Introduction
Recently, based on the results of genomic analysis,
endometrial cancer (EC) has been classified into four groups
molecular-pathologically: polymerase epsilon
(POLE)-ultra-mutated, microsatellite instability
(MSI)-hypermutated, copy-number-low (CN-L), and copy-number-high
(CN-H) groups. POLE-ultra-mutated tumors have the best
outcome, while CN-H tumors have the poorest outcome (1). Endometrioid carcinoma has been
categorized into the CN-L group, and serous carcinoma and
high-grade EC with TP53 mutation are frequently included in
CN-H group (1). The
MSI-hypermutated tumors appear associated with methylation of the
MLH1 promoter, and the prognosis of this type of tumor is moderate,
between POLE-ultra-mutated tumors and CN-H tumors (1). We recently reported that the rate of
MSI-H in sporadic EC was approximately 10% in Japanese women,
apparently lower than that in Western countries (2). Nevertheless, this classification has
not been applied in clinical practice, as, to date, there is no
immunohistochemistry (IHC) which can substitute for molecular
testing.
Patients with hormone receptor (HR)-positive early
breast cancer, which accounts for nearly 80% of all breast cancer
cases, are recommended to receive adjuvant endocrine therapy after
curative surgery (3). Tamoxifen
(TAM), a selective ER modulator, is one of the major endocrine
therapies along with aromatase inhibitors, but known to sometimes
be a cause of EC (TAM-related EC), although infrequently (4–6). The
partial agonist action of TAM promotes epithelial proliferation via
the ER and might be involved in EC development (7). However, the detailed mechanism has not
been fully elucidated, despite this disease having been recognized
for a considerable time and numerous studies having been conducted.
A study employing the Surveillance Epidemiology and End Results
(SEER) data indicated that TAM was not associated with
poor-prognosis disease (8), but
patients taking TAM have to frequently visit gynecologists for
screening and are forced to undergo invasive examinations
periodically, such as cytology of the endometrium. Furthermore,
based on the results of large clinical trials, the recommended
duration of TAM has been extended from five years to 10 years
(9). Moreover, recent advances in
chemo-regimens have reduced the recurrence rate of breast cancer,
thus the number of patients who complete adjuvant TAM treatment has
been increasing. It is likely that the number of patients who will
develop TAM-related EC will increase, and the establishment of
preventative methods will be necessary.
A number of basic investigations have attempted to
elucidate the characteristics of TAM-related EC. Employing
ChIP-seq, Droog et al (10)
identified that cross-talk between forkhead box A1 (FOXA1)
and ERα might contribute to the development of TAM-related EC.
Several oncogenes, such as PTEN and K-Ras, were also
investigated (11), however, due to
the relatively low occurrence rate, the nature of TAM-related EC is
far from fully understood. Therefore, we conducted copy number
variation (CNV) analysis by nCounter and investigated MSI status in
TAM-related EC and sporadic EC biopsies to assess the molecular
genetic characteristics of TAM-related EC.
Materials and methods
Patients
Among patients with HR-positive invasive breast
cancer who received TAM as adjuvant endocrine therapy after
curative surgery at our institute between 2013 and 2020, 10
patients developed EC after TAM administration (TAM-related EC). We
also analyzed 20 sporadic EC samples randomly selected from 98
patients, whom we investigated in our previous study (2), surgically treated during the same
period. The average age of TAM-related EC was 49.6 years, and those
with sporadic EC was 60.9 years. None of these patients had been
clinically diagnosed as having Lynch Syndrome (LS).
