Introduction
Breast cancer has overtaken lung cancer to become
the most prevalent malignancy worldwide, according to the latest
statistics (1). Human epidermal
growth factor receptor 2 (HER2)-positive breast cancer is one of
the most invasive subtypes, accounting for 15–20% of all breast
cancers (2). Beyond that,
HER2-positive status is considered an independent prognostic factor
and therapeutic target. In addition, anti-HER2 therapy has changed
the treatment paradigm and altered the natural history of
HER2-positive breast cancer (3,4).
For early-stage or locally advanced breast cancer,
neoadjuvant therapy has emerged as the most effective method for
decreasing advanced locoregional disease, which increases the
chance of successful surgical resection and provides an opportunity
for breast-preserving procedures in female patients (5). Moreover, response to neoadjuvant
therapy provides prognostic information relevant to follow-up
management. Neoadjuvant therapy with HER2-targeted agents has led
to a considerable increase in the pathological complete response
(pCR) rate in patients with HER2-positive breast cancer. Studies
that combined dual anti-HER2 inhibition with conventional
chemotherapy have shown improvements in survival compared with
single-drug targeted combinations (6). Although a controversial surrogate for
long-term survival, it is undeniable that pCR after neoadjuvant
treatment correlates positively with DFS and OS, especially in
triple-negative and HER2-positive subtypes (7).
Pyrotinib is an orally administered, small molecule,
irreversible pan-ErbB receptor tyrosine kinase inhibitor (TKI) that
can simultaneously target HER1/epidermal growth factor receptor
(EGFR), HER2, and HER4 (8). In the
PHENIX trial, PHOEBE trial, and the study of Ma et al
(9), 67–78.5% of patients in the
respective pyrotinib group achieved an objective response.
Furthermore, pyrotinib efficacy has been confirmed in patients with
advanced HER2-positive breast cancer who progressed after
trastuzumab and lapatinib treatment, as well as in those with brain
metastases. Certainly, the treatment-related adverse events were
inevitable, and the most common TRAEs caused by pyrotinib and
capecitabine were diarrhea, hand-foot syndrome, vomiting, decreased
white blood cell count, and decreased neutrophil count (9–14).
Therefore, pyrotinib has been approved for use in combination with
capecitabine in China for previously treated HER2-positive
metastatic or advanced breast cancer patients (15).
Importantly, although several relevant early trials
are underway, there is limited information on the use of pyrotinib
in a neoadjuvant setting. Therefore, a meta-analysis was conducted
to assess the safety and efficacy of pyrotinib in combination with
trastuzumab and chemotherapy in stage I–III HER2-positive breast
cancer.
Materials and methods
The present systematic review and meta-analysis was
conducted according to the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) guidelines (16).
Search strategy
A systematic search was conducted using databases
[PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science
(https://www.webofscience.com), Embase
(https://www.embase.com/) and Cochrane Central
(https://www.cochranelibrary.com/central/about-central)]
to identify eligible studies. The last search date was August 17,
2023. The search terms included: i) pyrotinib, ii) trastuzumab and
iii) breast cancer. Manual searches of reference lists in
identified reviews were performed to identify additional eligible
studies.
Outcomes
The primary outcome was treatment-related adverse
events (TRAEs), including any Grade and ≥3 Grade TRAEs. The pCR
rate was the secondary endpoint. pCR is defined as the absence of
microscopically invasive residual tumor cells in the breast and
axillary lymph nodes, with the possible presence of ductal
carcinoma in situ (ypT0/Tis ypN0).
Study selection
Inclusion criteria were as follows: i) Participants:
All patients had been newly diagnosed with early or local advanced
(stage I–III) HER2-positive breast cancer; ii) Intervention:
Patients were treated with pyrotinib-based dual-HER2 target
neoadjuvant therapy; iii) Outcome: Detailed treatment-related data,
such as TRAEs and/or pCR rate; iv) Study type: Prospective clinical
trials published in English. Retrospective studies, preclinical
studies, conference abstracts, case reports, reviews and
commentaries, as well as articles published in languages other than
English or without treatment data available were excluded.
Data extraction and quality
assessment
The following data were extracted from included
studies by two independent reviewers: Name of the first author,
publication year, study design, sample size, median age,
therapeutic strategy and toxicities. The quality of included
randomized controlled trials (RCTs) was assessed using the Cochrane
Risk of Bias Tool, and the quality of single-arm clinical trials
was assessed according to the methodological index for
non-randomized studies (17). The
details of quality evaluation are shown in Fig. S1 and Table SI.
Statistical analysis and risk of
bias
The incidence of TRAEs and pCR rates were analyzed
using R software (version 4.2.1; The R Foundation) and RevMan 5.4
(The Cochrane Collaboration). For single-arm data, a random-effects
model was applied to reduce the risk of bias. Relative risk was
used for the dichotomous outcomes of subgroup analysis.
