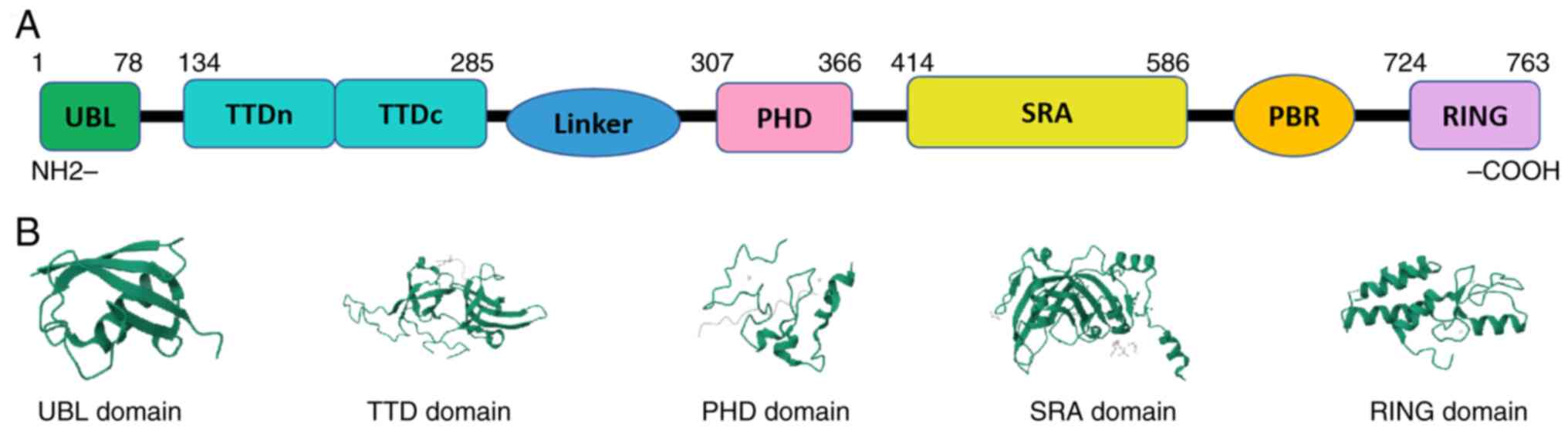
During tumorigenesis, abnormalities in key genes
and/or epigenetic events always occur in cell metabolism, survival
and proliferation. Epigenetic modification refers to genetic
changes in gene expression without altering the structure or
sequence of DNA. This includes DNA methylation, histone
modification, genomic imprinting, X chromosome inactivation and
microRNA regulation (1). Among
these, DNA methylation and histone modification are the most
significant (2). DNA methylation is
the process of transferring the methyl group to the C5 position of
cytosine to form 5-methylcytosine (5mC). This process typically
inhibits gene expression (3). DNA
methylation includes two forms: de novo methylation and
maintenance methylation or hemimethylation. Complete DNA
methylation involves three aspects: Recognition, establishment and
removal, and requires the cooperation of three molecules (writer,
eraser and reader). A number of studies have revealed that
ubiquitin like with PHD and ring finger domains 1 (UHRF1) is
involved in both de novo methylation and maintenance
methylation, which is mainly used as a reader to identify the DNA
to be methylated and then recruits writers to complete DNA
methylation (4), thus inhibiting
transcription.
The methylation effect of UHRF1 varies in different
types of cancer. DNA methylation of cancer cells (such as non-small
cell lung cancer, malignant pleural mesothelioma, endometrial
cancer and liver cancer) can be induced by UHRF1 (5–9). By
contrast, DNA methylation can also be inhibited by UHRF1 in
esophageal squamous cell carcinoma and glioma (10,11).
Additionally, UHRF1 has little effect on DNA methylation in
retinoblastoma (12). Moreover, a
number of studies have reported that UHRF1 is involved in the
proliferation of Treg cells (13)
and adult vascular smooth muscle cells by methylating promoters of
cyclin-dependent kinase suppressor genes (including P21 and P27)
(14). It has been revealed that
the overexpression of UHRF1 is related to the hypermethylation of
tumor suppressor genes (TSGs) in numerous cancers (15–21).
In addition to affecting TSGs, UHRF1 can also affect cancer
progression in immune, proliferative, apoptosis (including
ferroptosis) (22), and other
aspects through epigenetic modifications to proteins. A recent
study indicated that UHRF1 can affect the immune ability of
interferon against tumors by affecting the methylation of cyclic
GMP AMP synapse (23). Currently,
testing all-natural anticancer compounds involves the
downregulation of UHRF1 and the upregulation of TSG expression
(24–28). However, numerous regulatory
functions of UHRF1 involved in regulation have not been thoroughly
studied. Therefore, understanding the molecular mechanism of the
epigenetic modification of UHRF1 in tumors will help identify
targets for the inhibition of the expression and function of UHRF1,
which could thus play an anticancer role.
Generally, the clinical significance of UHRF1 has
two aspects. First, it can improve the prognosis and survival rate
as a therapeutic target for radiotherapy and chemotherapy (29). Second, it can serve as an effective
biomarker for diagnosis, prognosis and survival rate. The present
review aimed to comprehensively summarize the role of UHRF1 in DNA
methylation by clarifying the functions of its domains to provide
convenience for further exploration and clinical treatment of
cancers in the future.
DNA methylation mainly occurs on the 5′-carbon in
the cytosine of cytosine-phosphate-guanine (CpG) dinucleotide
(33), which varies greatly in
different human tissues (34). The
maintenance of methylation can be divided into two stages. The
first stage is the rapid replication-coupling stage, which occurs
within a few min after DNA double-strand bifurcation. This stage is
responsible for maintaining more than 80% of hemimethylation. The
other is the relatively slow replication-uncoupling stage that
occurs later (35), with UHRF1 and
DNMT1 involved. This stage mainly consists of five steps and two
conformations (Fig. 2).
At first, UHRF1 is in a closed conformation: SRA
combines with PHD. The diversity region (PBR) between SRA and RING
binds to TTD competitively with the linker (but mainly PBR), thus
preventing TTD from binding to H3k9me3. However, this state does
not hinder the interaction along UBL, RING, and ubiquitin-binding
enzyme (E2)-ubiquitin molecules (36).
