Introduction
The treatment of mRCC has undergone a paradigm shift
from conventional immunotherapy such as interferon and interleukin,
through molecular targeted drug therapy such as TKI and mTOR
inhibitors, to treatment using ICI such as anti-PD-1 and
anti-CTLA-4 antibodies. Currently, combination therapies such as
ICI plus ICI and ICI plus TKI have shown efficacy against mRCC
(1,2). Pembrolizumab, an anti-PD-1 monoclonal
antibody, and axitinib, a vascular endothelial growth factor
receptor TKI, demonstrated antitumor activity in patients with
untreated advanced RCC in the KEYNOTE-426 clinical trial (3).
ICI reactivates immune responses that are suppressed
by cancer cells, and results in the promotion of T cell attacking
cancer tissue (4,5). On the other hand, it has been noted
that activation of the immune response can cause immune related
adverse events. In pembrolizumab plus axitinib therapy, irAEs
(hypothyroidism, gastrointestinal disorders, and hepatitis) were
frequently reported in more than 50% of all patients in the
KEYNOTE-426 clinical trials (3).
Endocrine-related irAEs are common, and endocrine disorders such as
hypopituitarism, primary hypophysitis, thyroid dysfunction,
hypoparathyroidism, and type 1 diabetes mellitus can be severe, and
guideline-based treatment is recommended (6,7).
Hypopituitarism is irAE that should be taken care, because adrenal
crisis may develop and long-term hormone replacement may be
required. To our knowledge, there have been no prior literature of
hypopituitarism in patients with mRCC treated with pembrolizumab
plus axitinib. We report three cases of pembrolizumab plus axitinib
treated mRCC that resulted in clinically diagnosed hypopituitarism
which required constant steroid replacement therapy.
Case report
Case 1
A 73-year-old male had previously undergone
laparoscopic left nephrectomy and diagnosed clear cell carcinoma
pT1b. Lung and bone metastases were discovered 11 months after
surgery. The patient was diagnosed with mRCC and identified as
favorable risk according to the International mRCC Database
Consortium criteria. ECOG PS was 0, KPS was 100%. Pembrolizumab 400
mg every 6 weeks plus axitinib 5 mg twice daily was started. The
