Introduction
Lung cancer is a prevalent type of solid tumors
worldwide and non-small cell lung cancer (NSCLC) makes up ~85% in
lung cancer cases (1). In China
alone, there are almost 688,000 new cases and 604,000 deaths from
lung cancer each year (2). In
recent years, significant progress has been made in identifying
various driver genes in NSCLC and developing targeted drugs
specifically for these mutations. This has led to NSCLC with
positive driver gene mutations becoming one of the most successful
cancers to be treated with precision medicine (3). Furthermore, the use of programmed
death-1 (PD-1) blockades has shown promising and long-lasting
therapeutic effects for patients without driver gene mutations,
resulting in a significant increase in the 5-year survival rate of
advanced NSCLC patients ranging from 5 to >15% currently
(4). Over the past few years, the
use of PD-1 blockade monotherapy as standard treatment for patients
with advanced NSCLC in the second-line or subsequent-line therapy
has been established (5). The
combination of pembrolizumab, nivolumab and atezolizumab with
chemotherapy has emerged as the first-line therapy for patients
with advanced NSCLC; as evidenced by the Keynote, Checkmate, and
Impower series clinical trials (6).
Similarly in China, sintilimab, camrelizumab, tislelizumab and
other PD-1 blockades are also approved for patients with advanced
NSCLC (7) and widely used with
improved results (8). However, it
is important to note that the overall response of patients with
advanced NSCLC who received PD-1 blockades is still considered
disappointing. While PD-1 blockades have shown promise in some
patients, there is still need to identify efficacious biomarkers
that can help refine the selection of patients who are likely to
benefit from this therapy.
Nevertheless, accurately predicting the therapeutic
response of PD-1 blockades remained challenging, particularly in
cases where these blockades were administered with single agent, as
there were no specific biomarkers available in clinical practice
(9). A significant number of
patients with advanced NSCLC did not exhibit any response to PD-1
blockades, resulting in an ~20% objective response rate (ORR) for
patients without available biomarkers (10). Currently, only programmed
death-ligand 1 (PD-L1) expression, DNA mismatch repair (MMR) and
tumor mutation burden (TMB) status can potentially be beneficial
prediction markers for PD-1 blockades (11). Unfortunately, these are still not
satisfactory biomarkers because of genetic heterogeneity and
expression in tumors (12).
Therefore, ORR of pembrolizumab in patients with high PD-L1
expression (PD-L1 ≥50%) and dMMR patients was found to be below 45%
(44.8 and 43.8%) in the Keynote 024 and Keynote 177 clinical
trials, respectively (13,14). This indicated the need to further
investigate alternative biomarkers for more precise guidance in the
use of PD-1 blockades.
The PD-L1 gene, located at chromosome 9p24.1,
consists of 8 exons and demonstrates significant ethnic differences
even within the Chinese population (15). Furthermore, the level of PD-L1
expression can vary among different cancer patients, including
those with the same type of cancer (16). Previous studies have shown the
correlation of various PD-L1 mutations with susceptibility and
prognosis different cancers such as 901A>G with hepatocellular
carcinoma (17), rs822339,
rs1411262 rs2282055 and rs4143815 with prognosis of NSCLC patients
with NSCLC who received nivolumab (18,19).
This indicated that PD-L1 polymorphisms potentially contribute to
the clinical outcomes of patients with NSCLC who received PD-1
blockades therapy. However, there is currently no consensus on the
correlation between PD-L1 polymorphism and the effectiveness of
PD-1 blockades among Chinese patients with advanced NSCLC
currently.
Therefore, the present study aimed to
retrospectively examine the clinical outcomes of patients with
advanced NSCLC who received PD-1 blockades specifically focusing on
the clinical significance of PD-L1 polymorphisms.
Patients and methods
Design of the study and eligibility
criteria
In recent years, the Chinese National Medical
Products Administration has approved PD-1 blockades for the
treatment of patients with advanced NSCLC. Therefore, a significant
number of patients with advanced NSCLC have undergone PD-1 blockade
monotherapy in clinical practice. The present study aimed to
retrospectively include patients with advanced NSCLC who underwent
PD-1 blockade monotherapy (any PD-1 blockade licensed in China) at
the Department of Thoracic Surgery of the Affiliated Hospital of
Hebei University (Baoding, China) from July 2018 to June 2022. The
inclusion criteria were as follows: i) Diagnosis of NSCLC with
pathological staging of IIIb or IV confirmed by pathological
expert; ii) age ≥18 years; iii) patients with previously-treated
advanced NSCLC who received PD-1 blockades monotherapy for at least
one cycle in clinical practice; and iv) at least one measurable
target lesion. The main exclusion criteria were: i) Patients with a
history of autoimmune disease or clinical symptoms unsuitable for
PD-1 blockades therapy; ii) patients diagnosed with one or more
tumors or serious diseases that might compromise their living
status; and iii) insufficient availability of demographic
characteristics or efficacy assessment data according to the
investigators' judgment. Eventually, a total of 89 patients with
advanced NSCLC met the eligibility criteria and were included in
the present study. The primary objective of the present study was
to identify the association between PD-L1 polymorphisms and
clinical outcomes of PD-1 blockades, including ORR, disease
contrail rate (DCR), progression-free survival (PFS) and overall
survival (OS).
The protocol and additional materials for the
present study were approved (approval no. 2022-KY-11053) by the
Ethics Committee of the Affiliated hospital of Hebei university
(Baoding, China). Each enrolled patient provided written informed
consent in accordance to the recommendations of the Declaration of
Helsinki (1964).
Therapeutic regimens of PD-1 blockades
in clinical practice
All 89 patients with advanced NSCLC received PD-1
blockade monotherapy for a minimum of one cycle in clinical
practice. The PD-1 blockades utilized in the present study were
approved in mainland China and clinically available for Chinese
patients. These included nivolumab (Bristol-Myers Squibb Co.),
pembrolizumab (Merck KGaA), camrelizumab (Jiangsu Hengrui
Pharmaceuticals Co., Ltd.), sintilimab (Innovent) and tislelizumab
(BeiGene, Inc.). Camrelizumab, sintilimab, pembrolizumab and
tislelizumab were administered intravenously at a dose of 200 mg on
day 1, while nivolumab was given intravenously with a dose of 360
mg on day 1. A treatment cycle of 21 days was completed. (20). The administration of these five PD-1
blockades persisted until either disease progression or intolerable
adverse reactions in the patients.
Assessment of response and protocol of
follow-up
Since PD-1 blockades monotherapy was used in the
present study, the iRECIST criteria were utilized to evaluate the
therapeutic response of the patients (21). As aforementioned, all 89 patients
included in the present study had at least one measurable target
lesion, assessed through radiological scans such as CT or MRI at
baseline and throughout PD-1 blockades treatment. ORR and DCR were
calculated according to the best overall response during
administration of PD-1 blockades. Specifically, ORR was defined as
the proportion of patients with complete response (CR) and partial
response (PR) among the 89 patients. DCR was determined as the
proportion of patients with CR, PR and stable disease (SD) among
the 89 patients.
Furthermore, clinical and demographic
characteristics of the 89 patients were retrieved from the
electronic medical record system at the Affiliated Hospital of
Hebei University (Baoding). Additionally, the disease progression
status of each patient was assessed, and follow-up was conducted
through phone communication to gather prognostic data. The
therapeutic regimens of patients who experienced progression after
PD-1 blockades monotherapy were documented and their health status
was primarily obtained accordingly. PFS was defined as the duration
from the initiation of PD-1 blockade treatment to the date of
disease progression or death, whichever occurred first. OS was
defined as the initiation from the date of PD-1 blockades treatment
to the date of death from any cause.
