Introduction
Head and neck squamous cell carcinoma is the sixth
most common type of tumor worldwide, of which oral squamous cell
carcinoma (OSCC) is the most frequently occurring (1,2). Every
year, nearly 300,000 new cases of OSCC and ~140,000 associated
deaths occur worldwide. The 5-year survival rate of patients with
OSCC is ~50% (3–5). Maxillofacial defects and the loss of
food and language functions that may occur in cases of OSCC
severely affect the physiology and psychology of patients (6). OSCC is characterized by occult onset
with high aggressiveness and invasiveness (7). Numerous patients with OSCC receive
treatment in the middle or late clinical stages of the disease.
Histopathological biopsies remain the primary diagnostic method for
OSCC; however, there are currently no precise biomarkers for the
diagnosis and prognosis of OSCC.
Biomarkers are diagnostic and prognostic tools
comprising laboratory indicators associated with diagnosis or
outcome of a disease (8).
High-throughput sequencing technology has revealed a variety of
genes that are associated with the early diagnosis, treatment and
prognosis of OSCC (9). Various
databases collect sequencing data and make it available to
researchers; these include The Cancer Genome Atlas (TCGA) database,
which has molecularly characterized >20,000 primary cancers and
matched normal samples spanning 33 types of cancer (10). Differentially expressed genes (DEGs)
between primary OSCCs and matched normal samples have been screened
in a previous study using a dataset from TCGA (11). Furthermore, several bioinformatics
tools, including Gene Ontology (GO) Resource, Kyoto Encyclopedia of
Genes and Genomes (KEGG) and Gene Set Variation Analysis (GSVA),
have been used in other studies to investigate the functions,
pathways and associations with survival, DNA repair and immunocyte
infiltration of DEGs (12,13).
In the present study, several notable DEGs in OSCC
were identified using data from TCGA and Gene Expression Omnibus
(GEO) databases. A series of bioinformatics methods and tools were
used to analyze the expression of hub genes in OSCC and their
association with the tumor immune microenvironment, immune
checkpoints and DNA repair genes.
Materials and methods
Data collection
Gene expression profiles for human OSCC and normal
oral mucosa and clinical information were obtained from TCGA
database (portal.gdc.cancer.gov/). The respiratory module was used
to search RNA sequencing (RNA-seq) data. The search parameters used
were: Primary site: ‘Base of tongue’, ‘floor of mouth’ and ‘other
and ill-defined sites in lip, oral cavity and pharynx’; Program:
TCGA; Disease type: Squamous cell neoplasms; Data category:
Biospecimen; Experimental strategy: RNA-seq. The criteria used when
screening the data for download were as follows: Samples from oral
cancer sites (alveolar ridge, base of tongue, buccal mucosa, floor
of mouth, hard palate, oral cavity and oral tongue) were included;
samples of non-oral cavity cancer sites (hypopharynx, larynx, lip,
oropharynx and tonsil) were excluded. The mRNA expression data and
clinical information of the patients, as well as chart, manifest,
metadata, clinical and other files associated with the samples were
downloaded using the GDC Data Transfer Tool
(gdc.cancer.gov/access-data/gdc-data-transfer-tool). Finally, 361
patient samples, comprising 329 tumor samples and 32 controls with
complete clinical data were included in the study.
Identification of DEGs
The DEseq2 (version 1.26.0) package in R software
(version 3.6.3) (Microsoft, WA, USA) was used to identify DEGs. The
original RNA-seq data was corrected by normalization to transcripts
per million, using the limma package in R (version 3.6.3;
bioconductor.org/packages/release/bioc/html/limma.html) for data
filling, merging, correction and matrix fusion. The Ensembl gene
IDs were transformed to gene names by human gene annotation using
the GENCODE website (https://www.gencodegenes.org/). When identifying the
DEGs, an absolute log2 fold change
(|log2FC|)>2 and adjusted P<0.05 were set as the
cut-off criteria. Volcano plots were generated using the R package
ggplot2 (version 3.3.3). Heat maps were generated using the
ComplexHeatmap R package (version 2.2.0). The expression of DKK1 in
OSCC and normal tissue was analyzed based on data from TCGA
database using he R package DESeq2. Two OSCC GEO Dataset (GSE3524
and GSE37991) (14,15) were also selected for confirming the
expression of DKK1 in OSCC and normal tissues. The R package DESeq2
was used for differential expression analysis.
GO and KEGG enrichment analysis of
DEGs
To explore the potential functions of the DEGs,
functional enrichment analysis was performed. A conversion package
(org.Hs.eg.db; Version 3.8) from Bioconductor was
used to annotate the DEGs. The GO tool (GO;http://www.geneontology.org/) was used to categorize
the genes with regard to molecular function (MF), biological
pathway (BP) and cellular component (CC). GO enrichment analyses
were performed using the clusterProfiler package module (version
3.14.3) in R, and the top four results of each group were
identified. KEGG pathway enrichment analysis was also performed
using clusterProfiler. Terms with P<0.05 were considered to be
statistically significantly different and to meet the criteria and
thresholds for enriched pathways.
Key gene screening
The patient population was also screened for
potential prognostic genes affecting overall survival (OS) in OSCC.
The patients were divided into high and low expression groups based
on the median expression of the DEGs. Following Kaplan-Meier (KM)
analysis of the DEGs, several significantly expressed genes in OSCC
were obtained and potential key genes were searched through the
VennDiagram package in R (ggplot2 version: 3.3.3). To evaluate the
interactions between proteins, the STRING (http://string-db.org) online database was used. The
potentially key genes were input into the module ‘Multiple
proteins’, and protein interaction data with confidence >0.4
were selected as passing the threshold used to define an
interaction. The protein-protein interaction (PPI) networks were
then visualized and downloaded using Cytoscape software (version
3.7.2; Institute for Systems Biology).
Survival analysis
Univariate Cox analysis was used to analyze the
association between Dickkopf Wnt signaling pathway inhibitor 1
(DKK1) expression and OS for various types of cancer. The KM method
was used to investigate the relationship of DKK1 expression with OS
and disease-specific survival (DSS). The samples were divided into
DKK1 high and low expression groups based on the median expression
level of DKK1. Univariate Cox survival analysis was performed using
the R package survminer (version 0.4.9), and the results were
visualized using the R package ggplot2 (version 3.3.3) and forest
plots.
Immunocorrelation analysis
The correlations between DKK1 expression and immune
cell infiltration in OSCC were analyzed using the R package GSVA
(version 1.34.0). In addition, a correlation analysis of DKK1 with
immune checkpoint-associated genes in OSCC was performed using the
same R package.
DNA repair gene correlation
analysis
The correlation of DKK1 expression in OSCC with the
expression of five mismatch repair (MMR) genes, namely epithelial
cell adhesion molecule (EPCAM), MutL homolog 1 (MLH1), MutS homolog
2 (MSH2), MSH6 and PMS1 homolog 2 (PMS2), was evaluated using
expression profile data from TCGA. Visual analysis was performed
using ggplot 2 (version 3.3.3).
Construction of lentiviral
particles
Lentiviral particles carrying a short hairpin RNA
(shRNA) targeting DKK1 or a shRNA control (Lv-shDKK1 and Lv-shCon,
respectively) were designed and synthesized by Shanghai Genechem.
Briefly, shCon and shDKK1 were inserted into a GV248 lentiviral
vector (Shanghai GeneChem). The vector (1.5 µg) carrying shCon or
shDKK1 and Lenti-Easy Packaging Mix (Shanghai GeneChem) (1.5 µg)
were transfected into 293T cells (#bio-12947; Biobw) with Lipo6000™
reagent (#C0526; Beyotime Institute of Biotechnology) at 37°C for
24 h, and the lentiviral particles were collected by centrifugation
at 80,000 × g and 4°C for 2 h using an ultra-centrifuge (Beckman
Coulter, Inc.). The shRNA sequences were as follows: sh-DKK1,
5′-CACCGCTCTCATGGACTAGAAATATTTGATATCCGATATTTCTAGTCCATGAGAGC-3′; and
shCon,
5′-CACCTCTGTCAATTAGGACAAGCTTATGATATCCGTAAGCTTGTCCTAATTGACAGA-3′.
Subsequent experiments were performed 24 h later.
Cell culture
The OSCC cell lines CAL-27 and SCC15 were purchased
from the American Type Culture Collection. The cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; cat. no. BL304A;
Biosharp Life Sciences) containing 10% fetal bovine serum (FBS;
cat. no. AB-FBS0500; Animal Blood Ware) at 37°C with 5%
CO2.
