Introduction
It is not uncommon for hepatocellular carcinoma
(HCC) to recur in HCC patients after liver transplantation
(1). In contrast, reports of de
novo HCC developing in a healthy donor liver, transplanted to a
patient without a history of HCC, are extremely rare (2–16). A
review of the case reports of HCC in transplanted livers reveals
hepatitis B (5–7,10,15,17), C
(2,8,10–13,18)
and sclerosing cholangitis (18,19) as
underlying diseases. Chronic viral hepatitis is an important factor
in the development of HCC and, for both hepatitis B virus (HBV) and
hepatitis C virus (HCV), there are many cases in which HCC develops
after a long period of persistent infection. Especially for HBV, it
is generally accepted that most individuals develop hepatitis in
adulthood from a persistent infection following vertical
transmission, progressing to liver cirrhosis and the development of
HCC. In addition, the immunosuppressants used to prevent
post-transplant rejection are among the factors contributing to the
development of post-transplant HCC (20). Furthermore, it has been reported
that patients with glucose intolerance, such as diabetes, have a
high risk of developing malignancies. For example, patients with
diabetes are more than twice as likely as non-diabetics to develop
liver tumors (17,21–29).
In addition, the involvement of microsatellite instability in the
development of liver tumors is also important, and cases of
comorbidity with cancers in other organs also have been reported
(30–34).
This report describes the case of a poorly
controlled diabetes mellitus patient who underwent living-donor
liver transplantation, from an HBV-negative donor, for cirrhosis
attributable to HBV infection. The recipient developed not only HCC
but also colon cancer, both tumors having microsatellite
instabilities, the status MSI-high.
Case report
The patient was a woman in her sixties who had been
diagnosed with hepatitis B thirty years previously and given
continuous medical treatment. At that time, she was also diagnosed
with diabetes mellitus and started on insulin administration. In
August 1999, seven years after her first visit in April 1992 at
Toyama, Japan, she underwent a living-donor liver transplantation
from her husband at Matsumoto, Japan, who was HBV- and