Genotyping of PD-L1 gene
polymorphism
Concerning the analysis of PD-L1 gene polymorphism,
the DNA specimens of each patient were primarily extracted from
their respective peripheral blood or cancer tissue biopsies,
obtained before the initiation of PD-1 blockade treatment,
according to a previous study (22).
Additionally, single nucleotide polymorphisms in
PD-L1 gene of the present study were adopted from a previous study
(23), including rs2297136,
rs17718883, rs822339 and rs1411262. The preliminary analysis
comparing the genotype status of these polymorphisms and PFS of the
89 patients is presented in Table
I. Notably, only rs2297136 exhibited clinical significance.
Therefore, the present study primarily focused on the results of
rs2297136.
 | Table I.Association between genotypes status
of the four polymorphisms and PFS of the 89 patients with advanced
non-small cell lung cancer. |
Table I.
Association between genotypes status
of the four polymorphisms and PFS of the 89 patients with advanced
non-small cell lung cancer.
| Polymorphisms | Primer sequence
(5′→3′) | Location | Minor allele
frequency | Median PFS
(months) | P-value |
|---|
| rs2297136 |
GGAGGAGACGTAATCCAGCA | Non-coding | 0.19 | 2.95 vs. 5.3 | 0.038 |
|
|
CCAGGCTCCCTGTTTGACT | region |
| (AA vs. AG/GG) |
|
| rs17718883 |
GGACAGCATCAAGCTATGTACG | Coding region | 0.00 | NA | NA |
|
|
CTCTTGGAATTGGTGGTGGT |
|
|
|
|
| rs822339 |
TAACTCTGGCCCAAGGAAAA | Intron region | 0.39 | 3.2 vs. 3.5 | 0.315 |
|
|
TTTTGGTCTGTTTATGTCACTGG |
|
| (AA vs. AG/GG) |
|
| rs1411262 |
TGGTTTTGGGATTGAGTTCAG | Intron region | 0.42 | 3.5 vs. 3.1 | 0.536 |
|
|
TCCTGTGGGGAAGCTATGTT |
|
| (TT vs. TC/CC) |
|
Genotyping of rs2297136 polymorphism was carried out
using PCR-RFLP methods derived from a previous study (23). The forward primer for the PCR
products of rs2297136 was 5′-GGAGGAGACGTAATCCAGCA-3′, and the
reverse primer was 5′-CCAGGCTCCCTGTTTGACT-3′, resulting in a PCR
product of 216 bp. PCR product was disposed using PspOMI
restriction enzyme. The genotyping of rs2297136 was determined
based on the following criteria: AA (216 bp stripe); AG (216 bp
stripe, 104 bp stripe and 112 bp stripe); GG (104 bp stripe and 112
bp stripe).
Analysis of PD-L1 gene mRNA
expression
Aiming to investigate the potential association
between PD-L1 polymorphism and PD-L1 gene mRNA expression,
available fresh specimens of peripheral blood mononuclear cells
(PBMC) were initially collected from 89 patients with advanced
NSCLC. Unfortunately, 8 patients failed to obtain the qualified RNA
specimens. Ultimately, a total of 81 patients were included in
PD-L1 gene mRNA expression analysis. The methodology of PD-L1 mRNA
expression analysis was adopted from a previous study (23). Total RNA samples were extracted with
TRIzol® (Thermo Fisher Scientific, Inc.) using RNAiso
Plus reagents (Takara Biotechnology Co., Ltd.) as the RNA
extraction buffer according to the manufacturer's instructions, and
stored at −80°C for mRNA expression analysis. A total of 500 ng RNA
extracted from the PBMC was used as the templates for
reverse-transcription polymerase chain reaction to prepare the
first-stand of cDNA with the PrimeScript RT reagent Kit. Relative
quantitative analysis of PD-L1 gene mRNA expression was carried out
with Roche LightCycler 480 (Roche Diagnostics, Ltd.) using a SYBR
Premix EX Taq system. The forward primer of PD-L1 was
5′-TTCAATGTGACCAGCACACTGAG-3′, the reverse primer was
5′-TTTTCACATCCATCATTCTCCCT-3′. The amplification system was
comprised of a 20 µl containing 10 µl SYBR Premix EX Taq, 0.2 µl of
each primer (20 µM), 7.6 µl double distilled water (ddH2O) and 2 µl
cDNA. PD-L1 mRNA expression level was calculated by comparative Cq
(2−ΔΔCq) method (24),
with GAPDH mRNA expression serving as an endogenous control. The
forward primer sequences used for GAPDH mRNA expression was
5′-GAAGGTGAAGGTCGGAGTCAAC-3′, the reverse primer was
5′-CAGAGTTAAAAGCAGCCCTGGT-3′ (25).
The thermocycling conditions were as follows: 50°C for 2 min and
95°C for 2 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 30 sec.
Statistical analysis
The statistical analysis presented in the present
study was conducted using SPSS software version 25.0 (IBM Corp.).
The conformity of the genotype status of the rs2297136 polymorphism
with Hardy-Weinberg equilibrium was assessed through the chi-square
test (22). Regarding the analysis
of baseline characteristics, the distribution between proportion
variables and genotype status of rs2297136 was carried out using
the Mann-Whitney U non-parametric test, between the two groups.
Data in the present study were presented as median (range) and the
number of patients in percentages based on corresponding data
category. PFS and OS were defined as aforementioned. Survival
curves were generated using Stata 14.0 to illustrate survival data
according to rs2297136 genotype status, with a log-rank test used
to determine significant differences. Cox analysis was used for OS
in multivariate analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics and
genotyping of PD-L1 gene rs2297136 polymorphism
Baseline characteristics of the 89 patients with
advanced NSCLC were shown in Table
II. It was revealed that all the 89 patients included in the
present study were individuals commonly encountered in clinical
practice with previously treated advanced NSCLC. Notably, 38.2% of
the patients had an Eastern Cooperative Oncology Group (ECOG)
performance status score of 2–3. Interestingly, 18 cases (20.2%)
exhibited positive EGFR mutation, and 4 patients (4.5%) exhibited
anaplastic lymphoma kinase positive rearrangement. Among the
patients, 19 received PD-1 blockades as second-line therapy, while
70 underwent third-line or subsequent treatments. The present study
utilized five PD-1 blockades, including camrelizumab (28.1%),
sintilimab (23.6%), tislelizumab (22.5%), pembrolizumab (16.9%) and
nivolumab (8.9%).
 | Table II.Baseline characteristics of the 89
patients with advanced non-small cell lung cancer according to
genotype status of programmed death-ligand 1 rs2297136. |
Table II.
