/l, FT4
decreasing to 2.84 pmol/l and TSH increasing to 59.244 mIU/l.
Levothyroxine (12.5 mg daily, fasting, more than half an hour apart
from meals) was administered. The dose increased to 25 mg 3 days
later, 50 mg 1 week later and 75 mg 2 weeks later. On February 7,
2021, a fifth cycle of chemotherapy (240 mg liposomal paclitaxel on
day 1; 500 mg carboplatin on day 1) was administered. After
treatment with levothyroxine, FT3 returned to 3.19 pmol/l, FT4
returned to 14.69 pmol/l and TSH returned to 1.811 mIU/on February
7, 2021. The changes in thyroid function are shown in Fig. 2. The patient continued to take 75 mg
levothyroxine daily.
Enhanced chest CT (Fig.
1C) was performed on February 7, 2021, which demonstrated that
the cancer to the left lung had progressed with an invasion of the
left pulmonary artery. Second-line therapy treatment (240 mg
liposomal paclitaxel on day 1; 500 mg carboplatin on day 1)
demonstrated poor efficacy in the patient. Subsequently, treatment
was stopped and the patient died in July 2021. The timeline of the
patient's treatment course is shown in Fig. 3.
Discussion
Immunotherapy has changed the pattern of treatment
for NSCLC. Several trials exploring the efficacy and safety of
monotherapy ICI or in combination with chemotherapy in neoadjuvant
therapy of NSCLC have demonstrated promising results (5–7).
However, irAEs and poor response to immunotherapy have raised great
concerns (9,17). In the present study, a case of irAEs
and tumor progression after treatment with the PD-1 inhibitor,
pembrolizumab, in combination with chemotherapy was presented. To
the best of our knowledge, this is the first reported case of
thyroid dysfunction and tumor progression during neoadjuvant
immunotherapy for the treatment of NSCLC.
In trials reporting resectable NSCLC, hypothyroidism
was the most common ICI-related thyroid dysfunction (5,7,18–25).
The incidences ranged from 0.0–26.7% in anti-PD-1 therapy,
4.8–11.1% in dual-ICI treatment and 0.0–10.0% in ICI in combination
with chemotherapy (Table SI)
(5,7,18–25).
Furthermore, combination immunotherapy, baseline TSH, female sex
and preexisting thyroid disease were risk factors associated with
immunotherapy-related thyroid alterations (26–29).
The patient of the present study received immunotherapy in
combination with chemotherapy, which could be a personal risk
factor for thyroid dysfunction. The mechanism of ICI-related
thyroid dysfunction is not yet clear. The hypothesis that normal
organs, tissues and cells are damaged by ICIs may be a possible
mechanism (30,31).
Unfortunately, tumor progression occurred in the
patient of the present study without the opportunity for surgery.
In neoadjuvant immunotherapy trials, surgical failure rates ranged
from 0.0 to 16.7% in ICI monotherapy, from 0.0 to 45.8% in ICI in
combination with chemotherapy and from 19.0 to 33.3% in dual-ICI
treatment (Table SI) (5,7,18–24).
Disease progression, impaired lung function, unresectable disease,
adverse events, tumor location or refusal from the patient may be
reasons why patients cannot undergo resection (9). In the present study, the progression
of the disease contributed to the failure to undergo resection,
suggesting that a biomarker is of great need to predict the
efficacy of immunotherapy.
IrAEs may be associated with improved clinical
outcomes (32). Studies
investigating the association between thyroid dysfunction and
survival outcomes are shown in Table
I (13,14,33–41).
The development of thyroid dysfunction is associated with improved
outcomes and may serve as a predictive factor for the response to
therapy. This association may be due to the antigens shared between
melanoma cells and normal melanocytes (13,32,36).
 | Table I.Studies investigating the association
of thyroid dysfunction with survival rate outcomes. |
Table I.
