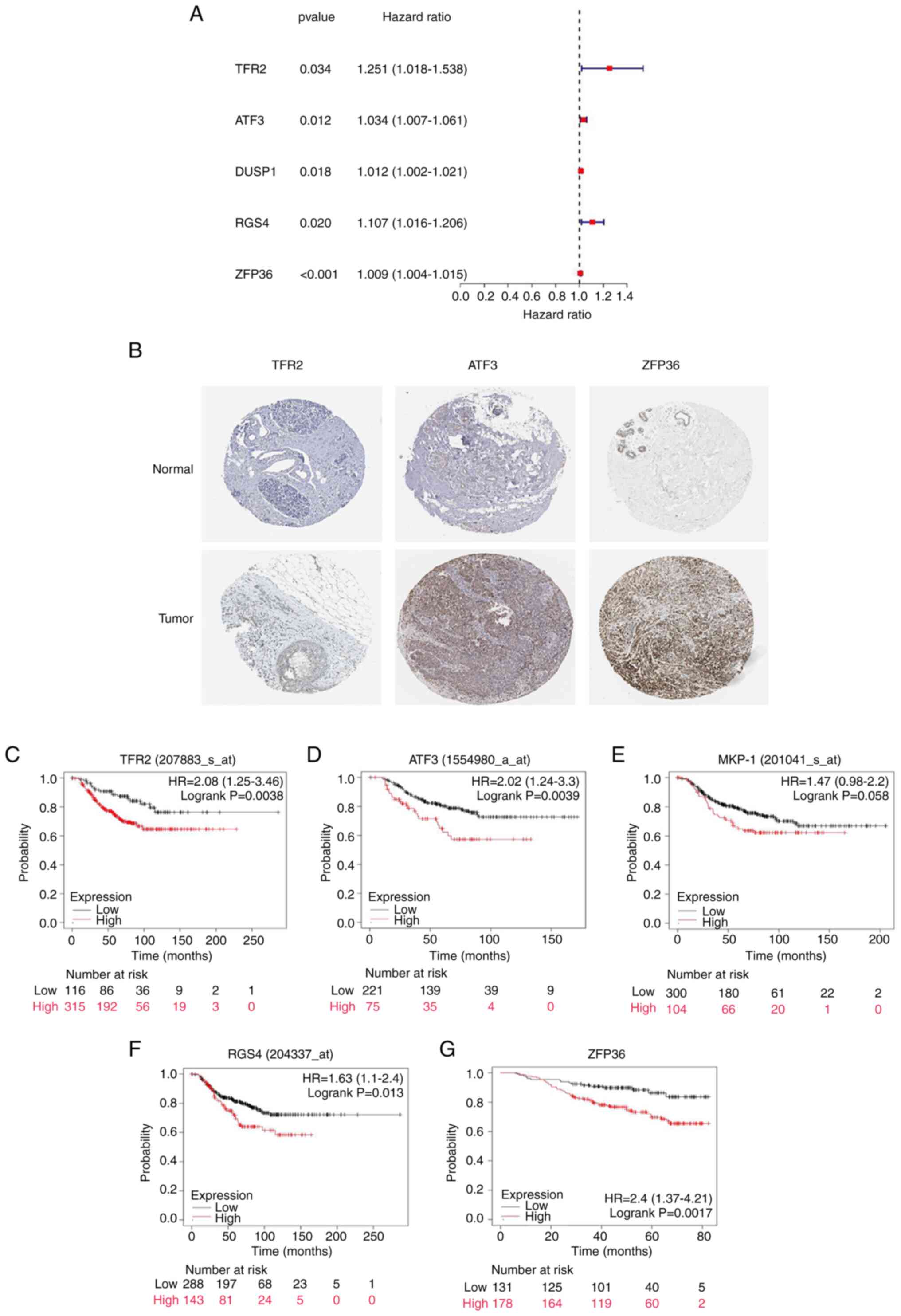
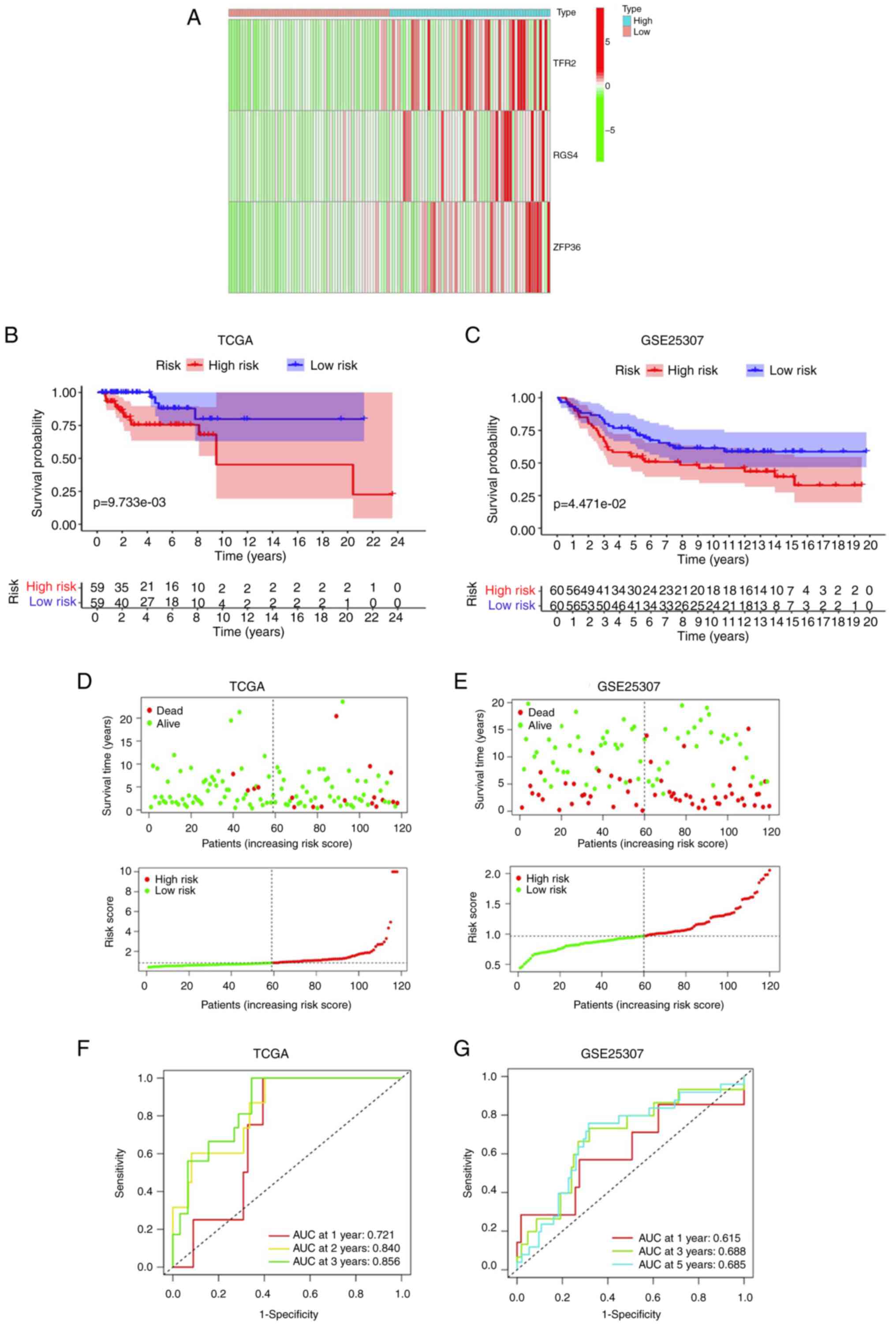
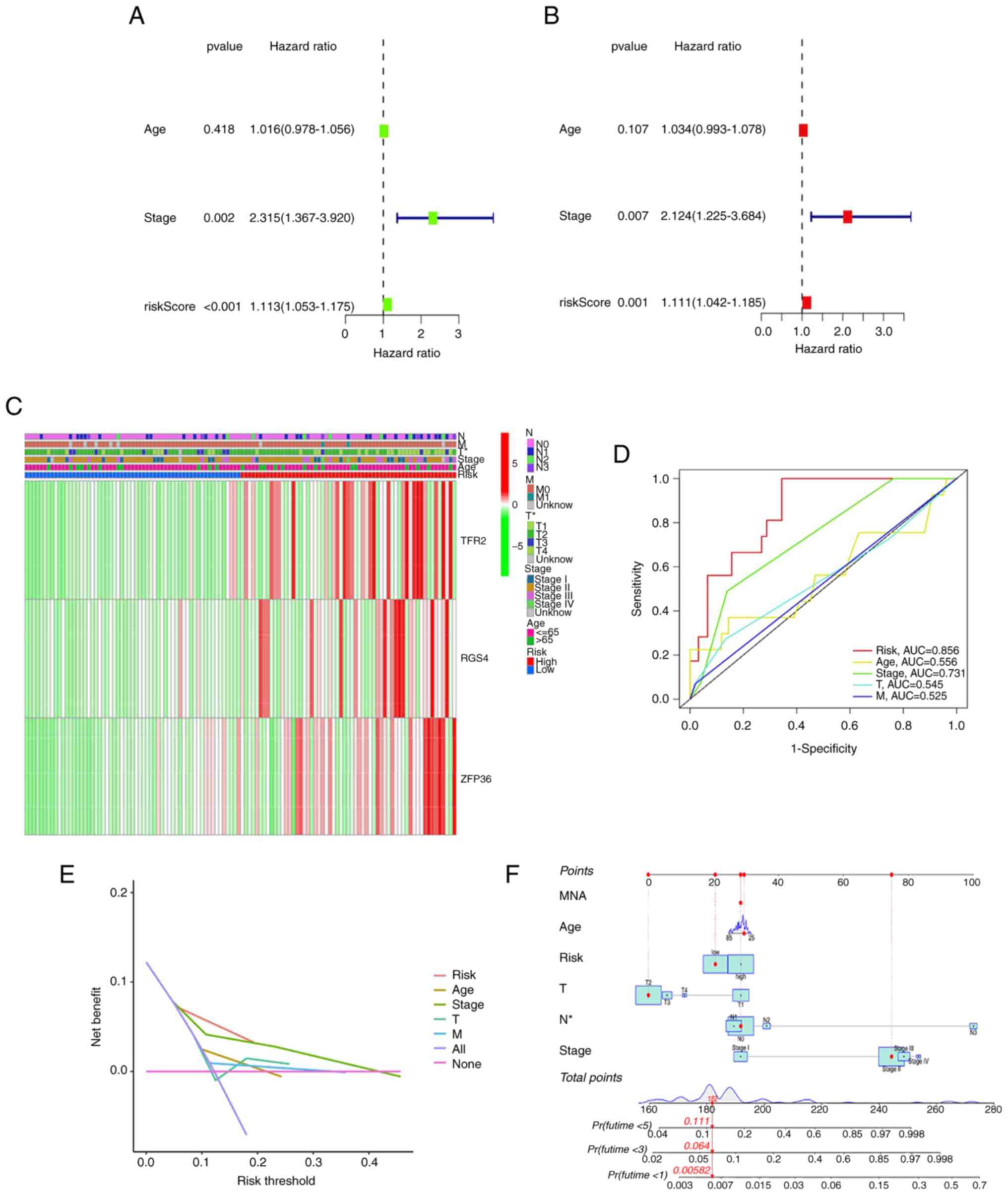
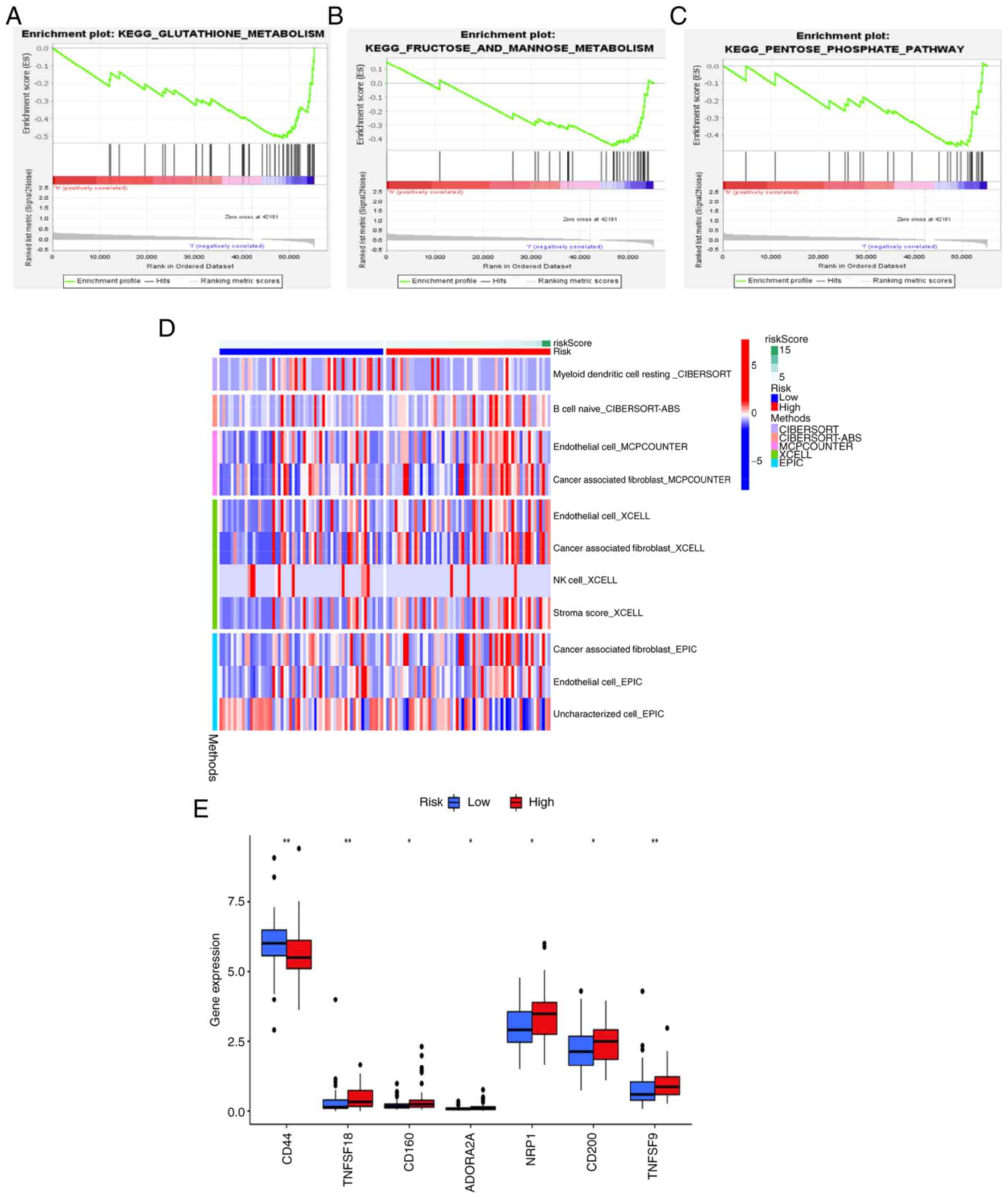
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma M, Turaga RC, Yuan Y, Satyanarayana
G, Mishra F, Bian Z, Liu W, Sun L, Yang J and Liu ZR:
Simultaneously targeting cancer-associated fibroblasts and
angiogenic vessel as a treatment for TNBC. J Exp Med.
218:e202007122021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marra A, Viale G and Curigliano G: Recent
advances in triple negative breast cancer: The immunotherapy era.
BMC Med. 17:902019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu B, Luo F, Sun B, Liu W, Shi Q, Cheng
SY, Chen C, Chen G, Li Y and Feng H: KAT6A Acetylation of SMAD3
regulates Myeloid-derived suppressor cell recruitment, metastasis,
and immunotherapy in Triple-Negative breast cancer. Adv Sci
(Weinh). 8:e21000142021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao W, Wang X, Zhou Y, Wang X and Yu Y:
Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor
immunotherapy. Signal Transduct Target Ther. 7:1962022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao Y, Shi Y, Gao Z, Sun Y, Yao F and Ma
L: Ferroptosis at the crossroads of tumor-host interactions,
metastasis, and therapy response. Am J Physiol Cell Physiol.