Lentiviral infection
CAL-27 and SCC15 cells were seeded into a 12-well
plate at 20,000 cells per well. When a confluence of 40–50% was
reached, Lv-shCon or Lv-shDKK1 at a multiplicity of infection of 10
was added to each well. After infection for 24 h, the medium
containing the lentivirus was replaced with DMEM without
lentivirus. At 72 h after infection, the cells infected with the
lentivirus were selected using 1 µg/ml puromycin (Beyotime
Institute of Biotechnology). Reverse transcription-quantitative PCR
(RT-qPCR) was used to confirm the knockdown of DKK1.
RNA extraction and RT-qPCR
Cells were lysed using TRIzol® reagent
(cat. no. 15596; Thermo Fisher Scientific, Inc.), and RNA was
extracted from the lysates using chloroform and separated using
isopropanol. The RNA concentration was determined using an
ultramicro spectrophotometer (Implen GmbH). The RNA was
reverse-transcribed into cDNA using a BeyoRT™ M-MuLV Reverse
Transcriptase kit (cat. no. 7268; Beyotime Institute of
Biotechnology) according to the manufacturer's instructions. The
cDNA was then subjected to qPCR using a BeyoFast™ SYBR Green qPCR
Mix kit (cat. no. 7260; Beyotime Institute of Biotechnology)
according to the manufacturer's instructions following the
thermocycling conditions: 95°C 10 min; (95°C, 15 sec; 60°C, 1 min)
×40 cycles. The primers used were as follows: DKK1 forward,
5′-GAATAAGTACCAGACCATTGAC-3′ and reverse,
5′-CCATTTTTGCAGTAATTCCC-3′; β-actin forward,
5′-CGGGAAATCGTGCGTGAC-3′ and reverse, 5′-CAGGCAGCTCGTAGCTCTT-3′.
The expression of target genes was quantified using β-actin as the
reference gene via the 2−ΔΔCq method (16).
Wound healing assay
CAL-27 and SCC-9 cells were seeded in 6-well plates
at a density of 500,000 cells/well. When the cells reached 90%
confluence, a wound was created by drawing a straight line through
the cells using a pipette tip. The cells were then cultured in DMEM
without FBS, and the wounds were observed and evaluated under an
inverted light microscope (IX53, Olympus, Tokyo, Japan) at 0, 24
and 48 h. The wound healing rate was calculated as follows:
(Original wound area-non-healing wound area)/original wound area
×100.
Transwell assay
The invasion capacity of the cells was evaluated
using a Transwell assay. Briefly, the upper Transwell membrane was
pre-coated with Matrigel (E6909; Sigma-Aldrich; Merck KGaA) at 37°C
for 4 h. CAL-27 and SCC-9 cells were starved for 24 h in serum-free
DMEM to stop cell proliferation. After that, 100,000 cells in 200
µl FBS-free DMEM were seeded in the upper chamber of the Transwell
system, while 500 µl medium containing 10% FBS was placed in the
lower chamber. After culture for 12 h at 37°C, cells crossing the
membrane were stained with crystal violet (cat. no. C0121; Beyotime
Institute of Biotechnology) for 3 min at 20°C. The cells were then
observed and counted under a light microscope (IX53, Olympus,
Tokyo, Japan).
CCK-8 assay
A CCK-8 assay was used to evaluate cell
proliferation ability. Briefly, the CAL-27 and SCC-9 cells were
seeded in a 96-well plate at 5,000 cells/well. After culture for 1,
24 and 48 h, cells were incubated in 10 µl CCK-8 reagents (cat. no.
C0037; Beyotime Institute of Biotechnology) for 1 h at 37°C. Cell
proliferation was determined by calculating the optical density at
450 nm.
Colony formation assay
The ability of the cells to form colonies was
determined using 6-well plates seeded with 1,000 cells/well. The
CAL-27 and SCC-9cells were incubated for 12 days, then fixed with
4% paraformaldehyde for 5 min at 20°C, washed with
phosphate-buffered saline and stained with crystal violet for 5 min
at 20°C. Clonogenicity was observed and the colonies were
photographed using an inverted microscope. The colonies larger than
0.3 mm were calculated using imageJ software (version 1.8.3)
(NIH).
Western blot analysis
Proteins were extracted from SCC-9 and CAL-27 cells
by lysis using RIPA buffer (cat. no. C1053; Applygen Technologies,
Inc.). Protein concentration was determined using a BCA kit (cat.
no. P0010; Beyotime Institute of Biotechnology) and total proteins
(30 µg/lane) were separated using 10% SDS-PAGE and then transferred
to a PVDF membrane (cat. no. FFP22; Beyotime Institute of
Biotechnology). The membrane was blocked with 5% fat-free milk
(cat. no. P0216; Beyotime Institute of Biotechnology) at 25°C for 1
h, incubated with primary antibodies [1:1,000 diluted in
Tris-buffered saline with 1% Tween 20 (TBST)] for 12 h at 4°C and
then incubated with secondary antibodies (1:10,000 diluted in TBST)
for 1 h at 20°C. Finally, the membranes were incubated in ECL
reagent (cat. no. P0018; Beyotime Institute of Biotechnology) for 1
min at 20°C and developed using a chemiluminescence imager (Tanon
Science & Technology Co., Ltd.). β-actin antibody (cat. no.
sc-8432) was purchased from Santa Cruz Biotechnology, Inc. Wnt-3a
antibody (cat. no. 2391), DKK1 antibody (cat. no. 4687) and
β-catenin (cat. no. 8480) antibody were purchased from Cell
Signaling Technology, Inc. Goat anti-rabbit HRP-secondary antibody
(cat. no. A0208) and goat anti-mouse HRP-secondary antibody (cat.
no. A0216) were purchased from Beyotime Institute of
Biotechnology.
ELISA
Secreted DKK1 in the culture supernatant was
detected using a Human DKK1 ELISA Kit (cat. no. EK0867; Wuhan
Boster Biological Technology, Ltd.) following the manufacturer's
instructions. Briefly, 100 µl supernatant or standards were added
to the plate pre-coated with a DKK1 antibody. Subsequently, 2 µl
DKK1 detector antibody was added and the plate was incubated in a
second antibody with horseradish peroxidase for 1 h at 37°C. After
washing the plate three times with wash solution, ABC working
reagent was added. The color reaction was performed by adding 90 µl
tetramethylbenzidine reagent for 20 min in the dark and terminated
by adding 100 µl stopping reagent. The optical density at 450 nm
was measured using a microplate reader (Molecular Devices, LLC). A
standard curve was established based on the optical density of the
standards and used to calculate the DKK1 concentration in the
supernatant.
Cell apoptosis
Cell apoptosis was detected using an AnnexinV/PI
staining kit (cat. no. APOAF-20TST; Sigma-Aldrich; Merck KGaA).
Briefly, 5×105 SCC-9 or CAL-27 cells were harvested and
suspended in 500 µl binding buffer. Then 5 µl Annexin V/PI staining
reagent was added and mixed with the cell suspension. Following
incubation for 10 min, cell apoptosis was determined using a flow
cytometer (BD LSRFortessa™; BD Biosciences) and analyze using the
FlowJo software (version 10.4.0) (FlowJo, OR, USA).
Statistical analysis
The difference in gene expression between the two
groups was analyzed using the Wilcoxon rank-sum test, except for
GSE37991 for which Welch's t-test was used. Cox regression analysis
was used to determine the survival probabilities of the two groups.
KM plots were drawn and the relationships between various factors
and survival outcomes were analyzed using Cox regression. The
correlation of DKK1 expression with immune factors and DNA repair
genes in OSCC was analyzed using Spearman's correlation method.