Baseline characteristics of the 89
patients with advanced non-small cell lung cancer according to
genotype status of programmed death-ligand 1 rs2297136.
|
|
| rs2297136 genotype
status |
|
|
|---|
|
|
|
|
|
|
|---|
| Baseline
characteristics | Total, N=89
(%) | AA (N=58) | AG/GG (N=31) | χ2 | P-value |
|---|
| Age, years |
|
|
|
|
|
| Median
(range) | 66 (21–79) | 66 (21–78) | 66 (24–79) | NA | 0.573 |
| Sex |
|
|
| 0.067 | 0.796 |
|
Male | 59 (66.3) | 39 (67.2) | 20 (64.5) |
|
|
|
Female | 30 (33.7) | 19 (32.8) | 11 (35.5) |
|
|
| ECOG PS score |
|
|
| 0.149 | 0.700 |
|
0-1 | 55 (61.8) | 35 (60.3) | 20 (64.5) |
|
|
|
2-3 | 34 (38.2) | 23 (39.7) | 11 (35.5) |
|
|
| Pathological
stage |
|
|
|
|
|
|
IIIb | 9 (10.1) | 6 (10.3) | 3 (9.7) | 0.010 | 0.920 |
| IV | 80 (89.9) | 52 (89.7) | 28 (90.3) |
|
|
| Smoking status |
|
|
| 1.384 | 0.239 |
|
Non-smoker | 17 (19.1) | 9 (15.5) | 8 (25.8) |
|
|
| Former
smoker/smoker | 72 (80.9) | 49 (84.5) | 23 (74.2) |
|
|
| EGFR mutation
status |
|
|
| 0.495 | 0.482 |
|
Positive | 18 (20.2) | 13 (22.4) | 5 (16.1) |
|
|
|
Negative | 71 (79.8) | 45 (77.6) | 26 (83.9) |
|
|
| Anaplastic lymphoma
kinase rearrangement |
|
|
| 0.608 | 0.434 |
|
Positive | 4 (4.5) | 2 (3.4) | 2 (6.5) |
|
|
|
Negative or not available | 85 (95.5) | 56 (96.6) | 29 (93.5) |
|
|
| History of surgical
resection |
|
|
| 0.021 | 0.885 |
|
Yes | 25 (28.1) | 16 (27.6) | 9 (29.0) |
|
|
| No | 64 (71.9) | 42 (72.4) | 22 (71.0) |
|
|
| Histological
category |
|
|
| 0.149 | 0.700 |
|
Adenocarcinoma | 55 (61.8) | 35 (60.3) | 20 (64.5) |
|
|
|
Squamous cell carcinoma | 34 (38.2) | 23 (39.7) | 11 (35.5) |
|
|
| Number of
metastatic lesions |
|
|
| 0.726 | 0.394 |
| ≤3 | 52 (58.4) | 32 (55.2) | 20 (64.5) |
|
|
|
>3 | 37 (41.6) | 26 (44.8) | 11 (35.5) |
|
|
| Therapeutic Lines
of PD-1 blockades |
|
|
| 0.563 | 0.453 |
|
Second-line | 19 (21.3) | 11 (19.0) | 8 (25.8) |
|
|
|
Third-line or more | 70 (78.7) | 47 (81.0) | 23 (74.2) |
|
|
| PD-1 blockades |
|
|
| 0.123 | 0.726 |
|
Camrelizumab | 25 (28.1) | 17 (29.3) | 8 (25.8) |
|
|
|
Sintilimab | 21 (23.6) | 14 (24.1) | 7 (22.6) |
|
|
|
Tislelizumab | 20 (22.5) | 13 (22.4) | 7 (22.6) |
|
|
|
Pembrolizumab | 15 (16.9) | 9 (15.5) | 6 (19.4) |
|
|
|
Nivolumab | 8 (8.9) | 5 (8.6) | 3 (9.7) |
|
|
As outlined in the methods section, only rs2297136
demonstrated clinical significance in the preliminary analysis, as
demonstrated in Table I. The
prevalence of rs2297136 among the 89 patients with advanced NSCLC
is detailed as follows: The AA genotype was observed in 58 cases
(65.2%), the AG genotype was found in 28 cases (31.5%), and the GG
genotype was noted in 3 cases (3.4%), resulting in a minor allele
frequency (MAF) of 0.19, consistent with Hardy-Weinberg Equilibrium
(P=0.865). Given the rarity of patients with GG genotype, patients
with GG and AG were combined into one group in the subsequent
analysis. The association between genotype status of rs2297136 and
baseline characteristics is presented in Table II. Evidently, baseline
characteristics of patients with AA and AG/GG genotypes were
comparable and well-balanced (P>0.05).
Association between efficacy of PD-1
blockades and genotype status of rs2297136
Radiological evidence for the target lesions of the
89 patients with advanced NSCLC who received PD-1 blockade
treatment was collected and assessed. According to iRECIST
criteria, the best overall response during PD-1 blockade treatment
indicated a CR in one patient (1.1%), PR in 19 patients (21.3%), SD
in 37 patients (41.6%) and progressive disease (PD) in 32 patients
(36.0%). This resulted in an ORR of 22.5% (95% CI: 14.3–32.6%) and
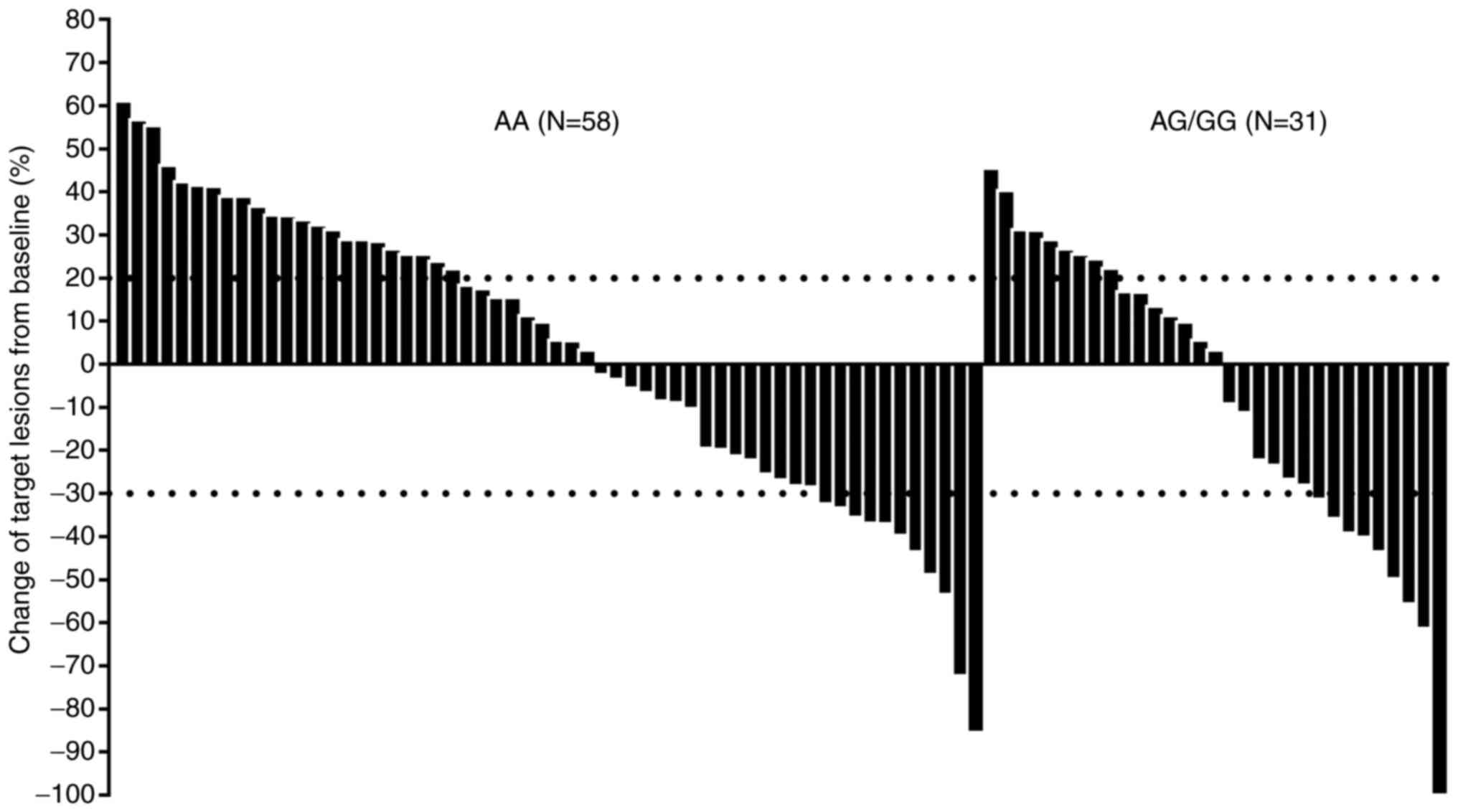
a DCR of 64.0% (95% CI: 53.2–73.9%; Fig. 1). Specifically, the changes in the
target lesions of the 89 patients after PD-1 blockade treatment
according to genotype status of rs2297136 are depicted in Fig. 1. Target lesions of some patients
shrunk significantly after the PD-1 blockade treatment. It was
noteworthy that even patients with AG/GG genotype numerically
demonstrated a higher ORR compared with patients with AA genotype
[ORR of AG/GG vs. AA: 29.0% (95% CI: 14.2–48.0%) vs. 19.0% (95% CI:
9.9–31.4%)]. However, this difference did not reach statistical
significance (χ2=1.18, P=0.278). Additionally, the DCR for patients
with AA and AG/GG genotype was 58.6% (95% CI: 44.9–71.4%) and 74.2%
(95% CI: 55.3–88.1%), respectively (χ2=2.13, P=0.145).
