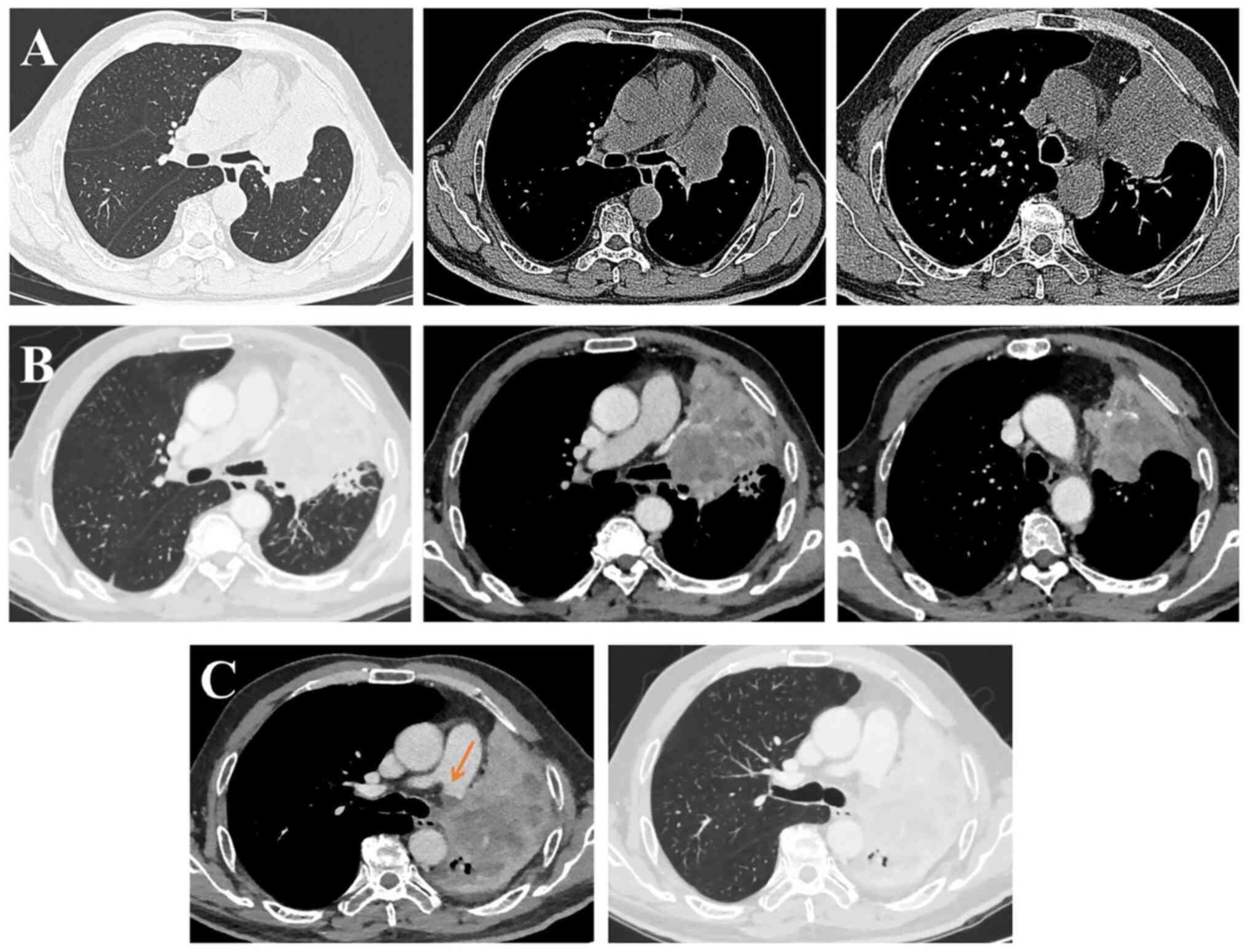
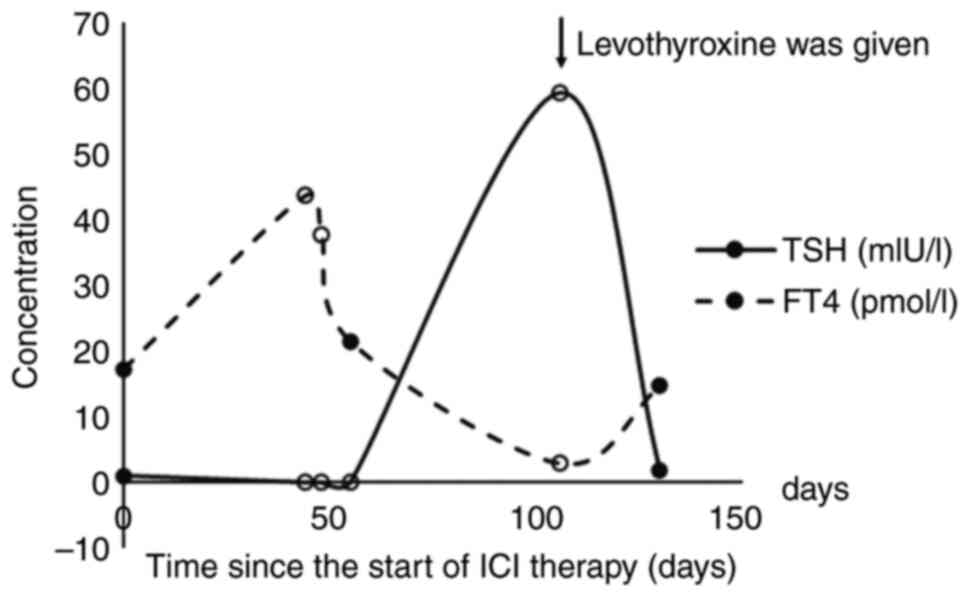
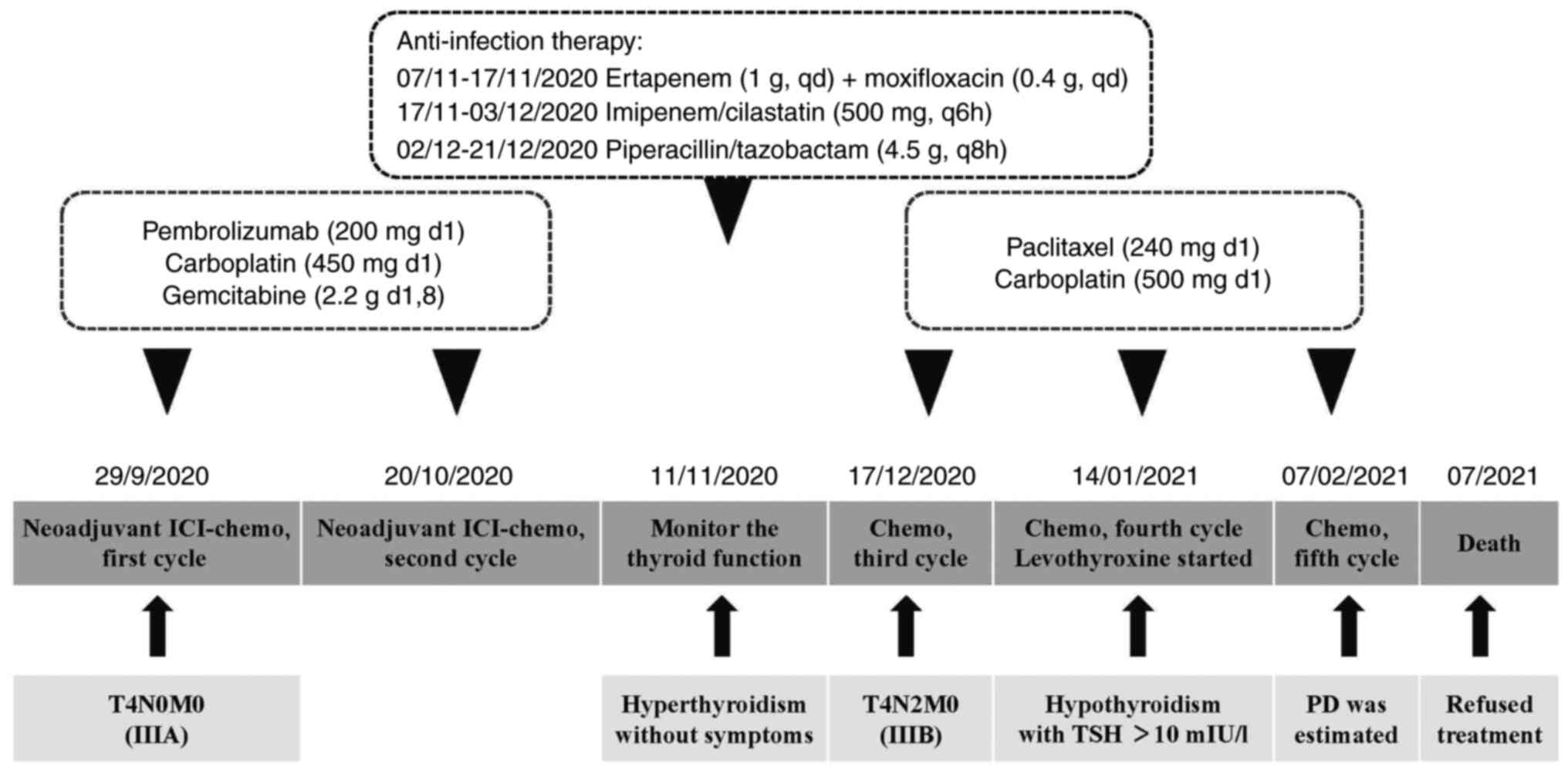
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-Year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 study. J Clin Oncol. 37:2518–2527. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8:5612017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forde PM, Chaft JE, Smith KN, Anagnostou
V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR,
Broderick S, et al: Neoadjuvant PD-1 blockade in resectable lung
cancer. N Engl J Med. 378:1976–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cascone T, William WN Jr, Weissferdt A,
Leung CH, Lin HY, Pataer A, Godoy MCB, Carter BW, Federico L,
Reuben A, et al: Neoadjuvant nivolumab or nivolumab plus ipilimumab
in operable non-small cell lung cancer: The phase 2 randomized