Data from the RT-qPCR, wound healing, Transwell, CCK-8 and colony
formation assays are presented as the mean ± SD and were analyzed
using unpaired Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
DEGs in OSCC
The clinical features of 361 samples, including 329
samples of OSCC tissue and 32 samples of normal control tissue,
were obtained from TCGA data portal. The total number of Gene IDs
was 56,494, and the number of those satisfying the threshold
|log2(FC)|>2 and P<0.01 was 3,226; the number with
higher expression in the tumor group than the normal group
(positive logFC) was 1,524, and the number with lower expression in
the tumor group than the normal group (negative logFC) was 1,702 as
determined using the DEseq2 package with
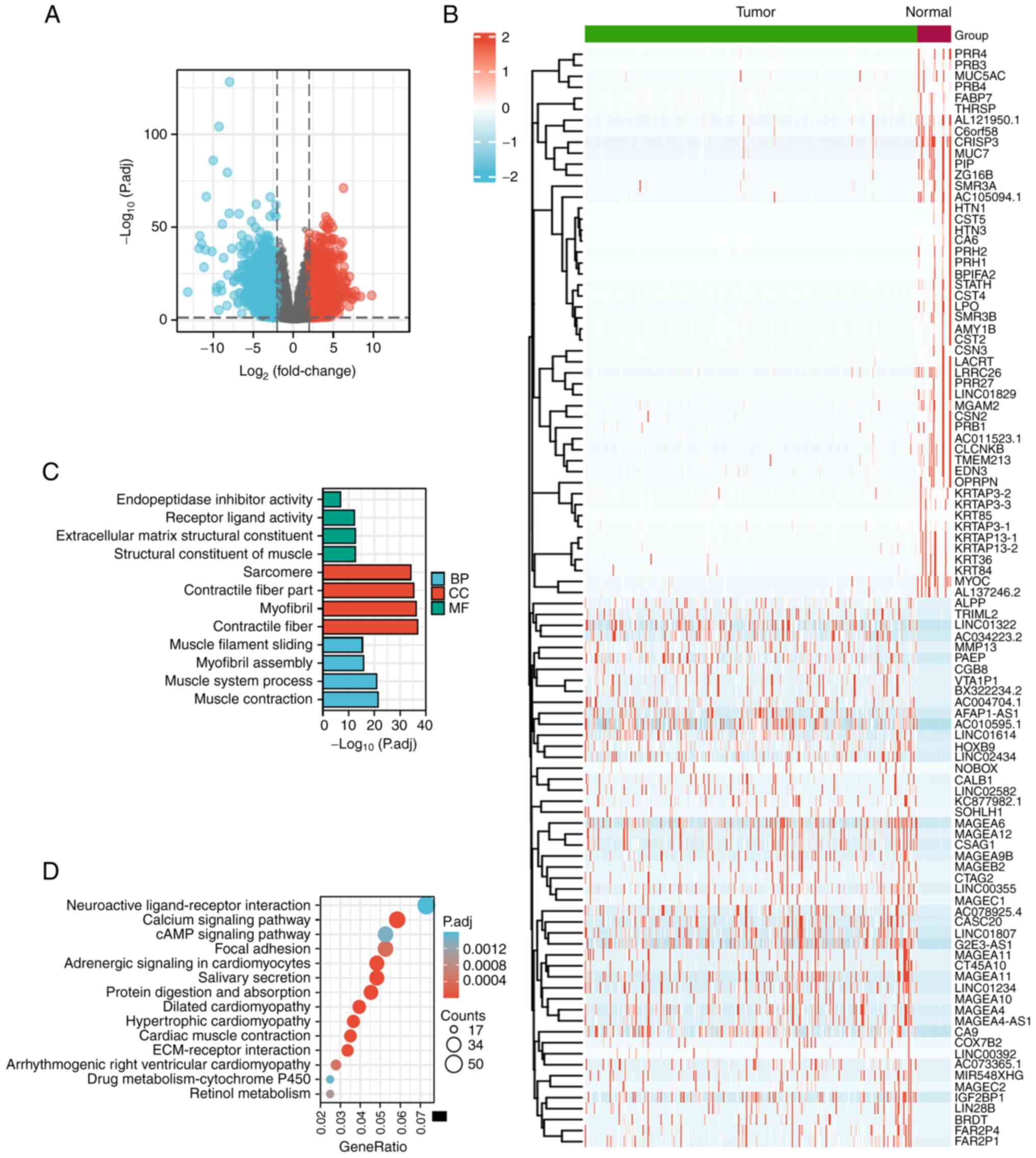
|log2(FC)|>2, P<0.05 (Fig. 1A).
The heat map in Fig.
1B shows the top 100 DEGs. These 100 DEGs were subjected to GO
enrichment analysis. The DEGs were significantly enriched in the BP
terms ‘muscle contraction’, ‘muscle system process’, ‘myofibril
assembly’ and ‘muscle filament sliding’. In addition, the enriched
MF terms comprised ‘structural constituent of muscle’,
‘extracellular matrix structural constituent’, ‘receptor ligand
activity’ and ‘endopeptidase inhibitor activity’ and the enriched
CC terms comprised ‘contractile fiber’, ‘myofibril’, ‘contractile
fiber part’ and ‘sarcomere’ (Fig.
1C). KEGG pathway analysis revealed that the DEGs were mainly
enriched in the pathways ‘salivary secretion’, ‘protein digestion
and absorption’, ‘dilated cardiomyopathy’, ‘hypertrophic
cardiomyopathy’, ‘cardiac muscle contraction’, ‘calcium signaling
pathway’ and ‘neuroactive ligand-receptor interaction’ (Fig. 1D).
Screening the prognostic genes of
OSCC
Through the univariate Cox analysis of genes in
OSCC, 2,130 protein-coding genes were found to be associated with
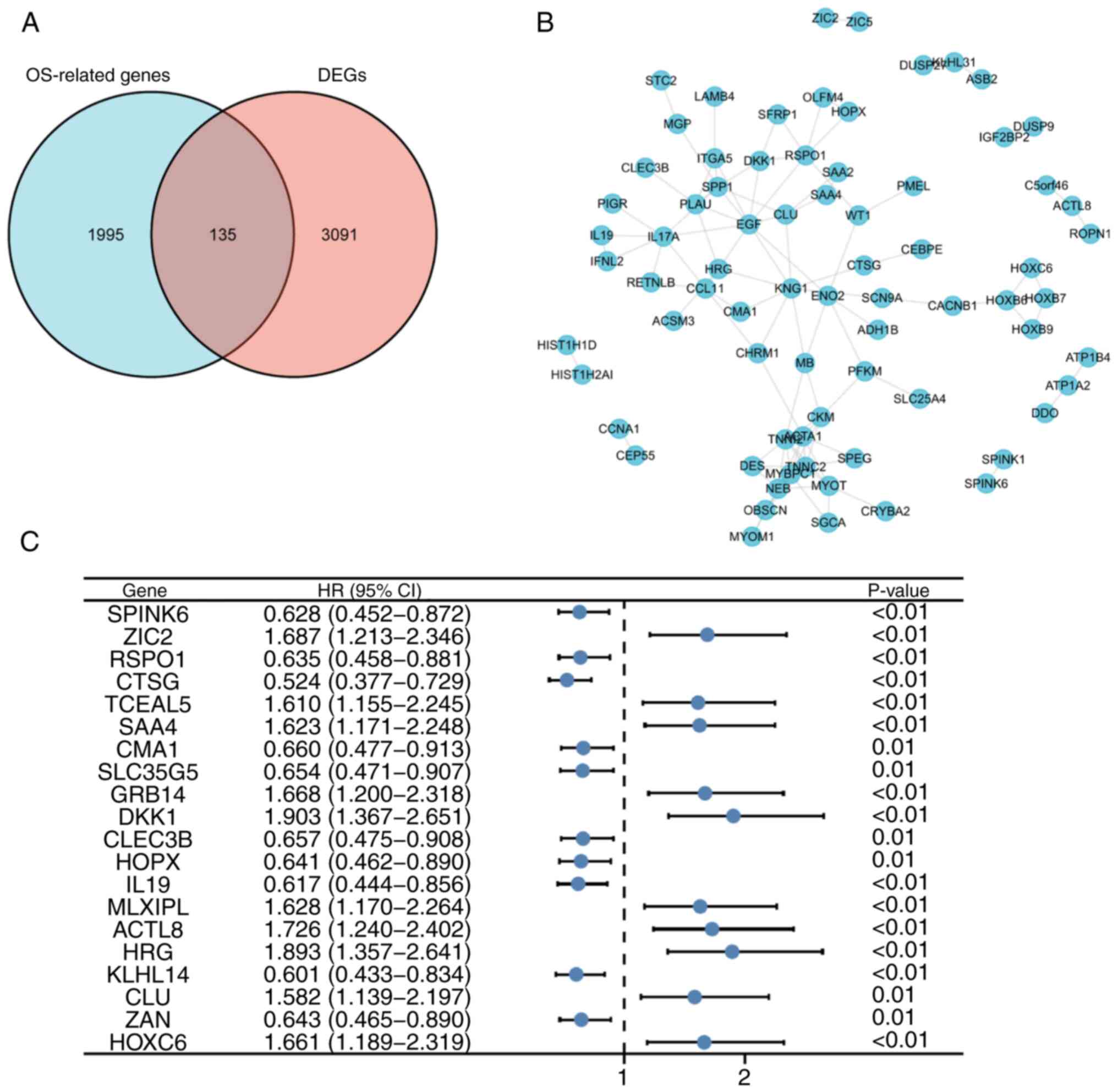
OS. Comparison of these 2,130 genes with the 3,226 DEGs identified
that 135 key DEGs in OSCC were common to both datasets (Fig. 2A). The STRING online database was
used to determine interactions among the proteins encoded by the
key DEGs, and a PPI network was constructed (Fig. 2B). Based the PPI network, the top 20
genes according to statistical significance (10 positive and 10
negative associations) were selected, among which DKK1 showed the
highest association with OSCC OS (hazard ratio=1.903; Fig. 2C).