UHRF1 undergoes a conformational change from a
closed state to an open conformation in the following three
scenarios: i) HAUSP binds to PBR of UHRF1 and hemi-methylated DNA
(hmDNA; where only one of the two complementary strands is
methylated); ii) SRA binds to hmDNA and iii) phosphorylation of
S651 by phosphatidyl-5-phosphate (PI5P) leads to the release of the
PBR's binding to TTD, allowing TTD to bind histone with the PHD
domain and linker (37). Since the
concentration of PI5P is different in the G1 and S phases, the
localization of UHRF1 in chromatin in the cell cycle can be
determined by analyzing the concentration of PI5P (38).
In the open state, TTD-PHD binds to H3K9me2/3, UBL
and histone H3 ubiquitinated by RING, and then UHRF1 recruits DNMT1
(39) in a cell cycle-dependent
manner (40–42) and relieves the autoinhibitory
activity of DNMT1 (37). In
addition, UBL and SRA also recruit DNMT1, releasing its catalytic
domain. Moreover, H3K27me3 can affect DNA methylation by inhibiting
UHRF1-mediated H3 ubiquitination in this process (43).
The binding of the replication foci targeting (RFT)
domain of DNMT1 with two monoubiquitinated histones H3 disrupts the
interaction between RFT and its C-terminal catalytic domain,
resulting in the conformational change of DNMT1, allowing hmDNA to
enter the catalytic center and undergo DNA methylation. It is also
considered that DNMT1 is unlikely to dissociate and bind repeatedly
from UHRF1 or the UHRF1 complex during methylation modification of
multiple methylation sites of hmDNA. Otherwise, the time for UHRF1
to recruit DNMT1 will be too long. Therefore, Bronner et al
(33) proposed a new possibility
that a macromolecular complex with DNMT1 formed after recruitment.
The complex slides along the newly synthesized DNA for DNA
replication and modification, controlled by the semi-methylation
state of DNA. If hmDNA is not encountered, the SRA domain may not
interact with the RFT sequencing (RFTS) domain of DNMT1, as DNMT1
has no enzymatic activity and abnormal DNA methylation will not
occur. At the same time, the two combination methods can be used as
a double check or double lock to ensure the fidelity of methylation
map transmission. However, there is no clear and recognized binding
mode, which needs to be confirmed through the interaction between
the two protein structures and in vitro experiments
(33).
HAUSP removes the ubiquitin labeling from histone H3
in the synthesized intact methylated DNA region after DNA
methylation modification. DNMT1 dissociates from ubiquitinated
histone H3 and repeats the process when it encounters new hmDNA
sites (44).
In the replication coupling stage of DNA
methylation, the arginine binding cavity of the TTD domain of UHRF1
can recognize the guanidine group in the Arg121 side chain of DNA
ligase 1 (LIG1), which helps the TTD domain to be recruited to the
replication site through the Lys126 di/trimethylation (45–47) on
LIG1. Subsequently, UHRF1 monoubiquitinates Lys15 and Lys24 of the
PCNA-associated factor 15 (PAF15) (48,49).
PAF15 with double monoubiquitin recruits DNMT1 into the new
replication chain (50).
Afterwards, PAF15ub2 may be deubiquitinated by HAUSP in two cases:
i) One case is the dissociation of UHRF1 from chromatin after the
semi methylated DNA is converted to fully methylated DNA. The
second case is the binding of DNMT1 to semi methylated DNA, which
induces conformational changes in USP7 or PAF15ub2, although the
mechanism is not yet fully clear (51). The two modes of methylation
complement each other and work together to maintain the stable
inheritance of epigenetic information.
TTD Domain includes two subdomains: i) TTDn and ii)
TTDc. The aromatic structures constructed by F152, Y188 and Y191 in
TTDn residues recognize dimethylated and trimethylated lysine
residues (H3K9me2/me3). During DNA methylation, TTD and PHD domains
read histone code information and transmit it to the SRA domain,
allowing the SRA domain to flip methylated cytosine out of the DNA
double-strand to locate the CpG site that needs to be methylated.
The combination of H3K9me2/me3 and LIG1 lysine 126 dimethylation
(LIG1K126me2) with UHRF1 may not play a vital role in maintaining
DNA methylation (52). However, a
previous study demonstrated that the arginine binding cavity in the
TTD domain is crucial for the interaction of LIG1. A specific
inhibitor, 5-amino-2,4-dimethylpyridine, was developed to target
the Arg binding cavity. This inhibitor binds with the Arg binding
cavity and effectively inhibits the binding of LIG1 and UHRF1
(53).
In addition, there is a number of studies targeting
the tight binding between TTD and H3K9me2 or H3K9me3, screening and
optimizing the small molecule antagonist, NV01, that disrupts this
binding. This has significant reference value for studying drugs
that target the structural domain (54). In addition, a recent study indicated
that TTD prefers binding to histone H3 tails containing K4me1 in
the context of H3K9me2/3. Moreover, the H3K4me1-K9me2/3 specific
binding of UHRF1-TTD to enhancers and promoters of transcription
factor binding sites downregulates these genes (55).
As one of the most common families of chromatin
reader domains, the PHD finger domain has a shallow acidic groove
used to identify the N-terminal of the ligand. Recognition of the
K4 methylation state of the H3N terminal tail is a relatively
stable and common recognition pattern in the PHD family (56–58).
TTD/PHD tandem module can stimulate H3K9 methyltransferase (H3K9MT)
and methylate adjacent H3K9 of adjacent nucleosomes. During this
process, UHRF1-related inhibitory complexes (including DNMT1,
H3K9MT, PCNA and HDAC1) may play a synergistic role (59,60),
while the phosphorylation of H3 threonine 3, symmetric or
asymmetric demethylation of H3R2, and acetylation of H3A1N terminal
may disrupt this binding (61).
In addition, the combination of H3K9me3 with TTD-PHD
also leads to a conformational change of TTD-PHD. However, this
conformational change does not affect its ubiquitination activity
or binding affinity with semi-methylated DNA. The potential
function of this conformational change requires further studies
(62). Moreover, the combination of
PHD and H3 could affect the combination of TTD and H3. When the PHD
domain mutates (mainly D334) or the N-terminal of H3 is modified,
the PHD separates from the H3 tail, thereby disrupting or weakening
the binding of TTD and methylated H3K9. Conversely, TTD mutation or
histone modification (such as H3K4me3) does not affect the
interaction between PHD and the unmodified H3 N-terminal (62–65).
This indicates that PHD is the critical domain to identify H3,
which is also consistent with the aforementioned situation of TTD
(66).