323:C95–C103. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sui S, Xu S and Pang D: Emerging role of
ferroptosis in breast cancer: New dawn for overcoming tumor
progression. Pharmacol Ther. 232:1079922022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh T, Beatty A and Peterson JR: The
AMPK-related kinase NUAK2 suppresses glutathione peroxidase 4
expression and promotes ferroptotic cell death in breast cancer
cells. Cell Death Discov. 8:2532022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sha R, Xu Y, Yuan C, Sheng X, Wu Z, Peng
J, Wang Y, Lin Y, Zhou L, Xu S, et al: Predictive and prognostic
impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer
treated with neoadjuvant chemotherapy. EBioMedicine. 71:1035602021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu G, Wang H, Li X, Huang R and Luo L:
Recent progress on targeting ferroptosis for cancer therapy.
Biochem Pharmacol. 190:1145842021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chemical Biol. 13:91–98. 2017. View Article : Google Scholar
|
|
13
|
Timmerman LA, Holton T, Yuneva M, Louie
RJ, Padró M, Daemen A, Hu M, Chan DA, Ethier SP, van't Veer LJ, et
al: Glutamine sensitivity analysis identifies the xCT antiporter as
a common triple-negative breast tumor therapeutic target. Cancer
Cell. 24:450–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen PH, Wu J, Ding CC, Lin CC, Pan S,
Bossa N, Xu Y, Yang WH, Mathey-Prevot B and Chi JT: Kinome screen
of ferroptosis reveals a novel role of ATM in regulating iron
metabolism. Cell Death Differ. 27:1008–1022. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang X, Ding CK, Wu J, Sjol J, Wardell S,
Spasojevic I, George D, McDonnell DP, Hsu DS, Chang JT and Chi JT:
Cystine addiction of triple-negative breast cancer associated with
EMT augmented death signaling. Oncogene. 36:43792017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu L, Tian Q, Jiang S, Gao H, Yu S, Zhou
Y, Yan Y, Ren Y, He J and Wang B: A novel Ferroptosis-Related gene
signature for overall survival prediction in patients with breast
cancer. Front Cell Dev Biol. 9:6701842021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Győrffy B: Survival analysis across the
entire transcriptome identifies biomarkers with the highest
prognostic power in breast cancer. Comput Struct Biotechnol J.
19:4101–4109. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie Y, Wolff DW, Wei T, Wang B, Deng C,
Kirui JK, Jiang H, Qin J, Abel PW and Tu Y: Breast cancer migration
and invasion depend on proteasome degradation of regulator of
G-protein signaling 4. Cancer Res. 69:5743–5751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan QH, Fan YH, Wang YZ, Li DM, Hu CE and
Li RX: Long noncoding RNA NNT-AS1 functions as an oncogene in
breast cancer via repressing ZFP36 expression. J Biol Regul Homeost
Agents. 34:795–805. 2020.PubMed/NCBI
|
|
21
|
Li D, Zhang J and Zhao X: Mechanisms and
molecular targets of artemisinin in cancer treatment. Cancer
Invest. 39:675–684. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng Y, Yu H, Zhang Y, Qu F, Tang Z, Qu C,
Tian J, Zong B, Wang Y, Ren H and Liu S: A ferroptosis-associated
gene signature for the prediction of prognosis and therapeutic
response in luminal-type breast carcinoma. Sci Rep. 11:176102021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Taie Z, Hannink M, Mitchem J,
Papageorgiou C and Shyu CR: Drug repositioning and subgroup
discovery for precision medicine implementation in triple negative
breast cancer. Cancers (Basel). 13:62782021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An Iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao M, Qin R, Huang W, Zhu HP, Peng F,
Han B and Liu B: Targeting regulated cell death (RCD) with
small-molecule compounds in triple-negative breast cancer: A
revisited perspective from molecular mechanisms to targeted
therapies. J Hematol Oncol. 15:442022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wen Y, Chen H, Zhang L, Wu M, Zhang F,
Yang D, Shen J and Chen J: Glycyrrhetinic acid induces
oxidative/nitrative stress and drives ferroptosis through
activating NADPH oxidases and iNOS, and depriving glutathione in
Triple-Negative breast cancer cells. Free Radic Biol Med.
173:41–51. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oliveira LJC, Amorim LC, Megid TBC, de
Resende CAA and Mano MS: Gene expression signatures in early breast
cancer: Better together with clinicopathological features. Crit Rev
Oncol Hematol. 175:1037082022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu ZH, Tang Y, Yu H and Li HD: The role of
ferroptosis in breast cancer patients: A comprehensive analysis.