Selection and validation of hub gene
signatures
The expression of DKK1 in OSCC and normal tissues
was analyzed based on data from TCGA database, which showed that
DKK1 expression levels were higher in OSCC tissue than in normal
control tissues (Fig. 3A). To
verify the expression of DKK1 in OSCC, DKK1 expression was analyzed
in two GEO gene expression datasets: GSE3524 and GSE37991. In
GSE3524, the expression of DKK1 in OSCC was higher than that in
normal oral mucosa, and the median difference between the two
groups was 1.285 (0.716–2.102), which was statistically significant
(Fig. 3B); the analysis of GSE37991
provided a similar result, as the median difference between the two
groups was 1.224 (0.978–1.47), which was also statistically
significant (Fig. 3C). The
expression of DKK1 in tumor and normal tissues was also compared in
33 tumor types based on TCGA data, as shown in Fig. 3D. The results revealed that DKK1
expression was significantly changed in 12 types of tumor, among
which DKK1 was upregulated in colon adenocarcinoma,
cholangiocarcinoma (CHOL), esophageal carcinoma (ESCA), head and
neck squamous cell carcinoma (HNSC), liver hepatocellular
carcinoma, lung squamous cell carcinoma (LUSC), stomach
adenocarcinoma (STAD) and thyroid carcinoma compared with the
corresponding normal tissues, and downregulated in bladder
urothelial carcinoma (BLCA), kidney chromophobe, kidney renal
papillary cell carcinoma and prostate adenocarcinoma (PRAD)
compared with the corresponding normal tissue (Fig. 3D).
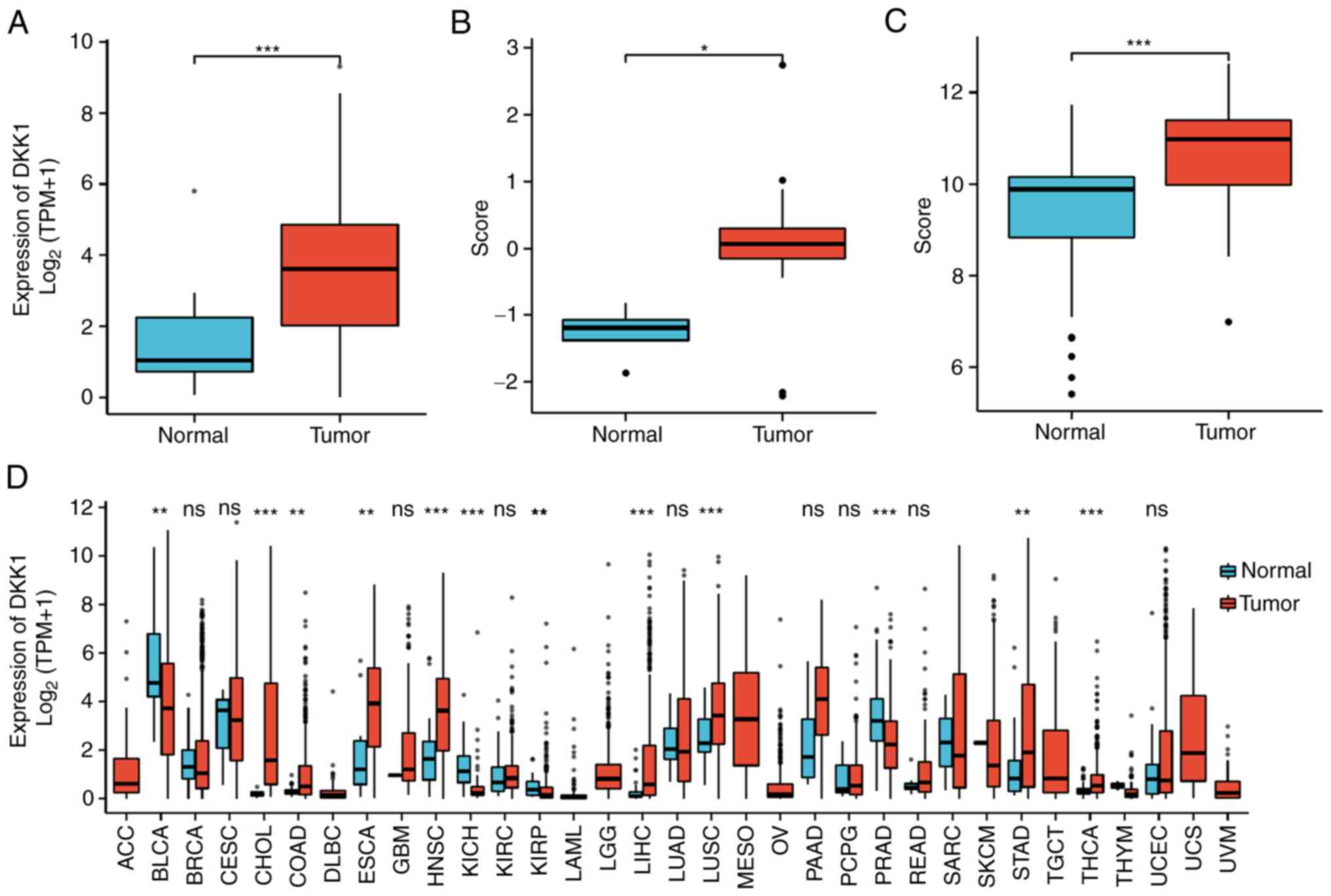 | Figure 3.Expression of DKK1 in various tumor
types. Expression levels of DKK1 in (A) The Cancer Genome Atlas,
(B) GSE3524 and (C) GSE37991 oral squamous cell carcinoma datasets.
(D) Expression level of DKK1 in 33 cancer types. *P<0.05,
**P<0.01, ***P<0.001. DKK1, Dickkopf Wnt signaling pathway
inhibitor 1; TPM, transcripts per million; ns, not significant;
ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma;
BRCA, breast invasive carcinoma; CESC, cervical squamous cell
carcinoma and endocervical adenocarcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid
neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma;
GBM, glioblastoma multiforme; HNSC, head and neck squamous cell
carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell
carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute
myeloid leukemia; LGG, brain lower grade glioma; LIHC, liver
hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung
squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous
cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG,
pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma;
READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous
melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell
tumors; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine
corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM,
uveal melanoma. |
Prognostic analysis of DKK1 expression
pan-cancer
The association of DKK1 expression with OS and DSS
in the 12 types of tumor was calculated using univariate survival
analysis. The results shown in Fig.
4A indicate that DKK1 was significantly associated with OS in
ESCA, HNSC and STAD. KM analysis shows that low DKK1 expression was
associated with poor OS prognosis in patients with ESCA (Fig. 4B), while high DKK1 expression was
associated with poor OS prognosis in patients with HNSC (Fig. 4C) and STAD (Fig. 4D). DKK1 expression was also found to
be significantly associated with DSS in HNSC and STAD (Fig. 4E), and KM analysis suggested that
high DKK1 expression was associated with poor prognosis in patients
with HNSC (Fig. 4F) and STAD
(Fig. 4G).