HCV-negative, to treat liver failure due to cirrhosis. Her resected
liver showed non-neoplastic complete cirrhosis and was confirmed
not to be included in the neoplastic lesions by the detailed
histopathological examination. Specifically, the paraffin sections
made from the samples of multiple sites from resected recipient
liver, whenever possible, were examined by several pathologists
subspecialized in liver pathology. After surgery, steroids
(methylprednisolone, 3 mg/day for half a year, prednisolone, 1
mg/every other day for nine years, and Tacrolimus, a calcineurin
inhibitor, from 1.2 mg to 0.4 mg/day gradually tapered down to
maintain blood levels of 4–6 ng/ml to present were started as
immunosuppressive drugs to suppress rejection. Lamivudine (100
mg/day from February 1999 to November 2015), Entecavir (0.5 mg/day
from November 2015 to February 2020), and Tenofovir alafenamide
fumarate (25 mg/day from February 2020 to present), an antiviral
agent, and immunoglobulin (Hepatitis B immunoglobulin, every week)
was started for HBV treatment. However, her diabetes was poorly
controlled and she was in and out of hospital. In February 2018,
nineteen years after liver transplantation, a mass lesion was noted
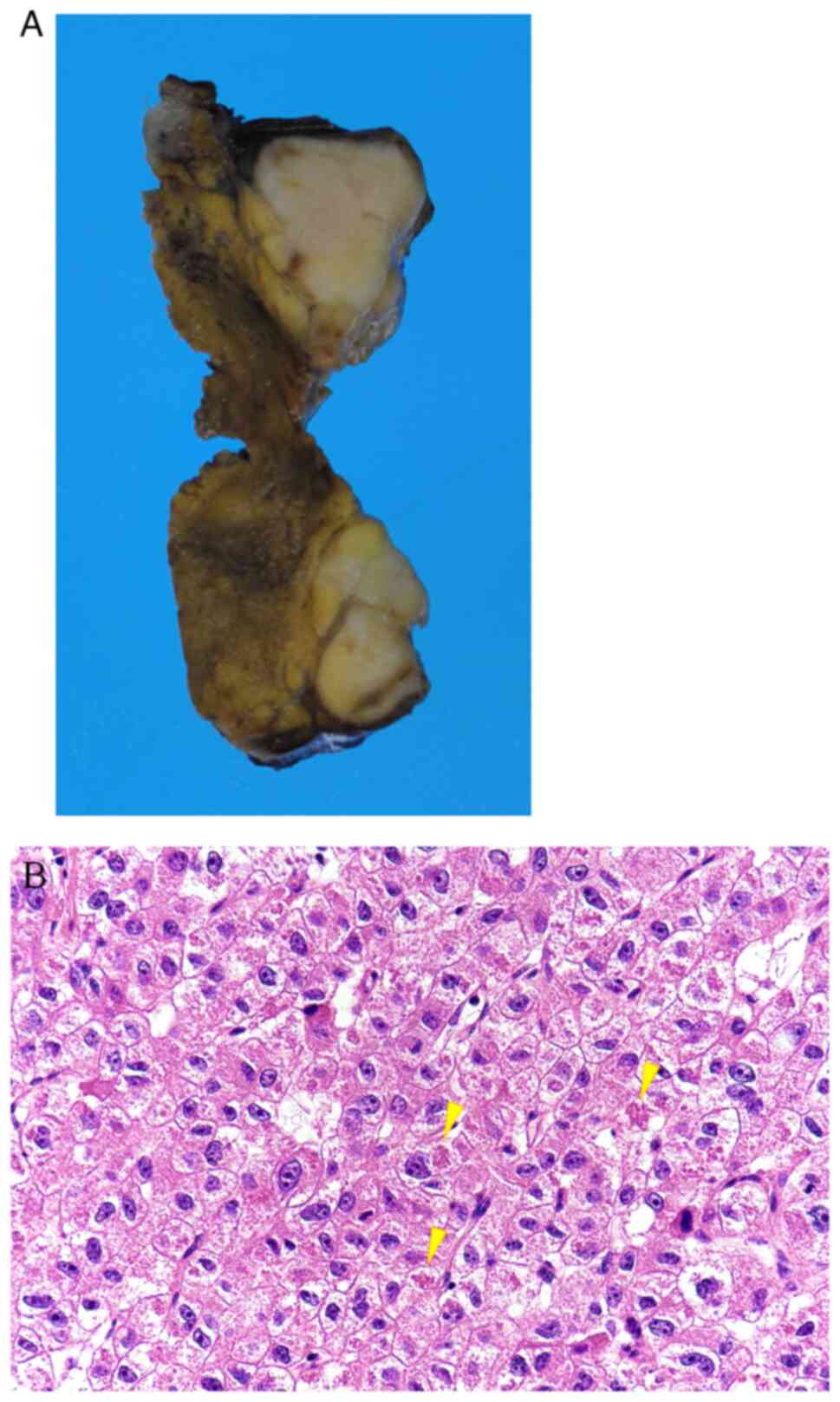
in segment four of the transplanted liver (Fig. 1A and B), and a subsegmental liver
resection was performed under the diagnosis of a liver tumor at
Toyama, Japan. The following year, she underwent a colonoscopy for
screening purposes; this revealed colon cancer in her cecum and she
underwent an ileocecal resection in March 2019 at the same
hospital. The following year, a mass lesion was found on the
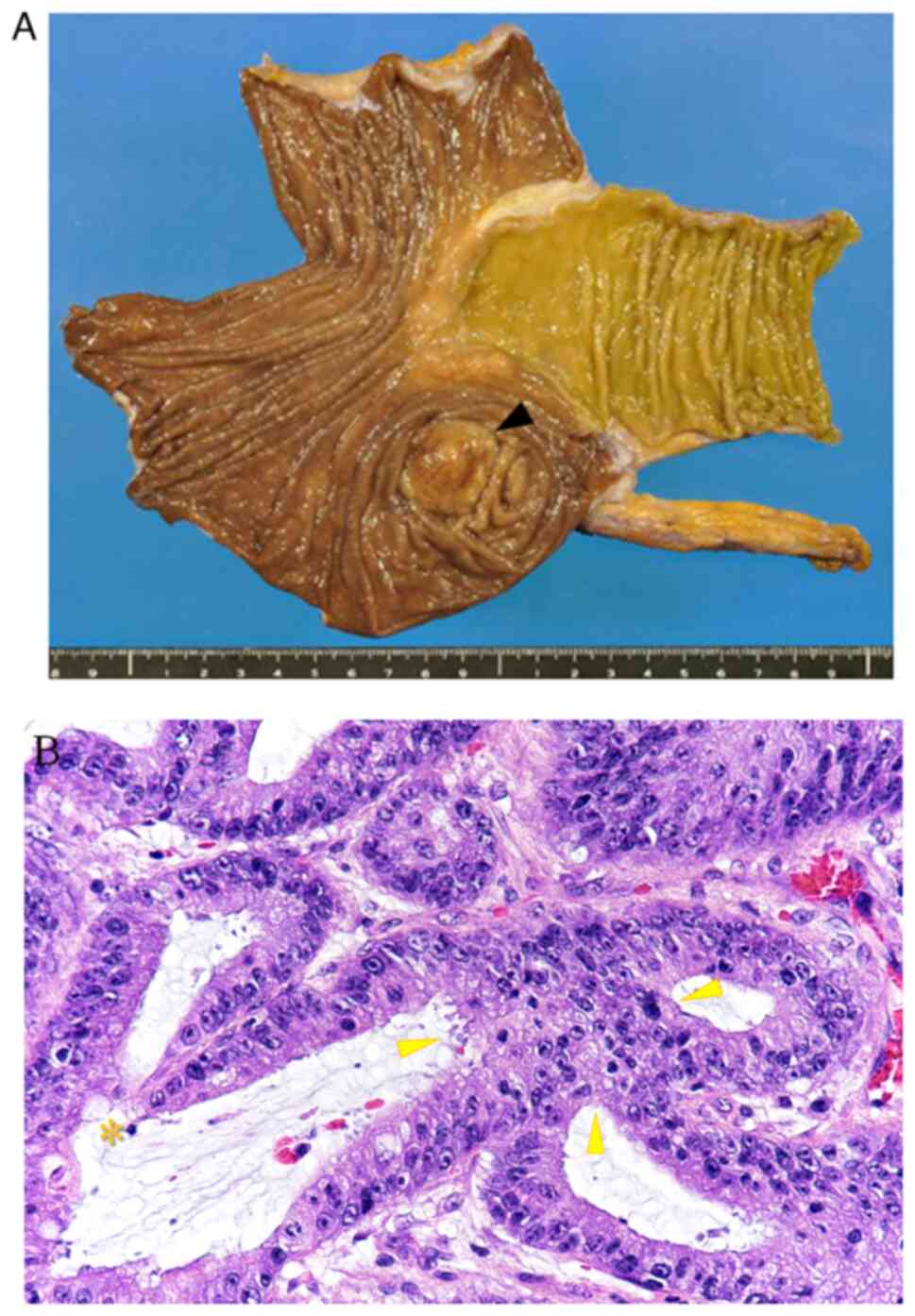
surface of the right liver lobe, segment 8 (Fig. 2A and B), and radiofrequency ablation
was performed on suspicion of recurrence of the hepatocellular
carcinoma, followed by additional resection in December 2020 at the
same hospital. Four years after the final operation, no recurrence
of the liver tumor or malignant tumors have been found, including
in other organs. The amount of HBV DNA in the serum before
transplantation was 3.8 logs IU/ml (TMA), after which hepatitis B
surface antigen (HBsAg) and HBV DNA became negative. To date,
HBsAg, anti-HBs, anti-HB core, anti-HB envelope, and HBV DNA by