Studies investigating the association
of thyroid dysfunction with survival rate outcomes.
| First author,
year | Cohort size, n | ICI | Cancer type | Outcome | (Refs.) |
|---|
| Basak et al,
2020 | 168 | Nivolumab or
pembrolizumab | Metastatic
melanoma; NSCLC; renal cell carcinoma | OS: HR, 0.18
(0.04–0.76), P=0.020; PFS: HR, 0.39 (0.15–0.998), P=0.050 | (33) |
| Luo et al,
2021 | 744 | Anti-PD-(L)1
monotherapy or anti-PD-(L)1 and CTLA-4 combination | NSCLC | PFS; HR, 0.68
(0.52–0.88), P=0.004 | (34) |
| Kim et al,
2017 | 58 | Nivolumab or
pembrolizumab | NSCLC | OS: HR, 0.11
(0.01–0.92), P=0.041; PFS: HR, 0.38 (0.17–0.85), P=0.018 | (35) |
| Osorio et
al, 2017 | 51 | Pembrolizumab | NSCLC | OS: HR, 0.29
(0.09–0.94), P=0.04 | (13) |
| Thuillier et
al, 2021 | 134 | Nivolumab | NSCLC | OS: HR, 0.32
(0.16–0.62), P<0.001; PFS: HR, 0.36 (0.21–0.62), P<0.001 | (36) |
| Zhou et al,
2021 | 191 | Nivolumab or
pembrolizumab | NSCLC | OS: HR, 0.356,
P<0.001; PFS: HR, 0.393, P<0.001 | (37) |
| D'Aiello et
al, 2021 | 205 | Pembrolizumab or
nivolumab or durvalumab or atezolizumab | Lung cancer | PFS: P=0.353 | (38) |
| Morimoto et
al, 2021 | 70 | Combination of
immunotherapy and chemotherapy | NSCLC | OS: HR, 0.53
(0.13–2.26), P=0.39; PFS: HR, 0.46 (0.17–1.29), P=0.14 | (41) |
| Koyama et
al, 2019 | 139 | Nivolumab or
pembrolizumab | NSCLC | OS: P=0.011; PFS:
P=0.012 | (40) |
| Grangeon et
al, 2019 | 270 | Anti-PD-(L)1
therapy | NSCLC | OS: HR, 0.46
(0.25–0.86), P=0.01; PFS: HR, 0.58 (0.39–0.85), P=0.005 | (39) |
| Percik et
al, 2021 | 208 | Anti-PD-(L)1
monotherapy or anti-PD-(L)1 and CTLA-4 combination | NSCLC | OS: HR, 0.87
(0.63–1.20), NA | (14) |
However, certain studies did not demonstrate a
significant trend towards improved survival rate in patients with
NSCLC and thyroid dysfunction (14,41).
In the present case report, the patient developed hypothyroidism
but had a poor prognosis. Furthermore, another study also did not
find significant differences in the mortality of patients with
thyrotoxicosis and those without thyroid dysfunction (42). By contrast, significant differences
have been observed in patients with overt or subclinical
hypothyroidism compared with those without thyroid irAE (42). The patient of the present study
developed thyrotoxicosis that developed to hypothyroidism, similar
to thyroiditis. The association between types of thyroid
dysfunction and prognosis in patients with cancer must therefore be
investigated in randomized and experimental studies.
The efficacy and safety of neoadjuvant immunotherapy
in resectable NSCLC should be monitored and assessed. Although
neoadjuvant immunotherapy has demonstrated potential pathological
benefits in patients with resectable NSCLC, irAEs should not be
ignored and tumor progression can still occur. The development of
thyroid dysfunction may not always predict a better response to ICI
therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by The Bethune Charitable Foundation of
Pharmaceutical Research Capacity Building Project (grant no.,
B-19-H-20200622).