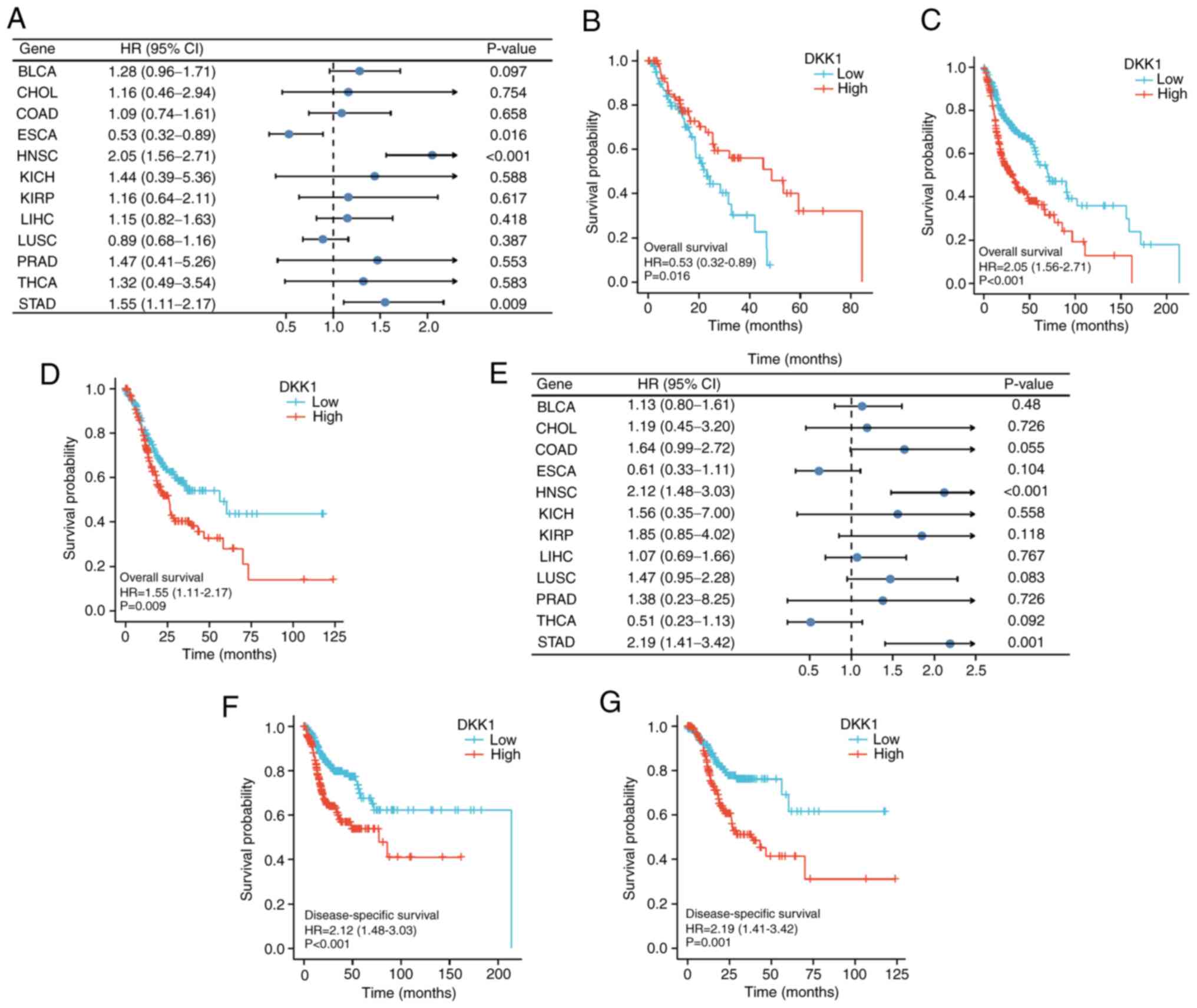 | Figure 4.Univariate survival analysis of the
association between DKK1 expression and survival time in 12 tumor
types. (A) Forest plot showing the relationship between DKK1
expression and OS. KM curves of high and low DKK1 expression in (B)
ESCA, (C) HNSC and (D) STAD reveal a significant association with
OS. (E) Forest plot showing the relationship between DKK1
expression and DSS. KM curves of high and low DKK1 expression in
(F) HNSC and (G) STAD show a significant association with DSS.
DKK1, Dickkopf Wnt signaling pathway inhibitor 1; OS, overall
survival; DSS, disease-specific survival; KM, Kaplan-Meier; BLCA,
bladder urothelial carcinoma; CHOL, cholangiocarcinoma; COAD, colon
adenocarcinoma; ESCA, esophageal carcinoma; HNSC, head and neck
squamous cell carcinoma; KICH, kidney chromophobe; KIRP, kidney
renal papillary cell carcinoma; LIHC, liver hepatocellular
carcinoma; LUSC, lung squamous cell carcinoma; PRAD, prostate
adenocarcinoma; STAD, stomach adenocarcinoma; THCA, thyroid
carcinoma; HR, hazard ratio. |
Correlation analysis of DKK1 and
immune cells
The correlations between DKK1 expression and the
infiltration of 24 immune cell types in OSCC were analyzed
(Fig. 5A). Among these immune
cells, T, regulatory T (TReg), plasmacytoid dendritic cell (pDC), T
helper 17 (Th17), T follicular helper (TFH), cytotoxic and B cells
showed a significant negative correlation with DKK1 expression.
However, Th2 cells and gdT (Tgd) cells showed a significant
positive correlation with DKK1 expression in OSCC (Fig. 5B-J).
 | Figure 5.Correlation analysis of DKK1 and
immune cell infiltration. (A) Correlation of DKK1 with the
infiltration of a panel of immune cells. Negative correlation of
DKK1 expression with the level of infiltration of (B) T cells, (C)
Treg cells, (D) pDC cells, (E) Th17 cells, (F) TFH cells, (G)
cytotoxic cells and (H) B cells. Positive correlation of DKK1
expression with the level of infiltration of (I) Th2 cells and (J)
Tgd cells. DKK1, Dickkopf Wnt signaling pathway inhibitor 1; DC,
dendritic cell; aDC, activated DC; iDC, inflammatory DC; pDC,
plasmacytoid DC; NK, natural killer; Tcm, T central memory; Tem, T
effector memory; TFH, T follicular helper; Tgd, γδT; Th, T helper;
TReg, regulatory T; TPM, transcripts per million. |
Correlation between DKK1 and immune
checkpoints
Correlation analysis between DKK1 and genes
associated with immune monitoring checkpoints in OSCC was performed
using the R package GSVA. In total, 15 immune checkpoint genes were
identified that correlated with DKK1 in the OSCC samples (Fig. 6). Among these genes, CD40,
CD44, v-set domain containing T cell activation inhibitor 1
(VTCN1), neuropilin 1 (NRP1), programmed cell death 1
ligand 2 (PDCD1LG2), CD276, CD80 and CD86 were
positively correlated with DKK1 expression, whereas TNF receptor
superfamily member 18 (TNFRSR18), CD27, T cell
immunoreceptor with Ig and ITIM domains (TIGIT), indoleamine
2,3-dioxygenase 2 (IDO2), CD48, CD244 and CD40 ligand
(CD40LG) were negatively correlated with DKK1.
 | Figure 6.Correlation between DKK1 and genes
associated with immune checkpoints in oral squamous cell carcinoma.
DKK1 expression was positively correlated with the expression
levels of (A) CD40, (B) CD44, (C) VTCN1, (D) NRP1, (E) PDCD1LG2,
(F) CD276, (G) CD80 and (H) CD86 and negatively correlated with
expression levels of (I) TNFRSR18, (J) CD27, (K) TIGIT, (L) IDO2,
(M) CD48, (N) CD244 and (O) CD40LG. DKK1, Dickkopf Wnt signaling
pathway inhibitor 1; VTCN1, v-set domain containing T cell
activation inhibitor 1; NRP1, neuropilin 1; PDCD1LG2, programmed
cell death 1 ligand 2; TNFRSR18, TNF receptor superfamily member
18; TIGIT, T cell immunoreceptor with Ig and ITIM domains; IDO2,
indoleamine 2,3-dioxygenase 2; CD40LG, CD40 ligand; TPM,
transcripts per million. |
DNA repair gene correlation
analysis
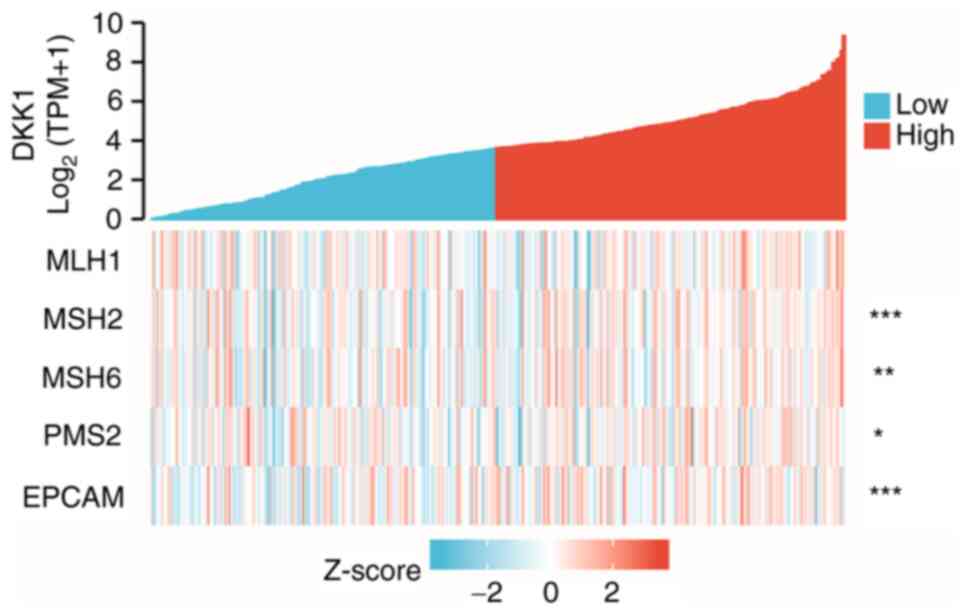
The correlation of DKK1 expression in OSCC with the
DNA MMR genes EPCAM, MLH1, MSH2, MSH6 and PMS2 was
evaluated using expression profile data from TCGA. As shown in
Fig. 7, DKK1 expression was
significantly correlated with the DNA repair genes MSH2, MSH6,
PMS2 and EPCAM but not with MLH1.