Previous studies have shown that the C-terminal
region of Stella (also known as Dppa3/PGC7) competitively inhibits
the binding of UHRF1 to H3K9me3 by binding to the PHD domain of
UHRF1, thereby damaging the DNA methylation function of UHRF1. In
addition, Stella can also inhibit DNA methylation by antagonizing
UHRF1 activity and isolating UHRF1 from the nucleus. Disrupting
Stella's interaction with UHRF1 may be a new potential direction
for developing UHRF1-targeted drugs (56,67).
In the process of DNA methylation modification
involved in UHRF1, SRA binds to PHD, which inhibits PHD from
recognizing H3R2 and keeps UHRF1 in a closed state. SRA has a high
affinity and specificity for hmDNA and could be released from PHD
when hmDNA exists. SRA binds to unmethylated cytosine in hmDNA
through N489 in the NKR finger (483–496 residues, named after the
abbreviation of asparagine, lysine and arginine) (41,68,69)
and converts UHRF1 into the open state. Previously, some
experiments pointed out that NKR combines with cytosine to
stimulate DNA deformation and turn out cytosine instead of SRA
directly (68).
P300/CBP-related factor, which is located in the NKR
of the SRA domain, and HDAC1 can acetylate and deacetylate UHRF1 at
K490, respectively. Acetylated UHRF1 hinders the binding of UHRF1
to hmDNA and the methylation modification of DNA, while the effect
of deacetylated UHRF1 is the opposite. This indicated that abnormal
DNA methylation can be eliminated by inducing the acetylation of
UHRF1 in some types of cancer, thus becoming a treatment option in
cancer therapy (70).
Based on the UM63 structure, some studies have
identified new inhibitors of UHRF1-SRA, such as AMSA2 and MPB7,
using multidisciplinary methods. Similar to UM63, they can inhibit
SRA-mediated base flipping at low concentrations but do not
intercalate into DNA. These inhibitors prevent the involvement of
UHRF1 and DNMT1 in DNA methylation. In addition, because they
prioritize affecting cells with high levels of UHRF1, they will
reduce damage to normal cells (77). Another inhibitor targeting the SRA
domain, UF146, has been shown to effectively eradicate
leukemia-initiating cells, confirming the potential of UHRF1
inhibitors in cancer treatment (78). However, due to UF146 being a
pan-assay interference compound (79), its specificity is not high, and it
may have unpredictable reactions with numerous biological targets,
resulting in false positive results (80). Through molecular docking, molecular
dynamics simulation and toxicity analysis, other inhibitors
targeting the SRA domain can also be screened, such as chicoric
acid. However, the specific therapeutic effects and indications
require extensive experiments for further verification and
screening (75).
UBL domain is also known as the N-terminal of a
novel Np95/icbp90-like ring finger protein, which is the target of
single- and multi-ubiquitination (81). Some studies have proved that UHRF1
cannot play its role in DNA methylation modification without the
UBL domain (47,82,83).
This highlights the two crucial functions of UBL in DNA methylation
inheritance (47): i) Cooperating
with the RING domain to ubiquitinate histone H3, recruiting the
ubiquitin-binding enzyme E2 to form a stable E2/ubiquitin ligase E3
/chromatin complex. Subsequently, ubiquitin is transferred from E2
to histone H3 (this binding is universal, meaning that other
proteins containing the UBL domain can also occur); ii) UBL
recruits DNMT1 into chromatin through a hydrophobic patch and
enhances DNMT activity by binding to DNMT1-621 (amino acids:
621–1,616) (47,82,83).
RING Domain has ubiquitin ligase activity, including
ubiquitination of UHRF1 itself. UHRF1 protects itself from
ubiquitination by interacting with HAUSP (69). The natural compound thymoquinone
(TQ) eliminates the protective effect on UHRF1 by reducing the
expression of HAUSP, leading to the ubiquitination of UHRF1 under
the action of ubiquitin ligase, reactivating TSG, inhibiting cell
proliferation, promoting cell cycle arrest and inducing apoptosis
(84). TQ can selectively induce
the degradation of UHRF1 in cancer cells without affecting its
expression level in normal cells (85). In addition to TQ, a recent study has
observed that diosgenin (DSG) induces the dissociation of UHRF1 and
HAUSP protein complexes by directly binding to UHRF1, inhibiting
the protective effect of HAUSP to UHRF1, thereby reducing the
expression of UHRF1 and increasing the expression of TSGs. However,
the specific binding sites between DSG and UHRF1 are not clear, and
the effect of DSG needs to be achieved at high drug concentrations
(86). Therefore, further research
is needed.
As a ubiquitin ligase, RING ubiquitinates the target
genes of DNMT1, which are histone H3Lys23 and Lys18 (87,88).
The ubiquitin ligase activity of the RING domain is crucial for the
growth of tumor cells, and inhibitors targeting this activity may
be a method to produce anticancer drugs (16). In addition to tumor cells, a
previous study indicated that the RING domain can interact with the
human immunodeficiency virus type 1 (HIV-1) protein Tat, which is
involved in virus replication, thus promoting the ubiquitination
degradation of Tat, inhibiting HIV-1 transcription and maintaining
HIV-1 latency (89).
In the methylation modification involving UHRF1, the
linker replaces the H3 tail in the TTD peptide binding tank so that
the H3 tail connects PHD at the N-terminal and the TTD domain at
K9me3. The two arginine and one lysine of the linker residue
(R295-R296-K297) are essential to stabilize this TTD-PHD
conformation (64,65). Additionally, phosphorylation of S298
in linker can change the interaction between UHRF1 and H3,
potentially serving as a functional switch for UHRF1 and
participating in a variety of regulatory pathways, including DNA
methylation maintenance, transcriptional inhibition and cell cycle
progression (17). In vitro
studies have confirmed that PIM1, an essential regulatory factor of
aging, can regulate the function of UHRF1 through Ser311
phosphorylation. This regulation inhibits the binding of TTD-PHD to
H3K9me3, thereby affecting the activation of DNMT1 and triggering
DNA hypomethylation (90).
PBR Domain has five kinds of functions: i) It binds
to TTD in the closed state of UHRF1, inhibiting its interaction
with H3K9me3; ii) it promotes the recognition and binding of SRA
and hmDNA; iii) it enhances the interaction between RFTS of DNMT1
and SRA (36); iv) it interacts
with the UBL1 and UBL2 domains of HAUSP, keeping UHRF1 in the open
state and facilitating the binding of UHRF1 to H3K9me3 (65,91,92);
and v) it binds to PI5P, opening the closed conformation of UHRF1
and increasing the affinity of H3K9me3 for TTD (38). All domains of UHRF2 and UHRF1 have a
very high sequence similarity, except for pRb (93). This may be why UHRF2 cannot replace
UHRF1 to maintain DNA methylation (94).