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
XL, YiL, RW, LH, YF, XX and LS made substantial
contributions to conception and design. XL and XW participated in
the anti-tumor treatment, obtained medical images, advised on
patient treatment and analyzed patient data. XL, XW, SW and YaL
contributed to the acquisition and interpretation of data. XL and
XW were involved in drafting the manuscript. XL, XW, YiL and XX
revised the manuscript critically for important intellectual
content and confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
The Peking University People's Hospital (approval no.,
2021PHB018-001) and was conducted in accordance with the
Declaration of Helsinki.
Patient consent for publication
Informed consent for publication was obtained from
the patient and the patient's relative.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
ECOG PS
|
Eastern Cooperative Oncology Group
Performance Status
|
|
FT3
|
free triiodothyronine
|
|
FT4
|
free thyroxine
|
|
ICIs
|
immune checkpoint inhibitors
|
|
irAEs
|
immune-related adverse events
|
|
NSCLC
|
non-small cell lung cancer
|
|
PD-1
|
programmed death protein 1
|
|
PD-L1
|
programmed death protein 1 ligand
|
|
TGAb
|
thyroglobulin antibodies
|
|
TSH
|
thyroid-stimulating hormone
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-Year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 study. J Clin Oncol. 37:2518–2527. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8:5612017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forde PM, Chaft JE, Smith KN, Anagnostou
V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR,
Broderick S, et al: Neoadjuvant PD-1 blockade in resectable lung
cancer. N Engl J Med. 378:1976–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cascone T, William WN Jr, Weissferdt A,
Leung CH, Lin HY, Pataer A, Godoy MCB, Carter BW, Federico L,
Reuben A, et al: Neoadjuvant nivolumab or nivolumab plus ipilimumab
in operable non-small cell lung cancer: The phase 2 randomized
NEOSTAR trial. Nat Med. 27:504–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao S, Li N, Gao S, Xue Q, Ying J, Wang S,
Tao X, Zhao J, Mao Y, Wang B, et al: Neoadjuvant PD-1 inhibitor
(Sintilimab) in NSCLC. J Thorac Oncol. 15:816–826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang J, Zhang C and Zhong WZ: Neoadjuvant
immunotherapy for non-small cell lung cancer: State of the art.
Cancer Commun (Lond). 41:287–302. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ulas EB, Dickhoff C, Schneiders FL, Senan
S and Bahce I: Neoadjuvant immune checkpoint inhibitors in
resectable non-small-cell lung cancer: A systematic review. ESMO
Open. 6:1002442021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wright JJ, Powers AC and Johnson DB:
Endocrine toxicities of immune checkpoint inhibitors. Nat Rev
Endocrinol. 17:389–399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morganstein DL, Lai Z, Spain L, Diem S,
Levine D, Mace C, Gore M and Larkin J: Thyroid abnormalities
following the use of cytotoxic T-lymphocyte antigen-4 and
programmed death receptor protein-1 inhibitors in the treatment of
melanoma. Clin Endocrinol (Oxf). 86:614–620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walters AGB and Braatvedt G: Endocrine
adverse effects of immune checkpoint inhibitors. Intern Med J.
51:1016–1020. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Osorio JC, Ni A, Chaft JE, Pollina R,
Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok
JD, et al: Antibody-mediated thyroid dysfunction during T-cell
checkpoint blockade in patients with non-small-cell lung cancer.
Ann Oncol. 28:583–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Percik R, Liel Y, Urban D, Bar J, Ben-Ami
E and Abu Tailakh M: Thyroid dysfunction and survival in cancer
patients treated with immune checkpoint inhibitors: Analyses from a
large single tertiary cancer center database. Acta Oncol.