lung metastasis had shrunken after 2 courses of pembrolizumab,
however, the patient claimed general fatigue and appetite loss. The
laboratory data showed liver dysfunction (aspartate
aminotransferase 288 U/l, alanine aminotransferase 638 U/l) CTCAE
ver 5.0 grade 3, so pembrolizumab plus axitinib was discontinued
and intravenous methylprednisolone succinate 125 mg daily was
initiated. The patient has been checked viral infection such as
HAV, HBV, HCV, HIV, however they were all negative. EBV was
negative by checking viral capsid antigen (VCA) IgA, Epstein-Barr
nuclear antigen (EBNA1) IgA. Cytomegalovirus was also negative by
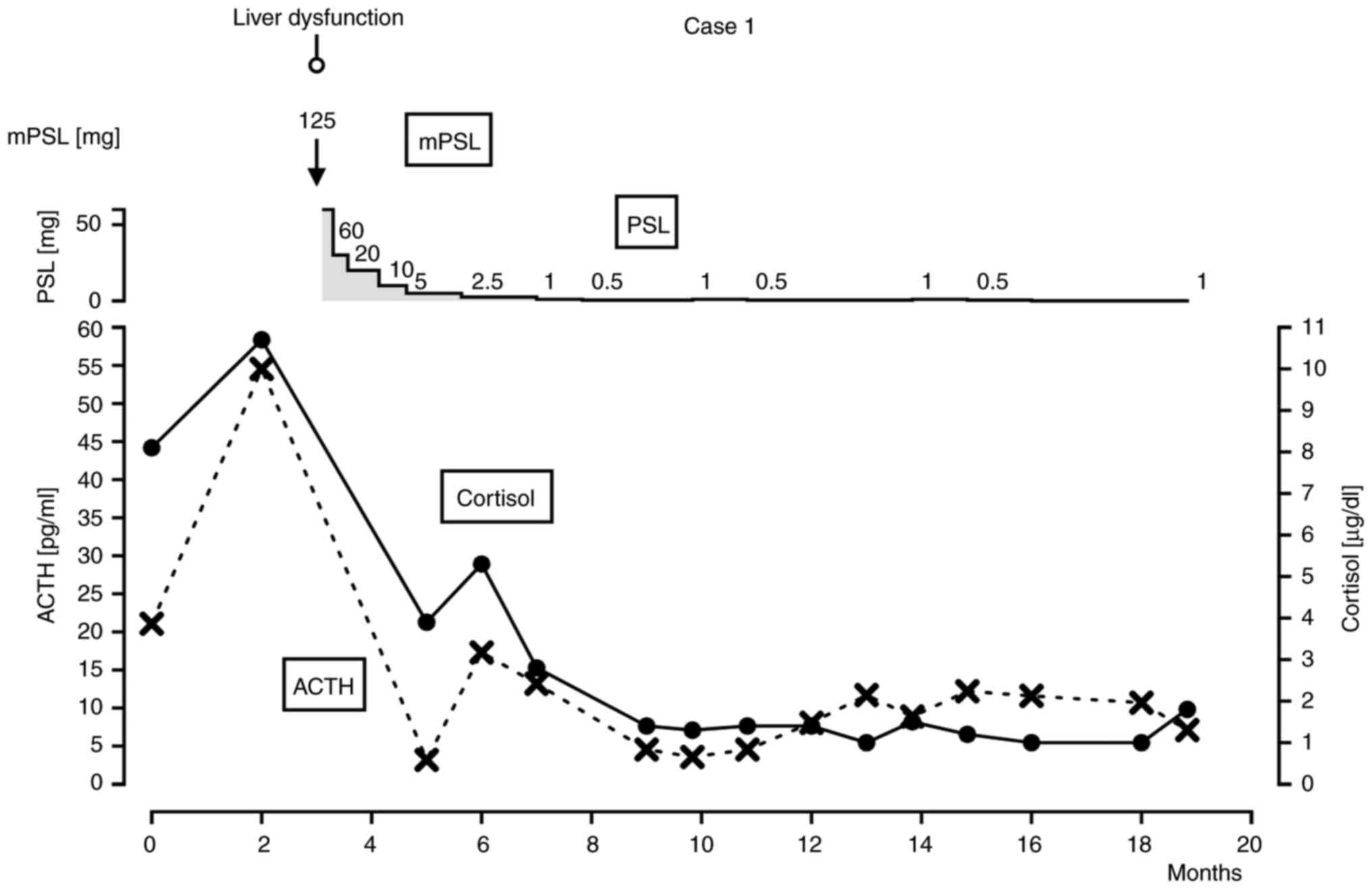
checking CMV IgG, IgM. With the decrease of liver enzymes, the dose
of prednisolone was gradually reduced over 2 months from 60 to 2.5
mg. When prednisolone was tapered off to 0.5 mg, fatigue and
decrease in appetite were observed. Laboratory data showed a
decrease in ACTH (3.5 pg/ml) and cortisol (1.3 µg/dl) but no
decrease in other hormone levels. Pituitary MRI to differentiate
from lymphocytic hypopituitarism or pituitary tumor was not
performed. The patient was diagnosed with hypopituitarism and CTCAE
ver 5.0 grade 3 based on clinical symptoms and regular laboratory
tests and was treated with steroid replacement. ACTH remained low
(3.5 to 8.6 pg/ml) after steroid replacement, and daily oral
prednisolone 1 mg was required to maintain clinical well-being of
the patient (Fig. 1). Regarding the
course of treatment, after discontinuation of pembrolizumab plus
axitinib, the patient was followed up with no treatment. However,
the disease has not progressed during the 20 months since the start
of treatment.
Case 2
A 77-year-old male had undergone laparoscopic left
nephrectomy and diagnosed clear cell carcinoma pT1b. Follow up CT
revealed a single metastatic site in the left lung 17 years after
nephrectomy. Lung metastasectomy was performed by thoracic surgery
unit. Nine months after the metastasectomy, sub bronchial lymph
node metastasis appeared. The patient was clinically diagnosed mRCC
identified as favorable risk. ECOG PS was 0, KPS was 100%.
Pembrolizumab 400 mg every 6 weeks plus axitinib 5 mg twice daily
was started and the size of the sub bronchial lymph node decreased.
