Introduction
A gastrointestinal stromal tumor (GIST) is a spindle
cell tumor of the gastrointestinal tract derived from mesenchymal
tissue and accounts for ~0.2% of gastrointestinal tumors worldwide
(1). In China, the incidence rate
of GIST is 1–2/100,000 individuals (2,3).
Clinical data have shown that GIST can occur in various parts of
the digestive tract; however, the stomach is the most common
location, accounting for 60–70% of cases, followed by the small
intestine accounting for 20–30% of cases, and the colon and rectum
accounting for 18.1% of cases. The disease may also present in the
esophagus, mesentery, momentum and retroperitoneum (4,5). Small
GISTs are rare with the incidence of small GISTs coexisting with
pancreatic cancer even rarer and in recent years, to the best of
our knowledge, only one case of pancreatic body cancer being
mistaken for GIST has been reported in Chinese and English
literature (6). In the present
study, a 54-year-old male patient with a primary small pelvic GIST
coexisting with pancreatic cancer was reported on for the first
time, to the best of our knowledge. The present case report
provides interesting clinical insights that may assist in future
diagnosis and treatment of similar cases.
Case report
A 54-year-old male patient was admitted to the Yiwu
Central Hospital (Yiwu, China) for diagnosis and treatment in June
2018, due to ‘right ureteral calculi found in physical examination
for 2 days’. A total of 2 days before admission, a B-ultrasound
examination of the patient's urinary system in Yiwu Second People's
Hospital (Yiwu, China) showed ‘right upper ureteral calculi and
pelvic space occupation’. The patient reported frequent and urgent
urination, pain during urination and occasional discomfort in the
lower abdomen. Physical examination upon admission revealed a body
temperature of 36.9°C, a pulse of 100 bpm, respiration of 19
breaths/min, blood pressure of 104/74 mmHg (1 mmHg=0.133 kPa) and
oxygen saturation of 99%. No notable abnormalities were found in
the cardiopulmonary examination. Urinary CT results indicated
‘right upper ureteral calculi, dilation of the upper ureter and
renal pelvis, low-density lesions of the left kidney and masses in
the adnexal right lower abdomen’. An auxiliary examination was
performed and routine blood testing revealed a white blood cell
count of 12.01×109/l, CRP of 161.10 mg/l and a
neutrophil count of 8.90×109/l. Coagulation parameter
assessment revealed fibrinogen levels of 6.767 g/l and D-dimer
levels of 2,330 mg/l, and the blood type of the patient was
Rh-positive B. Urine analysis revealed sedimentary white blood
cells at 102/ml and urinary sediment epithelial cells at 13/ml.
Biochemical analysis indicated g-glutamyl transferase levels of 353
U/l and alkaline phosphatase levels of 349 U/l. A color Doppler
ultrasound of the digestive system showed multiple calculi in the
upper right ureter with right hydronephrosis and a left renal cyst
with prostatic hyperplasia with calcification. Chest
posterior-anterior CT showed no notable substantial lesions. The
preliminary diagnoses were right ureteral calculi with
hydronephrosis and a pelvic mass. As the nature of the pelvic space
occupation was unknown and the routine blood test indicated an
inflammatory reaction, broad-spectrum antibacterial drugs for
anti-infection treatment were temporarily administered.
Subsequently, an enhanced CT of the urinary system showed a pelvic
space-occupying lesion, indicating the potential presence of a
stromal tumor; due to these data, a puncture biopsy was recommended
and space-occupying pancreatic cancer was considered (Fig. 1).
