Introduction
Liver cancer is predicted to be the sixth most
commonly diagnosed cancer and the fourth leading cause of
cancer-related death worldwide in 2018, with ~841,000 new cases and
782,000 deaths occurring annually (1). Hepatocarcinogenesis requires a number
of genetic regulations and multistep aberrant biological processes,
eventually leading to the malignant transformation of hepatocytes.
Surgical resection is the main treatment for liver cancer. However,
hepatocellular carcinoma (HCC), comprising 75–85% of primary liver
cancer cases (1), has a high
post-surgery recurrence rate (up to 70% in 5 years) (2) due to hematogenous metastasis,
especially intrahepatic hematogenous metastasis occurring in the
early stage of HCC. Moreover, surgical removal can be unachievable
due to the size and distribution of the tumor in the liver,
hypohepatia and extrahepatic metastasis. Some neoadjuvant
therapies, such as transarterial chemoembolization (TACE), has been
revealed to improve the overall disease-free survival period of
patients with liver cancer after resection (3). However, drug resistance, including
primary resistance and multidrug resistance, limits the
chemotherapeutic effect in some patients with liver cancer.
Additionally, most anticancer drugs have side effects that reduce
the quality of life of patients with liver cancer, thus it is
important to discover predictive factors to identify whether the
patients will be sensitive to chemotherapy or not.
Altered expression of miRNAs plays an important role
in the occurrence, development and drug resistance of human tumors
(4). miRNAs are small non-coding
RNA molecules of 20–23 nucleotides in length which regulate a
variety of biologic processes such as apoptosis, proliferation,
differentiation, development and metabolism. In a previous study
conducted by the authors, miRNA expression profiles were detected
and characterized in four types of liver cancer drug resistance
cell sublines, finding that miRNA-27b (miR-27b) expression was
upregulated in all four compared with the parental cell line Huh-7
(5).
miR-27b belongs to the miR-23b~27b~24-1 cluster,
which is localized at chromosome q22.32 within the C9orf3 gene. A
number of studies indicated that miR-27b is involved in the
development and progression of tumors (6–9).
According to previous findings of the authors, it was hypothesized
that altered expression of miR-27b might be involved in mediating
resistance to chemotherapy in HCC, a hypothesis which remains
unclear in present literature (5).
In addition, the role of miR-27b in the development and procession
of liver cancer remains elusive. In the present study, the authors
aimed to determine the influence of miR-27b in the occurrence,
development and drug resistance of liver cancer cells.
Materials and methods
Patients and samples
A total of 76 human HCC tissue samples and 46 paired
paracarcinoma tissue samples were obtained from adult patients
diagnosed with HCC in the Division of Hepatobiliary Surgery within
the Hepatic Disease Center of The First Affiliated Hospital of
Fujian Medical University (Fuzhou, China) between 2007.1–2010.11.
All patients with HCC underwent TACE after hepatectomy. Patients
ranged in age from 28 to 78 years, with a median age of 51 years.
There were 73 males and 3 females. All patients signed the informed
consent and the present study was approved by the Ethics Committee
of Fujian Medical University (approval no. FMU-2014-093; Fuzhou,
China).
Cell lines and culture
Human liver cancer cell lines Huh-7 and HepG2 were
obtained from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. Cells were cultured in DMEM
(high-glucose) (Gibco BRL Life Technologies Inc.) supplemented with
10% fetal bovine serum (Gibco BRL Life Technologies Inc.), 100 U/ml
penicillin, 100 µg/ml streptomycin (Sigma-Aldrich LLC.) in a
humidified incubator at 37°C with 5% CO2.
RNA isolation
Freshly resected tissue was immediately frozen in
liquid nitrogen for subsequent total RNA extraction. Total RNA was
extracted from cell lines and tissue samples using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to manufacturer's instructions. The concentration
of total RNA was quantitated by measuring the absorbance at 260 nm
while RNA integrity was determined using 2% gel electrophoresis 0.5
µg/ml ethidium bromide.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA samples were reverse transcribed into cDNA using
a Universal cDNA synthesis kit (Takara Bio, Inc., Code No.:RR037A)
according to the manufacturer's instructions. RT-qPCR amplification
was performed on an Applied Biosystems 7500 Real-Time PCR System
with the DNA-binding dye technique using SYBR Green (Takara Bio,
Inc.) according to the manufacturer's instructions. U6 snRNA was
used as a reference gene. The primers for miR-27b and U6 were
purchased from Takara Bio, Inc. The primer sequence for the genes
examined are presented in Table I.
Thermocycling conditions were as follows: Initial denaturation
temperature 95°C for 15 sec; annealing temperature 60°C for 30 sec.
The relative expression of miR-27b was calculated according to the
formula 2−ΔΔCq ΔΔCq=ΔCq of experimental sample-mean ΔCq
of control samples) (10). Each
RT-qPCR was performed in triplicates.
 | Table I.Primers for reverse
transcription-quantitative PCR. |
Table I.
Primers for reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5′→3′) |
|---|
|
microRNA-27ba | Forward:
TTCACAGTGGCTAAGTTCTGCAAA |
| U6 | Forward:
GGAACGATACAGAGAAGATTAGC |
|
| Reverse:
TGGAACGCTTCACGAATTTGCG |
Construction of stable miR-27b
overexpression liver cancer cell lines and control cell lines
Lentiviral transfer vector containing
miR-27bprecursorsequenggugcagagcuuagcugauuggugaacagugauugguuuccgcuuuguucacaguggcuaaguucugcaccugaagagaaggug)
(LPP-pEZX-MR03-eGFP-miR-27b) and lentiviral negative control vector
(LPP-pEZX-MR03-eGFP-NC) were purchased from GeneCopoeia, Inc. The
accuracy of the recombinant vector was verified by double digest
and gene sequencing method. Lentiviral transfer vector
(3.39×108TU/ml) and lentiviral negative control vector
(3.05×108TU/ml) infected into cell lines with
EndoFectin™ (GeneCopoeia Inc.) at 37°C for 15 h. At 72 h
post-transfection, 1 µg/ml puromycin (Sigma-Aldrich LLC.) was added
to screen for stable miR-27b overexpression and negative control
cell lines. The screening period is about 10 to 15 days.