Association between prognosis of PD-1
blockades and genotype status of rs2297136
Regular follow-up was performed for the 89 patients
with advanced NSCLC included in the present study, resulting in a
mature prognostic data ultimately. The data cut-off date of the
present study was November 15, 2022 and the median follow-up
duration was 10.2 months (follow-up range: 0.9–32.5 months). Among
these patients, 75 were observed to experience progression or death
events, providing a maturity of 84.3% for PFS data. The PFS
survival curve is presented in Fig.
2, revealing a median PFS of 3.4 months (95% CI: 1.80–5.00) for
the 89 patients treated with PD-1 blockade monotherapy. Notably, a
total of 15 patients experienced a sustained PFS benefit lasting
over 12 months.
Additionally, 61 patients were documented to have
succumbed, resulting in a maturity of OS data of 68.5%. The OS
survival curve, also depicted in Fig.
2, revealed a median OS of 11.3 months (95% CI: 7.93–14.67) for
the 89 patients with advanced NSCLC treated with PD-1 blockades.
Interestingly, 10 patients achieved a sustainable OS benefit
lasting over 24 months. Furthermore, as shown in Table SI, 49 patients received subsequent
treatment upon progression during PD-1 blockade therapy. Among
them, 21 received anlotinib regimen, 13 underwent chemotherapy, 9
were administered traditional Chinese medicine and the remaining 6
received PD-1/PD-L1 related therapy.
In exploring the connection between prognosis and
the genotype status of rs2297136, additional survival analysis was
conducted. As demonstrated in Fig.
3, patients with AG/GG genotype showed a tendency towards
improved PFS compared with those with AA genotype [median PFS: 5.30
months (95% CI: 3.42–7.18) vs. 2.95 months (95% CI: 2.58–3.32)],
reaching marginal statistical significance (χ2=4.30, P=0.038).
Furthermore, the association between OS and genotype status of
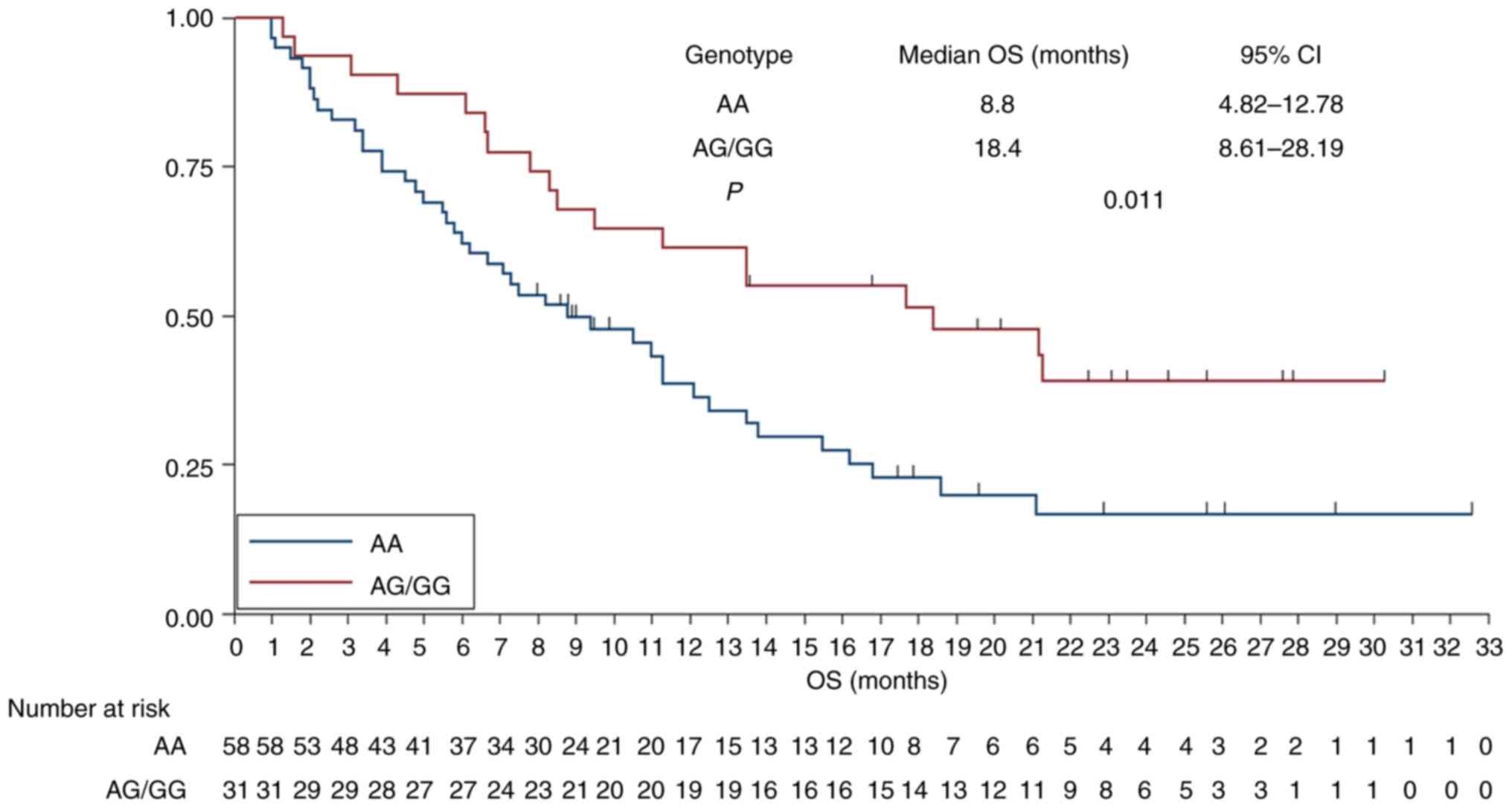
rs2297136 was separately examined. As illustrated in Fig. 4, patients with AG/GG genotype
exhibited a longer OS compared with those with AA genotype [median
OS: 18.4 months (95% CI: 8.61–28.19) vs. 8.8 months (95% CI:
4.82–12.78)] and this difference was statistically significant
(χ2=6.43, P=0.011).
Additionally, to investigate the independent
prognostic implication of rs2297136 for patients with advanced
NSCLC, multivariate Cox analysis for OS was adopted subsequently.