real-time PCR has been detected in serum.
The initial liver tumor showed circumscribed
encapsulization but it had no extracapsular invasion and was
diagnosed with moderately differentiated HCC, aligned in a
trabecular pattern, based on the histopathologically cellular and
structural atypia. Vascular invasion, exposure to the surgical
stump, and intrahepatic metastasis were not observed (pT1NXM0)
(Fig. 3A and B). The second hepatic
tumor had a similar histological appearance, without vascular
invasion (Fig. 4A and B). The colon
cancer was mainly located in the cecum and comprised a
well-differentiated tubular adenocarcinoma, invading the subserosal
layer and accompanied by regional lymph node metastasis (pT3N1MX,
pStage IIIb) (35) (Fig. 5A and B). The diagnosis of each of
the above tumor tissues was made histopathologically by two
pathologists (JI and AN) using the hematoxylin-eosin staining
formalin-fixed paraffin sections of each sample. Monitoring for the
recurrence of liver tumors and colon cancer was done every six
months using abdominal enhanced-computed tomography. The total
follow-up period is 30 years from the time of initial
administration.
In order to determine whether the initial liver
tumor was donor- or recipient-derived, genomic DNA was extracted
from both liver tumors and non-tumor tissue and analyzed for the
specific genes, SRT, STS, and Amelogenin, as genes
located on the Y chromosome. Amplification was performed by
polymerase chain reaction (PCR) and the products were confirmed by
capillary electrophoresis (C-EF) (36,37).
The presence of the SRT-a-Y, AMEL-Y, SRY-b-Y, and
STS-1-Y genes on the Y chromosome in both liver tumors and
non-liver tissues indicated that they originated from the liver
donated by the husband, a male (Fig.
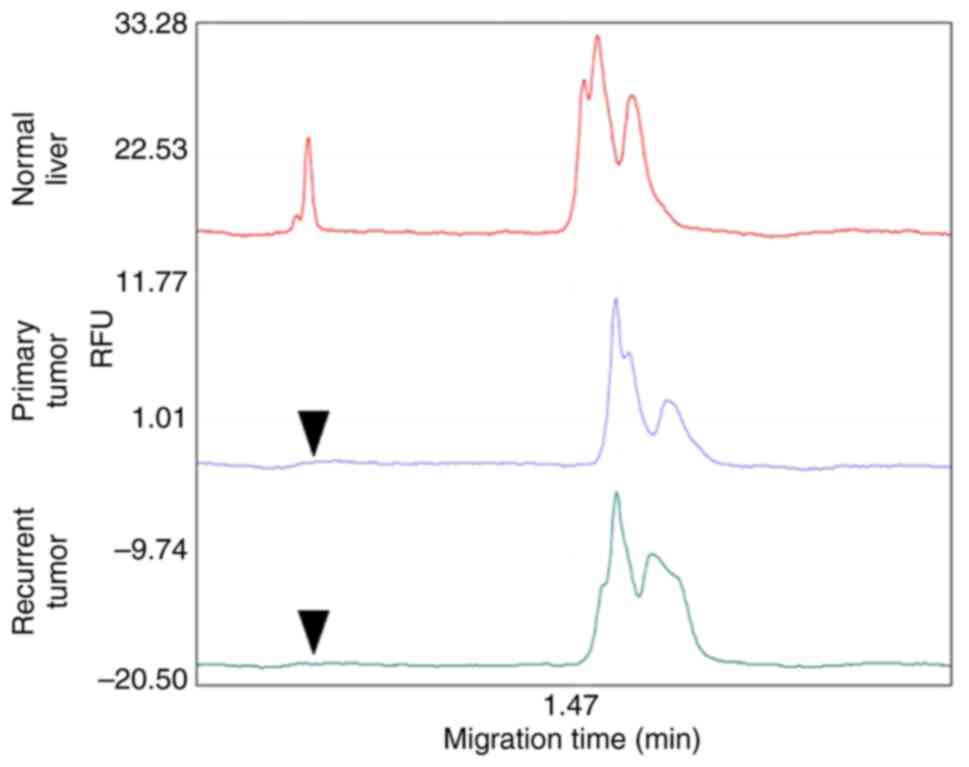
6). In addition, PCR was performed using a microsatellite
marker (D17S938) to determine whether the primary and recurrent
liver tumors were derived from the same clone (38). A peak, indicating expression in the
normal liver tissue (Fig. 7, upper
trace), was not detected in either tumor (Fig. 7, lower traces). Considering that the
site of recurrence was near the initial lesion, recurrence due to
intrahepatic metastasis was diagnosed. To confirm that the serum