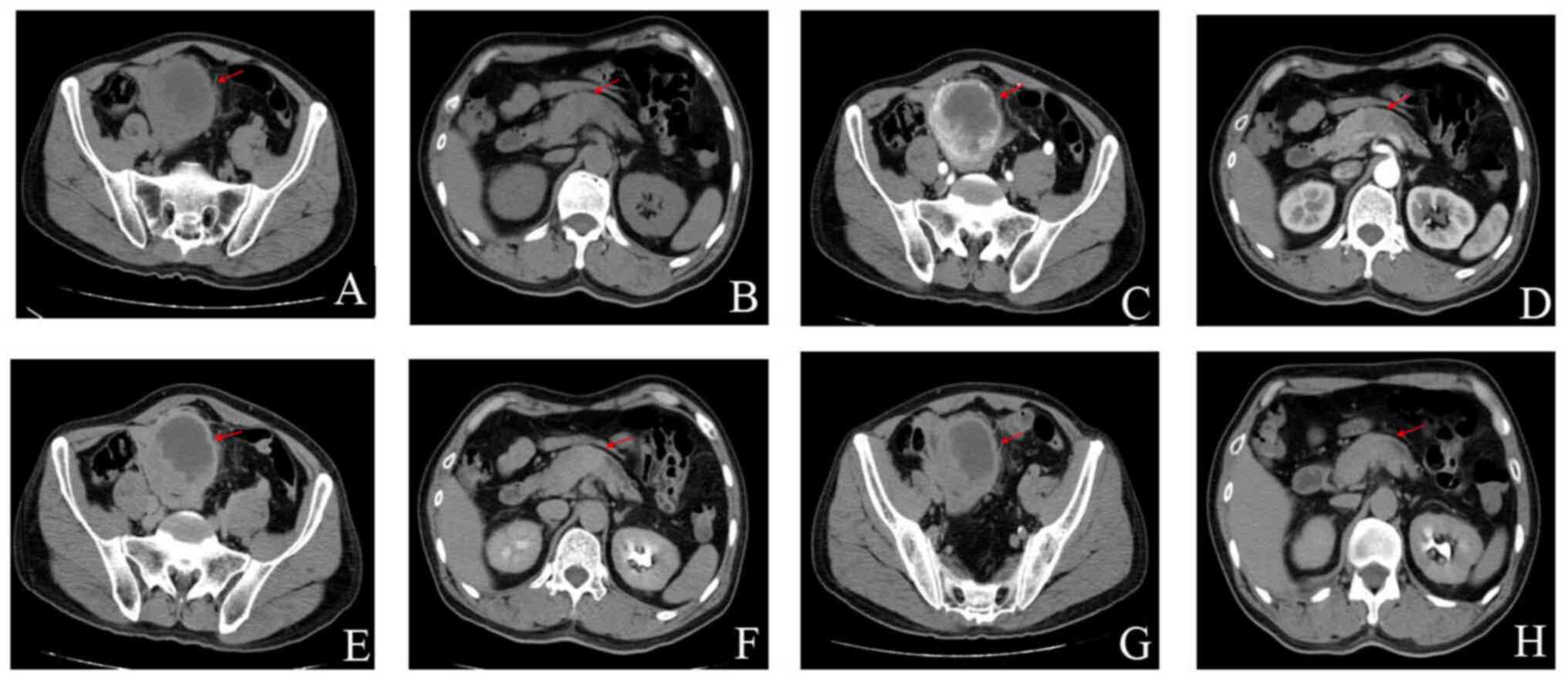 | Figure 1.Enhanced CT scan of the urinary
system. (A) Plain CT scan, Pelvic stromal tumor, (B) Plain CT scan,
pancreatic cancer, (C) CT enhanced scan of cortical phase, pelvic
stromal tumor, (D) CT enhanced scan of cortical phase, pancreatic
cancer, (E) CT enhanced scan of medullary phase, pelvic stromal
tumor, (F) CT enhanced scan of medullary phase, pancreatic cancer,
(G) CT enhanced scan of the excretion period, pelvic stromal tumor
and (H) CT enhanced scan of the excretion period, pancreatic
cancer. The red arrows indicate the location of the lesion. CT,
computerized tomography. |
Additionally, the enhanced CT of the urinary system
revealed that the distal pancreatic duct was dilated, with body and
tail atrophy observed. Calculi were identified in the ventral
segment of the right ureter with dilatation of the upper ureter and
right renal pelvis, indicating a possible bilateral renal cyst or
prostatic hyperplasia. In accordance with the opinion of experts in
hepatobiliary surgery, a right indwelling ureteral stent was
implanted after anti-infection treatment during urological surgery,
and the pancreatic and pelvic space-occupying lesion was treated
after infection control by hepatobiliary surgery. Implantation of
the right indwelling ureteral stent was successfully performed
under local anesthesia with antibiotics and fluid infusion
administered as the postoperative treatment for the stent
implantation. From these data, a postoperative diagnosis of right
ureteral calculus with hydronephrosis, pelvic mass and pancreatic
space-occupying lesion was made.