Cell proliferation assays
Cell Counting Kit-8 (CCK-8) assay
Exponentially growing cells were seeded into a
96-well plate at a density of 1×103 cells/well in 100 µl
of culture medium. Cells were cultured in a CO2
incubator at 37°C for 24, 48, 72, 96 and 120 h. CCK-8 solution was
added to wells at a concentration of 10 µl/well according to the
manufacturer's instructions (Dojindo Laboratories, Inc.). The plate
was incubated at 37°C for 2 h after which the absorbance [optical
density (OD) value] at 450 nm was measured using a microplate
reader (Model550; Omega Bio-Tek, Inc.). Independent experiments
were performed in triplicates. A cell viability curve was created,
plotting time is demonstrated in the X-axis coordinate and the
number of cells (OD value) in the Y-axis coordinate.
Clone formation assay
A total of 6×102 exponentially growing
cells were seeded onto a 60-mm petri dish. Cells were cultured in a
CO2 incubator at 37°C for 2–3 weeks until cells in
control plates have formed colonies that were of a substantially
good size (>50 cells/colony). At room temperature, cells were
fixed with −20°C precooled formaldehyde for 15 min and stained with
0.1% Giemsa solution (Beijing Leagene Biotech Co., Ltd.) for 20
min. The number of colonies was then counted and expressed as the
mean ± standard deviation of triplicate wells within the same
experiment.
Immunohistochemical staining (IHC)
with cell pellets
Exponentially growing cells were harvested by
trypsinization and washed with PBS. Cells were pelleted with 2%
agarose after fixation with 10% formalin at room temperature for 2
h. Cell pellets were processed into paraffin blocks. After being
dewaxed, hydrated, blocked with 3% H2O2
(Fuzhou Maixin Biotech Co., Ltd.) at 37°C for 15 min, paraffin
sections (thickness:5 um) were incubated overnight at 4°C with
mouse anti-human Ki-67 antibody (ready to use; cat. no. MAB-0672;
Fuzhou Maixin Biotech Co., Ltd.). After three washes of 5 min with
PBS, sections were incubated at room temperature with biotinylated
goat anti-mouse IgG/HRP secondary antibody (ready to use; cat. no.
KIT-9701; Fuzhou Maixin Biotech Co., Ltd.) for 1 h, followed by
three additional washes of 5 min with PBS. DAB solution was used
for visualization of the samples. As a negative control, the
primary antibody was replaced with PBS. Sections were observed
under a light microscope and images were captured with Olympus DP74
(Olympus Corporation). Image-Pro Plus 6.0 software (Media
Cybernetics, Inc.) was used to analyze and calculate the average
integrated OD (IOD) value.
Apoptosis assay using flow cytometry
(FCM)
Exponentially growing cells were harvested by
trypsinization and washed with PBS. Cells were resuspended with
binding buffer at a density of 6×106 cells/ml and 100 µl
of the cell suspension were transferred into a 1.5-ml Eppendorf
tube. Annexin V-PE (1.5 µl) and 5 µl 7-AAD were added in a step
wise manner. Finally, apoptosis was measured by flow cytometer (BD
FACSVerse™; BD Biosciences) after 1 h. Software FACSuite
(v1.0.5.3841; BD Biosciences) was used for data analysis. The
experiment was repeated three times.
MTT cell viability assay (drug
sensitivity assay)
Exponentially growing cells were seeded at
4×103 cells per well in 96-well plates with 100 µl of
culture medium/well and incubated at 37°C for 14 h. The cells were
then exposed to different concentrations of chemotherapy drugs such
as 5-fluorouracil (5-FU; 15.625, 62.5, 250 ng/ml, 1, 4, 16 µg/m,
64, 256 µg/ml; Jinyao Pharmaceutical Co., LTD, Tianjin), adriamycin
(ADR) (concentrations: 15.625, 62.5, 250 ng/ml, 1, 4, 16, 64, 256
µg/ml; Sigma-Aldrich LLC.), cisplatin (CDDP) (concentrations: 1.25,
2.5, 5, 10, 20, 40, 80, 160 µg/ml; Mackerlin Biochemical Technology
Co., LTD, Shanghai) and mitomycin-C (MMC; 9.765625, 39.0625,
156.25, 625 ng/ml, 2.5, 10, 40, 80 µg/ml; Roche, Switzerland) at
37°C for 48 h. At the end of the drug exposure period, 20 µl of MTT
(5 mg/ml in PBS) was added into each well and cells were cultured
at 37°C for an additional 4 h (approximate time for the formation
of formalin crystals). Subsequently, 150 µl DMSO were added to each
well to dissolve the crystals. The optical density values were then
measured at 570 nm using a microplate ELISA reader (Model550; Omega
Bio-Tek, Inc.). Each experiment was performed in quintuplicates and
repeated thrice. Resistance factors (RF) were calculated by
dividing the IC50 value (drug concentration results in
50% reduction in absorbance compared with the control) of drug
resistant cells with that of the parental control cells.
Statistical analysis
The ΔCq value (ΔCq=Cq value of miR-27b-Cq value of
U6) of all samples was calculated for RT-qPCR assessments. The
relative expression levels of miR-27b was calculated by the formula
2−ΔΔCq (ΔΔCq=ΔCq of experimental sample-mean ΔCq of
control samples). The samples were divided into the high miR-27b
expression group and the low miR-27b expression group according to
their miR-27b levels. The significance of differences was
determined with unpaired and paired Student's t-test, two-way ANOVA
and Bonferroni's post-hoc test, Cox regression and Kaplan-Meier
survival analysis (log-rank test) based on different data types.
Values were expressed as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant difference.
All statistical procedures were performed in SPSS version 16.0
(SPSS, Inc.) or in GraphPad Prism 7 (Dotmatics; GraphPad
Software).