During the third course of pembrolizumab plus axitinib, liver
dysfunction CTCAE ver 5.0 grade 3 was observed. Pembrolizumab plus
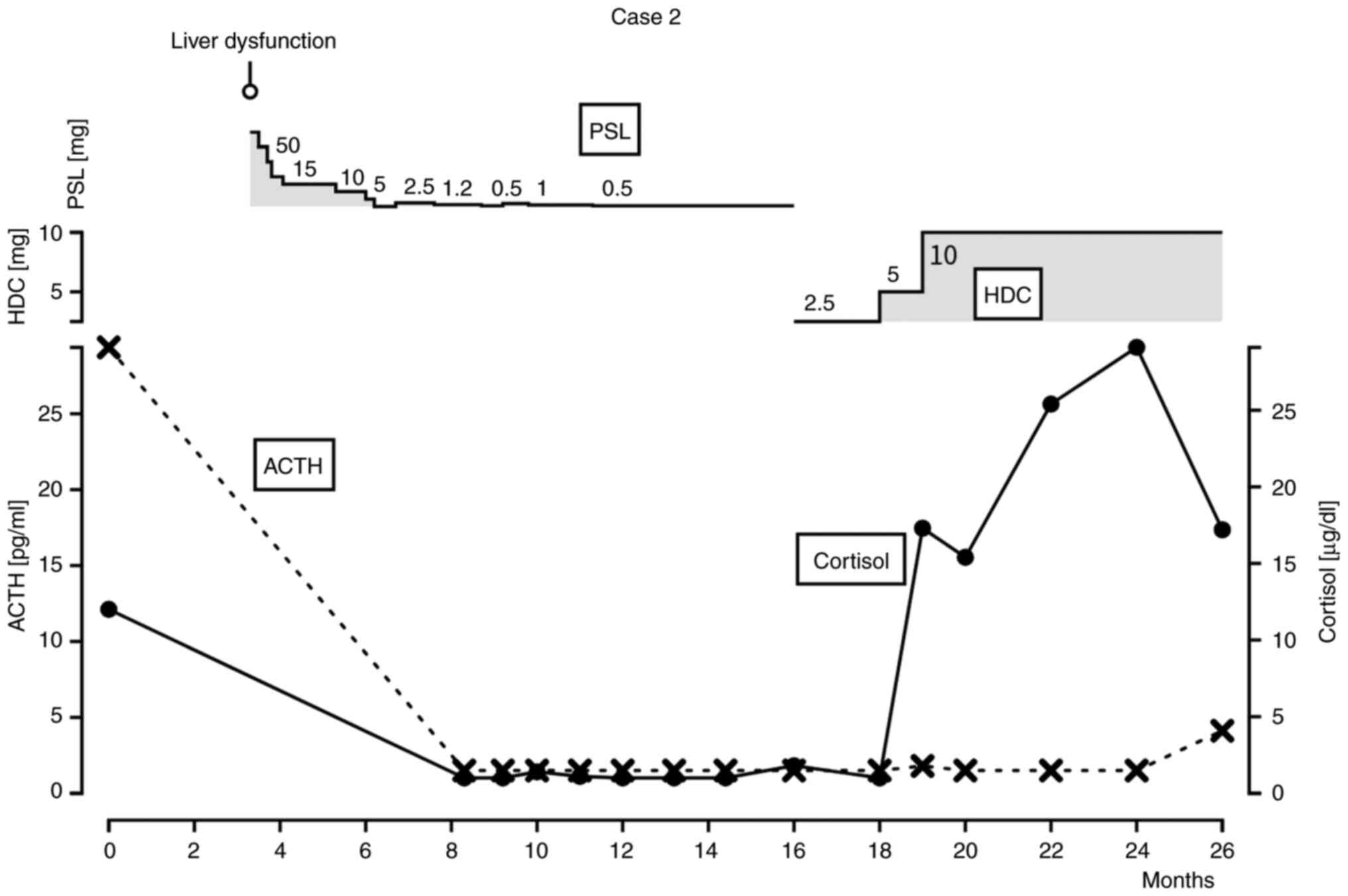
axitinib were stopped and oral daily prednisolone 50 mg/day was
started. The dose was gradually reduced 10 mg weekly to 10 mg/day,
and liver enzymes peaked out. As the dose was tapered off, the
patient complained general fatigue. Laboratory data showed a
decrease in ACTH and cortisol but no decrease in other hormone
levels. ACTH was below detection sensitivity and cortisol ranged
from l 1.0 to 2.4 pg/ml. Pituitary MRI was not performed in this
case either. The patient was diagnosed with hypopituitarism CTCAE
ver 5.0 grade 2 based on clinical symptoms and regular laboratory
tests. The patient was consulted to an endocrinologist, and
treatment was changed from prednisolone to hydrocortisone. The dose
of hydrocortisone had to be kept 10 mg daily as replacement therapy
(Fig. 2). After stopping
pembrolizumab and axitinib, the patient was followed up without
treatment, and the disease had not progressed for about 12 months
since the start of treatment. Then axitinib was started as a second
line therapy after the increase of sub bronchial lymph node
metastasis was observed.