Following this, the patient was transferred to the
hepatobiliary surgery department of the same hospital for further
evaluation; subsequently, a comprehensive examination using a
hepatobiliary 1.5T magnetic resonance imaging (MRI) protocol was
conducted. This imaging protocol included plain scans,
diffusion-weighted imaging and enhanced scans. The MRI findings
revealed the presence of a space-occupying lesion in the pancreatic
body, indicative of pancreatic cancer. Additionally, atrophy was
observed in the distal body and tail glands of the pancreas,
accompanied by pancreatic duct dilation. Furthermore, the
examination revealed multiple cysts in the liver with bilateral
renal cysts observed, bilateral renal cysts have no pathological
concern (Fig. 2). Diagnoses of a
pancreatic space-occupying lesion in the pelvic space, a hepatic
cyst, a renal cyst and a right ureteral calculus with
hydronephrosis were made. A definitive diagnosis was ascertained
for the patient following a multidisciplinary expert consultation
and the evaluation indicated the presence of a space-occupying
lesion in the pancreas, raising concern for possible pancreatic
cancer. The presence of a pancreatic tumor could not be
conclusively ruled out at this stage and additionally, a
space-occupying lesion in the pelvic region was identified,
warranting further investigation. Moreover, the expert consultation
considered the possibility of a small GIST being present. Surgery
was deemed the most appropriate primary treatment for pancreatic
cancer with the aim to remove the tumor, and keep the biliary and
pancreatic ducts unobstructed. General anesthesia was used in this
case as in accordance with more extensive operations in similar
cases.
The primary surgical method was laparoscopic distal
pancreatectomy and small intestinal tumor resection. The specific
surgical method was to be determined by the intraoperative
pathological results during the operation. The patient underwent
laparoscopic distal pancreatectomy, splenectomy, portal vein
repair, porta hepatis, parapancreatic and paravascular lymph node
dissection, and resection of the GIST, sigmoid colon and partial
bladder under general anesthesia. Following successful anesthesia,
the indwelling gastric tube and catheter were inserted, the ‘Y’
position was taken and the trocar laparoscopic guide hole was
placed 10 mm below the umbilicus. Additionally, a 10-mm trocar was
inserted into the midline of the left and right clavicle above the
umbilicus, and a 5-mm trocar was inserted into the anterior
axillary line on both sides. During the operation, the pelvic GIST
occupied ~15×10 cm. It was stiff and fixed to the anterior
abdominal wall of the pelvic cavity and invaded the sigmoid
bladder. Additionally, there was a palpable mass ~4 cm in diameter
in the neck of the pancreas, which was invaded the splenic vein and
surrounding tissue, and the tail of the pancreas was stiff. Distal
pancreatectomy and splenectomy combined with porta hepatis,
parapancreatic and mesenteric paravascular lymph node dissection
were performed.