Results
Establishment of two stable miR-27b
overexpression liver cancer cell lines and a control cell line
A total of two stable miR-27b overexpression liver
cancer cell lines and a control cell line were established. These
cells lines were named respectively as: Huh-7/miR-27b, Huh-7/NC,
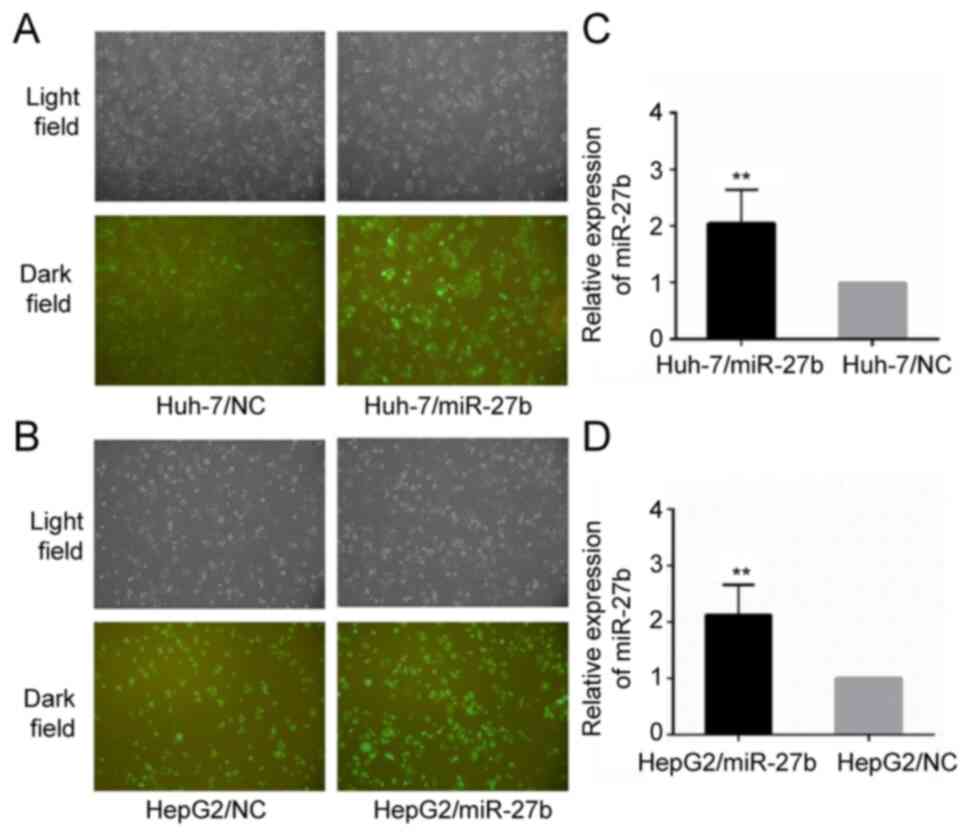
HepG2/miR-27b and HepG2/NC. The relative miR-27b level of
Huh-7/miR-27b and HepG2/miR-27b was upregulated 2.05±0.59 fold and
2.13±0.53 fold, respectively, compared with the control cell line
(all P-values were <0.01). Their cellular morphology and
relative miR-27b expression levels are demonstrated in Fig. 1.
The effect of miR-27b expression on
cell proliferation in liver cancer cells
CCK-8 assay
A CCK-8 commercial cell counting kit was used to
detect the cell proliferation of Huh-7/miR-27b, HepG2/miR-27b and
their control cell lines. The OD values of Huh-7/miR-27b at 24, 48,
72, 96 and 120 h were 0.3±0.003, 0.331±0.065, 0.551±0.034,
0.742±0.037 and 1.207±0.113, respectively (Fig. 2A). The OD values of Huh-7/NC at 24,
48, 72, 96 and 120 h were 0.364±0.066, 0.48±0.055, 0.763±0.086,
1.060±0.017 and 1.573±0.043, respectively (Fig. 2A). The OD values of HepG2/miR-27b at
24, 48, 72, 96 and 120 h were 0.388±0.009, 0.611±0.011,
0.957±0.043, 1.004±0.059 and 1.388±0.099, respectively (Fig. 2B). The OD values of HepG2/NC at 24,
48, 72, 96 and 120 h were 0.403±0.064, 0.696±0.039, 1.144±0.053,
1.259±0.05 and 1.544±0.044, respectively (Fig. 2B). The OD values of Huh-7/miR-27b
and HepG2/miR-27b at 24, 48, 72, 96 and 120 h were significantly
lower than the control cell lines (all P<0.01).
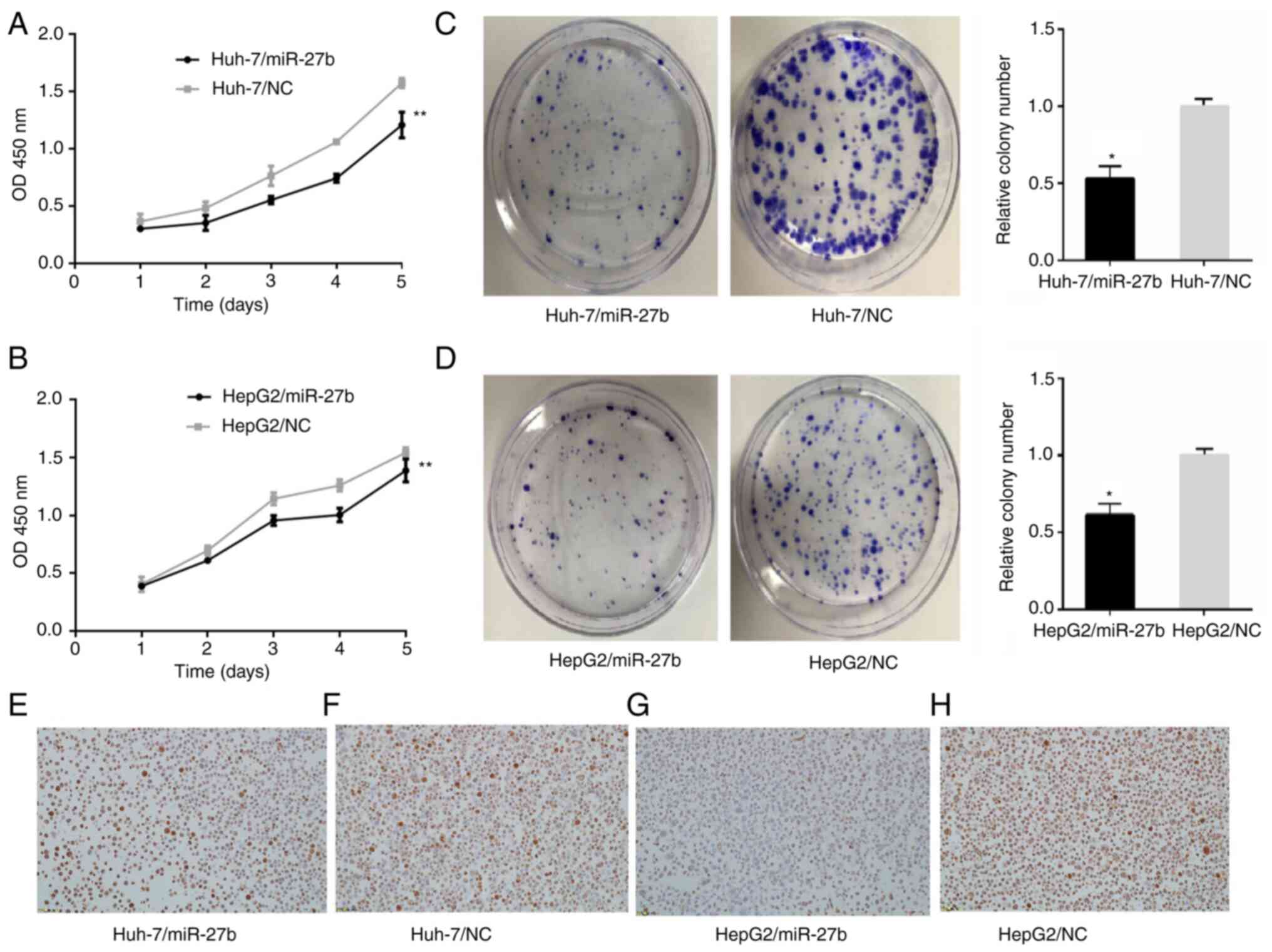 | Figure 2.Proliferation in miR-27b
overexpression liver cancer cell lines and control liver cancer
cell lines. (A and B) Cell proliferation in liver cancer cell lines
was measured with Cell Counting Kit-8 assay. (A) Cell viabilities
of Huh-7/miR-27b in 24, 48, 72, 96 and 120 h were all lower than
those of Huh-7/NC. (B) Cell viabilities of HepG2/miR-27b in 24, 48,
72, 96 and 120 h were all lower than those of HepG2/NC. (C and D)
Cell proliferation in liver cancer cell lines was measured with
clone formation assay. (C) The number of cell clones in
Huh-7/miR-27b (104±9) was less than that of Huh-7/NC (200±22). (D)
The number of cell clones in HepG2/miR-27b (111±40) was less than
that of HepG2/NC (180±53). (E-H) Ki-67 expression in HCC cell lines
(magnification, ×200). (E) Ki-67 expression in Huh-7/miR-27b, Ki-67
index was 70%. IOD was 361.15±58.50. (data not shown) (F) Ki-67