Pathological assessment and
immunohistochemistry
Pathological assessment for EC and breast cancer was
carried out at Juntendo University Hospital by two experienced
pathologists (HS and AA) based on the WHO Classification. The
histological subtype of all ECs was endometrioid carcinoma. Tissue
sections of 4 µm were prepared from formalin-fixed
paraffin-embedded blocks of EC surgical specimens and IHC was
performed. IHC staining for mismatch repair (MMR) proteins was
performed using primary monoclonal antibodies against MLH1 (clone
ES05; Dako, Carpinteria, CA), MSH2 (clone FE11; Dako), PMS2 (clone
EP51; Dako) and MSH6 (clone EP49; Dako). The staining procedures
were previously described in detail (12). All MMR proteins (MLH1, MSH2, PMS2
and MSH6) were assessed for positive staining in the nuclei of
cells. IHC for MMR proteins was also performed for breast cancer
and endometrial hyperplasia specimens as precursor lesions for EC
where available. Protein expression for the products of genes
identified as amplified by CNV analysis were also assessed by IHC
using the following antibodies: vascular endothelial growth factor
receptor 2 (VEGFR2; clone 55B11; Cell Signaling Technology,
Danvers, MA), tropomyosin receptor kinase (Trk) C (clone C44H5;
Cell Signaling Technology), platelet-derived growth factor receptor
β (PDGFRB; clone 28E1; Cell Signaling Technology), Notch1
(polyclonal, Santa Cruz Biotechnology, Dallas TX) and TrkA (clone
12G8; Cell Signaling Technology). Antigen retrieval was performed
by heating in tris-ethylenediamine tetraacetic acid buffer (pH 9.0)
for VEGFR2 and PDGFRB and in citrate buffer (pH 6.0) for TrkA, TrkC
and Notch1.
Nucleic acid extraction
Genomic DNA was extracted from TAM-related EC
surgical specimens as follows: 10 µm tissue sections were cut and
DNA was extracted using a QIAamp DNA Formalin-Fixed
Paraffin-Embedded Tissue kit (Qiagen Inc., Hilden, Germany). EC
lesions and non-neoplastic endometrium or simultaneously resected
ovary was separately dissected under a microscope for each case.
Nontumorous tissue from each patient was used as a control. As
nontumorous tissue, ovary and non-neoplastic endometrium were
employed for TAM-related EC and sporadic EC cases,
respectively.
Copy number variation and
microsatellite instability assessment
A total of 30 samples, including 10 TAM-related ECs
and 20 sporadic ECs, underwent CNV analysis using the NanoString
nCounter gene expression system (NanoString Technologies, Seattle,
WA). A customized panel including 28 genes encoding receptor
tyrosine kinases which we previously established (13) was employed. The copy number for
these genes for each EC lesion compared to the nonneoplastic lesion
were determined in nSolver according to the manufacturer's
instructions. Each copy number ratio was calculated with the score
of nonneoplastic lesion being 2. MSI status was assessed in all 10
TAM-related EC, including 10 surgical and one endometrial curettage
specimens. MSI testing was outsourced to the Takara Bio Inc.
(Shiga, Japan) and the Fasmac Co. Ltd. (Kanagawa, Japan) as
previously described (14). Using a
Promega MSI Multiplex System, five loci from the DNA sequence for
microsatellite markers (BAT-25, BAT-26, NR-21, NR-24, and MONO-27)
were amplified. MSI-high (MSI-H) was determined if instability was
detected at two or more markers, as recommended by the revised
Bethesda Guidelines (15). Tumors
with one or no unstable marker were classified as low levels of
microsatellite instability (MSI-L) or microsatellite stable (MSS),
respectively.
Methylation assay
Promoter-region methylation status of MLH1
was analyzed in two cases in which MLH1 protein expression was lost
by IHC. The detailed procedure has been described (16). The primers for methylated and
unmethylated alleles were designed as done by House et al
(17).
Ethical approval and informed
consent
This study was carried out with approval from the
Ethics Committee of Juntendo University (No. 2020281) and complies
with the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. All participants were informed that
the research policy was available on the homepage of the hospital
and that they had the opportunity to opt-out of the study at any
time later on, which was approved by the Ethics Committee. The
Ethics Committee approved of the opt-out method for the use of
specimens and clinical data under the condition that all data were
anonymized. Authors had access to information that could identify
individual participants during or after data collection. Only those
participants who had not opted-out from the study were included in
the data analysis.
Statistical analysis
Mann-Whitney U tests and unpaired t tests
were performed to analyze CNV data between TAM-related ECs and
sporadic ECs. In addition, the Fisher's exact test and unpaired
t-test was performed to compare clinicopathological data between
TAM-related ECs and sporadic ECs, and between the MSI-H and
MSI-L/MSS groups in TAM-related ECs. These data were evaluated
using a two-tailed test and P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological findings
Table I shows the
clinicopathological characteristics of TAM-related ECs and sporadic
ECs. Age was significantly younger in patients with TAM-related EC
than those with sporadic EC (P=0.004). There was no significant
difference in pathological stage based on the FIGO 2008
classification.
 | Table I.Clinicopathological data of patients
with TAM-related and sporadic EC. |
Table I.