Initially, association analysis between OS and baseline
characteristic subgroups in univariate analysis was carried out
separately. The median OS and 95% CI according to baseline
characteristic subgroups in univariate analysis were presented in
Table III. Notably, it appeared
that almost all patients might uniformly benefit from PD-1
blockades monotherapy uniformly. However, ECOG performance status
and number of metastatic lesions exhibited a significant
association with OS in the univariate analysis, as shown in
Table III. This suggested that
patients with ECOG performance status 0–1 score had a longer OS
than that of patients with 2–3 score (median OS: 15.5 vs. 9.5
months, P=0.008), and patients with number of metastatic lesions ≤3
demonstrated improved OS than those >3 metastatic lesions
(median OS: 13.5 vs. 9.5 months, P=0.019). Interestingly, patients
with EGFR positive mutation demonstrated a trend towards inferior
OS compared with those with EGFR negative mutation, although the
difference was not statistically significant (median OS: 9.5 vs.
13.8 months, P=0.131). Subsequently, variables significantly
associated with OS were incorporated into multivariate Cox analysis
furthermore. As illustrated in Table
III, after multivariate adjustment, the Cox multivariate
analysis demonstrated that ECOG performance status (HR=0.63,
P=0.011), the number of metastatic lesions (HR=0.73, P=0.026) and
PD-L1 rs2297136 genotype status (HR=2.01, P=0.018) were all
independent risk factors for OS.
 | Table III.OS of the 89 patients with advanced
non-small cell lung cancer according to baseline characteristic
subgroups in univariate analysis and multivariate Cox analysis. |
Table III.
OS of the 89 patients with advanced
non-small cell lung cancer according to baseline characteristic
subgroups in univariate analysis and multivariate Cox analysis.
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | Median OS (95%
CI) | Univariate analysis
P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age, years |
| 0.513 |
|
|
|
<66 | 12.5
(8.56–16.44) |
|
|
|
|
≥66 | 10.5
(8.02–12.98) |
|
|
|
| Sex |
| 0.331 |
|
|
|
Male | 11.3
(8.12–14.48) |
|
|
|
|
Female | 13.5
(9.35–17.65) |
|
|
|
| ECOG PS score |
| 0.008 | 0.63
(0.29–0.88) | 0.011 |
|
0-1 | 15.5
(10.12–20.88) |
|
|
|
|
2-3 | 9.5
(7.14–11.86) |
|
|
|
| Pathological
stage | 13.5
(7.81–19.19) | 0.637 |
|
|
|
IIIb |
|
|
|
|
| IV | 11.3
(8.02–14.58) |
|
|
|
| Smoking status |
| 0.535 |
|
|
|
Non-smoker | 11.3
(7.73–14.87) |
|
|
|
| Former
smoker/smoker | 11.3
(8.31–14.29) |
|
|
|
| EGFR mutation
status |
| 0.131 |
|
|
|
Positive | 9.5
(7.18–11.82) |
|
|
|
|
Negative | 13.8
(9.12–18.48) |
|
|
|
| History of surgical
resection |
| 0.618 |
|
|
|
Yes | 12.1
(9.22–14.98) |
|
|
|
| No | 11.0
(8.79–13.21) |
|
|
|
| Histological
category |
| 0.561 |
|
|
|
Adenocarcinoma | 10.5
(8.45–12.55) |
|
|
|
|
Squamous cell carcinoma | 12.5
(9.03–15.97) |
|
|
|
| Number of
metastatic lesions |
| 0.019 | 0.73
(0.41–0.92) | 0.026 |
| ≤3 | 13.5
(8.23–18.77) |
|
|
|
|
>3 | 9.5
(7.21–11.79) |
|
|
|
| Lines of PD-1
blockades |
| 0.572 |
|
|
|
Second-line | 12.5
(8.67–16.33) |
|
|
|
|
Third-line or later | 11.3
(8.83–13.77) |
|
|
|
| PD-1 blockades |
| 0.582 |
|
|
|
Camrelizumab | 10.5
(8.63–12.37) |
|
|
|
|
Sintilimab | 11.0
(7.91–14.09) |
|
|
|
|
Tislelizumab | 12.5
(9.51–15.49) |
|
|
|
|
Pembrolizumab | 11.3
(8.97–13.63) |
|
|
|
|
Nivolumab | 12.1
(9.34–14.86) |
|
|
|
| PD-L1 rs2297136
genotype status |
| 0.011 | 2.01
(1.12–3.32) | 0.018 |
| AA | 8.8
(4.82–12.78) |
|
|
|
|
AG/GG | 18.4
(8.61–28.19) |
|
|
|
Association between PD-L1 gene mRNA
expression and genotype status of rs2297136
Ultimately, mRNA analysis was conducted on a total
of 81 patients. The prevalence of rs2297136 polymorphism among
these patients was as follows: The AA genotype was observed in 53
cases (65.4%), the AG genotype was noted in 26 cases (32.1%), the
GG genotype was found in 2 cases (2.5%). The MAF was 0.19, aligning
with Hardy-Weinberg Equilibrium (P=0.567) and demonstrating
similarity with the genotype distribution frequency among the 89
patients with advanced NSCLC. The median relative expression level
of PD-L1 mRNA was 3.30 (ranging from 1.55 to 5.32) and the mean
relative expression level was 3.24±0.835 in 81 PBMC specimens.
Subsequently, the association between PD-L1 mRNA expression and
genotype status of rs2297136 is illustrated in Fig. 5. In comparison with AA genotype,
AG/GG genotypes of rs2297136 exhibited a higher relative expression
of PD-L1 mRNA in PBMC specimens (4.09±0.538 vs. 2.59±0.644),
demonstrating statistical significance (P<0.001).
PD-L1 immunohistochemical expression results in two
patients among the present study and matched with PD-L1 mRNA
expression status is shown in Fig.
S1. PD-L1 mRNA expression status was divided into PD-L1 high
expression (PD-L1 H) and PD-L1 low expression (PD-L1 L) according
to the median expression threshold value (3.30). Patients with
PD-L1 H and PD-L1 L were observed in 41 and 40 cases, respectively.
As exhibited in Fig. S2, patients
with PD-L1 H conferred a trend for superior PFS compared with those
with PD-L1 L (median PFS: 5.3 vs. 2.8 months), although the
difference was not statistically significant (χ2=3.438, P=0.064).
Furthermore, as shown in Fig. S3,
patients with PD-L1 H conferred a significantly improved OS
compared with those with PD-L1 L (median OS: 13.5 vs. 7.8 months),
demonstrating statistical significance (χ2=4.559, P=0.033).
Additionally, some patients with advanced NSCLC
examined the expression the of immunohistochemistry (IHC) of PD-L1
using biopsy cancer tissue samples in the third-party testing
agency to predict the efficacy of PD-1 blockades. These test
results were collected and matched with mRNA expression results
correspondingly. As shown in Fig.
S1, the relative mRNA expression level of PD-L1 gene was
correlated with the PD-L1 IHC expression consistently, the
relatively high mRNA expression level was associated with high TPS
score of PD-L1 IHC expression.
Discussion
The present study contributes real-world evidence
regarding the viability of PD-1 blockade monotherapy for patients
with previously treated advanced NSCLC, assessed retrospectively.
Simultaneously, the investigation of the present study highlights
the clinical significance of rs2297136 in the PD-L1 gene for
predicting the prognosis of the 89 patients. In aggregate,
rs2297136 in the PD-L1 gene holds potential as a biomarker for
prognostic prediction in clinical settings for patients with
advanced NSCLC undergoing PD-1 blockade monotherapy.