expression in Huh-7/NC, Ki-67 index was 82%. IOD was 589.02±143.39.
(data not shown) (G) Ki-67 expression in HepG2/miR-27b, Ki-67 index
was 50%. IOD was 363.61±80.94. (data not shown) (H) Ki-67
expression in HepG2/NC, Ki-67 index was 86%. IOD was 545.98±79.43.
(data not shown) *P<0.05 and **P<0.01. miR, microRNA; NC,
negative control. |
Clone formation assay
A clone formation assay was used to detect the cell
proliferation of Huh-7/miR-27b, HepG2/miR-27b and their control
cell lines. The number of cell clones corresponding to
Huh-7/miR-27b and Huh-7/NC were 104±9 and 200±22, respectively
(Fig. 2C). The differences between
them were statistically significant. The number of cell clones of
HepG2/miR-27b and HepG2/NC were 111±40 and 180±53, respectively
(Fig. 2D). The differences between
them were statistically significant.
Immunohistochemical staining with
Ki-67
Immunohistochemical staining with Ki-67 was employed
to detect the cell proliferation of Huh-7/miR-27b, HepG2/miR-27b
and their control cell lines. Expression of Ki-67 in Huh-7/miR-27b,
Huh-7/NC, HepG2/miR-27b and HepG2/NC are exhibited in Fig. 2E-H. Ki-67 index values (the number
of positive cells in 100 tumor cells) in Huh-7/miR-27b, Huh-7/NC,
HepG2/miR-27b and HepG2/NC were 70, 82, 50 and 86%, respectively
(data not shown). IOD of immunohistochemical staining with Ki-67 in
Huh-7/miR-27b, Huh-7/NC, HepG2/miR-27b and HepG2/NC were
361.15±58.50, 589.02±143.39, 363.61±80.94 and 545.98±79.43,
respectively (data not shown). IOD of immunohistochemical staining
with Ki-67 in Huh-7/miR-27b and HepG2/miR-27b were statistically
lower than the negative control cell line (unpaired Student's
t-test, P<0.05).
The effect of miR-27b on cell
apoptosis in liver cancer cells
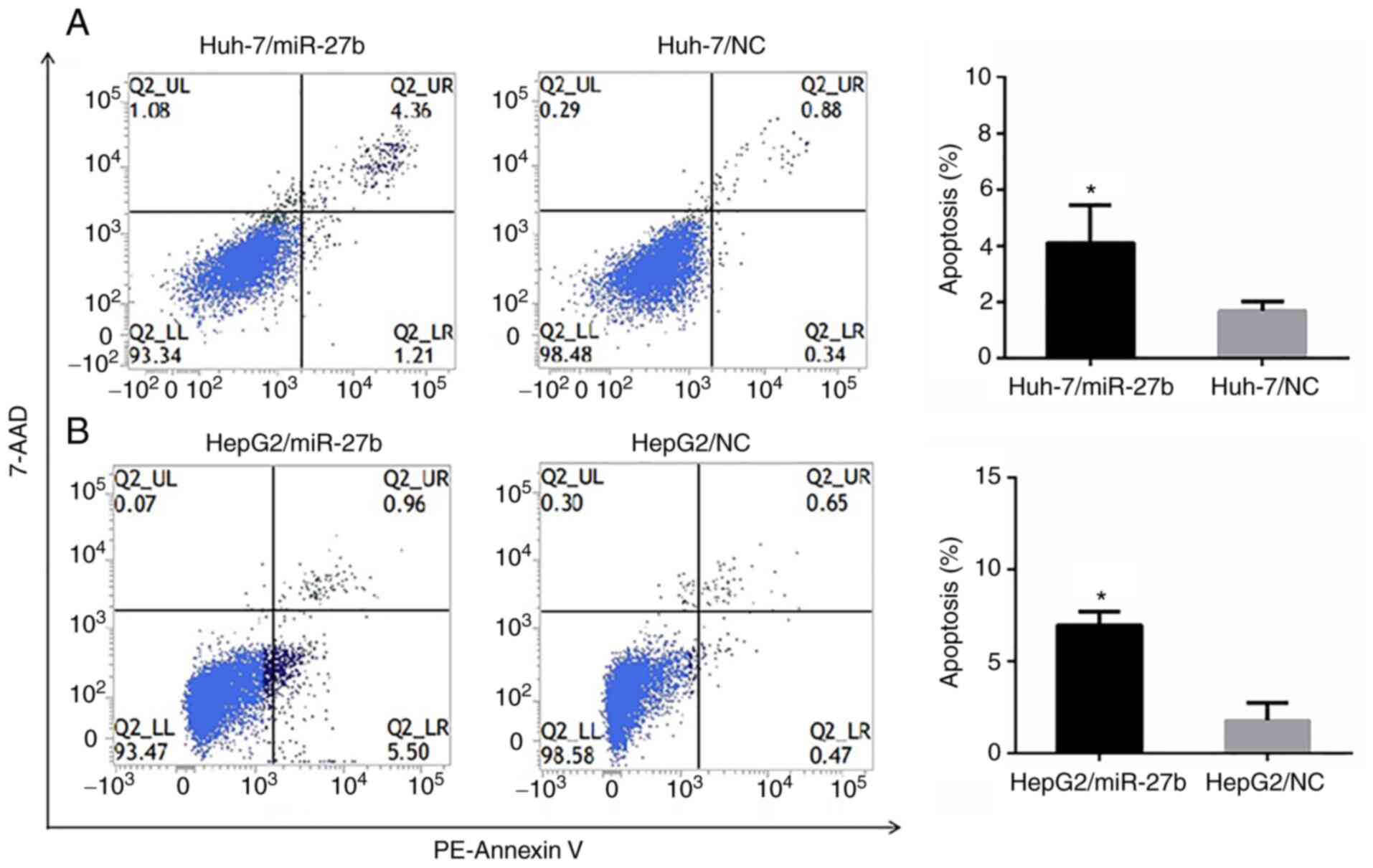
The FCM analysis evaluating apoptosis revealed that
the apoptotic rates (%) of Huh-7/miR-27b, Huh-7/NC, HepG2/miR-27b
and HepG2/NC were 4.11±1.35, 1.70±0.33, 6.98±0.73 and 1.79±0.95,
respectively. The apoptotic rates of Huh-7/miR-27b and
HepG2/miR-27b cell lines were significantly higher than the
negative control cell line (Fig. 3A and
B).
The effect of miR-27b on drug
sensitivity in liver cancer cells
The IC50 values and RF of cell lines,
presented in Table II, indicated
that compared with Huh-7/NC, Huh-7/miR-27b was less sensitive to
ADR and CDDP (Fig. 4A and B). There
was not a significant difference between Huh-7/miR-27b and Huh-7/NC
(Fig. 4C and D) in their
sensitivity to either 5-FU or MMC. Compared with HepG2/NC,