NEOSTAR trial. Nat Med. 27:504–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao S, Li N, Gao S, Xue Q, Ying J, Wang S,
Tao X, Zhao J, Mao Y, Wang B, et al: Neoadjuvant PD-1 inhibitor
(Sintilimab) in NSCLC. J Thorac Oncol. 15:816–826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang J, Zhang C and Zhong WZ: Neoadjuvant
immunotherapy for non-small cell lung cancer: State of the art.
Cancer Commun (Lond). 41:287–302. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ulas EB, Dickhoff C, Schneiders FL, Senan
S and Bahce I: Neoadjuvant immune checkpoint inhibitors in
resectable non-small-cell lung cancer: A systematic review. ESMO
Open. 6:1002442021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wright JJ, Powers AC and Johnson DB:
Endocrine toxicities of immune checkpoint inhibitors. Nat Rev
Endocrinol. 17:389–399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morganstein DL, Lai Z, Spain L, Diem S,
Levine D, Mace C, Gore M and Larkin J: Thyroid abnormalities
following the use of cytotoxic T-lymphocyte antigen-4 and
programmed death receptor protein-1 inhibitors in the treatment of
melanoma. Clin Endocrinol (Oxf). 86:614–620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walters AGB and Braatvedt G: Endocrine
adverse effects of immune checkpoint inhibitors. Intern Med J.
51:1016–1020. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Osorio JC, Ni A, Chaft JE, Pollina R,
Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok
JD, et al: Antibody-mediated thyroid dysfunction during T-cell
checkpoint blockade in patients with non-small-cell lung cancer.
Ann Oncol. 28:583–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Percik R, Liel Y, Urban D, Bar J, Ben-Ami
E and Abu Tailakh M: Thyroid dysfunction and survival in cancer
patients treated with immune checkpoint inhibitors: Analyses from a
large single tertiary cancer center database. Acta Oncol.