Clinicopathological data of patients
with TAM-related and sporadic EC.
| Characteristics | TAM-related EC
(n=10) | Sporadic EC
(n=20) | P-value |
|---|
| Mean age, years
(SD) | 49.5 (7.2) | 61.5 (10.0) | 0.004a |
| Stage, n |
|
|
|
| I | 8 | 17 |
>0.999b |
| II | 2 | 1 |
|
| III | 0 | 1 |
|
| IV | 0 | 1 |
|
| Histology, n |
|
|
|
|
Endometrioid carcinoma,
G1 | 9 | 11 | 0.101b |
|
Endometrioid carcinoma,
G2 | 1 | 7 |
|
|
Endometrioid carcinoma,
G3 | 0 | 2 |
|
Copy number variation and
immunohistochemistry between tamoxifen-related endometrial cancer
and sporadic endometrial cancer
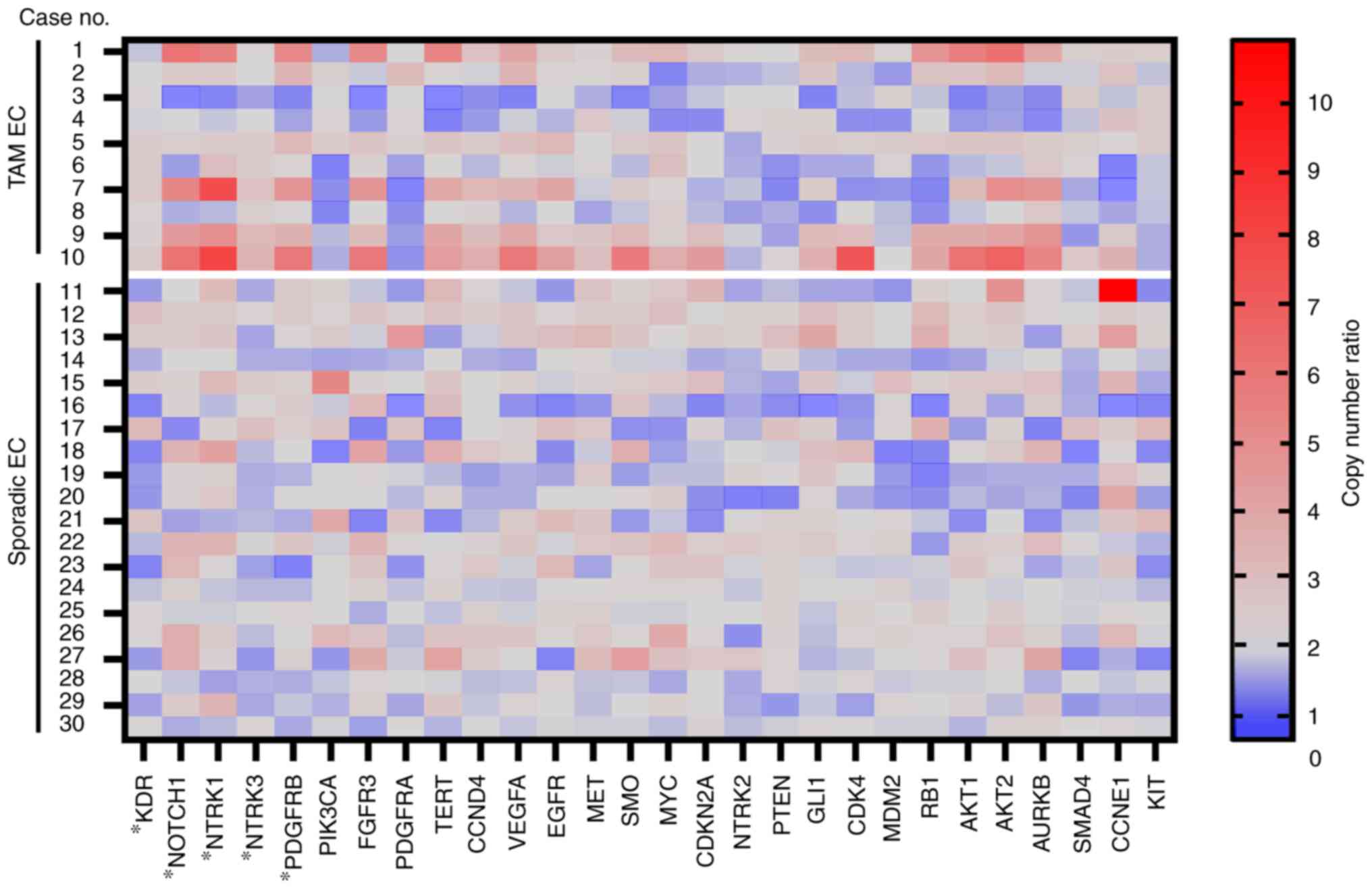
A summary of the results of CNV analysis is shown in
Fig. 1. In total, five genes,
KDR, NTRK3, PDGFRB, NOTCH1 and NTRK1, appeared as
frequently amplified genes in TAM-related EC, compared with
sporadic EC (KDR, P=0.039; NTRK3, P=0.006;
PDGFRB, P=0.035; NOTCH1, P<0.001; NTRK1,
P=0.011) by either Mann-Whitney U test or unpaired t-test.