During the operation, multiple gastrointestinal
surgery experts were consulted. In agreement with the consensus
recommendation, the small intestine and the sigmoid colon were cut
5 cm away from the mass and closed, and 15 cm of the small
intestine and 10 cm of the long intestine were removed. The long
sigmoid colon was removed and the mass was resected after removing
part of the bladder wall. Continuous suture was performed to repair
the bladder. The small intestine and sigmoid colon were sutured end
to end and the mesentery was repaired. The colon anastomosis was
~35 cm from the anus and the small intestine anastomosis was 85 cm
away from the ileocecal loop. After the operation, meticulous
hemostasis and abdominal cavity irrigation were performed, a
drainage tube was inserted and the trocar laparoscopic hole was
closed layer by layer. The operation lasted 97 min and the
intraoperative bleeding volume was 72 ml. The operation was smooth,
the anesthesia was satisfactory and the patient returned to the
ward safely. After the operation, the patient was treated with
antibiotics, stomach nourishment and fluid supplement. A plain
abdominal CT scan showed postoperative tumor occupation changes in
the pancreas and peripancreatic inflammatory exudation 8 days
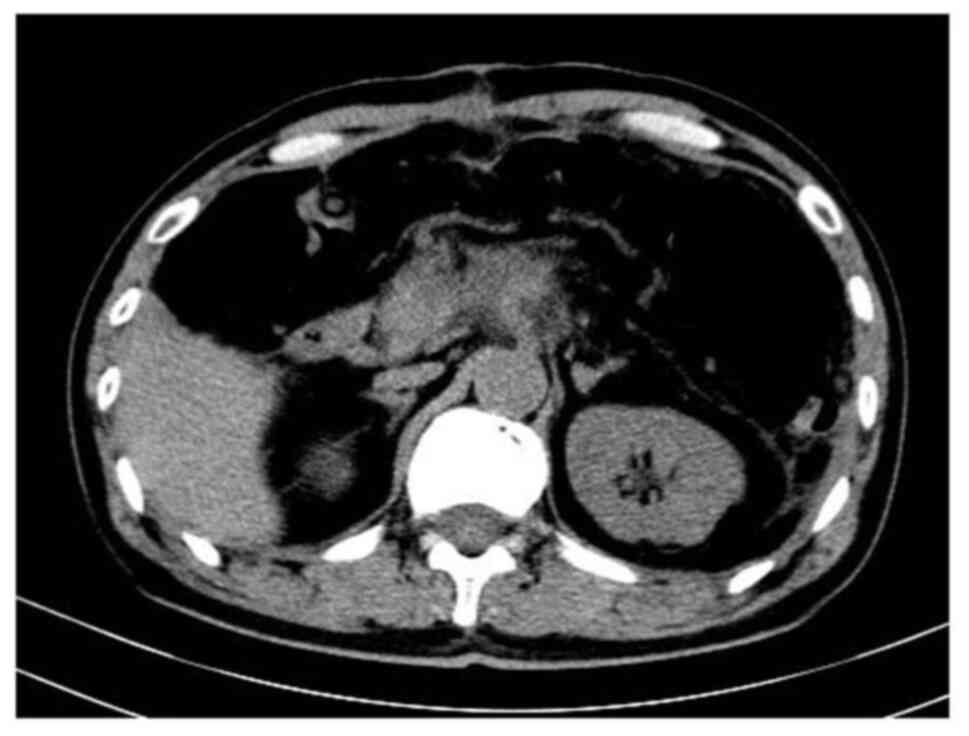
post-surgery (Fig. 3).