Knockdown of DKK1 inhibits cell
proliferation, clonogenicity, migration and invasion in OSCC
cells
To further elucidate the role of DKK1 in OSCC
progression, its influence on cell proliferation, migration and
invasion was investigated in vitro. DKK1 was knocked down in
the OSCC cell lines CAL-27 and SCC-9 using lentiviral infection. As
shown in Fig. 8A and B, the green
fluorescence signal indicated that the SCC-9 and CAL-27 cells were
infected with Lv-shCon or Lv-shDKK1. The knockdown efficiency was
evaluated by RT-qPCR (Fig. 8C); the
knockdown efficiencies of DKK1 in the SCC-9 and CAL-27 cells were
71.15 and 66.59%, respectively. Considering that DKK1 is a secreted
protein, the secreted form of DKK1 was detected by ELISA, and was
observed to decrease significantly after DKK1 knockdown (Fig. 8D). The role of DKK1 in cell
proliferation, colony formation, migration and invasion was
determined. The proliferation of SCC-9 and CAL-27 cells was
inhibited by DKK knockdown (Fig. 8E and
F), and the colony forming capacity of the cells was also
reduced (Fig. 8G). Furthermore, the
wound healing assay showed that the knockdown of DKK1 inhibited
cell migration (Fig. 8H-K), and the
Transwell assay indicated that the knockdown of DKK1 inhibited cell
invasion (Fig. 8L-N). Furthermore,
the expression of proteins associated with Wnt signaling downstream
of DKK1 was investigated, and the results indicated that Wnt-3a and
b-catenin levels increased after DKK1 knockdown (Fig. 8O).
 | Figure 8.Knockdown of DKK1 inhibits cell
proliferation, colony formation, migration and invasion in oral
squamous cell carcinoma cells. Bright-field and GFP fluorescence
images of (A) SCC-9 cells and (B) CAL-27 cells with Lv-shCon and
Lv-shDKK1 infection. Scale Bar=50 µm. (C) DKK1 mRNA expression and
(D) DKK1 secretion by SCC-9 cells and CAL-27 cells infected with
Lv-shCon and Lv-shDKK1. Cell proliferation rate of (E) SCC-9 and
(F) CAL-27 cells. (G) Colony formation of SCC-9 cells and CAL-27
cells. (H) Representative wound healing images of SCC-9 cells and
(I) quantification of the wound healing assay. (J) Representative
wound healing images of CAL-27 cells and (K) quantification of the
wound healing assay, magnification: 40×. Representative images of
the Transwell invasion assay for (L) SCC-9 and (M) CAL-27 cells,
magnification: 100×. (N) Cell counts of invaded SCC-9 and CAL-27
cells. (O) Western blot analysis of Wnt-3a, β-catenin and DKK1
expression in SCC-9 and CAL-27 cells infected with Lv-shCon and
Lv-shDKK1. *P<0.05 **P<0.01 and ***P<0.001 for shDKK1 vs.
shCon. DKK1, Dickkopf Wnt signaling pathway inhibitor 1; GFP, green
fluorescent protein; Lv, lentivirus; shCon, shRNA control; shDKK1,
shRNA targeting DKK1; shRNA, short hairpin RNA. |
Discussion
Accurate cancer biomarkers can be used to predict
the prognostic risk of patients and formulate individual
therapeutic strategies (17). The
present study aimed to identify the hub DEGs associated with OSCC
prognosis and explore their potential contributions to biological
processes and functions. Bioinformatic tools were used as they can
effectively compensate for the shortcomings of sequencing data
analysis and integrate various existing sequencing data to further
investigate their clinical significance (18).
In the present study, data on OSCC from TCGA
database were filtered and 3,226 DEGs were identified. In addition,
2,130 genes associated with OSCC OS-were identified using
univariate Cox analysis. Furthermore, by examining the intersection
of the DEGs with the OS-related genes, 135 key genes were
identified, among which DKK1 showed the greatest prognostic
association with OS.
According to the dataset from TCGA and the two GEO
datasets, GSE3524 and GSE37991, DKK1 is upregulated in OSCC
tissues. However, DKK1 is not upregulated in all tumor types; as
TCGA database suggests, DKK1 is upregulated in several tumors,
including CHOL, ESCA, LUSC and STAD, whereas it is downregulated in
other tumors, including BLCA and PRAD. These results indicate that
the expression of DKK1 may be tumor type-specific. Subsequently,
the prognostic value of DKK1 was analyzed in various types of
cancer, and the results showed that the expression of DKK1 was
significantly increased in HNSC and STAD compared with normal
tissue, and that the OS and DSS of the high-expression DKK1 group
were significantly lower than those of the low-expression DKK1
group in HNSC and STAD, indicating the prognostic value of DKK1 in
these tumors. In the in vitro experiments performed in the
current study, DKK1 knockdown decreased OSCC cell migration and
invasion. These results are inconsistent with those in a previous
study (19), which showed that
DKK1-positive cases were significantly associated with a low risk
of regional lymph node metastasis, and cellular migration and
invasion were negatively regulated by DKK1 knockdown. However, a
report by Wang et al (20)
supports the present study, as it showed that the proliferation and
migration of OSCC cells were inhibited by the inhibition of DKK1.
Therefore, the role of DKK1 in OSCC cell migration and invasion is
unclear. In the study by Ogoshi et al, it was shown that
DKK1 regulates the phosphorylation of β-catenin in cell nuclei;
therefore, the authors speculated that DKK1 regulates cellular
migration and invasion through the regulation of β-catenin
phosphorylation in nuclei. In the present study, it was found that
the knockdown of DKK1 increased the expression of β-catenin.
Notably, Sa3 and H1 cell lines were used in the study of Ogoshi
et al, while the SCC-4 cell line was used in the study of
Wang et al and CAL-27 and SCC-9 cells were used in the
present study. Different cell lines are derived from different
patients, who have individual differences. Disparities in the
reaction of β-catenin to DKK1 in different cells may be the reason
for the conflicting roles observed for DKK1 in OSCC cell migration
and invasion. Although it has been widely reported that DKK1 is
associated with cancer cell migration and invasion, the function of
DKK1 in different tumor types may vary. For example, the reduction
of DKK1 expression in ovarian cancer cells has been shown to induce
cell migration and invasion via activation of the
serine/threonine-protein kinase 3/FOXO3 pathway (21). This may be due to DKK1 interacting
with Wnt receptors, thereby inhibiting the classical Wnt signaling
pathway and promoting tumor invasion and migration (22,23).
However, DKK1 has also been shown to inhibit the invasion and
migration of breast cancer cells by suppressing the
β-catenin/matrix metalloproteinase 7 pathway (24). The reason that DKK1 functions
differently in different tumor types requires further analysis.
The results of the CCK-8 assay in the present study
indicated that the proliferation of the DKK1 knockdown cells was
decreased compared with that of the control cells; therefore, it
was important to avoid the effect of growth suppression on cell
migration and invasion during the Transwell assay. Notably, in the
CCK-8 assay, the cells were cultured in DMEM with 10% FBS. However,
in the Transwell assay, the cells were cultured in serum-free DMEM
for 24 h to stop cell proliferation. Therefore, during the
Transwell assay, there was no marked cell proliferation. Also,
duration of the Transwell assay was limited to 12 h. In addition,
during cell culture, DKK1 knockdown was not observed to induce any
obvious cell death when viewed under a microscope (Fig. S1A). Cell death was also analyzed
using flow cytometry, and the knockdown of DKK1 was found to have
no effect on cell death (Fig.