was HBV DNA negative before transplantation and to determine
whether the viral genome was present in the liver tumors and
non-tumor liver tissues, PCR was performed using specific HBV DNA
primers and confirmed by C-EF. The conditions for PCR amplification
of HBV DNA and the used primer sequences were as followed. To
obtain total HBV DNA, amplification was performed by PCR
conditioning for 3 min at 94°C and then 30 sec at 94°C, 45 sec at
58°C, and 30 sec at 72°C to be repeated 40 cycles, using primers of
HBV forward CAACCTCCAATCACTCACCAAC and HBV reverse
ACGGGCAACATACCTTGGTAG (39). No
amplified product was observed, confirming that no HBV DNA was
present in any of the tissues. To investigate the causal
relationship between the development of the three tumors of the
liver and the colon and microsatellite instability,
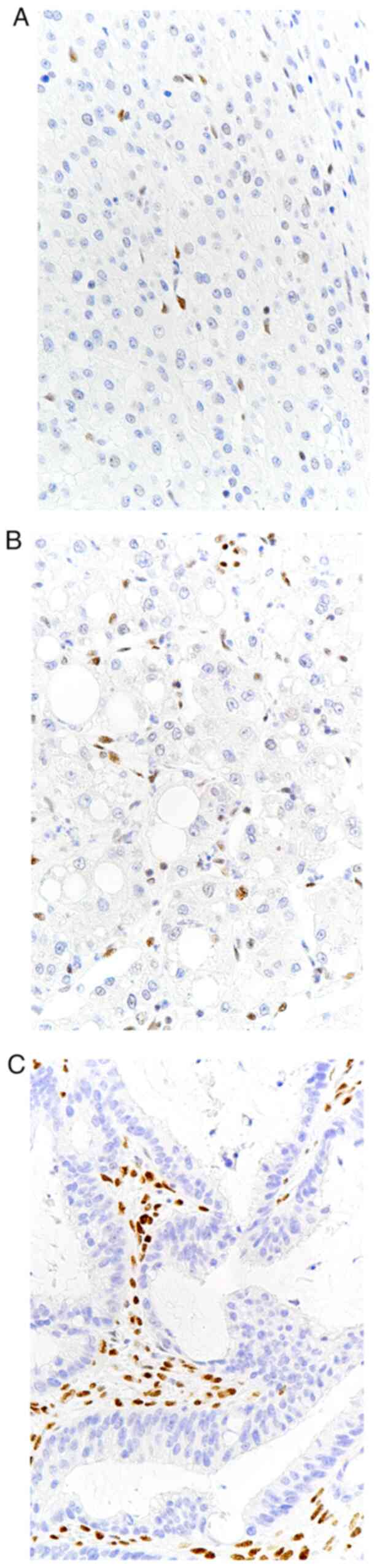
immunohistochemical studies were performed on four microsatellite
regions in mutL homolog 1 (MLH1), postmeiotic segregation increased
2 (PMS2), mutS homolog 2 (MSH2) and MSH6 (40). Of these four factors, MLH1 and PMS2
confirmed the absence of nuclear expression in the liver and
colorectal tumors, and MSH2 and MSH6 were retained in the nucleus,
indicating that the three lesions were tumors with microsatellite
instability-high (MSI-H) (Fig.
8A-C).
Discussion
Hepatocarcinogenesis appears to be a multistep
process, involving disruption of genetic stability, and in which
normal hepatocytes are transformed into liver cancer via chronic
hepatitis, with persistent HBV or HCV infection, and subsequent
cirrhotic conditions (32,33,41).
It is generally accepted that the hepatitis viruses are strongly
implicated in liver cancer development. However, the serological
findings suggested the patient's HBV infection had not persisted
after the antiviral treatment, possibly due to the remarkable
efficacy of the antiviral drugs in this case. Although it cannot be
shown that HBV has completely disappeared from the patient's body,
at least it cannot be considered that HBV DNA remained detectable
in the liver tissue. Therefore, although it cannot be completely
ruled out that HBV was somehow involved in the development of liver
cancer in this case, there is a high possibility that other
triggers were involved.