To the best of the authors' knowledge, PD-1
blockades have demonstrated enduring responses and promising
efficacy in patients with previously-treated advanced NSCLC,
establishing themselves as the standard second-line treatment for
patients with advanced NSCLC over the past years (26). However, the overall response to PD-1
blockade monotherapy in patients with advanced NSCLC remains
suboptimal. Despite the clinical significance of factors such as
PD-L1 expression, DNA MMR status and TMB in predicting PD-1
blockade efficacy to some extent, a substantial number of patients
still do not respond to these regimens (27). There is an ongoing need to explore
additional potential biomarkers to identify patients who may
benefit from subsequent PD-1 blockade administration. (28). In this context, other potential
biomarkers were observed, such as the neutrophil-to-lymphocyte
ratio and gut microbiota, have recently emerged as potentially
clinically significant predictors of PD-1 blockade efficacy
(29). However, these alternatives
also lack conclusive evidence.
Among the 89 patients enrolled in the present study,
a total of 19 were administered PD-1 blockades as second-line
therapy, while the remaining 70 received PD-1 blockades as
third-line treatment or beyond. Considering that certain PD-1
blockades (specifically, tislelizumab and nivolumab) had
indications for use as second-line therapy in patients with
advanced NSCLC in China, the administration of PD-1 blockades
monotherapy in the present study appears to be reasonable and
ethical. All 89 NSCLC patients included in the present study were
typical cases of advanced NSCLC, making the sample representative
(30).
Overall, the therapeutic outcomes exhibited that the
ORR and DCR for the 89 patients with advanced NSCLC treated with
PD-1 blockades monotherapy were 22.5 and 64.0%, respectively. The
median PFS was 3.4 months. The efficacy of the present study and
PFS outcomes closely aligned with the ORR and PFS of Checkmate-017
and Checkmate-057 trials, where nivolumab served as second-line
treatment for squamous cell and non-squamous cancers, respectively
(ORR was ~20%, median PFS was almost 3 months) (31,32).
Additionally, the present study's therapeutic outcomes were
consistent with the ORR and DCR observed in the RATIONALE-303
trial, where tislelizumab was utilized as second-line therapy for
advanced NSCLC (ORR=22.6%, DCR=55.7%) (33). Interestingly, it is noteworthy that
the PFS and OS in RATIONALE-303 trial were slightly longer than
those in the present study. It was hypothesized that this
discrepancy may be attributed in two aspects: Firstly, all patients
in the RATIONALE-303 trial had an ECOG performance status of 0–1,
whereas the present study included 38.2% of patients with a status
of 2–3. Clearly, ECOG performance status emerged as an independent
factor influencing the prognosis of patients with advanced NSCLC
(34). Additionally, the present
study's retrospective design may have impacted the management of
patients compared with well-designed phase III clinical trials,
potentially compromising the efficacy and prognosis to some extent.
This notion is supported by a prior retrospective study among
advanced NSCLC patients (35).
These two factors could potentially explain why the prognosis in
the present study was inferior to that in RATIONALE-303 trial.
Significantly, the present study included 18 patients with positive
EGFR mutation who received PD-1 blockades as third-line or
subsequent treatment. These patients, having undergone extensive
prior treatments with EGFR-TKI and chemotherapy, had limited
therapeutic options, making immunotherapy a viable consideration
(36). An association analysis
between EGFR mutation status and OS suggested that patients with
positive EGFR mutation might not benefit significantly from PD-1
blockades monotherapy, even though the statistical difference was
not significant (P=0.131). However, caution is warranted in
interpreting this finding. All 18 patients with positive EGFR
mutation underwent intensive treatment and received PD-1 blockades
as third-line or subsequent therapy, indicating a relatively worse
prognosis regardless of the therapeutic regimens (37). A recent study indicated that a
subset of patients with positive EGFR mutation and high PD-L1
expression may derive benefits from PD-1 blockades administration
(38). Another study suggested that
subjects with a short PFS on EGFR tyrosine kinase inhibitor (TKI)
might exhibit an improved response to immunotherapy, and combined
PD-1 blockades treatment might be a promising option compared with
chemotherapy in second-line setting for patients with worse PFS on
EGFR TKI therapy and no T790M mutation (39). In conclusion, the question whether
patients with EGFR positive mutation might benefit from PD-1
blockades treatment should be thoroughly explored in prospective
clinical trials.
Remarkably, a recent study indicated that genetic
variation in the pathogenic gene might contribute to the
therapeutic outcomes of PD-1 blockades in metastatic melanoma
(40). In a recent investigation
led by Parakh et al (40),
comprising 318 patients undergoing PD-1/PD-L1 blockade treatment,
the clinical significance of key genes associated with tumor
immunity was explored. Their findings identified immunogenetic
polymorphisms including ATG7 rs7625881, CD274 rs2297136 and TLR4
rs1927911 as potential predictors of response to PD-1/PD-L1
blockade in tumor patients. These studies suggested that gene
polymorphism may play a role in the clinical outcomes of PD-1/PD-L1
blockades. The conclusion drawn from the present study regarding
PD-L1 gene polymorphism suggested that AG/GG genotype of rs2297136
is associated to a relatively favorable prognosis among Chinese
patients with advanced NSCLC undergoing PD-1 blockades, aligning
with the previous study initiated by Yoshida et al (18). The aforementioned study, which
included 133 patients treated with nivolumab, identified an
association between prognostic outcomes and PD-L1 polymorphisms.
Among the 7 polymorphisms investigated, rs822339 and rs1411262 were
suggested to predict the prognosis of patients receiving nivolumab
therapy but not those undergoing non-PD-1 blockades therapy. While
the concept and design of the aforementioned study are consistent
to the present study, the present study did not establish the
clinical significance of rs822339 and rs1411262, as outlined in the
preliminary analysis in Table I.
This discrepancy was attributed to ethnic variations in the two
polymorphisms, where the MAF of rs822339 and rs1411262 ranged from
0.11 to 0.55 among different population, potentially contributing
to the differences in efficacy of PD-1 blockades (41). Additionally, another exploratory
study initiated by Nomizo et al (19), also investigated the influence of
polymorphism in PD-L1 on the response to nivolumab among patients
with advanced NSCLC. Involving 50 patients who received nivolumab
monotherapy, the aforementioned study identified rs2282055 and
rs4143815 as associated with distinct ORR and PFS among NSCLC
patients treated with nivolumab. Despite the alignment in study
design with the present study, it is important to note that the
sample size of Nomizo's et al study was limited,
necessitating confirmation of their conclusions in a larger patient
cohort. Furthermore, the present study is in line with another
previous study initiated by Minari et al (42), which investigated the clinical
significance of PD-L1 polymorphism as potential biomarker,
predicting the prognosis of 166 patients with advanced NSCLC who
received PD-1/PD-L1 blockades. The findings of the aforementioned
study indicated that rs4143815 in PD-L1 gene appeared to be
marginally correlated with clinical outcomes in NSCLC undergoing
PD-1/PD-L1 blockades. Collectively, all these studies suggested
that PD-L1 polymorphisms may contribute to the potential
interactions between PD-1 and PD-L1, thereby influencing the
therapeutic efficacy of PD-1/PD-L1 blockades clinically.
Additionally, PD-L1 gene mRNA expression analysis
suggested that AG/GG genotype of rs2297136 was associated to higher
PD-L1 mRNA expression, consistent with findings of a previous study
initiated by Su et al (23).
The aforementioned study, which involved 86 PBMC specimens, aimed
to uncover the association between the genotype status of rs2297136
and PD-L1 mRNA expression, confirming that patients with the AG/GG
genotype of rs2297136 exhibited elevated PD-L1 mRNA expression.
Interestingly, the present study shared a similar conclusion to
previous research, indicating that higher expression of PD-L1 mRNA
could predict superior efficacy for patients undergoing PD-1
blockades. This is in contrast to studies suggesting that higher
PD-L1 mRNA expression predicts worse prognosis for patients
receiving capecitabine-based adjuvant chemotherapy (43,44).