S1B). Therefore, in the present study, the possibility that the
inhibitory effects of shDKK1 on invasion and migration were due to
cell death may be discounted. In addition, Transwell assays have
been widely performed in numerous high-quality studies (25–27),
without consideration of the effects of cell proliferation and cell
death.
Immune cells in the tumor microenvironment play
important roles in the occurrence and development of the tumor and
are significantly associated with prognosis (28,29).
They participate in remodeling the microenvironment, and regulating
tumor progression; the tumor microenvironment affects immune cell
infiltration (30), and targeting
genes involved in this process is a promising strategy for tumor
therapy. In a mouse model of ovarian cancer, the overexpression of
DKK1 was found to decrease the infiltration of CD45+
leukocytes into the peritoneum and omentum, and to reduce the
numbers of natural killer and CD8 T cells and the expression of
interferon-g on activated CD8 T cells (31). In other studies, DKK1 was
demonstrated to be associated with antitumor immunity and serve as
a potential predictive marker and target for immunotherapy in
several types of tumors, including lung adenocarcinoma and
endometrial carcinoma (32–35). However, whether DKK1 has potential
as a biomarker and target for OSCC is not clear. Therefore, the
correlation between DKK1 expression and 24 immune cell types in
OSCC was analyzed in the present study. The results showed that
DKK1 was negatively correlated with the infiltration of T, TReg,
pDC, Th17, TFH, cytotoxic and B cells, whereas it was positively
correlated with the infiltration of Th2 and Tgd cells in OSCC. In
addition, DKK1 positively correlated with the expression of several
immune checkpoint genes, including CD40, CD44, VTCN1, NRP1,
PDCD1LG2, CD276, CD80 and CD86, and negatively
correlated with TNFRSR18, CD27, TIGIT, IDO2, CD48, CD244 and
CD40LG. These findings suggest that DKK1 may affect the
immune status of the tumor in OSCC via the regulation of specific
immune checkpoint genes. Notably, DKK1 is an inhibitor of Wnt
signaling, which can affect the tumor immune status in various
types of tumor (36–38). However, studies on the relationship
between Wnt signaling and the DKK1-related immune checkpoint genes
screened in the present study are lacking. It may be hypothesized
that the Wnt signaling pathway is responsible for the association
of DKK1 with tumor immunity.
The DNA MMR system recognizes and corrects
occasional DNA base mismatches in non-homologous chromosomes during
DNA replication, and this correction ensures the stability and
integrity of the genome (39).
Deficiencies in the MMR system can lead to genetic mutations and
induce tumorigenesis (40). Various
genes participate in DNA MMR, including members of the MSH family,
BRAF, PMS2 and EPCAM (41,42).
In the present study, DKK1 expression was found to significantly
correlate with the expression of the DNA repair genes MSH2,
MSH6, PMS2 and EPCAM, suggesting that DKK1 participates
in the regulation of DNA repair in OSCC. MSH2, MSH6, PMS2
and EPCAM have been reported to be associated with the Wnt
signaling pathway (43–45). Therefore, it is important to verify
whether DKK1 participates in DNA MMR through the Wnt signaling
pathway.
Although a search of the literature found that
several of the DNA repair genes and immune checkpoints screened in
the present study are associated with the Wnt pathway
(Wnt/β-catenin signaling induces DNA damage repair in ameliorating
radio-resistance (46), no
mechanistic studies were found. In future studies, detection of the
mechanism by which DKK1/Wnt regulates the transcription of these
genes will be investigated.
One shortcoming of the present study is that it
predominantly involves bioinformatics analysis. Further in
vivo and in vitro studies are required to verify the
role of DKK1 in OSCC progression and to reveal the mechanism by
which DKK1 participates in OSCC immunity, DNA MMR, cell
proliferation, cell migration and invasion. Another shortcoming is
that only DKK1 knockdown was performed; the DKK1 overexpression
experiments that could provide a more convincing conclusion were
omitted. In addition, the expression levels of Wnt3a and β-catenin
were detected in cells with DKK1 knockdown. β-catenin can transfer
into the nucleus to affect the transcription of target genes
(47). Therefore, in addition to
showing that DKK1 knockdown upregulates β-catenin expression, it
would also be interesting to investigate whether β-catenin is also
activated by DKK1 knockdown.
In summary, the present study explored the
relationship between DKK1 and OSCC prognosis and the possible
underlying mechanisms. The results demonstrated that DKK1 is
upregulated in OSCC, and associated with survival, tumor immunity,
DNA MMR and cell proliferation, migration and invasion. These
findings indicate that DKK1 is a candidate gene for OSCC therapy
and prognosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was supported by the National Natural Science
Foundation of China (grant no. 81602374), the Natural Science
Foundation of Shandong Province (grant no. ZR2021MH176), the China
Postdoctoral Science Foundation (grant no. 2021M701538) and the
Natural Science Foundation of Liaocheng People's Hospital (grant
no. LYQN201903).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Figshare repository (https://figshare.com/search?q=10.6084%2Fm9.figshare.21671213).
Authors' contributions
ZM and GXZ designed the study. YJL and CCW performed
the cell culture and bioinformatics analysis. SW performed
lentiviral infection and drafted the manuscript. SXD performed the
RT-qPCR experiments. YGL and DPZ performed CCK-8 and transwell
assay and confirm the authenticity of the raw data. YNL performed
the ELISA and western blot analysis. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zibelman M and Mehra R: Overview of
current treatment options and investigational targeted therapies
for locally advanced squamous cell carcinoma of the head and neck.
Am J Clin Oncol. 39:396–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jayanthi P, Varun BR and Selvaraj J:
Epithelial-mesenchymal transition in oral squamous cell carcinoma:
An insight into molecular mechanisms and clinical implications. J
Oral Maxillofac Pathol. 24:1892020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thomson PJ: Perspectives on oral squamous
cell carcinoma prevention-proliferation, position, progression and
prediction. J Oral Pathol Med. 47:803–807. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zaid KW, Nhar BM, Ghadeer Alanazi SM,
Murad R, Domani A and Alhafi AJ: Lack of effects of recombinant
human bone morphogenetic Protein2 on angiogenesis in oral squamous
cell carcinoma induced in the syrian hamster cheek pouch. Asian Pac
J Cancer Prev. 17:3527–3531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Silva NJ, Perez-Pacheco C and Schmitd
LB: The 3D's of neural phenotypes in oral cancer: Distance,
diameter, and density. Adv Biol (Weinh). 7:e22001882023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Torres-Ferrús M, Ursitti F, Alpuente A,
Brunello F, Chiappino D, de Vries T, Di Marco S, Ferlisi S,
Guerritore L, Gonzalez-Garcia N, et al: From transformation to
chronification of migraine: Pathophysiological and clinical
aspects. J Headache Pain. 21:422020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Serafini MS, Lopez-Perez L, Fico G,
Licitra L, De Cecco L and Resteghini C: Transcriptomics and
Epigenomics in head and neck cancer: Available repositories and
molecular signatures. Cancers Head Neck. 5:22020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hede K: Superhighway or blind alley? The
cancer genome atlas releases first results. J Natl Cancer Inst.
100:1566–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu X, Cheng S, Wang W, Shi O, Gao F, Li Y
and Wang Q: TCGA dataset screening for genes implicated in
endometrial cancer using RNA-seq profiling. Cancer Genet. 254–255.
40–47. 2021.
|
|
12
|
Gene Ontology Consortium: The gene
ontology resource: Enriching a GOld mine. Nucleic Acids Res.
49(D1): D325–D334. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu M, Yang F and Xu Y: Identification of
potential drug therapy for dermatofibrosarcoma protuberans with
bioinformatics and deep learning technology. Curr Comput Aided Drug
Des. 18:393–405. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toruner GA, Ulger C, Alkan M, Galante AT,
Rinaggio J, Wilk R, Tian B, Soteropoulos P, Hameed MR, Schwalb MN
and Dermody JJ: Association between gene expression profile and
tumor invasion in oral squamous cell carcinoma. Cancer Genet
Cytogenet. 154:27–35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheu JJ, Lee CC, Hua CH, Li CI, Lai MT,
Lee SC, Cheng J, Chen CM, Chan C, Chao SC, et al: LRIG1 modulates
aggressiveness of head and neck cancers by regulating
EGFR-MAPK-SPHK1 signaling and extracellular matrix remodeling.
Oncogene. 33:1375–1384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen Y, Dong S, Liu J, Zhang L, Zhang J,
Zhou H and Dong W: Identification of potential biomarkers for
thyroid cancer using bioinformatics strategy: A study based on GEO
datasets. Biomed Res Int. 2020:97104212020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wooller SK, Benstead-Hume G, Chen X, Ali Y
and Pearl FMG: Bioinformatics in translational drug discovery.