The involvement of immunosuppressants, administered
to this patient for an extended period of time, also should be
considered as a potential carcinogenesis-inducing factor. Many
transplant patients have to take immunosuppressants for an extended
period of time to prevent rejection. The most typical side effect
of immunosuppressants is an increased susceptibility to infection,
but they have also been reported to increase the risk of
carcinogenesis, albeit in a small number of cases (20). Azathioprine is well-known as an
immunosuppressant used after transplantation and there is a report
confirming its oncogenicity to normal cells, as a result of
long-term exposure in an in vivo experimental model.
Tacrolimus, a calcineurin inhibitor administered to the patient in
this case, is widely used worldwide as an immunosuppressant for
transplant patients. The mechanism by which Tacrolimus promotes the
onset and progression of cancer is not yet well understood. Reports
suggest that inhibition of the immune system not only weakens the
recipient's immune response but also impairs the ability of the
tumor immune system to respond to early-stage tumor cells and their
associated antigens (42). An
indirect correlation between the ability to prevent rejection and
the risk of developing tumors may support this notion. There is
also evidence that Tacrolimus may directly promote the
aggressiveness and invasiveness of tumor cells, including DNA
damage induced by immunosuppressants and activation of cytokines
such as TGF-β (43). The patient in
the present case also has been administered immunosuppressive drugs
for nearly 20 years. Although a definitive basis cannot be proved,
the patient's immunosuppression may have been involved in her
developing liver cancer. In the future, it may be worthwhile
considering managing withdrawal from immunosuppressive drugs,
reducing the dose, or switching to other medications.
It has been reported that diabetes is a risk factor
for developing malignant tumors. The mechanism by which impaired
glucose tolerance causes carcinogenesis remains a matter of
speculation, but it has been suggested that diabetes is strongly
involved in carcinogenesis in many organs (24,29,44,45).
Especially in liver cancer, type 2 diabetes mellitus is an
established independent risk factor for both nonalcoholic fatty
liver disease and HCC. Independent of the coexistence of cirrhosis
or another etiology, patients with type 2 diabetes have been
reported to have a 2.5- to 4-fold increased risk of developing HCC
(3,23,25,27).
There also appears to be an increased risk of developing HCC in
patients with long-standing uncontrolled diabetes mellitus
(5,17). Furthermore, 10 to 15% of liver
transplant recipients are said to have type 2 diabetes, and
patients with underlying diabetes mellitus are considered at a
higher risk of developing HCC (46). In these previous reports, because
insulin produced in the islets of the pancreas is transported to
the liver via the portal vein, the long-term exposure of hepatic
cells to insulin secreted in a hyperglycemic state may increase the
risk of developing liver cancer (21). In addition, hyperglycemia acts to
promote tumor growth and there are many factors that promote
carcinogenesis, such as hyperinsulinemia through effects on insulin
and/or insulin-like growth factor-1 (IGF-1) receptors and
inflammatory cytokines secreted from adipose tissue. Furthermore,
insulin resistance and activation of the insulin receptor and IGF-1
have been implicated as one of the major determinants in the
initiation and progression of the multistep carcinogenic process of
colorectal cancer (22). Diabetes
is also known to increase the risk of developing colorectal cancer
(47). It is believed that
hyperinsulinemia in diabetes promotes the development and
progression of colorectal cancer by delaying the intestinal transit
time of feces and increasing the concentration of bile acids in the
feces. This patient also had had diabetes mellitus since her
hepatic failure and was receiving insulin therapy, but her diabetes
was poorly controlled and she had persistent hyperglycemia, as
shown by her high glycated hemoglobin (HbA1c) level (11.1%). It may
not be a coincidence that HCC and colon cancer developed in such a
state. In the future, potent diabetes treatment may be necessary,
along with monitoring the immunosuppressive drugs.
Microsatellite instability is one of the factors
that triggers carcinogenesis. It is common knowledge that MSI is
closely involved in familial colon and endometrial cancer, typified
by Lynch syndrome (48). Recent
studies have revealed multiple genetic alterations in
hepatocarcinogenesis, the most important of which involve mismatch
repair (MMR) genes (30). It has
been confirmed immunohistologically that microsatellite markers,
such as MLH1 and PMS2, are lost from tumor cells in both primary
and recurrent liver and colorectal cancer. Without a family history
of Lynch syndrome in the patient's background, the development of
MSI-positive cancer may be described as sporadic. However,
MSI-positive liver cancer has been reported to occur, albeit less
commonly than in colorectal cancer (34). Although immunohistochemical studies
of MMR genes in HCC are scarce (31,40,49,50),
this is an accurate method for identifying MMR defects, with
sensitivity and specificity of 97 and 100%, respectively (51). Helal et al reported that the
proportions of hMSH2 and hMLH1 protein downregulation in the HCC
cases studied were 64 and 70%, respectively (40). On the other hand, in Japanese
patients, hMSH2 and hMLH1 are implicated in around 20% (31,50)
but, in the United States, no reduction or disappearance has been
reported (49). The difference in
the association between HCC and MSI in Japan/United States and
Egypt may be due to racial differences or differences in the HBV
genotypes, but the possibility that HCC development is affected by
factors other than MSI cannot be ruled out. However, considering
that the instability of the MSI gene is a somatic mutation, it is
not unreasonable to speculate that MSI could have been caused in
the donor's liver by some other trigger, such as the
immunosuppressive drugs or abnormal glucose tolerance mentioned
above.