However, it should be noted that the present study highlighted that
higher expression of PD-L1 mRNA might predict superior efficacy of
patients who received PD-1 blockades. It was hypothesized that this
discrepancy may attribute to the therapeutic regimens received. To
the best of the authors' knowledge, PD-L1 gene was a hot spot gene
in the field of tumor immunotherapy at present and considerable
clinical trials confirmed that higher expression level of PD-L1
could predict the superior efficacy of PD-1/PD-L1 blockades
(45). Unfortunately, since the IHC
results of PD-L1 expression were not available in the present
study, an analysis of the association between PD-L1 mRNA expression
and IHC expression could not be performed. Fortunately, PD-L1 IHC
expression results in two patients matched with the PD-L1 mRNA
expression correspondingly, suggesting that the results of PD-L1
mRNA expression in the present study might also reflect the results
of PD-L1 IHC expression to some extent. Therefore, further in-depth
investigations are necessary to validate the clinical significance
of PD-L1 polymorphism and PD-L1 mRNA expression. Given that
rs2297136 is located at the 3′-untranslated regions of PD-L1 gene,
potentially modifying miRNA binding and altering the interaction
between miRNAs and target mRNAs, could result in increased mRNA
expression of PD-L1. Previous studies have validated that
miR-324-5p and miR-632 possess the potential to bond to rs2297136,
altering mRNA expression and influencing susceptibility to cancer
occurrence (46,47). Therefore, it was hypothesized that
the genotype status of rs2297136 may have different binding
capacities to miR-324-5p and miR-632, leading to changes in PD-L1
mRNA expression. Regarding the association between PD-L1 expression
and efficacy of PD-1 blockades, Keynote-010 and Checkmate 057
clinical trials have previously affirmed that increased PD-L1
expression predicts superior clinical outcomes for both
pembrolizumab and nivolumab among patients with advanced NSCLC
(32,48). As a result, increased PD-L1 mRNA
expression might serve as a positive prognostic biomarker for PD-1
blockades in the present study.
The present study, however, does have certain
limitations. Firstly, due to its retrospective nature, the sample
size in the present study was relatively small with only 89
patients included in polymorphism analysis. The conclusion that
rs2297136 is associated with effectiveness of PD-1 blockades
requires further clarification in larger subject cohorts. Secondly,
various PD-1 blockades were used in the present study, potentially
resulting in heterogeneous and diverse efficacy outcomes. Thirdly,
the present study was unable to detect the PD-L1 IHC expression,
compromising the utility of PD-L1 mRNA expression to some extent.
Nonetheless, the present study highlights the potential
significance of rs2297136 in predicting the effectiveness of PD-1
blockades for patients with advanced NSCLC, suggesting that
rs2297136 in the PD-L1 gene could be a valuable biomarker for
predicting therapeutic outcomes in clinical practice.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QG and HTJ designed the study, conducted data
analysis and drafted the manuscript. SYD collected data, performed
the experiment and participated in the patients' follow-up. HLQ and
HTJ provided guidance in designing the study and supervised the
study's result. QG and HTJ confirm the authenticity of all the raw
data. All authors have read and approved the manuscript, agreeing
to be accountable for all aspects of the research and ensuring that
the accuracy and integrity of the work were appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved (approval no.
2022-KY-11053) by the Ethics Committee of the Affiliated Hospital
of Hebei University (Baoding, China). Written informed consent was
obtained from each enrolled patient according to the
recommendations of the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu L, Wan S, Li J, Xu Y, Lou X, Sun M and
Wang S: Expression and prognostic value of E2F3 transcription
factor in non-small cell lung cancer. Oncol Lett. 21:4112021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jahanzeb M, Lin HM, Pan X, Yin Y, Baumann
P and Langer CJ: Immunotherapy treatment patterns and outcomes
among ALK-positive patients with non-small-cell lung cancer. Clin
Lung Cancer. 22:49–57. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borghaei H, Gettinger S, Vokes EE, Chow
LQM, Burgio MA, de Castro Carpeno J, Pluzanski A, Arrieta O,
Frontera OA, Chiari R, et al: Five-year outcomes from the
randomized, phase III trials checkmate 017 and 057: Nivolumab
versus docetaxel in previously treated non-small-cell lung cancer.
J Clin Oncol. 39:723–733. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qu J, Mei Q, Liu L, Cheng T, Wang P, Chen
L and Zhou J: The progress and challenge of anti-PD-1/PD-L1
immunotherapy in treating non-small cell lung cancer. Ther Adv Med
Oncol. 13:17588359219929682021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, Bai H, Wang C, Seery S, Wang Z,
Duan J, Li S, Xue P, Wang G, Sun Y, et al: Efficacy and safety of
first-line immunotherapy combinations for advanced NSCLC: A
systematic review and network meta-analysis. J Thorac Oncol.
16:1099–1117. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Yang Y, Yu J, Zhang S, Li X, Wu X,
Nie X, Liu W, Zhang P, Li Y, et al: Efficacy and safety of
anti-PD-1/PD-L1 in combination with chemotherapy or not as
first-line treatment for advanced non-small cell lung cancer: A
systematic review and network meta-analysis. Thorac Cancer.
13:322–337. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xue Y, Gao S, Gou J, Yin T, He H, Wang Y,
Zhang Y, Tang X and Wu R: Platinum-based chemotherapy in
combination with PD-1/PD-L1 inhibitors: Preclinical and clinical
studies and mechanism of action. Expert Opin Drug Deliv.
18:187–203. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia L, Liu Y and Wang Y: PD-1/PD-L1
blockade therapy in advanced non-small-cell lung cancer: Current
status and future directions. Oncologist. 24:S31–S41. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patil NS, Nabet BY, Müller S, Koeppen H,
Zou W, Giltnane J, Au-Yeung A, Srivats S, Cheng JH, Takahashi C, et
al: Intratumoral plasma cells predict outcomes to PD-L1 blockade in
non-small cell lung cancer. Cancer Cell. 40:289–300.e284. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y,
Lin D, Gao Q, Zhou H, Liao W and Yao H: Association of survival and
immune-related biomarkers with immunotherapy in patients with
non-small cell lung cancer: A meta-analysis and individual
patient-level analysis. JAMA Netw Open. 2:e1968792019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai X, Wu DH, Ma SC, Wang J, Tang XR, Kang
S, Fu QJ, Cao CH, Luo HS, Chen YH, et al: Development and
validation of a genomic mutation signature to predict response to
PD-1 inhibitors in non-squamous NSCLC: A multicohort study. J
Immunother Cancer. 8:e0003812020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Zhou ZW, Lu J, Luo H, Wang SN, Peng
Y, Deng MS, Song GB, Wang JM, Wei X, et al: PD-L1(P146R) is
prognostic and a negative predictor of response to immunotherapy in
gastric cancer. Mol Ther. 30:621–631. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumagai S, Togashi Y, Kamada T, Sugiyama
E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y,
Matsui S, et al: The PD-1 expression balance between effector and
regulatory T cells predicts the clinical efficacy of PD-1 blockade
therapies. Nat Immunol. 21:1346–1358. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie Q, Chen Z, Xia L, Zhao Q, Yu H and
Yang Z: Correlations of PD-L1 gene polymorphisms with
susceptibility and prognosis in hepatocellular carcinoma in a
Chinese Han population. Gene. 674:188–194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshida H, Nomizo T, Ozasa H, Tsuji T,
Funazo T, Yasuda Y, Ajimizu H, Yamazoe M, Kuninaga K, Ogimoto T, et
al: PD-L1 polymorphisms predict survival outcomes in advanced
non-small-cell lung cancer patients treated with PD-1 blockade. Eur
J Cancer. 144:317–325. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nomizo T, Ozasa H, Tsuji T, Funazo T,
Yasuda Y, Yoshida H, Yagi Y, Sakamori Y, Nagai H, Hirai T and Kim
YH: Clinical impact of single nucleotide polymorphism in PD-L1 on
response to nivolumab for advanced non-small-cell lung cancer
patients. Sci Rep. 7:451242017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun X, Xu J, Xie L and Guo W:
Effectiveness and tolerability of anlotinib plus PD-1 inhibitors
for patients with previously treated metastatic soft-tissue
sarcoma. Int J Gen Med. 15:7581–7591. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song ZZ, Zhao LF, Zuo J, Fan ZS, Wang L
and Wang YD: Clinical outcomes and safety of apatinib mesylate in
the treatment of advanced non-squamous non-small cell lung cancer
in patients who progressed after standard therapy and analysis of
the KDR gene polymorphism. Onco Targets Ther. 13:603–613. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su J, Dai B, Yuan W, Wang G, Zhang Z, Li
Z, Liu J and Song J: The influence of PD-L1 genetic variation on
the prognosis of R0 resection colorectal cancer patients received
capecitabine-based adjuvant chemotherapy: A long-term follow-up,
real-world retrospective study. Cancer Chemother Pharmacol.