60:1466–1471. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soh J, Hamada A, Fujino T and Mitsudomi T:
Perioperative therapy for non-small cell lung cancer with immune
checkpoint inhibitors. Cancers (Basel). 13:40352021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eichhorn F, Klotz LV, Kriegsmann M,
Bischoff H, Schneider MA, Muley T, Kriegsmann K, Haberkorn U,
Heussel CP, Savai R, et al: Neoadjuvant anti-programmed death-1
immunotherapy by pembrolizumab in resectable non-small cell lung
cancer: First clinical experience. Lung Cancer. 153:150–157. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tong BC, Gu L, Wang X, Wigle DA, Phillips
JD, Harpole DH Jr, Klapper JA, Sporn T, Ready NE and D'Amico TA:
Perioperative outcomes of pulmonary resection after neoadjuvant
pembrolizumab in patients with non-small cell lung cancer. J Thorac
Cardiovasc Surg. 163:427–436. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen D, Wang J, Wu J, Chen S, Li J, Liu J,
Chen Q and Jiang Y: Neoadjuvant pembrolizumab with chemotherapy for
the treatment of stage IIB-IIIB resectable lung squamous cell
carcinoma. J Thorac Dis. 13:1760–1768. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Provencio M, Nadal E, Insa A,
García-Campelo MR, Casal-Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, et al:
Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell
lung cancer (NADIM): An open-label, multicentre, single-arm, phase
2 trial. Lancet Oncol. 21:1413–1422. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shu CA, Gainor JF, Awad MM, Chiuzan C,
Grigg CM, Pabani A, Garofano RF, Stoopler MB, Cheng SK, White A, et
al: Neoadjuvant atezolizumab and chemotherapy in patients with
resectable non-small-cell lung cancer: An open-label, multicentre,
single-arm, phase 2 trial. Lancet Oncol. 21:786–795. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tfayli A, Al Assaad M, Fakhri G, Akel R,
Atwi H, Ghanem H, El Karak F, Farhat F, Al Rabi K, Sfeir P, et al:
Neoadjuvant chemotherapy and Avelumab in early stage resectable
nonsmall cell lung cancer. Cancer Med. 9:8406–8411. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang CJ, McSherry F, Mayne NR, Wang X,
Berry MF, Tong B, Harpole DH Jr, D'Amico TA, Christensen JD, Ready
NE and Klapper JA: Surgical outcomes after neoadjuvant chemotherapy
and ipilimumab for non-small cell lung cancer. Ann Thorac Surg.
105:924–929. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reuss JE, Anagnostou V, Cottrell TR, Smith
KN, Verde F, Zahurak M, Lanis M, Murray JC, Chan HY, McCarthy C, et
al: Neoadjuvant nivolumab plus ipilimumab in resectable non-small
cell lung cancer. J Immunother Cancer. 8:e0012822020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Presotto EM, Rastrelli G, Desideri I,
Scotti V, Gunnella S, Pimpinelli N, Vaccher E, Bearz A, Di Costanzo
F, Bruggia M, et al: Endocrine toxicity in cancer patients treated
with nivolumab or pembrolizumab: Results of a large multicentre
study. J Endocrinol Invest. 43:337–345. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li M, Hou X, Chen J, Yu J, Chen M, Wang N,
Zhang B and Chen L: Comparing organ-specific immune-related adverse
events for immune checkpoint inhibitors: A Bayesian network
meta-analysis. Clin Transl Med. 11:e2912021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rubino R, Marini A, Roviello G, Presotto
EM, Desideri I, Ciardetti I, Brugia M, Pimpinelli N, Antonuzzo L,
Mini E, et al: Endocrine-related adverse events in a large series
of cancer patients treated with anti-PD1 therapy. Endocrine.
74:172–179. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muir CA, Clifton-Bligh RJ, Long GV,
Scolyer RA, Lo SN, Carlino MS, Tsang VHM and Menzies AM: Thyroid
immune-related adverse events following immune checkpoint inhibitor
treatment. J Clin Endocrinol Metab. 106:e3704–e3713. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhan L, Feng HF, Liu HQ, Guo LT, Chen C,
Yao XL and Sun SR: Immune checkpoint inhibitors-related thyroid
dysfunction: Epidemiology, clinical presentation, possible
pathogenesis, and management. Front Endocrinol (Lausanne).