Targeting the highly expressed oncoproteins or genes
is a novel approach to anticancer drugs research (66). As a multifunctional epigenetic
modifier, UHRF1 has significant differential expression between
tumor and normal tissues and is a potential cancer treatment
target. Overexpression of UHRF1 can silence TSGs, inhibit DNA
repair and apoptosis and promote tumor growth and migration.
Conversely, the deletion of UHRF1 leads to DNA demethylation and
histone acetylation, which promotes tumor cell apoptosis and
inhibits tumor proliferation and invasion through TSGs reactivation
and DNA repair (44).
Moreover, the expression of UHRF1 was an independent
factor affecting overall survival (OS) and progression-free
survival (PFS) (OS, P=0.038; PFS, P=0.014) (95). A previous study reported that
patients with elevated UHRF1 levels had lower OS and PFS (96). The high expression of UHRF1 may also
be related to tumor size, stage and metastasis (97). It has been reported that UHRF1 can
be used as a cancer diagnostic tool, stem cell marker and
therapeutic tool (98). With the
progress of research on the function and mechanism of UHRF1 in
cancer, the clinical research on UHRF1 will be more extensive and
profound.
There are three directions of targeted drugs for
UHRF1. One direction aims for the complex composed of UHRF1, such
as DNMT1, HDAC1 and HAUSP in ECReM. For example, combining UHRF1
and low-dose DNMT inhibitors can effectively reduce DNA methylation
and reactivate TSGs (99). Due to
the expression level of UHRF1 in all normal tissues being 5–70-fold
lower than that of HDAC1 and DNMT1, the side effects of UHRF1
inhibitors are moderate compared with current HDAC and DNMT
inhibitors. This can help reduce the tolerance of the patients to
DNMT inhibitors. In addition, a previous study revealed that loss
of UHRF1, combined with HDAC inhibition, can reactivate TSGs and
inhibit the proliferation of colorectal cancer cells (100). The second direction is targeted at
UHRF1 domains. Since UHRF1 has multiple functional domains, it is
necessary to clarify the function of each domain to determine which
of the several domains can achieve sufficient tumor inhibition
effect (66). At present, the
regulation mechanism of UHRF1 has been detected in a variety of
cancers (40), which plays an
enlightening role in clinical treatment, especially in personalized
therapies. The last method is to indirectly inhibit the expression
of UHRF1 by affecting the upstream molecules. For example, in
prostate cancer, especially in cases that are resistant to
abiraterone, UHRF1 and p-AKT are abnormally overexpressed. AKT
phosphorylation inhibitor MK2206 can induce the degradation of
UHRF1 protein, thereby increasing the expression of epigenetic
silenced TSGs (such as p21), and reducing the typical biomarkers of
neuroendocrine prostate cancer in prostate neuroendocrine
carcinoma, SYP and NCAM1, thereby improving the therapeutic effect
of abiraterone on tumors (101).
The present review aimed to provide ideas for drug research and
direction for the functional identification of other proteins with
similar structures by clarifying the function and regulatory
pathway of UHRF1 domains.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 81802761) and Natural Science
Foundation of Shandong Province (grant no. ZR2023MH258).
Not applicable.
YZ conceptualized the study and approved the final
version of the manuscript. YS made substantial contributions in
writing the original draft. HL responded to the questions raised by
the reviewers, made systematic revisions to the article, and gave
the final approval of the version to be published. QX, CG and MX
were mainly involved in the interpretation of the data through the
figures. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Probst AV, Dunleavy E and Almouzni G:
Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell
Biol. 10:192–206. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zafon C, Gil J, Pérez-González B and Jordà
M: DNA methylation in thyroid cancer. Endocr Relat Cancer.
26:R415–R439. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abhishek S, Nakarakanti NK, Deeksha W and
Rajakumara E: Mechanistic insights into recognition of symmetric
methylated cytosines in CpG and non-CpG DNA by UHRF1 SRA. Int J
Biol Macromol. 170:514–522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bronner C, Krifa M and Mousli M:
Increasing role of UHRF1 in the reading and inheritance of the
epigenetic code as well as in tumorogenesis. Biochem Pharmacol.
86:1643–1649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheng Y, Wang H, Liu D and Zhang C, Deng
Y, Yang F, Zhang T and Zhang C: Methylation of tumor suppressor
gene CDH13 and SHP1 promoters and their epigenetic regulation by
the UHRF1/PRMT5 complex in endometrial carcinoma. Gynecol Oncol.
140:145–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daskalos A, Oleksiewicz U, Filia A,
Nikolaidis G, Xinarianos G, Gosney JR, Malliri A, Field JK and
Liloglou T: UHRF1-mediated tumor suppressor gene inactivation in
nonsmall cell lung cancer. Cancer. 117:1027–1037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mudbhary R, Hoshida Y, Chernyavskaya Y,
Jacob V, Villanueva A, Fiel MI, Chen X, Kojima K, Thung S, Bronson
RT, et al: UHRF1 overexpression drives DNA hypomethylation and
hepatocellular carcinoma. Cancer Cell. 25:196–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhuo H, Tang J, Lin Z, Jiang R, Zhang X,
Ji J, Wang P and Sun B: The aberrant expression of MEG3 regulated
by UHRF1 predicts the prognosis of hepatocellular carcinoma. Mol
Carcinog. 55:209–219. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reardon ES, Shukla V, Xi S, Gara SK, Liu
Y, Straughan D, Zhang M, Hong JA, Payabyab EC, Kumari A, et al:
UHRF1 is a novel druggable epigenetic target in malignant pleural
mesothelioma. J Thorac Oncol. 16:89–103. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakamura K, Baba Y, Kosumi K, Harada K,
Shigaki H, Miyake K, Kiyozumi Y, Ohuchi M, Kurashige J, Ishimoto T,
et al: UHRF1 regulates global DNA hypomethylation and is associated
with poor prognosis in esophageal squamous cell carcinoma.