HepG2/miR-27b was less sensitive to ADR, CDDP and 5-FU (Fig. 4E-G). There was no significant
difference in sensitivity to MMC between HepG2/miR-27b and HepG2/NC
(Fig. 4H).
 | Table II.The IC50 values and RF to
four drugs of miR-27b overexpression liver cell lines and their
control cell lines. |
Table II.
The IC50 values and RF to
four drugs of miR-27b overexpression liver cell lines and their
control cell lines.
|
| IC50
(µg/ml)/RF |
|---|
|
|
|
|---|
| Cell line | ADR | CDDP | MMC | 5-FU |
|---|
| Huh-7/NC | 2.35±1.01 | 8.80±9.8 | 3.02±4.92 | 32.9±53.46 |
| Huh-7/miR-27b |
8.72±2.22a |
23.24±8.07a | 2.44±4.1 | 31.9±55.47 |
| RF | 3.71 | 3.15 | 0.81 | 0.97 |
| HepG2/NC | 4.92±3.22 | 6.44±1.48 | 1.38±2.08 | 20.68±5.72 |
| HepG2/miR-27b |
8.39±1.84a |
9.51±2.29a | 1.66±2.3 |
52.28±8.68a |
| RF | 1.71 | 1.48 | 1.2 | 2.53 |
miR-27b expression in HCC and
paracarcinoma tissue
Association between miR-27b level in HCC and
paired paracarcinoma tissues
The expression of miR-27 in HCC tissues was found to
be lower than paired paracarcinoma tissues. Specifically, the
relative level of miR-27b in HCC tissues and paired paracarcinoma
tissues was 0.73±0.49 and 1.14±0.7, respectively. The miR-27b level
in HCC tissues was significantly lower than in liver tissues
adjacent to the tumor (P<0.01). The relative miR-27b expression
levels in tissues are demonstrated in Fig. 5.
The relationship of miR-27b expression
and clinicopathological parameters in patients with HCC
The association between miR-27b expression and the
clinicopathological parameters of patients with HCC is presented in
Table III. The miR-27b level in
samples from HCC patients with cirrhosis was remarkably lower
compared with patients without cirrhosis. Notably, HCC tissues from
patients <40 years old had higher miR-27b expression levels than
patients >40 years old. Furthermore, the level of miR-27b in
patients with abnormal liver function [alanine aminotransferase
(ALT) ≥40 U/l] was lower than in the normal group (ALT <40 U/l).
Conversely, the level of miR-27b in the high serum α-fetoprotein
(AFP) expression group was higher than that in the low AFP
expression group. However, there were no statistically significant
differences in miR-27b level and nodule number, tumor size,
vascular invasion, degree of differentiation, TNM stage,
extrahepatic metastasis, serum HBsAg, total bilirubin,
Y-glutamyltranspeptidase, or albumin.
 | Table III.The relationship of miR-27b
expression with clinicopathological parameters in HCC. |
Table III.
The relationship of miR-27b
expression with clinicopathological parameters in HCC.