Case 3
A 67-year-old male had previously undergone
laparoscopic right nephrectomy and diagnosed clear cell carcinoma
pT3b. The patient's left lung metastatic lesion was discovered 15
years after the primary surgery and metastasectomy was performed.
Multiple pancreatic metastases appeared 9 months after lung
metastasectomy. The patient was diagnosed mRCC identified as
favorable risk. ECOG PS was 0, KPS was 100%. Pembrolizumab 400 mg
every 6 weeks plus axitinib 5 mg twice daily were started. The
sizes of the pancreatic metastases decreased after the first
course. The dose of pembrolizumab plus axitinib were decreased
according to symptoms of adverse events such gastrointestinal
disorder and general fatigue. Blood tests and pituitary MRI were
used to diagnose hypopituitarism. Blood tests showed a decrease in
ACTH to 17.4 pg/ml and cortisol to below detection sensitivity, and
no decrease in other hormone levels. Based on clinical symptoms and
various test findings, a clinically diagnosis of hypopituitarism
CTCAE ver 5.0 grade 3 was made. The patient started hydrocortisone
50 mg daily administered orally. When hydrocortisone was tapered
off to 10 mg daily, the patient's symptoms showed remission and
worsening. The dose of hydrocortisone was then increased to 20 mg
daily due to a decrease of ACTH to 3.93 pg/ml. The patient
continued hydrocortisone 20 mg daily with no general fatigue
(Fig. 3). Regarding the course of
treatment, after 4 courses of treatment, metastases in the pancreas
and liver became increased, and metastases in the left lung
appeared. However, pembrolizumab plus axitinib were continued for
about 17 months with volume adjustment according to the degree of
adverse events, despite the increase of pancreatic and liver
metastases. Meanwhile, there was no apparent worsening of
hypopituitarism. After that, the patient was started on pazopanib
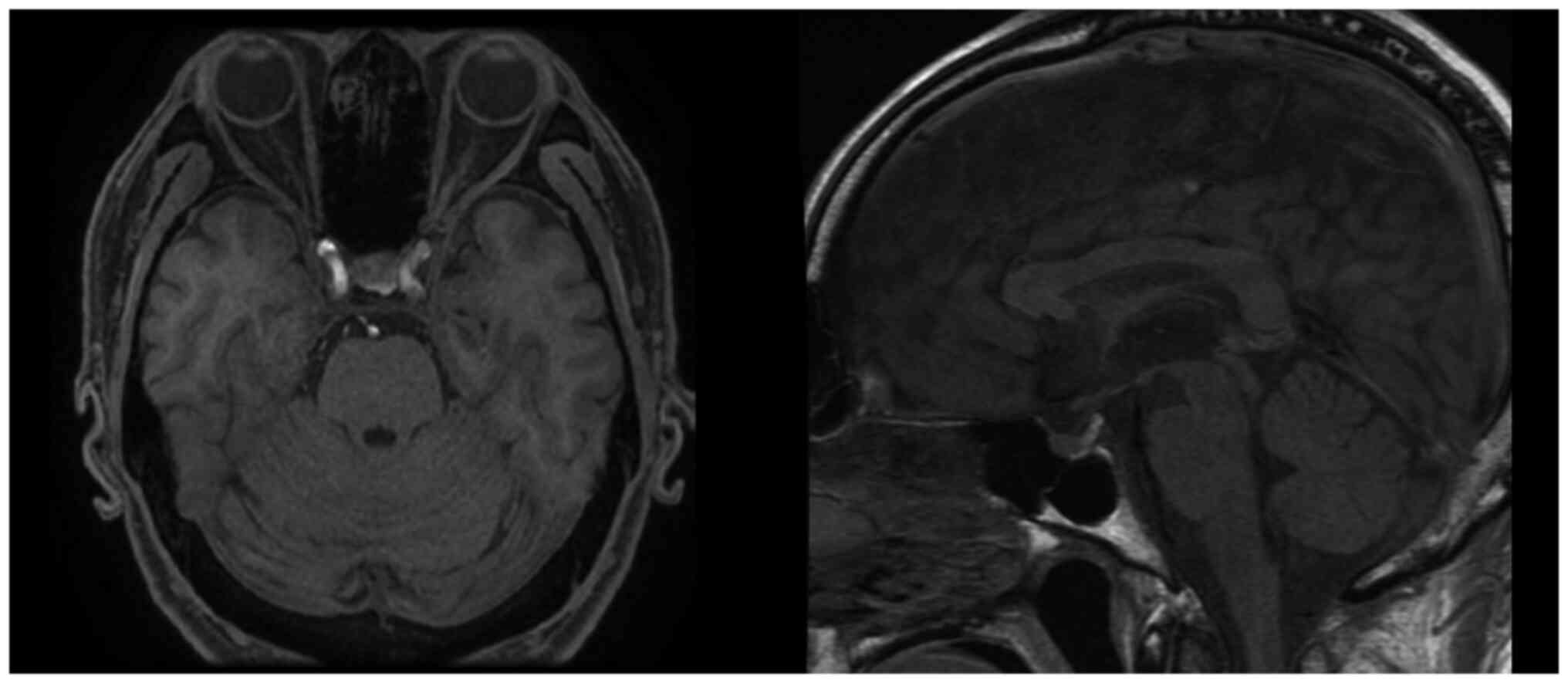
as second line. Contrast-enhanced pituitary MRI was performed in
the present case at the diagnosis of hypopituitarism (Fig. 4). MRI showed no abnormalities in the