Oncotarget. 7:57821–57831. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hervouet E, Lalier L, Debien E, Cheray M,
Geairon A, Rogniaux H, Loussouarn D, Martin SA, Vallette FM and
Cartron PF: Disruption of Dnmt1/PCNA/UHRF1 interactions promotes
tumorigenesis from human and mice glial cells. PLoS One.
5:e113332010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kan G, He H, Zhao Q, Li X, Li M, Yang H
and Kim JK: Functional dissection of the role of UHRF1 in the
regulation of retinoblastoma methylome. Oncotarget. 8:39497–39511.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Obata Y, Furusawa Y, Endo TA, Sharif J,
Takahashi D, Atarashi K, Nakayama M, Onawa S, Fujimura Y, Takahashi
M, et al: The epigenetic regulator Uhrf1 facilitates the
proliferation and maturation of colonic regulatory T cells. Nat
Immunol. 15:571–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elia L, Kunderfranco P, Carullo P,
Vacchiano M, Farina FM, Hall IF, Mantero S, Panico C, Papait R,
Condorelli G and Quintavalle M: UHRF1 epigenetically orchestrates
smooth muscle cell plasticity in arterial disease. J Clin Invest.
128:2473–2486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Unoki M, Nishidate T and Nakamura Y:
ICBP90, an E2F-1 target, recruits HDAC1 and binds to methyl-CpG
through its SRA domain. Oncogene. 23:7601–7610. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jenkins Y, Markovtsov V, Lang W, Sharma P,
Pearsall D, Warner J, Franci C, Huang B, Huang J, Yam GC, et al:
Critical role of the ubiquitin ligase activity of UHRF1, a nuclear
RING finger protein, in tumor cell growth. Mol Biol Cell.
16:5621–569. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Unoki M, Brunet J and Mousli M: Drug
discovery targeting epigenetic codes: The great potential of UHRF1,
which links DNA methylation and histone modifications, as a drug
target in cancers and toxoplasmosis. Biochem Pharmacol.
78:1279–1288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mousli M, Hopfner R, Abbady AQ, Monté D,
Jeanblanc M, Oudet P, Louis B and Bronner C: ICBP90 belongs to a
new family of proteins with an expression that is deregulated in
cancer cells. Br J Cancer. 89:120–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Unoki M, Kelly JD, Neal DE, Ponder BA,
Nakamura Y and Hamamoto R: UHRF1 is a novel molecular marker for
diagnosis and the prognosis of bladder cancer. Br J Cancer.
101:98–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crnogorac-Jurcevic T, Gangeswaran R,
Bhakta V, Capurso G, Lattimore S, Akada M, Sunamura M, Prime W,
Campbell F, Brentnall TA, et al: Proteomic analysis of chronic
pancreatitis and pancreatic adenocarcinoma. Gastroenterology.
129:1454–1463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lorenzato M, Caudroy S, Bronner C, Evrard
G, Simon M, Durlach A, Birembaut P and Clavel C: Cell cycle and/or
proliferation markers: What is the best method to discriminate
cervical high-grade lesions? Hum Pathol. 36:1101–1107. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Cheng D, Wang Y, Xi S, Wang T, Sun
W, Li G, Ma D, Zhou S, Li Z and Ni C: UHRF1-mediated ferroptosis
promotes pulmonary fibrosis via epigenetic repression of GPX4 and
FSP1 genes. Cell Death Dis. 13:10702022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang L, Hao Y, Yu H, Gu X, Peng Q, Zhuo H,
Li Y, Liu Z, Wang J, Chen Y, et al: Methionine restriction promotes
cGAS activation and chromatin untethering through demethylation to
enhance antitumor immunity. Cancer Cell. 41:1118–1133. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Achour M, Mousli M, Alhosin M, Ibrahim A,
Peluso J, Muller CD, Schini-Kerth VB, Hamiche A, Dhe-Paganon S and
Bronner C: Epigallocatechin-3-gallate up-regulates tumor suppressor
gene expression via a reactive oxygen species-dependent
down-regulation of UHRF1. Biochem Biophys Res Commun. 430:208–212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alhosin M, Abusnina A, Achour M, Sharif T,
Muller C, Peluso J, Chataigneau T, Lugnier C, Schini-Kerth VB,
Bronner C and Fuhrmann G: Induction of apoptosis by thymoquinone in
lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent
pathway which targets the epigenetic integrator UHRF1. Biochem
Pharmacol. 79:1251–1260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alhosin M, Sharif T, Mousli M,
Etienne-Selloum N, Fuhrmann G, Schini-Kerth VB and Bronner C:
Down-regulation of UHRF1, associated with re-expression of tumor
suppressor genes, is a common feature of natural compounds
exhibiting anti-cancer properties. J Exp Clin Cancer Res.
30:412011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krifa M, Alhosin M, Muller CD, Gies JP,
Chekir-Ghedira L, Ghedira K, Mély Y, Bronner C and Mousli M:
Limoniastrum guyonianum aqueous gall extract induces apoptosis in
human cervical cancer cells involving p16 INK4A re-expression
related to UHRF1 and DNMT1 down-regulation. J Exp Clin Cancer Res.
32:302013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abusnina A, Alhosin M, Keravis T, Muller
CD, Fuhrmann G, Bronner C and Lugnier C: Down-regulation of cyclic
nucleotide phosphodiesterase PDE1A is the key event of p73 and
UHRF1 deregulation in thymoquinone-induced acute lymphoblastic
leukemia cell apoptosis. Cell Signal. 23:152–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang C, Wang Y, Zhang F, Sun G, Li C, Jing
S, Liu Q and Cheng Y: Inhibiting UHRF1 expression enhances
radiosensitivity in human esophageal squamous cell carcinoma. Mol
Biol Rep. 40:5225–5235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao L, Tan XF, Zhang S, Wu T, Zhang ZM, Ai
HW and Song J: An intramolecular interaction of uhrf1 reveals dual
control for its histone association. Structure. 26:304–311. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong X, Chen J, Xie W, Brown SM, Cai Y, Wu
K, Fan D, Nie Y, Yegnasubramanian S, Tiedemann RL, et al: Defining
UHRF1 domains that support maintenance of human colon cancer DNA
methylation and oncogenic properties. Cancer Cell. 35:633–648.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bronner C, Achour M, Arima Y, Chataigneau
T, Saya H and Schini-Kerth VB: The UHRF family: Oncogenes that are
drugable targets for cancer therapy in the near future? Pharmacol
Therap. 115:419–434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bronner C, Alhosin M, Hamiche A and Mousli
M: Coordinated dialogue between UHRF1 and DNMT1 to ensure faithful
inheritance of methylated DNA patterns. Genes (Basel). 10:652019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schultz MD, He Y, Whitaker JW, Hariharan
M, Mukamel EA, Leung D, Rajagopal N, Nery JR, Urich MA, Chen H, et
al: Human body epigenome maps reveal noncanonical DNA methylation
variation. Nature. 523:212–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ming X, Zhang Z, Zou Z, Lv C, Dong Q, He
Q, Yi Y, Li Y, Wang H and Zhu B: Kinetics and mechanisms of mitotic
inheritance of DNA methylation and their roles in aging-associated
methylome deterioration. Cell Res. 30:980–996. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harrison JS, Cornett EM, Goldfarb D,
DaRosa PA, Li ZM, Yan F, Dickson BM, Guo AH, Cantu DV, Kaustov L,
et al: Hemi-methylated DNA regulates DNA methylation inheritance
through allosteric activation of H3 ubiquitylation by UHRF1. ELife.