Biosci Rep. 37:BSR201601802017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ogoshi K, Kasamatsu A, Iyoda M, Sakuma K,
Yamatoji M, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H and Uzawa K:
Dickkopf-1 in human oral cancer. Int J Oncol. 39:329–336.
2011.PubMed/NCBI
|
|
20
|
Wang Z, Wang J, Chen Z, Wang K and Shi L:
MicroRNA-1-3p inhibits the proliferation and migration of oral
squamous cell carcinoma cells by targeting DKK1. Biochem Cell Biol.
96:355–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huo Q, Xu C, Shao Y, Yu Q, Huang L, Liu Y
and Bao H: Free CA125 promotes ovarian cancer cell migration and
tumor metastasis by binding Mesothelin to reduce DKK1 expression
and activate the SGK3/FOXO3 pathway. Int J Biol Sci. 17:574–588.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chi C, Li M, Hou W, Chen Y, Zhang Y and
Chen J: Long noncoding RNA SNHG7 activates Wnt/β-catenin signaling
pathway in cervical cancer cells by epigenetically silencing DKK1.
Cancer Biother Radiopharm. 35:329–337. 2020.PubMed/NCBI
|
|
23
|
Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu
ZY, Shi W, Jiang J, Yao PP and Zhu HP: Risk factors and preventions
of breast cancer. Int J Biol Sci. 13:1387–1397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niu J, Li XM, Wang X, Liang C, Zhang YD,
Li HY, Liu FY, Sun H, Xie SQ and Fang D: DKK1 inhibits breast
cancer cell migration and invasion through suppression of
β-catenin/MMP7 signaling pathway. Cancer Cell Int. 19:1682019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goetz JG, Minguet S, Navarro-Lérida I,
Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibáñez T,
Pellinen T, Echarri A, et al: Biomechanical remodeling of the
microenvironment by stromal caveolin-1 favors tumor invasion and
metastasis. Cell. 146:148–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue M, Zhu Y, Jiang Y, Han L, Shi M, Su R,
Wang L, Xiong C, Wang C, Wang T, et al: Schwann cells regulate
tumor cells and cancer-associated fibroblasts in the pancreatic
ductal adenocarcinoma microenvironment. Nat Commun. 14:46002023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo G, Zhang L, Wu W, Zhang L, Lin J, Shi
H, Wu X, Yu Y, Qiu W, Chen J, et al: Upregulation of ubiquitin
carboxy-terminal hydrolase 47 (USP47) in papillary thyroid
carcinoma ex vivo and reduction of tumor cell malignant behaviors
after USP47 knockdown by stabilizing SATB1 expression in vitro.
Oncol Lett. 26:3702023.PubMed/NCBI
|
|
28
|
Zheng W, Qian C, Tang Y, Yang C, Zhou Y,
Shen P, Chen W, Yu S, Wei Z, Wang A, et al: Manipulation of the
crosstalk between tumor angiogenesis and immunosuppression in the
tumor microenvironment: Insight into the combination therapy of
anti-angiogenesis and immune checkpoint blockade. Front Immunol.
13:10353232022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnson A, Townsend M and O'Neill K: Tumor
microenvironment immunosuppression: A roadblock to CAR T-cell
advancement in solid tumors. Cells. 11:36262022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suresh K, Naidoo J, Lin CT and Danoff S:
Immune checkpoint immunotherapy for non-small cell lung cancer:
Benefits and pulmonary toxicities. Chest. 154:1416–1423. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Betella I, Turbitt WJ, Szul T, Wu B,
Martinez A, Katre A, Wall JA, Norian L, Birrer MJ and Arend R: Wnt
signaling modulator DKK1 as an immunotherapeutic target in ovarian
cancer. Gynecol Oncol. 157:765–774. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Zhao M, Lin S, Han Q, Ye H, Peng
F and Li L: Prediction of prognosis and immunotherapy response in
lung adenocarcinoma based on CD79A, DKK1 and VEGFC. Heliyon.
9:e185032023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arend R, Dholakia J, Castro C, Matulonis
U, Hamilton E, Jackson CG, LyBarger K, Goodman HM, Duska LR, Mahdi
H, et al: DKK1 is a predictive biomarker for response to DKN-01:
Results of a phase 2 basket study in women with recurrent
endometrial carcinoma. Gynecol Oncol. 172:82–91. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chu HY, Chen Z, Wang L, Zhang ZK, Tan X,
Liu S, Zhang BT, Lu A, Yu Y and Zhang G: Dickkopf-1: A promising
target for cancer immunotherapy. Front Immunol. 12:6580972021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wall JA, Klempner SJ and Arend RC: The
anti-DKK1 antibody DKN-01 as an immunomodulatory combination
partner for the treatment of cancer. Expert Opin Investig Drugs.
29:639–644. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Phillips C, Bhamra I, Eagle C, Flanagan E,
Armer R, Jones CD, Bingham M, Calcraft P, Edmenson Cook A, Thompson
B and Woodcock SA: The Wnt pathway inhibitor RXC004 blocks tumor
growth and reverses immune evasion in Wnt ligand-dependent cancer
models. Cancer Res Commun. 2:914–928. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lyu H, Zhang J, Wei Q, Huang Y, Zhang R,
Xiao S, Guo D, Chen XZ, Zhou C and Tang J: Identification of
Wnt/β-catenin- and autophagy-related lncRNA signature for
predicting immune efficacy in pancreatic adenocarcinoma. Biology
(Basel). 12:3192023.PubMed/NCBI
|
|
38
|
Muto S, Enta A, Maruya Y, Inomata S,
Yamaguchi H, Mine H, Takagi H, Ozaki Y, Watanabe M, Inoue T, et al:
Wnt/β-catenin signaling and resistance to immune checkpoint
inhibitors: From non-small-cell lung cancer to other cancers.
Biomedicines. 11:1902023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
D'Souza LC, Shekher A, Challagundla KB,
Sharma A and Gupta SC: Reprogramming of glycolysis by chemical
carcinogens during tumor development. Semin Cancer Biol.
87:127–136. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taieb J, Svrcek M, Cohen R, Basile D,
Tougeron D and Phelip JM: Deficient mismatch repair/microsatellite
unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur
J Cancer. 175:136–157. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lamb NA, Bard JE, Loll-Krippleber R, Brown
GW and Surtees JA: Complex mutation profiles in mismatch repair and
ribonucleotide reductase mutants reveal novel repair substrate
specificity of MutS homolog (MSH) complexes. Genetics.
221:iyac0922022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kašubová I, Holubeková V, Janíková K,
Váňová B, Sňahničanová Z, Kalman M, Plank L and Lasabová Z: Next
generation sequencing in molecular diagnosis of lynch syndrome-a
pilot study using new stratification criteria. Acta Medica (Hradec
Kralove). 61:98–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Castiglia D, Bernardini S, Alvino E,
Pagani E, De Luca N, Falcinelli S, Pacchiarotti A, Bonmassar E,
Zambruno G and D'Atri S: Concomitant activation of Wnt pathway and
loss of mismatch repair function in human melanoma. Genes
Chromosomes Cancer. 47:614–624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Suzuki H, Hirata Y, Suzuki N, Ihara S,
Sakitani K, Kobayashi Y, Kinoshita H, Hayakawa Y, Yamada A, Watabe
H, et al: Characterization of a new small bowel adenocarcinoma cell
line and screening of anti-cancer drug against small bowel
adenocarcinoma. Am J Pathol. 185:550–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie X, Chen J, Wo D, Ma E, Ning Y, Peng J,
Zhu W and Ren DN: Babao Dan is a robust anti-tumor agent via
inhibiting wnt/β-catenin activation and cancer cell stemness. J
Ethnopharmacol. 280:1144492021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hashemi M, Hasani S, Hajimazdarany S,
Ghadyani F, Olyaee Y, Khodadadi M, Ziyarani MF, Dehghanpour A,
Salehi H, Kakavand A, et al: Biological functions and molecular
interactions of Wnt/β-catenin in breast cancer: Revisiting
signaling networks. Int J Biol Macromol. 232:1233772023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Alshahrani SH, Rakhimov N, Rana A, Alsaab
HO, Hjazi A, Adile M, Abosaooda M, Abdulhussien Alazbjee AA,
Alsalamy A and Mahmoudi R: Dishevelled: An emerging therapeutic
oncogene in human cancers. Pathol Res Pract. 250:1547932023.
View Article : Google Scholar : PubMed/NCBI
|