Next, IHC for the five proteins encoded by these
genes was performed (Table SI).
Representative images are shown in Fig. S1. One TAM-related EC case was
diffusely and strongly positive for VEGFR2 and weakly positive for
PDGFRB. Two sporadic EC cases were focally and weakly positive for
TrkC and one sporadic EC case was focally and weakly positive for
PDGFRB. Notch1 was positive for all cases and various intensity was
seen from 1+ (weakly positive) to 3+ (strongly positive). Overall,
intensity of IHC did not correlate with the ratio of copy
number.
Microsatellite instability status in
tamoxifen-related endometrial cancers
Next, we investigated MSI status in all 10
TAM-related ECs. Of the 10 TAM-related EC cases, four were MSI-H
(40%) and six cases were MSS (60%). The clinicopathological
findings according to MSI status are shown in Table II. There were no differences in
age, pathological stage, EC tumor grade, or the length of time
after initiating TAM administration, according to MSI status.
 | Table II.Clinicohistological data of patients
with TAM-related endometrial cancer. |
Table II.
Clinicohistological data of patients
with TAM-related endometrial cancer.
|
Characteristics | MSI-H (n=4) | MSS (n=6) | P-value |
|---|
| Mean age, years
(SD) | 48.5 (10.0) | 54.0 (3.7) | 0.244a |
| Stage, n |
|
|
|
| I | 3 | 5 |
>0.999b |
| II | 1 | 1 |
|
|
III | 0 | 0 |
|
| IV | 0 | 0 |
|
| Histology, n |
|
|
|
|
Endometrioid carcinoma,
G1 | 4 | 5 |
>0.999c |
|
Endometrioid carcinoma,
G2 | 0 | 1 |
|
| Mean time
after | 42.0 (11.0) | 46.2 (7.6) | 0.397a |
| TAM administration,
months (SD) |
|
|
|
Immunohistochemistry for mismatch
repair proteins
Since we observed some MSI-H cases in TAM-related
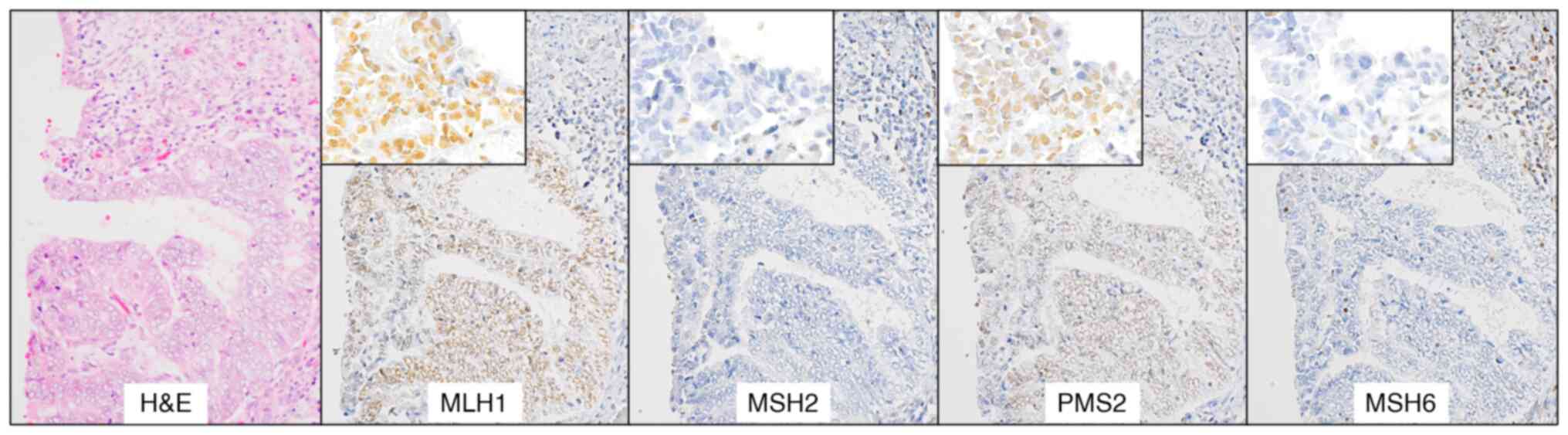
EC, we next conducted IHC for MMR proteins. As shown in Table III, four cases lost some MMR
proteins and these corresponded to the MSI-H cases. All of MSI-H
cases were endometrioid carcinoma, Grade 1 and one case was
classified as FIGO Stage II due to an invasion to cervical stroma.