60:1466–1471. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soh J, Hamada A, Fujino T and Mitsudomi T:
Perioperative therapy for non-small cell lung cancer with immune
checkpoint inhibitors. Cancers (Basel). 13:40352021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eichhorn F, Klotz LV, Kriegsmann M,
Bischoff H, Schneider MA, Muley T, Kriegsmann K, Haberkorn U,
Heussel CP, Savai R, et al: Neoadjuvant anti-programmed death-1
immunotherapy by pembrolizumab in resectable non-small cell lung
cancer: First clinical experience. Lung Cancer. 153:150–157. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tong BC, Gu L, Wang X, Wigle DA, Phillips
JD, Harpole DH Jr, Klapper JA, Sporn T, Ready NE and D'Amico TA:
Perioperative outcomes of pulmonary resection after neoadjuvant
pembrolizumab in patients with non-small cell lung cancer. J Thorac
Cardiovasc Surg. 163:427–436. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen D, Wang J, Wu J, Chen S, Li J, Liu J,
Chen Q and Jiang Y: Neoadjuvant pembrolizumab with chemotherapy for
the treatment of stage IIB-IIIB resectable lung squamous cell
carcinoma. J Thorac Dis. 13:1760–1768. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Provencio M, Nadal E, Insa A,
García-Campelo MR, Casal-Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, et al:
Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell
lung cancer (NADIM): An open-label, multicentre, single-arm, phase
2 trial. Lancet Oncol. 21:1413–1422. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shu CA, Gainor JF, Awad MM, Chiuzan C,
Grigg CM, Pabani A, Garofano RF, Stoopler MB, Cheng SK, White A, et
al: Neoadjuvant atezolizumab and chemotherapy in patients with
resectable non-small-cell lung cancer: An open-label, multicentre,
single-arm, phase 2 trial. Lancet Oncol. 21:786–795. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tfayli A, Al Assaad M, Fakhri G, Akel R,
Atwi H, Ghanem H, El Karak F, Farhat F, Al Rabi K, Sfeir P, et al:
Neoadjuvant chemotherapy and Avelumab in early stage resectable
nonsmall cell lung cancer. Cancer Med. 9:8406–8411. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang CJ, McSherry F, Mayne NR, Wang X,
Berry MF, Tong B, Harpole DH Jr, D'Amico TA, Christensen JD, Ready
NE and Klapper JA: Surgical outcomes after neoadjuvant chemotherapy
and ipilimumab for non-small cell lung cancer. Ann Thorac Surg.
105:924–929. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reuss JE, Anagnostou V, Cottrell TR, Smith
KN, Verde F, Zahurak M, Lanis M, Murray JC, Chan HY, McCarthy C, et
al: Neoadjuvant nivolumab plus ipilimumab in resectable non-small
cell lung cancer. J Immunother Cancer. 8:e0012822020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Presotto EM, Rastrelli G, Desideri I,
Scotti V, Gunnella S, Pimpinelli N, Vaccher E, Bearz A, Di Costanzo
F, Bruggia M, et al: Endocrine toxicity in cancer patients treated
with nivolumab or pembrolizumab: Results of a large multicentre
study. J Endocrinol Invest. 43:337–345. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li M, Hou X, Chen J, Yu J, Chen M, Wang N,
Zhang B and Chen L: Comparing organ-specific immune-related adverse
events for immune checkpoint inhibitors: A Bayesian network
meta-analysis. Clin Transl Med. 11:e2912021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rubino R, Marini A, Roviello G, Presotto
EM, Desideri I, Ciardetti I, Brugia M, Pimpinelli N, Antonuzzo L,
Mini E, et al: Endocrine-related adverse events in a large series
of cancer patients treated with anti-PD1 therapy. Endocrine.
74:172–179. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muir CA, Clifton-Bligh RJ, Long GV,
Scolyer RA, Lo SN, Carlino MS, Tsang VHM and Menzies AM: Thyroid
immune-related adverse events following immune checkpoint inhibitor
treatment. J Clin Endocrinol Metab. 106:e3704–e3713. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhan L, Feng HF, Liu HQ, Guo LT, Chen C,
Yao XL and Sun SR: Immune checkpoint inhibitors-related thyroid
dysfunction: Epidemiology, clinical presentation, possible
pathogenesis, and management. Front Endocrinol (Lausanne).