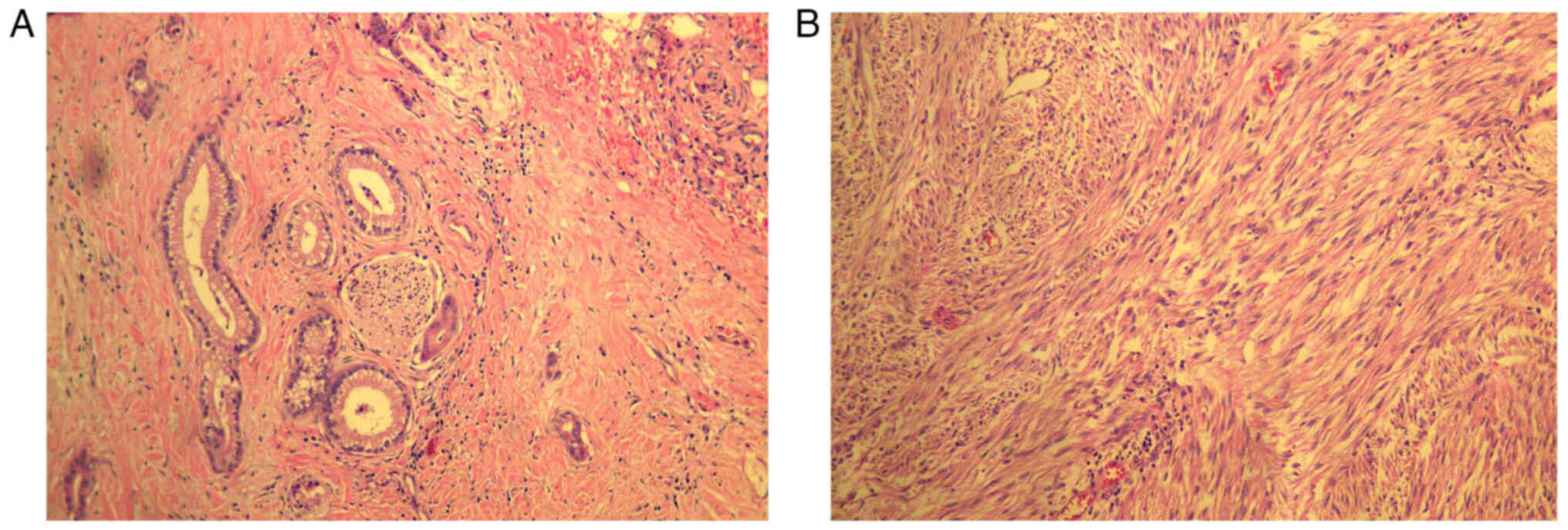
Postoperative hematoxylin and eosin (H&E)
staining pathology findings showed: i) Moderately differentiated
adenocarcinoma (pancreas) ~4.5×4×2 cm in size, invaded
peripancreatic fibrous adipose tissue and invaded nerves (Fig. 4A); ii) no lymph node metastasis was
observed with 0/8 lymph nodes (near the splenic portal) and 0/4
lymph nodes affected; iii) a stromal (small intestine) tumor ~8×7×5
cm in size (Fig. 4B), with
necrosis, mitosis <5/50 high power field, moderate risk, invaded
the myometrium of the sigmoid colon and mesentery of the small
intestine and sigmoid. A small amount of bladder muscle wall
adhered to and fused with the fibers and adipose tissue around the
tumor; iv) negative margins of the pancreas, small intestine and
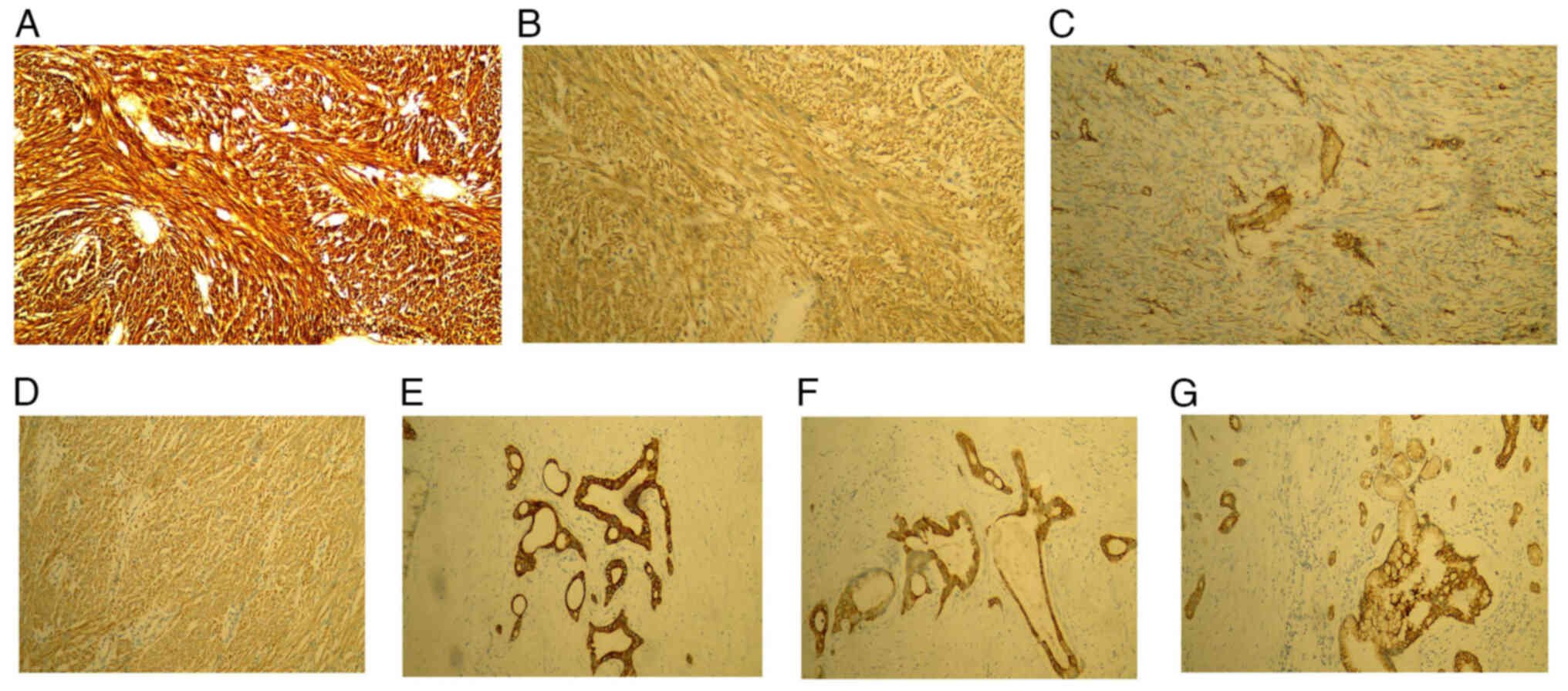
sigmoid colon; and v) spleen tissue immunohistochemistry (Fig. 5). GIST1 and pancreatic cancer
specimens were fixed with 4% neutral buffered formalin (at 35°C for
24 h), dehydrated and cleared with 70% ethanol for 3 h, 30% ethanol
for 3 h, 90% ethanol for 2 h, 95% ethanol for 2.5 h, 100% ethanol I
for 1.5 h and 100% ethanol II for 1.5 h before being embedded in
paraffin and sectioned at 5 µm. Immunohistochemistry was performed
using EnVision (OriGene Technologies, Inc.) two-step method and DAB
staining. Each specimen was subjected to immunohistochemical