Representative images of one case (Case #1) are shown in Fig. 2. Among the 10 patients with
TAM-related EC, surgical specimens of primary breast cancer were
available for IHC in six cases. All six breast cancer specimens
retained MMR protein expression, including one MSH-H tumor (Case
#2).
 | Table III.MSI status and MMR protein expression
based on immunohistochemistry in tamoxifen-related endometrial
cancer. |
Table III.
MSI status and MMR protein expression
based on immunohistochemistry in tamoxifen-related endometrial
cancer.
|
| EC | BC |
|
|
|---|
|
|
|
|
|
|
|---|
| Case no. | Age, years | MSI status | Loss of MMR
proteins | Tumor grade | Vessel invasion
(Ly/V) | Lymph node
metastasis | Loss of MMR
proteins | Past history | Family history |
|---|
| 1 | 49 | MSI-H | MSH2, MSH6 | G1 | - | - | N.E. | None | BC (mother) |
| 2 | 47 | MSI-H | MLH1, PMS2 | G1 | +/+ | - | - | BLC | None |
| 3 | 50 | MSI-H | MSH6 | G1 | +/- | - | N.E. | None | CC (mother), BLC
and LC (father) |
| 4 | 45 | MSI-H | MLH1, PMS2 | G1 | -/- | N.E. | N.E. | None | None |
| 5 | 54 | MSS | - | G1 | -/- | N.E. | - | None | None |
| 6 | 54 | MSS | - | G2 | -/- | - | N.E. | None | CC (mother) |
| 7 | 36 | MSS | - | G1 | -/- | - | - | None | BC (grandmother,
aunt) |
| 8 | 43 | MSS | - | G1 | -/- | N.E. | - | None | BC (sister), CC and
PC (uncle) |
| 9 | 54 | MSS | - | G1 | -/- | N.E. | - | None | None |
| 10 | 64 | MSS | − | G1 | -/- | N.E. | - | None | GC (mother) |
Microsatellite instability status and
mismatch repair protein expressions in previous benign biopsy
specimens
Among MSI-H cases of TAM-related ECs, Case #2
patient underwent histological examinations of the endometrium
several times before developing EC and was diagnosed with
endometrial hyperplasia without atypia. Hence, we also examined MSI
status and MMR protein expressions in this biopsy specimen of
hyperplasia. The benign lesion was MSI-H, and MLH1 and PMS2
expressions were deficient, similar to the surgical specimen of
subsequent EC.