| Parameter | Δcq value of
miR-27b | Cases | P-value |
|---|
| Age, years |
|
| 0.047a |
|
<40 | 13.35±2.1 | 16 |
|
|
≥40 | 14.33±1.63 | 60 |
|
| Vascular
invasion |
|
| 0.400 |
|
Yes | 13.96±2.05 | 40 |
|
| No | 14.3±1.4 | 36 |
|
| Extrahepatic
metastasis |
|
| 0.593 |
|
Yes | 13.91±2.01 | 16 |
|
| No | 14.18±1.71 | 60 |
|
| Cirrhosis |
|
| 0.008b |
|
Yes | 14.34±1.61 | 65 |
|
| No | 12.83±2.16 | 11 |
|
| Number of
tumors |
|
| 0.210 |
|
Single | 14.03±1.85 | 66 |
|
|
More | 14.78±0.89 | 10 |
|
| Differentiated |
|
| 0.245 |
|
Well/moderately | 13.97±1.61 | 52 |
|
|
Poorly | 14.48±2.06 | 24 |
|
| TNM stage |
|
| 0.650 |
| I/II
phase | 14.18±1.75 | 57 |
|
| III/IV
phase | 13.96±1.86 | 19 |
|
| Tumor size, cm |
|
| 0.070 |
|
<7 | 14.52±1.66 | 35 |
|
| ≥7 | 13.79±1.81 | 41 |
|
| AFP |
|
| 0.035a |
|
<292.5 | 14.55±1.33 | 38 |
|
|
≥292.5 | 13.7±2.05 | 38 |
|
| HBSAg |
|
| 0.426 |
|
Negative | 14.7±1.11 | 7 |
|
|
Positive | 14.07±1.82 | 69 |
|
| TBIL |
|
| 0.440 |
|
<20 | 14.28±1.67 | 49 |
|
|
≥20 | 13.87±1.86 | 13 |
|
| GGT |
|
| 0.185 |
|
<40 | 13.71±2.07 | 16 |
|
|
≥40 | 14.37±1.55 | 46 |
|
| ALT |
|
| 0.034a |
|
<40 | 13.76±1.58 | 32 |
|
|
≥40 | 14.67±1.74 | 30 |
|
| ALB |
|
| 0.102 |
|
<35 | 13.39±0.91 | 10 |
|
|
≥35 | 14.36±1.79 | 52 |
|
The relationship of miR-27b expression
and disease prognosis
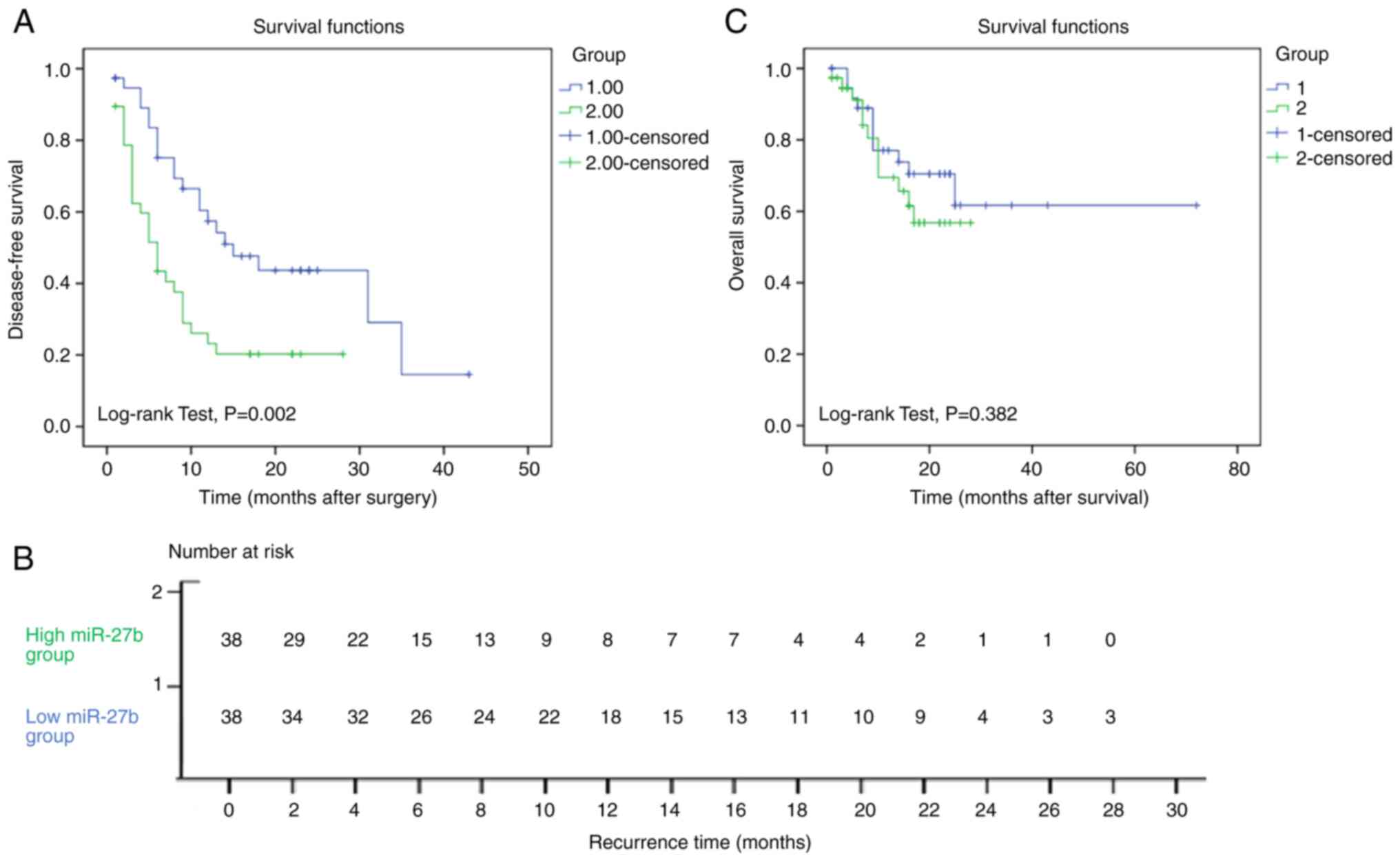
Bounded by the median miR-27b level, HCC samples
were divided into high miR-27b expression group (38 cases) and low
miR-27b expression group (38 cases). The recurrence time
(disease-free survival time) of the low miR-27b expression group
(20.8±2.7 months) was longer than that of the high miR-27b
expression group, the recurrence time of which was 9.7±1.6 months.
This doubling of disease-free survival time was statistically
significant (P=0.002). The Kaplan-Meier survival curve is
demonstrated in Fig. 6A. The number
of subjects at risk is shown in Fig.
6B. To identify variables with potential prognostic
significance, univariate and multivariate analyses for each
variable in relation to the disease-free survival time of patients
with HCC were conducted. Clinicopathologic factors with a
significant impact on the recurrence time in univariate Cox
regression analysis included miR-27b (P=0.004), vascular invasion
(P=0.006), extrahepatic metastasis (P<0.001), number of tumors
(P=0.014), TNM stages (P<0.001), tumor size (P=0.024) and serum
ALT (P=0.009). In multivariate analyses, miR-27b expression
(P=0.012), extrahepatic metastasis (P<0.001), number of tumors
(P=0.003), differentiated status (P=0.033), serum AFP (P=0.034) and
serum ALT (P=0.004) were significant independent factors for tumor
recurrence (Table IV).
 | Table IV.Univariate and multivariate Cox
regression analyses of recurrence-free survival in HCC patients
underwent TACE after hepatectomy. |
Table IV.