5:e171012016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xue B, Zhao J, Feng P, Xing J, Wu H and Li
Y: Epigenetic mechanism and target therapy of UHRF1 protein complex
in malignancies. Onco Targets Ther. 12:549–559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gelato KA, Tauber M, Ong MS, Winter S,
Hiragami-Hamada K, Sindlinger J, Lemak A, Bultsma Y, Houliston S,
Schwarzer D, et al: Accessibility of different histone H3-binding
domains of UHRF1 is allosterically regulated by
phosphatidylinositol 5-phosphate. Mol Cell. 54:905–919. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Greiner VJ, Kovalenko L, Humbert N,
Richert L, Birck C, Ruff M, Zaporozhets OA, Dhe-Paganon S, Bronner
C and Mély Y: Site-selective monitoring of the interaction of the
SRA domain of UHRF1 with target DNA sequences labeled with
2-aminopurine. Biochemistry. 54:6012–6020. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hashimoto H, Horton JR, Zhang X, Bostick
M, Jacobsen SE and Cheng X: The SRA domain of UHRF1 flips
5-methylcytosine out of the DNA helix. Nature. 455:826–829. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Avvakumov GV, Walker JR, Xue S, Li Y, Duan
S, Bronner C, Arrowsmith CH and Dhe-Paganon S: Structural basis for
recognition of hemi-methylated DNA by the SRA domain of human
UHRF1. Nature. 455:822–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arita K, Ariyoshi M, Tochio H, Nakamura Y
and Shirakawa M: Recognition of hemi-methylated DNA by the SRA
protein UHRF1 by a base-flipping mechanism. Nature. 455:818–821.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, Liu Y, Xie Y, Zhu Y, Liu J and Lu
F: H3K27me3 shapes DNA methylome by inhibiting UHRF1-mediated H3
ubiquitination. Sci China Life Sci. 65:1685–1700. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xie S and Qian C: The growing complexity
of UHRF1-mediated maintenance DNA methylation. Genes (Basel).
9:6002018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ferry L, Fournier A, Tsusaka T, Adelmant
G, Shimazu T, Matano S, Kirsh O, Amouroux R, Dohmae N, Suzuki T, et
al: Methylation of DNA ligase 1 by G9a/GLP recruits UHRF1 to
replicating DNA and regulates DNA methylation. Mol Cell.
67:550–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kori S, Ferry L, Matano S, Jimenji T,
Kodera N, Tsusaka T, Matsumura R, Oda T, Sato M, Dohmae N, et al:
Structure of the UHRF1 tandem tudor domain bound to a methylated
non-histone protein, LIG1, reveals rules for binding and
regulation. Structure. 27:485–496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li T, Wang L, Du Y, Xie S, Yang X, Lian F,
Zhou Z and Qian C: Structural and mechanistic insights into
UHRF1-mediated DNMT1 activation in the maintenance DNA methylation.
Nucl Acids Res. 46:3218–3231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Povlsen LK, Beli P, Wagner SA, Poulsen SL,
Sylvestersen KB, Poulsen JW, Nielsen ML, Bekker-Jensen S, Mailand N
and Choudhary C: Systems-wide analysis of ubiquitylation dynamics
reveals a key role for PAF15 ubiquitylation in DNA-damage bypass.
Nat Cell Biol. 14:1089–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Karg E, Smets M, Ryan J, Forné I, Qin W,
Mulholland CB, Kalideris G, Imhof A, Bultmann S and Leonhardt H:
Ubiquitome analysis reveals PCNA-associated factor 15 (PAF15) as a
specific ubiquitination target of UHRF1 in embryonic stem cells. J
Mol Biol. 429:3814–3824. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
González-Magaña A, de Opakua AI, Merino N,
Monteiro H, Diercks T, Murciano-Calles J, Luque I, Bernadó P,
Cordeiro TN, Biasio A and Blanco FJ: Double monoubiquitination
modifies the molecular recognition properties of p15(PAF) promoting
binding to the reader module of Dnmt1. ACS Chem Biol. 14:2315–2326.
2019.PubMed/NCBI
|
|
51
|
Miyashita R, Nishiyama A, Qin W, Chiba Y,
Kori S, Kato N, Konishi C, Kumamoto S, Kozuka-Hata H, Oyama M, et
al: The termination of UHRF1-dependent PAF15 ubiquitin signaling is
regulated by USP7 and ATAD5. Elife. 12:e790132023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bronner C, Krifa M and Mousli M:
Increasing role of UHRF1 in the reading and inheritance of the
epigenetic code as well as in tumorogenesis. Biochem Pharmacol.
86:1643–1649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kori S, Shibahashi Y, Ekimoto T, Nishiyama
A, Yoshimi S, Yamaguchi K, Nagatoishi S, Ohta M, Tsumoto K,
Nakanishi M, et al: Structure-based screening combined with
computational and biochemical analyses identified the inhibitor
targeting the binding of DNA Ligase 1 to UHRF1. Bioorg Med Chem.