Methylation assay for MLH1 promoter
region in MLH1 protein-deficient tumors
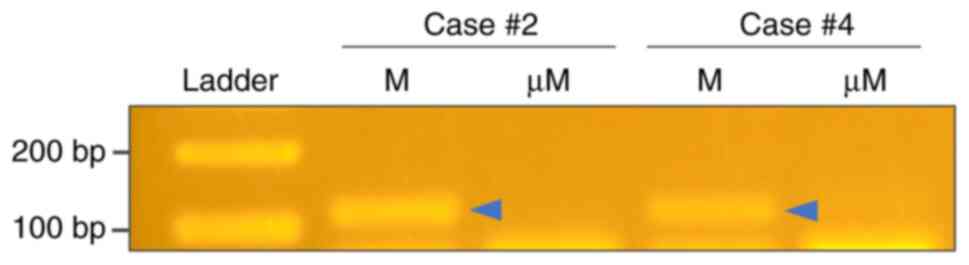
For two cases in which MLH1 protein was lost by IHC,
we further conducted MLH1 methylation-specific polymerase
chain reaction. Methylation of the MLH1 promoter region was
detected in both cases (Fig.
3).
Discussion
Our CNV analysis revealed that the amplification of
five receptor tyrosine kinase genes, KDR, NOTCH1, NTRK1,
NTRK3 and PDGFRB, were more frequently detected in
TAM-related EC, compared with sporadic EC. Meanwhile, the
expression of corresponding proteins for these genes, assessed by
IHC, did not reflect the results of the CNV analysis. This
discrepancy might be caused by some deficiency or modification in
the process of transcription. Yet, the biological significance of
these genes for TAM-related EC cannot be ruled out and may merit
further investigation.
According to the cBioPortal for Cancer Genomics
across TCGA pan cancer atlas (18),
the frequency of amplification for each gene in endometrioid
carcinoma or mixed carcinoma of EC is low (KDR none, NOTCH1
1.04–4.76%, NTRK1 1.83–19.05%, NTRK3 0.26% and
PDGFRB none). Our cases might show the characteristics of
TAM-related EC different from sporadic EC. The frequency of MSI-H
in TAM-related EC was 40%, higher than what we previously reported
in sporadic EC in Japanese patients (2). There have been a few reports on MSI
status in TAM-related EC and their results differed to the current
study, as the frequency of MSI-H in TAM-related EC was not
different from (19) or, rather,
was lower than that in sporadic EC (11). However, these studies employed
relatively older methods (e.g., with less microsatellite markers)
while we followed the revised Bethesda Guidelines. It is unclear
whether TAM promotes MSI or not. In the current study, we were
unable to perform a functional analysis. Further investigations
employing cell lines to assess changes in MSI status with TAM
treatment and analysis of endometrial tissue over time before
developing TAM-related EC are also warranted.
The high frequency of MSI-H in TAM-related ECs
indicates that the prognosis of these tumors may be better than
that of sporadic ECs. However, one report suggests a worse
prognosis among LS patients with EC occurring in the group taking
TAM (20). Considering the recent
trend of extension of TAM duration after breast cancer surgery, we
believe that it is necessary to continue to investigate the
characteristics of this tumor in more detail.
In our study, patients in the TAM-related EC group
were significantly younger than those in the sporadic EC group,
while the risk of EC due to TAM is known to be higher in older
patients (4,21). The main reason for the lower age in
our cohort is possibly due to the low frequency of TAM
administration in postmenopausal women. Compared to a few decades
ago, aromatase inhibitors rather than TAM are now given to most
postmenopausal patients as adjuvant endocrine therapy for breast
cancer in Japan. Thus, the number of postmenopausal breast cancer
patients taking TAM has decreased. Unfortunately, we were unable to
collect detailed data on the type and duration of endocrine therapy
for all of the patients who underwent curative surgery for breast
cancer. Therefore, it was not possible to assess the exact
frequency of TAM-related EC development.
We could not completely exclude the possibility that
patients with TAM-related EC had a background of LS. For instance,
Case #2 developed urinary tract cancer after breast cancer and EC
but had no relevant family history. Case #3 had a family history as
her mother suffered colon cancer and father experienced urinary
tract and liver cancers. Only one primary breast cancer sample was
available among the MSI-H cases, and the expression of MMR proteins
were retained. However, some reports indicate that breast cancer in
patients with LS is not necessarily MSI-H (22,23),
hence LS cannot be determined by MMR protein expressions in breast
cancer. Nevertheless, no patients in our cohort had undergone
genetic testing for LS diagnosis.