12:6498632021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Delivanis DA, Gustafson MP, Bornschlegl S,
Merten MM, Kottschade L, Withers S, Dietz AB and Ryder M:
Pembrolizumab-Induced thyroiditis: Comprehensive clinical review
and insights into underlying involved mechanisms. J Clin Endocrinol
Metab. 102:2770–2780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haratani K, Hayashi H, Chiba Y, Kudo K,
Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M and
Nakagawa K: Association of immune-related adverse events with
nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol.
4:374–378. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Basak EA, van der Meer JWM, Hurkmans DP,
Schreurs MWJ, Oomen-de Hoop E, van der Veldt AAM, Bins S, Joosse A,
Koolen SLW, Debets R, et al: Overt thyroid dysfunction and
anti-thyroid antibodies predict response to anti-PD-1 immunotherapy
in cancer patients. Thyroid. 30:966–973. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo J, Martucci VL, Quandt Z, Groha S,
Murray MH, Lovly CM, Rizvi H, Egger JV, Plodkowski AJ, Abu-Akeel M,
et al: Immunotherapy-Mediated thyroid dysfunction: Genetic risk and
impact on outcomes with PD-1 blockade in non-small cell lung
cancer. Clin Cancer Res. 27:5131–5140. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HI, Kim M, Lee SH, Park SY, Kim YN,
Kim H, Jeon MJ, Kim TY, Kim SW, Kim WB, et al: Development of
thyroid dysfunction is associated with clinical response to PD-1
blockade treatment in patients with advanced non-small cell lung
cancer. Oncoimmunology. 7:e13756422017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thuillier P, Joly C, Alavi Z, Crouzeix G,
Descourt R, Quere G, Kerlan V and Roudaut N: Thyroid dysfunction
induced by immune checkpoint inhibitors is associated with a better
progression-free survival and overall survival in non-small cell
lung cancer: An original cohort study. Cancer Immunol Immunother.
70:2023–2033. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou Y, Xia R, Xiao H, Pu D, Long Y, Ding
Z, Liu J and Ma X: Thyroid function abnormality induced by PD-1
inhibitors have a positive impact on survival in patients with
non-small cell lung cancer. Int Immunopharmacol. 91:1072962021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
D'Aiello A, Lin J, Gucalp R, Tabatabaie V,
Cheng H, Bloomgarden NA, Tomer Y and Halmos B: Thyroid dysfunction
in lung cancer patients treated with immune checkpoint inhibitors
(ICIs): Outcomes in a multiethnic urban cohort. Cancers (Basel).
13:14642021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grangeon M, Tomasini P, Chaleat S, Jeanson
A, Souquet-Bressand M, Khobta N, Bermudez J, Trigui Y, Greillier L,
Blanchon M, et al: Association between immune-related adverse
events and efficacy of immune checkpoint inhibitors in
non-small-cell lung cancer. Clin Lung Cancer. 20:201–207. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koyama J, Horiike A, Yoshizawa T, Dotsu Y,
Ariyasu R, Saiki M, Sonoda T, Uchibori K, Nishikawa S, Kitazono S,
et al: Correlation between thyroid transcription factor-1
expression, immune-related thyroid dysfunction, and efficacy of
anti-programmed cell death protein-1 treatment in non-small cell
lung cancer. J Thorac Dis. 11:1919–1928. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morimoto K, Yamada T, Takumi C, Ogura Y,
Takeda T, Onoi K, Chihara Y, Taniguchi R, Yamada T, Hiranuma O, et
al: Immune-Related adverse events are associated with clinical
benefit in patients with non-small-cell lung cancer treated with
immunotherapy plus chemotherapy: A retrospective study. Front
Oncol. 11:6301362021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baek HS, Jeong C, Shin K, Lee J, Suh H,
Lim DJ, Kang MI and Ha J: Association between the type of thyroid
dysfunction induced by immune checkpoint inhibitors and prognosis
in cancer patients. BMC Endocr Disord. 22:892022. View Article : Google Scholar : PubMed/NCBI
|