detection of antibodies against CD117 (cat. no. ZA-0523), Dog-1
(cat. no. ZM-0371), CD34 (cat. no. ZM-0046), Ki-67 (cat. no.
ZM-0166), S-100 (cat. no. ZM-0224), Vim (cat. no. TA801297), SMA
(cat. no. ZM-0003), Desmin (cat. no. ZM-0610), CK7 (cat. no.
ZM-0071), CK19 (cat. no. ZA-0670), CK20 (cat. no. ZA-0574), P53
(cat. no. ZM-0408), TTF-1 (cat. no. ZM-0270), PSA (cat. no.
ZM-0218), and CAM5.2 (cat. no. ZM-0316). All antibody reagents were
purchased from OriGene Technologies, Inc. with a dilution of 1:200.
Goat anti IgG was the secondary antibody (1:2,000; Abcam; cat. no.
K006153P, K000328P). Section E (E is the serial number code in
pathology): Discovered on GIST1 (DOG-1; +), CD117 (+), CD34 (+),
Ki-67 (10%+), S-100 (−), vimentin (+), SMA (+) and desmin (−);
section R (R is another serial number code in pathology): CK7 (+),
CK19 (+), CK20 (+), Ki-67 (5%+), P53 (−), thyroid transcription
factor-1 (−), prostate specific antigen (−), S-100 (+) and CAM5.2
(+). The patient had a cough, low percutaneous arterial oxygen
saturation and a pulmonary infection in the first 2 days after
surgery. On day 4 after surgery, the patient passed gas via the
anus. the patient did not show signs of distension, nausea,
vomiting or fever after eating. The patient was discharged from the
hospital 22 days post-surgery.