size or shape and no nodular structures.
Discussion
ICI has been demonstrated to be effective in the
treatment of advanced cancers of various types and improved the
treatment for malignant tumors. Some prior literatures have
reported that hypopituitarism associated with anti-CTLA-4
antibodies is 1.5 to 17%, whereas that associated with anti-PD-1
antibodies is less frequent, less than 1.5% (6,8,9). In
the KEYNOTE-426 study, hypopituitarism and hypophysitis induced by
pembrolizumab plus axitinib occurred in only 2 (0.5%) and 13 (3.0%)
of 429 patients, respectively (3,10). The
frequency of hypopituitarism is reported to be very rare, however,
we have experienced in 3 out of 5 patients treated with
pembrolizumab plus axitinib in our hospital. This may have been
associated with multiple factors such as clinical course, patient
background, environmental factors, or genetic predisposition. There
might be chances that the patients' status is different from that
in the clinical trials which diverge from actual clinical practice.
In terms of patient background, all patients were male, their
medical history was unremarkable except for hypertension and
hyperuricemia, which were already treated. What was uncommon in the
clinical course was the steroid treatment for liver dysfunction
which were prescribed in two of the three patients before the onset
of hypopituitarism.
Hypopituitarism induced different hormone type
defects, depending on the type of ICI used. CTLA-4 inhibitor
therapy often results in impaired secretion of thyroid-stimulating
hormone (TSH), luteinizing hormone (LH)/follicle-stimulating
hormone (FSH), as well as impaired ACTH secretion (11,12).
On the other hand, anti-PD-1 antibodies induce IAD more commonly
(13–15). In fact, in a report including 16
cases of hypopituitarism caused by anti-PD-1 antibodies alone, 14
cases induced IAD (13). The
detailed mechanism of hypopituitarism is unknown, but previous
studies have hypothesized that ICI itself directly attacks
secretory cells in the pituitary gland, or that ICI activates
autoimmunity and induces autoantibodies (11,13).