Many case reports of the development of HCC after
liver transplantation involve the use of fluorescent in situ
hybridization to the Y chromosome to determine whether the tumor is
derived from the original or grafted liver (2,18).
Instead of that method, this report features a pathomolecular
study, amplifying the SRT-a-Y, AMEL-Y, SRY-b-Y, and
STS-1-Y genes located on the Y chromosome, an approach used
in the field of forensic medicine (36,37).
It was confirmed that these genes were present in the normal liver
and its tumors and proved that the patient's liver tissue was
derived from the donor. This method can be considered a highly
sensitive and specific modality for analyzing even a very small
sample. However, the disadvantage of this method is that the
recipient and donor must be of opposite sexes. This report is of a
case in which HCC developed in liver tissue derived from the donor,
but if the gene located on the Y chromosome had not been detected,
the tumor might have been derived from the recipient. The patient's
liver had been resected entirely, so that no remnant liver was
present. However, the possibility of liver cell renewal by
hepatocytes derived from bone marrow stem cells cannot be
completely ruled out.
In summary, the carcinogenic process of HCC in the
grafted liver and colon cancer in the present case is related to
immunosuppressants administered after transplantation, poor control
of diabetes mellitus, MSI, and the interaction of these
factors.
Acknowledgements
The authors would like to thank Mrs. Akiko
Shimomura, Mr. Takeshi Nishida and Mr. Hideki Hatta (Department of
Diagnostic Pathology, Faculty of Medicine, Academic Assembly,
University of Toyama) for their advice and assistance in the
pathological and molecular analysis throughout this case
report.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated and analyzed during this study
are included in this published article.
Authors' contributions
SS and JI conceptualized this case report. TT, MM
and KT carried out the internal medicine treatment and follow-up.
KS and TF performed the surgery. SS, AN, KH and JI performed the
histopathological and molecular examinations, and analyzed the data
from the patient's cancer tissue. SS and JI wrote and prepared the
original draft; TT, KS, AN, KT, MM, TF and KH reviewed the
manuscript and confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of any data and/or accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
HBV
|
hepatitis B virus
|
|
HCV
|
hepatitis C virus
|
|
HBsAg
|
hepatitis B surface antigen
|
|
PCR
|
polymerase chain reaction
|
|
C-EF
|
capillary electrophoresis
|
|
MLH1
|
mutL homolog 1
|
|
PMS2
|
postmeiotic segregation increased
2
|
|
MSH
|
mutS homolog
|
|
MSI-H
|
microsatellite instability-high
|
|
IGF-1
|
insulin-like growth factor-1
|
|
MMR
|
mismatch repair
|
References
|
1
|
Hoffman D and Mehta N: Recurrence of
hepatocellular carcinoma following liver transplantation. Expert
Rev Gastroenterol Hepatol. 15:91–102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Joundi T, Gibson S, Brunt EM, Shakil O,
Lee RS and Di Bisceglie AM: Delayed recurrence of hepatocellular
carcinoma after liver transplantation: Detection of origin by
chromosomal analysis. Liver Transpl. 6:374–375. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allaire M and Nault JC. Type 2
diabetes-associated hepatocellular carcinoma: A molecular profile.
Clin Liver Dis (Hoboken). 8:53–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Croitoru A, Schiano TD, Schwartz M,
Roayaie S, Xu R, Suriawinata A and Fiel MI: De novo hepatocellular
carcinoma occurring in a transplanted liver: Case report and review
of the literature. Dig Dis Sci. 51:1780–1782. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Domiaty N, Saliba F, Sebagh M, Salloum
C, Vibert E, Azoulay D, Hamelin J, Cherqui D, Adam R and Samuel D:
De novo hepatocellular carcinoma in a non-cirrhotic allograft 27
years after liver transplantation: A case report. Am J Transplant.
21:1953–1958. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flemming P, Tillmann HL, Barg-Hock H,
Kleeberger W, Manns MP, Klempnauer J and Kreipe HH: Donor origin of
de novo hepatocellular carcinoma in hepatic allografts.