Discussion
To the best of our knowledge, the present case
report describes the first case of small GIST coexisting with
pancreatic cancer. GIST has no specific clinical symptoms with
symptoms generally related to the location and size of the tumor,
the relationship between the tumor and the intestinal wall, and the
benign or malignant nature of the tumor (7). Notably, the location and size of the
tumor are the main factors that determine the change in symptoms
(8). The most common symptoms are
gastrointestinal bleeding, upper abdominal discomfort and dysphagia
(9). In the present study, the
patient had repeated right upper abdominal pain for 2 months, right
lower back pain with hematuria for 20 days and was admitted to the
hospital for examination after right ureteral calculi were found 2
days following physical examination. The diagnosis of GIST is
generally difficult, and is mainly based on tumor location,
histology and immunohistochemistry examination (10). In the present study, the case was
complex with ureteral calculi, pancreatic cancer, pelvic small
intestinal stromal tumor and hepatorenal cysts reported. During
diagnosis and treatment, experts in imaging, urology, hepatobiliary
surgery and gastrointestinal surgery made cooperative,
comprehensive evaluations, which provided strong evidence for
obtaining a more accurate diagnosis before the operation.
Immunohistochemical CD117 (+) and DOG-1 (+) protein expression are
the main criteria for the diagnosis of GIST (11). CD117 is highly consistent with
DOG-1, with the positive rate of GIST diagnosis by CD117 being
94–98% and the positive diagnosis rate of DOG-1 being 94–96%
(12). Other positive antigens
indicative of GIST include: CD34 (positive rate, 70%), SMA
(positive rate, 30%), S-100 (positive rate, 5%) and desmin
(positive rate, 2%) (13,14). The immunohistochemical results of
the case in the present study were DOG-1 (+), CD117 (+), CD34 (+),
S-100 (−), SMA (+) and desmin (−). These clinical data were
consistent with the immunohistochemical diagnostic criteria of GIST
(10).
GIST possesses the potential for malignant
transformation and its biological behavior is assessed through
tumor pathological classification. The 2017 edition of the Expert
Consensus on the Diagnosis and Treatment of Gastrointestinal
Stromal Tumor in China (15)
divides the risk of GIST into four levels: Extremely low, low,
medium and high. The risk of a tumor is directly related to its
size with tumors <5 cm observed to constitute a low or
moderate-low risk, and tumors >10 cm constituting a high risk
(16). In the present study, the
size of the small GIST tumor was 8×7×5 cm and was considered a
moderate risk. During the operation, it was found that cancer cells
had invaded the muscle layer of the sigmoid colon, and the
mesentery of the small intestine and sigmoid colon. Additionally, a
small portion of the bladder muscle wall had adhered and was fused
with the fibers and adipose tissue around the tumor. Previous
clinical experience has shown that surgical resection is the
preferred treatment for small GISTs and pancreatic cancer (17,18).
However, due to multiple complications, the case in the present
study was treated with laparoscopic distal pancreatectomy,
splenectomy, portal vein repair, porta hepatis, parapancreatic and
paravascular lymph node dissection, and resection of the small
GIST, sigmoid colon and partial bladder under general
anesthesia.
The diagnosis and treatment of the present case
offers a distinct advantage as it involved cooperation among
multidisciplinary experts and the successful removal of multiple
lesions through laparoscopic surgery. However, it is essential to
acknowledge certain limitations, such as the rarity of the
condition, resulting in limited diagnosis and treatment experience.
Furthermore, the intricacies associated with diagnosis and
treatment of this condition were compounded, making it challenging
to arrive at a definitive diagnosis until after the surgical
procedure. The results of the present study show that laparoscopic
surgery for patients with small GISTs has a good clinical effect,
which has clinical reference value.