Our data indicate that TAM-related ECs are
frequently MSI-high. If further study confirms our finding,
gynecological routine screening during TAM medication may be less
critical than expected because MSI-high EC is known to have a
relatively favorable prognosis (1).
As such, unnecessary testing may be reduced in the future in
clinical practice. Furthermore, for treatment of TAM-related EC,
MSI status should be actively tested for to determine the optimal
individualized postoperative treatment.
A major limitation of this study is, as with other
studies, the small sample size. Another difficulty of analyzing
this disease is that presumably not all TAM-related EC develop due
to TAM. Considering the low occurrence rate of TAM-associated EC,
there is even the possibility that it only arises from biologically
altered-endometrium by TAM, but that TAM does not ‘promote’ the
development of cancer per se. Given the timing of TAM
administration, i.e., duration of the treatment and time after
completion of treatment, we believe that it is also necessary to
continue to accumulate more patients over a longer period.
In our study, we investigated the molecular
characteristics of TAM-related ECs and revealed several genes with
specific changes in TAM-related ECs. Furthermore, the frequency of
MSI was higher in TAM-related EC. Our findings may lead to a
reduction of unnecessary gynecologic testing in clinical practice,
and also encourage the testing of MSI status for optimal
individualized treatment. We believe that researchers need to
continue to analyze TAM-related ECs from a variety of
perspectives.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Ms. Tomomi Ikeda and
Ms. Takako Ikegami (Laboratory of Molecular and Biochemical
Research, Biomedical Research Core Facilities, Juntendo University
Graduate School of Medicine, Tokyo, Japan) for the technical
assistance with microsatellite instability analysis, and Dr
Hidetaka Eguchi (Intractable Disease Research Center, Juntendo
University Graduate School of Medicine, Tokyo, Japan) for technical
assistance with the NanoString nCounter gene expression system for
copy number variation analysis.
Funding
The present study was supported through grants from the
Grant-in-Aid of the Japan Society for the Promotion of Science
KAKENHI (grant nos. 20K17594 and 21K15409) and a grant from the
Institute for Environmental & Gender-specific Medicine,
Juntendo University (grant no. E2011).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HS, YH and TS designed the study. HS, YH, MTH, YM,
LR, YI, TU, EY and YT conducted data acquisition. YH, YI, TU, EY
and YT treated patients. HS, YH, AA and TY performed pathological
assessment and histological analysis. HS, YH, YM and TS conducted
data analysis and statistical analysis. HS and YH drafted the
original manuscript and TS and TY substantially revised it. HS and
YH confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Ethics Committee of Juntendo University (approval no. 2020281;
Tokyo, Japan) and with the 1964 Helsinki declaration and its later
amendments or comparable ethical standards. The requirement for
informed consent was waived by the Ethics Committee of Juntendo
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cancer Genome Atlas Research Network, .
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saeki H, Hlaing MT, Horimoto Y, Kajino K,
Ohtsuji N, Fujino K, Terao Y and Hino O: Usefulness of
immunohistochemistry for mismatch repair protein and microsatellite
instability examination in adenocarcinoma and background
endometrium of sporadic endometrial cancer cases. J Obstet Gynaecol
Res. 45:2037–2042. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Davies C, Godwin J, Gray R, Clarke
M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, et al: Relevance
of breast cancer hormone receptors and other factors to the
efficacy of adjuvant tamoxifen: Patient-level meta-analysis of
randomised trials. Lancet. 378:771–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher B, Costantino JP, Redmond CK,
Fisher ER, Wickerham DL and Cronin WM: Endometrial cancer in
tamoxifen-treated breast cancer patients: Findings from the
national surgical adjuvant breast and bowel project (NSABP) B-14. J
Natl Cancer Inst. 86:527–537. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braithwaite RS, Chlebowski RT, Lau J,
George S, Hess R and Col NF: Meta-analysis of vascular and
neoplastic events associated with tamoxifen. J Gen Intern Med.
18:937–947. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang Y and Brown M: Molecular
determinants for the tissue specificity of SERMs. Science.