Univariate and multivariate Cox
regression analyses of recurrence-free survival in HCC patients
underwent TACE after hepatectomy.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| miR-27b (Low vs.
High expression) | 2.345 | 1.308–4.207 | 0.004b | 2.147 | 1.186–3.887 | 0.012a |
| Age, years (<40
vs. ≥40) | 1.015 | 0.518–1.990 | 0.965 |
|
|
|
| Vascular invasion
(Yes vs. No) | 2.271 | 1.260–4.094 | 0.006b |
|
|
|
| Extrahepatic
metastasis (Yes vs. No) | 4.247 | 2.254–8.000 |
<0.001c | 4.181 | 2.271–8.053 |
<0.001c |
| Cirrhosis (Yes vs.
No) | 0.797 | 0.373–1.706 | 0.560 |
|
|
|
| Number of tumor
(Single vs. More) | 2.672 | 1.224–5.833 | 0.014a | 3.899 | 1.592–9.549 | 0.003b |
| Differentiated
(Well/moderately vs. Poorly) | 0.878 | 0.484–1.592 | 0.669 | 0.440 | 0.207–0.936 | 0.033a |
| TNM Stages (I/II
vs. III/IV phase) | 3.949 | 2.160–7.217 |
<0.001c |
|
|
|
| Tumor size, cm (≤7
vs. >7) | 1.946 | 1.093–3.465 | 0.024a |
|
|
|
| HBSAg (Positive vs.
Negative) | 3.698 | 0.864–15.825 | 0.078 |
|
|
|
| AFP (<292.5 vs.
≥292.5 ng/ml) | 1.563 | 0.892–2.739 | 0.118 | 2.019 | 1.054–3.867 | 0.034a |
| TBIL (<20 vs.
≥20 µmol/l) | 1.453 | 0.689–3.067 | 0.327 |
|
|
|
| ALB (<35 vs. ≥35
g/l) | 0.620 | 0.271–1.417 | 0.257 |
|
|
|
| ALT (<40 vs. ≥40
U/l) | 2.322 | 1.234–4.370 | 0.009b | 2.854 | 1.392–5.852 | 0.004b |
| GGT (<40 vs. ≥40
U/l) | 1.533 | 0.728–3.226 | 0.260 |
|
|
|
However, there was no statistically significant
difference between the overall survival time of the low miR-27b
expression group and the high miR-27b expression group. The
Kaplan-Meier survival curve is illustrated in Fig. 6C.
Discussion
In the present study, miR-27b expression in patients
with HCC who underwent TACE after hepatectomy was assessed. It was
found that miR-27b expression in HCC tissues was lower than in
paired paracarcinoma liver tissues. Upon analyzing the relationship
between miR-27b expression and various clinicopathological
parameters, the authors observed that miR-27b levels were
associated with age, cirrhosis, serum AFP and ALT levels before the
operation. In addition, the results revealed that the disease-free
survival time of the low miR-27b expression group was twice as long
as that in the high miR-27b expression group. At the same time,
in vitro cell experiments demonstrated that upregulation of
miR-27b inhibited cell proliferation, promoted cell apoptosis and
reduced chemosensitivity in liver cancer cell lines.
Recent studies have demonstrated aberrant miR-27b
expression levels in various cancers, indicating its potential role
in cancer progression. For instance, Li et al (7) indicated that miR-27b was significantly
downregulated in tongue squamous cell carcinoma (TSCC) tissues and
overexpression of miR-27b led to diminished proliferation,
migration and invasion.
Numerous researchers have demonstrated that miR-27b
can play a suppressive role in several cancers including TSCC
(7), breast cancer (11), gastric cancer (12,13),
lung cancer (14), esophageal
squamous cell carcinoma (8) and
colorectal cancer (15). As for
liver cancer, Liang et al (16) reported that the expression of
miR-27b in tumor tissues is lower than that in adjacent non-tumor
tissues. This finding opposed the results of Sun et al
(17) and He et al (18), who suggested that miR-27b serves as
an oncogenic miRNA in HCC by modulating proliferation, cell cycle
progression and apoptosis. In the present study, miR-27b level was
found to be lower in tumor tissues. Meanwhile, the findings of the
present study indicated that upregulation of miR-27b in liver
cancer cells suppressed cell proliferation and promoted cell
apoptosis. This suggested that miR-27b acts as a liver cancer
suppressor. The results of the present study also indicated that
the expression level of miR-27b in the low age group was higher
than that in the high age group, which is consistent with the fact
that HCC tends to occur in middle-aged and elderly people.
In addition, miR-27b may play an important role in
chemosensitivity of cancers, though its effect on chemotherapeutic
resistance of tumors is also controversial. Zhang et al
(9) revealed that miR-27b and
miR-34a overexpression enhanced docetaxel sensitivity of prostate
carcinoma partly through inhibiting epithelial-to-mesenchymal
transition (EMT) by targeting zinc finger E-Box binding homeobox 1.
On the contrary, Xu et al (19) observed that downregulation of
miR-27b can increase the chemosensitivity of the doxorubicin (Dox)
resistant cell lines of human anaplastic thyroid cancer,
specifically, SW1736/Dox and 8305C/Dox cells. In the present study,
upregulation of miR-27b significantly reduced the sensitivity to
chemotherapeutic drugs (ADR, CDDP and 5-FU) in liver cancer Huh-7
and HepG2 cells. Moreover, after analyzing the association of
miR-27b levels with clinicopathological parameters in patients with
HCC, it was demonstrated that the period of disease recurrence of
the low miR-27b expression group was longer than that of the high
miR-27b expression group. As all patients with HCC in the present
study underwent TACE after hepatectomy, it can be assumed that
patients with HCC who had low miR-27b expression levels experienced
improved chemotherapeutic effects due to higher sensitivity to the
drugs. Collectively, these results indicated that miR-27b may act
as a biomarker to estimate the curative effect of chemotherapeutics
and as a determinant of the appropriate treatment course for
patients with HCC.