In summary, GISTs are rare in clinical practice and
even rarer when coinciding with pancreatic cancer. Collaborative
diagnosis and treatment by multidisciplinary experts was present
throughout the whole treatment process of the present case. It is
hypothesized that with continuous improvement of the understanding
of this disease, the ongoing in-depth study of the pathogenesis, as
well as the development of more clinical studies, a more scientific
basis can be provided, and ultimately reduce the recurrence rate,
prolong the overall survival time and improve the overall efficacy
of patients with GIST and pancreatic cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC carried out the study methodology, investigation,
data curation and wrote the original draft. YT carried out the
study investigation, and wrote and edited the review.AG conceived
the idea for the study, supervised the study, and wrote and edited
the review. XC, YT and AG confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of any data and/or accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blay JY, Kang YK, Nishida T and von Mehren
M: Gastrointestinal stromal tumours. Nat Rev Dis Primers. 7:222021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mazur MT and Clark HB: Gastric stromal
tumors. Reappraisal of histogenesis. Am J Surg Pathol. 7:507–519.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sikai W: A national study on the incidence
rate of gastrointestinal stromal tumors in urban population of
China. Chin J Med. 102:4672022.
|
|
4
|
Miettinen M, Sarlomo-Rikala M and Lasota
J: Gastrointestinal stromal tumors: Recent advances in
understanding of their biology. Hum Pathol. 30:1213–1220. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miettinen M, Majidi M and Lasota J:
Pathology and diagnostic criteria of gastrointestinal stromal
tumors (GISTs): A review. Eur J Cancer. 38 (Suppl 5):S39–S51. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bejiga G: Pancreatic body cancer
presenting with dysphagia and palpable abdominal mass being
mistaken for gastric gastrointestinal stromal tumor: ‘Case report’.
Int J Surg Case Rep. 92:1068352022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhijun L, Mi Z and Shangming Y: Clinical
analysis of extraabdominal and pelvic metastasis of
gastrointestinal stromal tumors. Shenzhen J Integrated Traditional
Chin Western Med. 27:143–144. 2017.
|
|
8
|
Wang YP, Li YI and Song C:
Clinicopathological features and prognosis of small
gastrointestinal stromal tumors outside the stomach. Oncol Lett.
10:2723–2730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan X, Han H, Sun Z, Zhang L, Chen G, Mzee
SAS, Yang H and Chen J: Prognostic value of bleeding in
gastrointestinal stromal tumors: A meta-analysis. Technol Cancer
Res Treat. 20:153303382110342592021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirota S: Differential diagnosis of
gastrointestinal stromal tumor by histopathology and
immunohistochemistry. Transl Gastroenterol Hepatol. 3:272018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu CE, Tzen CY, Wang SY and Yeh CN:
Clinical diagnosis of gastrointestinal stromal tumor (GIST): From
the molecular genetic point of view. Cancers (Basel). 11:6792019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Junjie Y, Yehong Y, Jinhua G, et al: A
case of giant stromal tumor of the duodenum with cystic
transformation. Guangdong Med J. 38:26162017.
|
|
13
|
Arpaci T, Tokat F, Arpaci RB, Akbas T,
Ugurluer G and Yavuz S: Primary pericardial extragastrointestinal
stromal tumor: A case report and literature review. Oncol Lett.
9:2726–2728. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu D, Duan Y, Chen Y, Li B, Du Y and Shi
S: A case report of gastrointestinal stromal tumor of the duodenum.
Am J Transl Res. 15:8279–8285. 2022.PubMed/NCBI
|
|
15
|
Gastrointestinal stromal tumor expert
committee of the Chinese Society of Clinical Oncology Chinese
Consensus on the Diagnosis and Treatment of Gastrointestinal
Stromal Tumors (2017 Edition). Electronic J Comprehensive Tumor
Ther. 4:31–43. 2018.
|
|
16
|
Shi P, Zhang J, Li Y and Ma L: Comparative
study of ultrasound findings and pathological risk classification
of gastrointestinal stromal tumors. Modern Oncol Med. 2795–2797.
2015.(In Chinese).
|
|
17
|
Madhavan A, Phillips AW, Donohoe CL,
Willows RJ, Immanuel A, Verril M and Griffin SM: Surgical
management of gastric gastrointestinal stromal tumours: Comparison
of outcomes for local and radical resection. Gastroenterol Res
Pract. 2018:21402532018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masiak-Segit W, Rawicz-Pruszyński K,
Skórzewska M and Polkowski WP: Surgical treatment of pancreatic
cancer. Pol Przegl Chir. 90:45–53. 2018. View Article : Google Scholar : PubMed/NCBI
|