295:2465–2468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jordan VC and Assikis VJ: Endometrial
carcinoma and tamoxifen: Clearing up a controversy. Clin Cancer
Res. 1:467–472. 1995.PubMed/NCBI
|
|
9
|
Davies C, Pan H, Godwin J, Gray R,
Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A,
Bonfill X, et al: Long-term effects of continuing adjuvant
tamoxifen to 10 years versus stopping at 5 years after diagnosis of
oestrogen receptor-positive breast cancer: ATLAS, a randomised
trial. Lancet. 381:805–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Droog M, Nevedomskaya E, Kim Y, Severson
T, Flach KD, Opdam M, Schuurman K, Gradowska P, Hauptmann M, Dackus
G, et al: Comparative cistromics reveals genomic cross-talk between
FOXA1 and ERα in tamoxifen-associated endometrial carcinomas.
Cancer Res. 76:3773–3784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turbiner J, Moreno-Bueno G, Dahiya S,
Sánchez-Estevez C, Hardisson D, Prat J, Oliva E and Palacios J:
Clinicopathological and molecular analysis of endometrial carcinoma
associated with tamoxifen. Mod Pathol. 21:925–936. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki O, Eguchi H, Chika N, Sakimoto T,
Ishibashi K, Kumamoto K, Tamaru JI, Tachikawa T, Akagi K, Arai T,
et al: Prevalence and clinicopathologic/molecular characteristics
of mismatch repair-deficient colorectal cancer in the
under-50-year-old Japanese population. Surg Today. 47:1135–1146.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasa K, Saito T, Kurihara T, Hasegawa N,
Sano K, Kubota D, Akaike K, Okubo T, Hayashi T, Takagi T, et al:
Establishment of rapid and accurate screening system for molecular
target therapy of osteosarcoma. Technol Cancer Res Treat.
21:153303382211382172022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horimoto Y, Thinzar Hlaing M, Saeki H,
Kitano S, Nakai K, Sasaki R, Kurisaki-Arakawa A, Arakawa A, Otsuji
N, Matsuoka S, et al: Microsatellite instability and mismatch
repair protein expressions in lymphocyte-predominant breast cancer.
Cancer Sci. 111:2647–2654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Umar A, Boland CR, Terdiman JP, Syngal S,
de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ,
Hamelin R, et al: Revised Bethesda guidelines for hereditary
nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite
instability. J Natl Cancer Inst. 96:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki Y, Fukumura Y, Asahina M, Fujimaki
M, Ohba S, Matsumoto F, Kurahayashi I, Yao T and Ikeda K: EGFR
protein expression relates with tumor histology, methylation status
of EGFR and HPV16 E6 viral load in oropharyngeal carcinoma. Head
Neck Pathol. 15:743–756. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
House MG, Guo M, Iacobuzio-Donahue C and
Herman JG: Molecular progression of promoter methylation in
intraductal papillary mucinous neoplasms (IPMN) of the pancreas.
Carcinogenesis. 24:193–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prasad M, Wang H, Douglas W, Barakat RR
and Ellenson LH: Molecular genetic characterization of
tamoxifen-associated endometrial cancer. Gynecol Oncol. 96:25–31.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johnatty SE, Stewart CJR, Smith D,
Buchanan D, Leung Y, Oehler MK, Brand A, Webb PM and Spurdle AB:
Risk and prognostic factors for endometrial carcinoma after
diagnosis of breast or Lynch-associated cancers-A population-based
analysis. Cancer Med. 7:6411–6422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fisher B, Costantino JP, Wickerham DL,
Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins
JN, Margolese RG, et al: Tamoxifen for the prevention of breast
cancer: Current status of the national surgical adjuvant breast and
bowel project P-1 study. J Natl Cancer Inst. 97:1652–1662. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Latham A, Srinivasan P, Kemel Y, Shia J,
Bandlamudi C, Mandelker D, Middha S, Hechtman J, Zehir A,
Dubard-Gault M, et al: Microsatellite instability is associated
with the presence of Lynch syndrome pan-cancer. J Clin Oncol.
37:286–295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanaya N, Tanakaya K, Yamasaki R, Arata T,
Shigeyasu K, Aoki H, Morito T, Sanaii H, Akagi K and Fujiwara T:
Clinicopathological features of breast cancer in Japanese female
patients with Lynch syndrome. Breast Cancer. 26:359–364. 2019.
View Article : Google Scholar : PubMed/NCBI
|