It is also thought that anti-CTLA-4 antibodies and
anti-PD-1 antibodies may have different mechanisms (13). A study on the mechanism of
hypopituitarism induced by anti-CTLA-4 antibodies reported that one
of the causes is that anti-CTLA-4 antibodies induce a direct
interaction with CTLA-4 ectopically expressed on secretory cells in
the pituitary gland, inducing complement-dependent cell damage to
the pituitary gland (11). On the
other hand, PD-1 has not been reported to be ectopically expressed
on secretory cells in the pituitary gland, making it unlikely that
anti-PD-1 antibodies act directly on the pituitary gland to cause
hypopituitarism. Kanie et al (13) hypothesized that ectopic ACTH
expression in tumors can evoke autoreactive T-cell activation and
ICI administration can enhance the autoimmunity, ultimately
resulting in the specific injury of corticotrophs and ACTH
deficiency. This hypothesis is consistent with reports of a higher
frequency of IAD in hypopituitarism caused by anti-PD-1 antibodies
(15,16) and with this report. To evaluate the
three cases in this report for ectopic expression of ACTH from
tumor cells, immunostaining for ACTH was performed on specimens of
the primary renal tumor in Case 1 and on specimens of lung
metastases in Cases 2 and 3, however, no ectopic expression of ACTH
was found in either case. In Case 2 and 3, the expression in the
primary tumor could not be evaluated because the kidney specimens
were not available due to the time since the surgery of the primary
tumor. Kanie et al (13)
also reported that some cases of hypopituitarism did not show
ectopic expression of ACTH, and it is possible that the ectopic
expression was transient and difficult to detect.
The irAE in our cases were treated the appropriate
medication volume (16), and it is
unlikely that the suppression of the hypothalamic-pituitary-adrenal
axis due to long-term high-dose steroid administration is the
primary cause of hypopituitarism. Considering previous studies, it
is likely that there is some relationship, such as the pituitary
gland receiving negative feedback from steroid therapy was more
prone to hypopituitarism due to the involvement of autoimmunity
induced by anti-PD-1 antibodies. When comparing the incidence of
irAE in our hospital with the general incidence, we can hypothesize
that hypopituitarism may occur more frequently after the treatment
of irAE by steroid. A retrospective study of 22 patients with mRCC
treated with ipilimumab plus Nivolumab combination therapy reported
hypopituitarism in 41% of all patients (17). The incidence of hypophysitis and
hypopituitarism, which may be included in hypopituitarism in the
checkmate 214 study, is 5 and 3%, respectively, indicating that
some racial difference might be related (18). Another concern is that most patients
with hypopituitarism has previously experienced irAEs in Takagi's
report when compared with Checkmate 214 study. Although there is no
description of therapies of irAE in Takagi's report (17). It can be suggested that steroid
therapy might be used to treat irAEs in this report. Limitation of
this report include the number of patients in this report is small
and does not include statistical analysis and pituitary MRI was not
performed in two of three cases. The two cases had no symptom of
headache, visual disturbance and no hormonal disruption other than
ACTH, therefore we have not considered hypophysitis and MRI were
not taken. MRI was taken only in case 3 and showed no sign of
hypophysitis. The reason of not performing MRI is that the patients
did not show pituitary tumor related symptoms such as headache or
visual disturbances. A prospective study of the relationship
between steroid therapy and hypopituitarism for irAE is ideal, but
it is unrealistic to conduct such studies with the addition of MRI
findings and anti-pituitary antibodies.
In conclusion, the present study reports three cases
of hypopituitarism in combination therapy with pembrolizumab plus
axitinib for mRCC. All patients developed IAD, and two patients
developed hypopituitarism after steroid therapy for irAE.
Hypopituitarism is more likely to develop in patients previously
prescribed steroids for irAE due to pembrolizumab plus
axitinib.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH, NS, SK and TM conceived and designed the study.
JA, YO, YK, KM, HS and TM made substantial contributions to
acquisition of data, and analysis and interpretation of data. KH
and NS confirm the authenticity of all the raw data and drafted the
manuscript. HS, SK, and TM critically revised the manuscript. All
authors agreed to the journal to which the article was submitted
and agreed to take all the responsibility of the article. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of University of Yamanashi Hospital (approval no. 2616;
Yamanashi, Japan).