Transplantation. 76:1625–1627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kita Y, Klintmalm G, Kobayashi S and
Yanaga K: Retransplantation for de novo hepatocellular carcinoma in
a liver allograft with recurrent hepatitis B cirrhosis 14 years
after primary liver transplantation. Dig Dis Sci. 52:3392–3393.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levitsky J, Faust TW, Cohen SM and Te HS:
Group G streptococcal bacteremia and de novo hepatocellular
carcinoma after liver transplantation. Liver Transpl. 8:5722002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morita K, Taketomi A, Soejima Y, Ikegami
T, Fukuhara T, Iguchi T, Nagata S, Sugimachi K, Gion T, Shirabe K
and Maehara Y: De novo hepatocellular carcinoma in a liver graft
with sustained hepatitis C virus clearance after living donor liver
transplantation. Liver Transpl. 15:1412–1416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Navarro Burgos JB, Lee KW, Shin YC, Lee
DS, Lee KB, Yi NJ and Suh KS: Inexplicable outcome of early
appearance of hepatocellular carcinoma in the allograft after
deceased donor liver transplantation: A case report. Transplant
Proc. 47:3012–3015. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramadori G, Bosio P, Moriconi F and Malik
IA: Case report: 8 years after liver transplantation: De novo
hepatocellular carcinoma 8 months after HCV clearance through
IFN-free antiviral therapy. BMC Cancer. 18:2572018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saab S, Zhou K, Chang EK and Busuttil RW:
De novo Hepatocellular Carcinoma after Liver Transplantation. J
Clin Transl Hepatol. 3:284–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saxena R, Ye MQ, Emre S, Klion F, Nalesnik
MA and Thung SN: De novo hepatocellular carcinoma in a hepatic
allograft with recurrent hepatitis C cirrhosis. Liver Transpl Surg.
5:81–82. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sotiropoulos GC, Frilling A, Molmenti EP,
Brokalaki EI, Beckebaum S, Omar OS, Broelsch CE and Malagó M: De
novo hepatocellular carcinoma in recurrent liver cirrhosis after
liver transplantation for benign hepatic disease: Is a deceased
donor re-transplantation justified? Transplantation. 82:11122006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Torbenson M, Grover D, Boitnott J, Klein A
and Molmenti E: De novo hepatocellular carcinoma in a liver
allograft associated with recurrent hepatitis B. Transplant Proc.
37:2205–2206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu S, Guo H, Zhuang L, Yu J, Yan S, Zhang
M, Wang W and Zheng S: A case report of de novo hepatocellular
carcinoma after living donor liver transplantation. World J Surg
Oncol. 11:1762013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoo JJ, Cho EJ, Han K, Heo SS, Kim BY,
Shin DW and Yu SJ: Glucose variability and risk of hepatocellular
carcinoma in patients with diabetes: A nationwide population-based
study. Cancer Epidemiol Biomarkers Prev. 30:974–981. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tame M, Calvanese C, Cucchetti A,
Gruppioni E, Colecchia A and Bazzoli F: The onset of de novo
hepatocellular carcinoma after liver transplantation can be both of
donor and recipient origin. A case report. J Gastrointestin Liver
Dis. 24:387–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Allaire M, Seree O, Coilly A,
Houssel-Debry P, Neau-Cransac M, Pageaux GP, Dumortier J and
Altieri M: De novo primary liver cancer after liver
transplantation: A French National Study on 15803 Patients. Exp
Clin Transplant. 16:779–780. 2018.PubMed/NCBI
|
|
20
|
Vajdic CM and van Leeuwen MT: Cancer
incidence and risk factors after solid organ transplantation. Int J
Cancer. 125:1747–1754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giovannucci E, Harlan DM, Archer MC,
Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG and
Yee D: Diabetes and cancer: A consensus report. Diabetes Care.
33:1674–1685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hung CH, Wang JH, Hu TH, Chen CH, Chang
KC, Yen YH, Kuo YH, Tsai MC, Lu SN and Lee CM: Insulin resistance
is associated with hepatocellular carcinoma in chronic hepatitis C
infection. World J Gastroenterol. 16:2265–2271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inoue M, Iwasaki M, Otani T, Sasazuki S,
Noda M and Tsugane S: Diabetes mellitus and the risk of cancer:
Results from a large-scale population-based cohort study in Japan.
Arch Intern Med. 166:1871–1877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin CM, Huang HL, Chu FY, Fan HC, Chen HA,
Chu DM, Wu LW, Wang CC, Chen WL, Lin SH and Ho SY: Association
between gastroenterological malignancy and diabetes mellitus and
anti-diabetic therapy: A nationwide, population-based cohort study.
PLoS One. 10:e01254212015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mantovani A and Targher G: Type 2 diabetes
mellitus and risk of hepatocellular carcinoma: Spotlight on
nonalcoholic fatty liver disease. Ann Transl Med. 5:2702017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Kruijsdijk RC, van der Wall E and
Visseren FL: Obesity and cancer: The role of dysfunctional adipose
tissue. Cancer Epidemiol Biomarkers Prev. 18:2569–2578. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen
Y, Li G and Wang L: Increased risk of hepatocellular carcinoma in
patients with diabetes mellitus: A systematic review and
meta-analysis of cohort studies. Int J Cancer. 130:1639–1648. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wojciechowska J, Krajewski W, Bolanowski
M, Krecicki T and Zatonski T: Diabetes and Cancer: A Review of
Current Knowledge. Exp Clin Endocrinol Diabetes. 124:263–275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Pelzer AM, Kiang DT and Yee D:
Down-regulation of type I insulin-like growth factor receptor
increases sensitivity of breast cancer cells to insulin. Cancer
Res. 67:391–397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Macdonald GA, Greenson JK, Saito K,
Cherian SP, Appelman HD and Boland CR: Microsatellite instability
and loss of heterozygosity at DNA mismatch repair gene loci occurs
during hepatic carcinogenesis. Hepatology. 28:90–97. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsukura S, Soejima H, Nakagawachi T,
Yakushiji H, Ogawa A, Fukuhara M, Miyazaki K, Nakabeppu Y,
Sekiguchi M and Mukai T: CpG methylation of MGMT and hMLH1 promoter
in hepatocellular carcinoma associated with hepatitis viral
infection. Br J Cancer. 88:521–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suriawinata A and Xu R: An update on the
molecular genetics of hepatocellular carcinoma. Semin Liver Dis.