52:1165002021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Senisterra G, Zhu HY, Luo X, Zhang H, Xun
G, Lu C, Xiaon W, Hajian T, Loppnau P, Chau I, et al: Discovery of
small-molecule antagonists of the H3K9me3 binding to UHRF1 tandem
tudor domain. SLAS Discov. 23:930–940. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Choudalakis M, Kungulovski G, Mauser R,
Bashtrykov P and Jeltsch A: Refined read-out: The hUHRF1
Tandem-Tudor domain prefers binding to histone H3 tails containing
K4me1 in the context of H3K9me2/3. Protein Sci. 32:e47602023.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hata K, Kobayashi N, Sugimura K, Qin W,
Haxholli D, Chiba Y, Yoshimi S, Hayashi G, Onoda H, Ikegami T, et
al: Structural basis for the unique multifaceted interaction of
DPPA3 with the UHRF1 PHD finger. Nucleic Acids Res. 50:12527–12542.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jain K, Fraser CS, Marunde MR, Parker MM,
Sagum C, Burg JM, Hall N, Popova IK, Rodriguez KL, Vaidya A, et al:
Characterization of the plant homeodomain (PHD) reader family for
their histone tail interactions. Epigenetics Chromatin. 13:32020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Musselman CA and Kutateladze TG:
Handpicking epigenetic marks with PHD fingers. Nucleic Acids Res.
39:9061–9071. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xie S, Jakoncic J and Qian C: UHRF1 double
tudor domain and the adjacent PHD finger act together to recognize
K9me3-containing histone H3 tail. J Mol Biol. 415:318–328. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Morinière J, Rousseaux S, Steuerwald U,
Soler-López M, Curtet S, Vitte AL, Govin J, Gaucher J, Sadoul K,
Hart DJ, et al: Cooperative binding of two acetylation marks on a
histone tail by a single bromodomain. Nature. 461:664–668. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rajakumara E, Wang Z, Ma H, Hu L, Chen H,
Lin Y, Guo R, Wu F, Li H, Lan F, et al: PHD finger recognition of
unmodified histone H3R2 links UHRF1 to regulation of euchromatic
gene expression. Mol Cell. 43:275–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cheng J, Yang Y, Fang J, Xiao J, Zhu T,
Chen F, Wang P, Li Z, Yang H and Xu Y: Structural insight into
coordinated recognition of trimethylated histone H3 lysine 9
(H3K9me3) by the plant homeodomain (PHD) and tandem tudor domain
(TTD) of UHRF1 (ubiquitin-like, containing PHD and RING finger
domains, 1) protein. J Biol Chem. 288:1329–1339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rothbart SB, Krajewski K, Nady N, Tempel
W, Xue S, Badeaux AI, Barsyte-Lovejoy D, Martinez JY, Bedford MT,
Fuchs SM, et al: Association of UHRF1 with methylated H3K9 directs
the maintenance of DNA methylation. Nat Struct Mol Biol.
19:1155–1160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Arita K, Isogai S, Oda T, Unoki M, Sugita
K, Sekiyama N, Kuwata K, Hamamoto R, Tochio H, Sato M, et al:
Recognition of modification status on a histone H3 tail by linked
histone reader modules of the epigenetic regulator UHRF1. Proc Natl
Acad Sci USA. 109:12950–1295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rothbart SB, Dickson BM, Ong MS, Krajewski
K, Houliston S, Kireev DB, Arrowsmith CH and Strahl BD: Multivalent
histone engagement by the linked tandem Tudor and PHD domains of
UHRF1 is required for the epigenetic inheritance of DNA
methylation. Genes Dev. 27:1288–1298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Unoki M: Current and potential anticancer
drugs targeting members of the UHRF1 complex including epigenetic
modifiers. Recent Pat Anticancer Drug Discov. 6:116–130. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Du W, Dong Q, Zhang Z, Liu B, Zhou T, Xu
RM, Wang H, Zhu B and Li Y: Stella protein facilitates DNA
demethylation by disrupting the chromatin association of the RING
finger-type E3 ubiquitin ligase UHRF1. J Biol Chem. 294:8907–8917.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vaughan RM, Rothbart SB and Dickson BM:
The finger loop of the SRA domain in the E3 ligase UHRF1 is a
regulator of ubiquitin targeting and is required for the
maintenance of DNA methylation. J Biol Chem. 294:15724–15732. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Alhosin M, Omran Z, Zamzami MA, Al-Malki
AL, Choudhry H, Mousli M and Bronner C: Signalling pathways in
UHRF1-dependent regulation of tumor suppressor genes in cancer. J
Exp Clin Cancer Res. 35:1742016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hahm JY, Park JW, Kang JY, Park J, Kim CH,
Kim JY, Ha NC, Kim JW and Seo SB: Acetylation of UHRF1 regulates
hemi--methylated DNA binding and maintenance of Genome-wide DNA
methylation. Cell Rep. 32:1079582020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Achour M, Jacq X, Rondé P, Alhosin M,
Charlot C, Chataigneau T, Jeanblanc M, Macaluso M, Giordano A,
Hughes AD, et al: The interaction of the SRA domain of ICBP90 with
a novel domain of DNMT1 is involved in the regulation of VEGF gene
expression. Oncogene. 27:2187–2197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bashtrykov P, Jankevicius G, Jurkowska RZ,
Ragozin S and Jeltsch A: The UHRF1 protein stimulates the activity
and specificity of the maintenance DNA methyltransferase DNMT1 by
an allosteric mechanism. J Biol Chem. 289:4106–4115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Taniue K, Hayashi T, Kamoshida Y, Kurimoto
A, Takeda Y, Negishi L, Iwasaki K, Kawamura Y, Goshima N and
Akiyama T: UHRF1-KAT7-mediated regulation of TUSC3 expression via
histone methylation/acetylation is critical for the proliferation
of colon cancer cells. Oncogene. 39:1018–1030. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Luo G, Li Q, Yu M, Wang T, Zang Y, Liu Z,
Niu Z, Yang H and Lai J: UHRF1 modulates breast cancer cell growth
via estrogen signaling. Med Oncol. 39:1112022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Awal MA, Nur SM, Al Khalaf AK, Rehan M,
Ahmad A, Hosawi SBI, Choudhry H and Khan MI: Structural-guided
identification of small molecule inhibitor of UHRF1
methyltransferase activity. Front Gene. 13:9288842022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zaayter L, Mori M, Ahmad T, Ashraf W,
Boudier C, Kilin V, Gavvala K, Richert L, Eiler S, Ruff M, et al: A
molecular tool targeting the base-flipping activity of human UHRF1.
Chemistry. 25:13363–13375. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ciaco S, Mazzoleni V, Javed A, Eiler S,
Ruff M, Mousli M, Mori M and Mély Y: Inhibitors of UHRF1 base
flipping activity showing cytotoxicity against cancer cells. Bioorg
Chem. 137:1066162023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hu CL, Chen BY, Li Z, Yang T, Xu CH, Yang
R, Yu PC, Zhao J, Liu T, Liu N, et al: Targeting UHRF1-SAP30-MXD4
axis for leukemia initiating cell eradication in myeloid leukemia.