85:969–978. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barber RD, Harmer DW, Coleman RA and Clark
BJ: GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression
in a panel of 72 human tissues. Physiol Genomics. 21:389–395. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Liu Z, Wen H, Guo Y, Xu F, Zhu Q,
Yuan W, Luo R, Lu C, Liu R, et al: Immunosuppressive TREM2(+)
macrophages are associated with undesirable prognosis and responses
to anti-PD-1 immunotherapy in non-small cell lung cancer. Cancer
Immunol Immunother. 71:2511–2522. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin J, Wu Y, Yang X, Gan L and Xue J:
Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy
in non-small-cell lung cancer: Occurrence and mechanism. Front
Immunol. 13:8306312022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou C, Wang Y, Zhao J, Chen G, Liu Z, Gu
K, Huang M, He J, Chen J, Ma Z, et al: Efficacy and biomarker
analysis of camrelizumab in combination with apatinib in patients
with advanced nonsquamous NSCLC previously treated with
chemotherapy. Clin Cancer Res. 27:1296–1304. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bie F, Tian H, Sun N, Zang R, Zhang M,
Song P, Liu L, Peng Y, Bai G, Zhou B and Gao S: Research progress
of anti-PD-1/PD-L1 immunotherapy related mechanisms and predictive
biomarkers in NSCLC. Front Oncol. 12:7691242022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Z, Li T, Hu X, Hao X, Xing P and Li J:
Efficacy and safety profile of combining programmed cell death-1
(PD-1) inhibitors and antiangiogenic targeting agents as subsequent
therapy for advanced or metastatic non-small cell lung cancer
(NSCLC). Thorac Cancer. 12:2360–2368. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou C, Huang D, Fan Y, Yu X, Liu Y, Shu
Y, Ma Z, Wang Z, Cheng Y, Wang J, et al: Tislelizumab versus
docetaxel in patients with previously treated advanced NSCLC
(RATIONALE-303): A phase 3, open-label, randomized controlled
trial. J Thorac Oncol. 18:93–105. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Geng N, Su J, Liu Z, Ding C, Xie S and Hu
W: The influence of KDR genetic variation on the efficacy and
safety of patients with advanced NSCLC receiving first-line
bevacizumab plus chemotherapy regimen. Technol Cancer Res Treat.
20:153303382110194332021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li XP, Zhang WD, Li MJ, Wang J, Lian J and
Zhou HG: Effectiveness and safety of PD-1 inhibitor monotherapy for
elderly patients with advanced non-small cell lung cancer: A
real-world exploratory study. J Oncol. 2022:17102722022.PubMed/NCBI
|
|
36
|
Qiao M, Jiang T, Liu X, Mao S, Zhou F, Li
X, Zhao C, Chen X, Su C, Ren S and Zhou C: Immune checkpoint
inhibitors in EGFR-mutated NSCLC: Dusk or dawn? J Thorac Oncol.
16:1267–1288. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee CK, Man J, Lord S, Cooper W, Links M,
Gebski V, Herbst RS, Gralla RJ, Mok T and Yang JC: Clinical and
molecular characteristics associated with survival among patients
treated with checkpoint inhibitors for advanced non-small cell lung
carcinoma: A systematic review and meta-analysis. JAMA Oncol.
4:210–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Masuda K, Horinouchi H, Tanaka M,
Higashiyama R, Shinno Y, Sato J, Matsumoto Y, Okuma Y, Yoshida T,
Goto Y, et al: Efficacy of anti-PD-1 antibodies in NSCLC patients
with an EGFR mutation and high PD-L1 expression. J Cancer Res Clin
Oncol. 147:245–251. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu S, Wu F, Li X, Zhao C, Jia Y, Jia K,
Han R, Qiao M, Li W, Yu J, et al: Patients with short PFS to
EGFR-TKIs predicted better response to subsequent anti-PD-1/PD-L1
based immunotherapy in EGFR common mutation NSCLC. Front Oncol.
11:6399472021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parakh S, Musafer A, Paessler S, Witkowski
T, Suen C, Tutuka CSA, Carlino MS, Menzies AM, Scolyer RA, Cebon J,
et al: PDCD1 polymorphisms may predict response to anti-PD-1
blockade in patients with metastatic melanoma. Front Immunol.
12:6725212021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Geng N, Ding CM, Liu ZK, Song S and Hu WX:
Influence of VEGFR2 gene polymorphism on the clinical outcomes of
apatinib for patients with chemotherapy-refractory extensive-stage
SCLC: A real-world retrospective study. Int J Clin Oncol.
26:670–683. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Minari R, Bonatti F, Mazzaschi G, Dodi A,
Facchinetti F, Gelsomino F, Cinquegrani G, Squadrilli A, Bordi P,
Buti S, et al: PD-L1 SNPs as biomarkers to define benefit in
patients with advanced NSCLC treated with immune checkpoint
inhibitors. Tumori. 108:47–55. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Enkhbat T, Nishi M, Takasu C, Yoshikawa K,
Jun H, Tokunaga T, Kashihara H, Ishikawa D and Shimada M:
Programmed cell death ligand 1 expression is an independent
prognostic factor in colorectal cancer. Anticancer Res.
38:3367–3373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou C, Che G, Zheng X, Qiu J, Xie Z, Cong
Y, Pei X, Zhang H, Sun H and Ma H: Expression and clinical
significance of PD-L1 and c-Myc in non-small cell lung cancer. J
Cancer Res Clin Oncol. 145:2663–2674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Q, Xu Z, Zheng L, Zhang L, You Q and
Sun J: Multimodal detection of PD-L1: Reasonable biomarkers for
immune checkpoint inhibitor. Am J Cancer Res. 8:1689–1696.
2018.PubMed/NCBI
|
|
46
|
Wu Y, Zhao T, Jia Z, Cao D, Cao X, Pan Y,
Zhao D, Zhang B and Jiang J: Polymorphism of the programmed
death-ligand 1 gene is associated with its protein expression and
prognosis in gastric cancer. J Gastroenterol Hepatol. 34:1201–1207.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Song X, Zhong H, Zhou J, Hu X, Zhou Y, Ye
Y, Lu X, Wang J, Ying B and Wang L: Association between
polymorphisms of microRNA-binding sites in integrin genes and
gastric cancer in Chinese Han population. Tumour Biol.
36:2785–2792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|