Patient consent for publication
The patients provided written informed consent for
the publication regarding their related data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACTH
|
adrenocorticotropic hormone
|
|
CT
|
computed tomography
|
|
CTCAE
|
Common Terminology Criteria for
Adverse Events
|
|
CTLA-4
|
cytotoxic T-lymphocyte associated
antigen-4
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
HDC
|
hydrocortisone
|
|
IAD
|
Isolated ACTH deficiency
|
|
ICI
|
immune checkpoint inhibitors
|
|
irAE
|
immune-related adverse events
|
|
KPS
|
Karnofsky Performance status
|
|
PSL
|
prednisolone
|
|
mPSL
|
methylprednisolone
|
|
mRCC
|
metastatic renal cell carcinoma
|
|
MRI
|
magnetic resonance imaging
|
|
PD-1
|
programmed cell death protein 1
|
|
PS
|
Performance status
|
|
RCC
|
renal cell carcinoma
|
|
TKI
|
tyrosine kinase inhibitor
|
References
|
1
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Makker V, Colombo N, Casado Herráez A,
Santin AD, Colomba E, Miller DS, Fujiwara K, Pignata S, Baron-Hay
S, Ray-Coquard I, et al: Lenvatinib plus pembrolizumab for advanced
endometrial cancer. N Engl J Med. 386:437–448. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Shao C, Shi Y and Han W: Lessons
learned from the blockade of immune checkpoints in cancer
immunotherapy. J Hematol Oncol. 11:312018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Li F, Jiang F, Lv X, Zhang R, Lu A
and Zhang G: A mini-review for cancer immunotherapy: Molecular
understanding of PD-1/PD-L1 pathway & translational
blockade of immune checkpoints. Int J Mol Sci. 17:11512016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cukier P, Santini FC, Scaranti M and Hoff
AO: Endocrine side effects of cancer immunotherapy. Endocr Relat
Cancer. 24:T331–T347. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arima H, Iwama S, Inaba H, Ariyasu H,
Makita N, Otsuki M, Kageyama K, Imagawa A and Akamizu T: Management
of immune-related adverse events in endocrine organs induced by
immune checkpoint inhibitors: clinical guidelines of the Japan
endocrine society. Endocr J. 66:581–586. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertrand A, Kostine M, Barnetche T,
Truchetet ME and Schaeverbeke T: Immune related adverse events
associated with anti-CTLA-4 antibodies: Systematic review and
meta-analysis. BMC Med. 13:2112015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barroso-Sousa R, Barry WT, Garrido-Castro
AC, Hodi FS, Min L, Krop IE and Tolaney SM: Incidence of endocrine
dysfunction following the use of different immune checkpoint
inhibitor regimens: A systematic review and meta-analysis. JAMA
Oncol. 4:173–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Powles T, Plimack ER, Soulières D, Waddell
T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko
I, et al: Pembrolizumab plus axitinib versus sunitinib monotherapy
as first-line treatment of advanced renal cell carcinoma
(KEYNOTE-426): Extended follow-up from a randomised, open-label,
phase 3 trial. Lancet Oncol. 21:1563–1573. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwama S, De Remigis A, Callahan MK, Slovin
SF, Wolchok JD and Caturegli P: Pituitary expression of CTLA-4
mediates hypophysitis secondary to administration of CTLA-4
blocking antibody. Sci Transl Med. 6:230ra452014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caturegli P, Di Dalmazi G, Lombardi M,
Grosso F, Larman HB, Larman T, Taverna G, Cosottini M and Lupi I:
Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein
4 blockade: Insights into pathogenesis from an autopsy series. Am J
Pathol. 186:3225–3235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanie K, Iguchi G, Bando H, Urai S, Shichi
H, Fujita Y, Matsumoto R, Suda K, Yamamoto M, Fukuoka H, et al:
Mechanistic insights into immune checkpoint inhibitor-related
hypophysitis: A form of paraneoplastic syndrome. Cancer Immunol
Immunother. 70:3669–3677. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohara N, Ohashi K, Fujisaki T, Oda C,
Ikeda Y, Yoneoka Y, Hashimoto T, Hasegawa G, Suzuki K and Takada T:
Isolated adrenocorticotropin deficiency due to nivolumab-induced
hypophysitis in a patient with advanced lung adenocarcinoma: A case
report and literature review. Intern Med. 57:527–535. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takagi T, Yoshida K, Kondo T, Fukuda H,
Ishihara H, Kobayashi H, Iizuka J, Ishida H and Tanabe K:
Hypopituitarism in patients with metastatic renal cell carcinoma
treated with ipilimumab and nivolumab combination therapy. Jpn J
Clin Oncol. 51:1744–1750. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Motzer RJ, Rini BI, McDermott DF, Arén
Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B,
Beuselinck B, Amin A, et al: Nivolumab plus ipilimumab versus
sunitinib in first-line treatment for advanced renal cell
carcinoma: Extended follow-up of efficacy and safety results from a
randomised, controlled, phase 3 trial. Lancet Oncol. 20:1370–1385.
2019. View Article : Google Scholar : PubMed/NCBI
|