Cell Res. 32:1105–1123. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Baell JB and Holloway GA: New substructure
filters for removal of pan assay interference compounds (PAINS)
from screening libraries and for their exclusion in bioassays. J
Med Chem. 53:2719–2740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Baell J and Walters MA: Chemistry:
Chemical con artists foil drug discovery. Nature. 513:481–483.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nady N, Lemak A, Walker JR, Avvakumov GV,
Kareta MS, Achour M, Xue S, Duan S, Allali-Hassani A, Zuo X, et al:
Recognition of multivalent histone states associated with
heterochromatin by UHRF1 protein. J Biol Chem. 286:24300–24311.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
DaRosa PA, Harrison JS, Zelter A, Davis
TN, Brzovic P, Kuhlman B and Klevit RE: A bifunctional role for the
UHRF1 UBL domain in the control of hemi-methylated DNA-dependent
histone ubiquitylation. Mol Cell. 72:753–765. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Foster BM, Stolz P, Mulholland CB, Montoya
A, Kramer H, Bultmann S and Bartke T: Critical role of the UBL
domain in stimulating the E3 ubiquitin ligase activity of UHRF1
toward chromatin. Mol Cell. 72:739–752. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Abdullah O, Omran Z, Hosawi S, Hamiche A,
Bronner C and Alhosin M: Thymoquinone is a multitarget single
epidrug that inhibits the UHRF1 protein complex. Genes (Basel).
12:6222021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Alhosin M, Ibrahim A, Boukhari A, Sharif
T, Gies JP, Auger C and Schini-Kerth VB: Anti-neoplastic agent
thymoquinone induces degradation of α and β tubulin proteins in
human cancer cells without affecting their level in normal human
fibroblasts. Invest New Drugs. 30:1813–1819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Peng Y, Tang R, Ding L, Zheng R, Liu Y,
Yin L, Fu Y, Deng T and Li X: Diosgenin inhibits prostate cancer
progression by inducing UHRF1 protein degradation. Eur J Pharmacol.
942:1755222023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Nishiyama A, Yamaguchi L, Sharif J,
Johmura Y, Kawamura T, Nakanishi K, Shimamura S, Arita K, Kodama T,
Ishikawa F, et al: Uhrf1-dependent H3K23 ubiquitylation couples
maintenance DNA methylation and replication. Nature. 502:249–253.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Qin W, Wolf P, Liu N, Link S, Smets M, La
Mastra F, Forné I, Pichler G, Hörl D, Fellinger K, et al: DNA
methylation requires a DNMT1 ubiquitin interacting motif (UIM) and
histone ubiquitination. Cell Res. 25:911–929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liang T, Zhang Q, Wu Z, Chen P, Huang Y,
Liu S and Li L: UHRF1 suppresses HIV-1 transcription and promotes
HIV-1 latency by competing with p-TEFb for
ubiquitination-proteasomal degradation of tat. mBio.
12:e01625212021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yang J, Liu K, Yang J, Jin B, Chen H, Zhan
X, Li Z, Wang L, Shen X, Li M, et al: PIM1 induces cellular
senescence through phosphorylation of UHRF1 at Ser311. Oncogene.
36:4828–4842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chu J, Loughlin EA, Gaur NA, SenBanerjee
S, Jacob V, Monson C, Kent B, Oranu A, Ding Y, Ukomadu C and Sadler
KC: UHRF1 phosphorylation by cyclin A2/cyclin-dependent kinase 2 is
required for zebrafish embryogenesis. Mol Biol Cell. 23:59–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang ZM, Rothbart SB, Allison DF, Cai Q,
Harrison JS, Li L, Wang Y, Strahl BD, Wang GG and Song J: An
allosteric interaction links USP7 to deubiquitination and chromatin
targeting of UHRF1. Cell Rep. 12:1400–1406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fang J, Cheng J, Wang J, Zhang Q, Liu M,
Gong R, Wang P, Zhang X, Feng Y, Lan W, et al: Hemi-methylated DNA
opens a closed conformation of UHRF1 to facilitate its histone
recognition. Nat Commun. 7:111972016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhang J, Gao Q, Li P, Liu X, Jia Y, Wu W,
Li J, Dong S, Koseki H and Wong J: S phase-dependent interaction
with DNMT1 dictates the role of UHRF1 but not UHRF2 in DNA
methylation maintenance. Cell Res. 21:1723–1739. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Geng Y, Gao Y, Ju H and Yan F: Diagnostic
and prognostic value of plasma and tissue ubiquitin-like,
containing PHD and RING finger domains 1 in breast cancer patients.
Cancer Sci. 104:194–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chen X, Cheung ST, So S, Fan ST, Barry C,
Higgins J, Lai KM, Ji J, Dudoit S, Ng IO, et al: Gene expression
patterns in human liver cancers. Mol Biol Cell. 13:1929–1939. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhu M, Xu Y, Ge M, Gui Z and Yan F:
Regulation of UHRF1 by microRNA-9 modulates colorectal cancer cell
proliferation and apoptosis. Cancer Sci. 106:833–839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yang H, Cheung S and Churg A: UHRF1
Immunohistochemical staining separates benign reactive spindle cell
mesothelial proliferations from sarcomatoid mesotheliomas. Am J
Surg Pathol. 46:840–845. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cai Y, Tsai HC, Yen RC, Zhang YW, Kong X,
Wang W, Xia L and Baylin SB: Critical threshold levels of DNA
methyltransferase 1 are required to maintain DNA methylation across
the genome in human cancer cells. Genome Res. 27:533–544. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Niinuma T, Kitajima H, Kai M, Yamamoto E,
Yorozu A, Ishiguro K, Sasaki H, Sudo G, Toyota M, Hatahira T, et
al: UHRF1 depletion and HDAC inhibition reactivate epigenetically
silenced genes in colorectal cancer cells. Clin Epigenetics.
11:702019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fu Y, Cao T, Zou X, Ye Y, Liu Y, Peng Y,
Deng T, Yin L and Li X: AKT1 regulates UHRF1 protein stability and
promotes the resistance to abiraterone in prostate cancer.
Oncogenesis. 12:12023. View Article : Google Scholar : PubMed/NCBI
|