24:77–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang SH, Cong WM, Xian ZH and Wu MC:
Clinicopathological significance of loss of heterozygosity and
microsatellite instability in hepatocellular carcinoma in China.
World J Gastroenterol. 11:3034–3039. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brierly JD, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. Eighth Edition.
Whiley-Blackwell; Hoboken, N, USA: pp. 73–76. 2017
|
|
36
|
Morikawa T, Yamamoto Y and Miyaishi S: A
new method for sex determination based on detection of SRY, STS and
amelogenin gene regions with simultaneous amplification of their
homologous sequences by a multiplex PCR. Acta Med Okayama.
65:113–122. 2011.PubMed/NCBI
|
|
37
|
Wang LJ, Chen YM, George D, Smets F, Sokal
EM, Bremer EG and Soriano HE: Engraftment assessment in human and
mouse liver tissue after sex-mismatched liver cell transplantation
by real-time quantitative PCR for Y chromosome sequences. Liver
Transpl. 8:822–828. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sakuraoka Y, Kubota K, Imura J, Yamagishi
H, Aoki T, Matsumoto T, Arakawa T, Suzuki T, Tanaka G, Shimizu T,
et al: Microsatellite analysis of recurrent lesions confirms merit
of anatomical liver resection for hepatocellular carcinoma.
Anticancer Res. 39:4315–4324. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong Y, Han J, Zou Z, Liu S, Tang B, Ren
X, Li X, Zhao Y, Liu Y, Zhou D, et al: Quantitation of HBV
covalently closed circular DNA in micro formalin fixed
paraffin-embedded liver tissue using rolling circle amplification
in combination with real-time PCR. Clin Chim Acta. 412:1905–1911.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Helal TE, Khamis NS, El-Sharkawy TM, Nada
OH and Radwan NA: Immunohistochemical expression of mismatch repair
genes (hMSH2 and hMLH1) in hepatocellular carcinoma in Egypt.
APMIS. 118:934–940. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feitelson MA, Sun B, Satiroglu Tufan NL,
Liu J, Pan J and Lian Z: Genetic mechanisms of
hepatocarcinogenesis. Oncogene. 21:2593–2604. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Frey AB and Monu N: Signaling defects in
anti-tumor T cells. Immunol Rev. 222:192–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maluccio M, Sharma V, Lagman M, Vyas S,
Yang H, Li B and Suthanthiran M: Tacrolimus enhances transforming
growth factor-beta1 expression and promotes tumor progression.
Transplantation. 76:597–602. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Giovannucci E: Insulin, insulin-like
growth factors and colon cancer: A review of the evidence. J Nutr.
131 (11 Suppl):3109S–3120S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pollak M: Insulin and insulin-like growth
factor signalling in neoplasia. Nat Rev Cancer. 8:915–928. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Azhie A, Sheth P, Hammad A, Woo M and Bhat
M: Metabolic complications in liver transplantation recipients: How
we can optimize long-term survival. Liver Transpl. 27:1468–1478.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Seow A, Yuan JM, Koh WP, Lee HP and Yu MC:
Diabetes mellitus and risk of colorectal cancer in the Singapore
Chinese Health Study. J Natl Cancer Inst. 98:135–138. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roudko V, Cimen Bozkus C, Greenbaum B,
Lucas A, Samstein R and Bhardwaj N: Lynch Syndrome and MSI-H
Cancers: From mechanisms to ‘Off-The-Shelf’ cancer vaccines. Front
Immunol. 12:7578042021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang L, Bani-Hani A, Montoya DP, Roche PC,
Thibodeau SN, Burgart LJ and Roberts LR: hMLH1 and hMSH2 expression
in human hepatocellular carcinoma. Int J Oncol. 19:567–570.
2001.PubMed/NCBI
|
|
50
|
Wani Y, Notohara K, Tsukayama C and Okada
S: Reduced expression of hMLH1 and hMSH2 gene products in
high-grade hepatocellular carcinoma. Acta Med Okayama. 55:65–71.
2001.PubMed/NCBI
|
|
51
|
Marcus VA, Madlensky L, Gryfe R, Kim H, So
K, Millar A, Temple LK, Hsieh E, Hiruki T, Narod S, et al:
Immunohistochemistry for hMLH1 and hMSH2: A practical test for DNA
mismatch repair-deficient tumors. Am J Surg Pathol. 23:1248–1255.
1999. View Article : Google Scholar : PubMed/NCBI
|