Cell Death Discov. 7:932021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang W, Xu F, Zhao M and Zhang S:
Ferroptosis regulators, especially SQLE, play an important role in
prognosis, progression and immune environment of breast cancer. BMC
Cancer. 21:11602021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guda MR, Velpula KK, Asuthkar S, Cain CP
and Tsung AJ: Targeting RGS4 Ablates Glioblastoma proliferation.
Int J Mol Sci. 21:33002020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu Y, Zheng M, Wang S, Gao L, Gou R, Liu
O, Dong H, Li X and Lin B: Identification of a five-gene signature
of the RGS gene family with prognostic value in ovarian cancer.
Genomics. 113:2134–2144. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park HJ, Kim SH and Moon DO: Growth
inhibition of human breast carcinoma cells by overexpression of
regulator of G-protein signaling 4. Oncol Lett. 13:4357–4363. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yau C, Esserman L, Moore DH, Waldman F,
Sninsky J and Benz CC: A multigene predictor of metastatic outcome
in early stage hormone receptor-negative and triple-negative breast
cancer. Breast Cancer Res. 12:R852010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding Y, Li Y, Duan Y, Wang W, Zheng W,
Cheng W, Qi Y, Feng J, Chen Z, Yu T, et al: LncRNA MBNL1-AS1
represses proliferation and cancer Stem-Like properties of breast
cancer through MBNL1-AS1/ZFP36/CENPA Axis. J Oncol.
2022:99993432022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fang S, Zhao Y and Hu X: LncRNA
ADAMTS9-AS1 restrains the aggressive traits of breast carcinoma
cells via sponging miR-513a-5p. Cancer Manag Res. 12:10693–10703.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong X, Yang Y, Xu G, Tian Z, Yang Q, Gong
Y and Wu G: The initial expression alterations occurring to
transcription factors during the formation of breast cancer:
Evidence from bioinformatics. Cancer Med. 11:1371–1395. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Calzolari A, Deaglio S, Maldi E, Cassoni
P, Malavasi F and Testa U: TfR2 expression in human colon
carcinomas. Blood Cells Mol Dis. 43:243–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Calzolari A, Larocca LM, Deaglio S,
Finisguerra V, Boe A, Raggi C, Ricci-Vitani L, Pierconti F,
Malavasi F, De MariaR, et al: Transferrin receptor 2 is frequently
and highly expressed in glioblastomas. Transl Oncol. 3:123–134.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao QF, Ji J, Cai Q, Wang C, Shi M, Zhou
CF, Zhu ZG and Zhang J: Low expression of transferrin receptor 2
predict poor prognosis in gastric cancer patients. Kaohsiung J Med
Sci. 36:1014–1020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cao Y, Chen C, Tao Y, Lin W and Wang P:
Immunotherapy for Triple-Negative Breast Cancer. Pharmaceutics.
13:20032021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu X, Wang D, Chen W, Li N, Suwinski R,
Rossi A, Rosell R, Zhong J and Fan Y: A nomogram model based on
peripheral blood lymphocyte subsets to assess the prognosis of
non-small cell lung cancer patients treated with immune checkpoint
inhibitors. Transl Lung Cancer Res. 10:4511–4525. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Adams S, Schmid P, Rugo HS, Winer EP,
Loirat D, Awada A, Cescon DW, Iwata H, Campone M, Nanda R, et al:
Pembrolizumab monotherapy for previously treated metastatic
triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086
study. Ann Oncol. 30:397–404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hsu SK, Li CY, Lin IL, Syue WJ, Chen YF,
Cheng KC, Teng YN, Lin YH, Yen CH and Chiu CC: Inflammation-related
pyroptosis, a novel programmed cell death pathway, and its
crosstalk with immune therapy in cancer treatment. Theranostics.
11:8813–8835. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu S, Pan R, Lu J, Wu X, Xie J, Tang H and
Li X: Development and verification of a prognostic
Ferroptosis-Related gene model in Triple-Negative breast cancer.
Front Oncol. 12:8969272022. View Article : Google Scholar : PubMed/NCBI
|