12:6498632021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Delivanis DA, Gustafson MP, Bornschlegl S,
Merten MM, Kottschade L, Withers S, Dietz AB and Ryder M:
Pembrolizumab-Induced thyroiditis: Comprehensive clinical review
and insights into underlying involved mechanisms. J Clin Endocrinol
Metab. 102:2770–2780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haratani K, Hayashi H, Chiba Y, Kudo K,
Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M and
Nakagawa K: Association of immune-related adverse events with
nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol.
4:374–378. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Basak EA, van der Meer JWM, Hurkmans DP,
Schreurs MWJ, Oomen-de Hoop E, van der Veldt AAM, Bins S, Joosse A,
Koolen SLW, Debets R, et al: Overt thyroid dysfunction and
anti-thyroid antibodies predict response to anti-PD-1 immunotherapy
in cancer patients. Thyroid. 30:966–973. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo J, Martucci VL, Quandt Z, Groha S,
Murray MH, Lovly CM, Rizvi H, Egger JV, Plodkowski AJ, Abu-Akeel M,
et al: Immunotherapy-Mediated thyroid dysfunction: Genetic risk and
impact on outcomes with PD-1 blockade in non-small cell lung
cancer. Clin Cancer Res. 27:5131–5140. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HI, Kim M, Lee SH, Park SY, Kim YN,
Kim H, Jeon MJ, Kim TY, Kim SW, Kim WB, et al: Development of
thyroid dysfunction is associated with clinical response to PD-1
blockade treatment in patients with advanced non-small cell lung
cancer. Oncoimmunology. 7:e13756422017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thuillier P, Joly C, Alavi Z, Crouzeix G,
Descourt R, Quere G, Kerlan V and Roudaut N: Thyroid dysfunction
induced by immune checkpoint inhibitors is associated with a better
progression-free survival and overall survival in non-small cell
lung cancer: An original cohort study. Cancer Immunol Immunother.
70:2023–2033. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou Y, Xia R, Xiao H, Pu D, Long Y, Ding
Z, Liu J and Ma X: Thyroid function abnormality induced by PD-1
inhibitors have a positive impact on survival in patients with
non-small cell lung cancer. Int Immunopharmacol. 91:1072962021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
D'Aiello A, Lin J, Gucalp R, Tabatabaie V,
Cheng H, Bloomgarden NA, Tomer Y and Halmos B: Thyroid dysfunction
in lung cancer patients treated with immune checkpoint inhibitors
(ICIs): Outcomes in a multiethnic urban cohort. Cancers (Basel).
13:14642021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grangeon M, Tomasini P, Chaleat S, Jeanson
A, Souquet-Bressand M, Khobta N, Bermudez J, Trigui Y, Greillier L,
Blanchon M, et al: Association between immune-related adverse
events and efficacy of immune checkpoint inhibitors in
non-small-cell lung cancer. Clin Lung Cancer. 20:201–207. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koyama J, Horiike A, Yoshizawa T, Dotsu Y,
Ariyasu R, Saiki M, Sonoda T, Uchibori K, Nishikawa S, Kitazono S,
et al: Correlation between thyroid transcription factor-1
expression, immune-related thyroid dysfunction, and efficacy of
anti-programmed cell death protein-1 treatment in non-small cell
lung cancer. J Thorac Dis. 11:1919–1928. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morimoto K, Yamada T, Takumi C, Ogura Y,
Takeda T, Onoi K, Chihara Y, Taniguchi R, Yamada T, Hiranuma O, et
al: Immune-Related adverse events are associated with clinical
benefit in patients with non-small-cell lung cancer treated with
immunotherapy plus chemotherapy: A retrospective study. Front
Oncol. 11:6301362021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baek HS, Jeong C, Shin K, Lee J, Suh H,
Lim DJ, Kang MI and Ha J: Association between the type of thyroid
dysfunction induced by immune checkpoint inhibitors and prognosis
in cancer patients. BMC Endocr Disord. 22:892022. View Article : Google Scholar : PubMed/NCBI
|