miR-27b acts as both a tumor suppressor and a
biomarker of chemoresistance, although the underlying mechanism has
not been fully investigated. Li et al (7) detected that miR-27b inhibited TSCC
proliferation and migration via suppressing the EMT process by
targeting integrin subunit alpha 5. The study of Han et al
(8) indicated that miR-27b-3p
suppresses cell proliferation, migration, invasion and EMT via
suppressing nuclear factor erythroid 2-related factor 2. In
addition, Bai et al (20)
reported that miR-27b-3p overexpression can inhibit EMT and
alleviate renal fibrosis via suppressing STAT1 both in vivo
and in vitro. The etiology and pathogenesis of HCC are not
clear, but numerous studies have demonstrated that cirrhosis is a
high-risk factor for HCC (21). The
results of the present study revealed that patients with cirrhosis
have lower miR-27b levels compared with the patients without
cirrhosis. This suggested that reduced miR-27b expression may
induce EMT and lead to cirrhosis and then promote the occurrence of
liver cancer. The mechanism of miR-27b in inhibiting proliferation,
promoting apoptosis and conferring chemotherapeutic resistance in
HCC is slowly coming to light. However, more has to be discovered
through follow-up research. Pending aims include the screening of
miR-27b target genes by using bioinformatics analysis software and
detection of liver cancer tissues, miR-27b overexpression HCC cell
lines and a control cell line. Subsequently, the target genes would
be verified by double luciferase reporting assays and functional
experiments.
Acknowledgements
The authors would like to thank Mr. Junjin Lin
(Public Technology Service Center, Fujian Medical University) for
technical assistance with the flow cytometry.
Funding
The present study was supported from the Natural Science
Foundation of Fujian (grant nos. 2015J01310 and 2019J01298).
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
AMH, LJZ and WMZ designed the study. LJZ and LLZ
conducted most of the experiments and HC conducted part of the
experiments. LJZ and LLZ analyzed the data. LJZ and AMH wrote the
manuscript. All authors have read and approved the final version of
the manuscript. LJZ and LLZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Ethical approval for this study was obtained from
the Ethics Committee of Fujian Medical University (approval no.
FMU-2014-093; Fuzhou, China). Written informed consent was obtained
from all participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saraiya N, Yopp AC, Rich NE, Odewole M,
Parikh ND and Singal AG: Systematic review with meta-analysis:
Recurrence of hepatocellular carcinoma following direct-acting
antiviral therapy. Aliment Pharmacol Ther. 48:127–137. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lohitesh K, Chowdhury R and Mukherjee S:
Resistance a major hindrance to chemotherapy in hepatocellular
carcinoma: An insight. Cancer Cell Int. 18:442018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhuo L, Liu J, Wang B, Gao M and Huang A:
Differential miRNA expression profiles in hepatocellular carcinoma
cells and drug-resistant sublines. Oncol Rep. 29:555–562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bao CH and Guo L: Retracted: miR-27b-3p
inhibits invasion, migration and epithelial-mesenchymal transition
in gastric cancer by targeting RUNX1 and activation of the hippo
signaling pathway. Anticancer Agents Med Chem. 22:864–873. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li T, Wu Q, Liu D and Wang X: miR-27b
suppresses tongue squamous cell carcinoma epithelial-mesenchymal
transition by targeting ITGA5. Onco Targets Ther. 13:11855–11867.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han M, Li N, Li F, Wang H and Ma L:
MiR-27b-3p exerts tumor suppressor effects in esophageal squamous
cell carcinoma by targeting Nrf2. Hum Cell. 33:641–651. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang G, Tian X, Li Y, Wang Z, Li X and
Zhu C: miR-27b and miR-34a enhance docetaxel sensitivity of
prostate cancer cells through inhibiting epithelial-to-mesenchymal
transition by targeting ZEB1. Biomed Pharmacother. 97:736–744.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen D, Si W, Shen J, Du C, Lou W, Bao C,
Zheng H, Pan J, Zhong G, Xu L, et al: miR-27b-3p inhibits
proliferation and potentially reverses multi-chemoresistance by
targeting CBLB/GRB2 in breast cancer cells. Cell Death Dis.
9:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Cui Y, Xie X, Xing Y, Yuan Z and
Wei Y: Functional role of miR-27b in the development of gastric
cancer. Mol Med Rep. 17:5081–5087. 2018.PubMed/NCBI
|
|
13
|
Feng Q, Wu X, Li F, Ning B, Lu X, Zhang Y,
Pan Y and Guan W: miR-27b inhibits gastric cancer metastasis by
targeting NR2F2. Protein Cell. 8:114–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Y, Xu T, Cao YW and Ding XQ: Antitumor
effect of miR-27b-3p on lung cancer cells via targeting Fzd7. Eur
Rev Med Pharmacol Sci. 21:4113–4123. 2017.PubMed/NCBI
|
|
15
|
Luo Y, Yu SY, Chen JJ, Qin J, Qiu YE,
Zhong M and Chen M: MiR-27b directly targets Rab3D to inhibit the
malignant phenotype in colorectal cancer. Oncotarget. 9:3830–3841.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang H, Ai-Jun J, Ji-Zong Z, Jian-Bo H,
Liang Z, Yong-Xiang Y and Chen Y: Clinicopathological significance
of miR-27b targeting Golgi protein 73 in patients with
hepatocellular carcinoma. Anticancer Drugs. 30:186–194. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun XF, Sun JP, Hou HT, Li K, Liu X and Ge
QX: MicroRNA-27b exerts an oncogenic function by targeting Fbxw7 in
human hepatocellular carcinoma. Tumour Biol. 37:15325–15332. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He S, Zhang J, Lin J, Zhang C and Sun S:
Expression and function of microRNA-27b in hepatocellular
carcinoma. Mol Med Rep. 13:2801–2808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Y, Han YF, Ye B, Zhang YL, Dong JD, Zhu
SJ and Chen J: miR-27b-3p is involved in doxorubicin resistance of
human anaplastic thyroid cancer cells via targeting peroxisome
proliferator-activated receptor gamma. Basic Clin Pharmacol
Toxicol. 123:670–677. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai L, Lin Y, Xie J, Zhang Y, Wang H and
Zheng D: MiR-27b-3p inhibits the progression of renal fibrosis via
suppressing STAT1. Hum Cell. 34:383–393. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma SA, Kowgier M, Hansen BE, Brouwer
WP, Maan R, Wong D, Shah H, Khalili K, Yim C, Heathcote EJ, et al:
Toronto HCC risk index: A validated scoring system to predict
10-year risk of HCC in patients with cirrhosis. J Hepatol.
S0168-8278(17)32248-1. 2017.(Epub ahead of print).
|