Heterogeneity among the included studies was measured using the
I2 statistic. Based on the percentage of I2,
heterogeneity was defined as low level (I2≤50%) and high
level (I2>50%) (18).
Egger's tests, funnel plots, and sensitivity analyses were used to
evaluate publication bias.
Results
Eligible studies and basic
characteristics
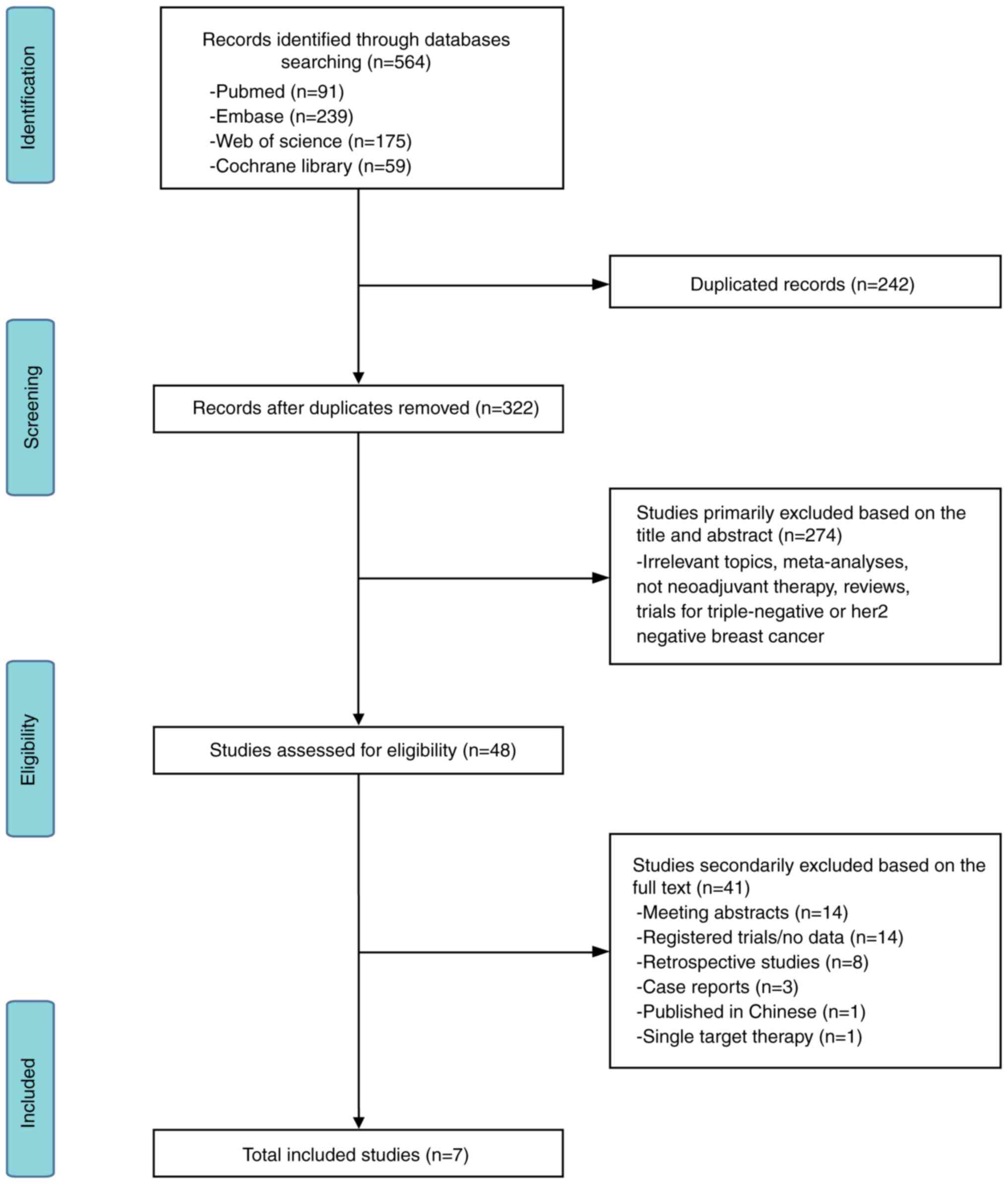
In total, 564 relevant records were identified, of
which 91 records were from PubMed, 175 from Web of Science, 239
from Embase and 59 records from Cochrane Library. Overall, 242
duplicate records were excluded, and a further 274 records were
excluded due to irrelevancy. A further sum of 41 articles were
excluded in the following categories: Meeting abstracts (n=14);
registered trials/no data (n=14); single target therapy (n=1); case
report (n=3); retrospective studies (n=8); and non-English language
(n=1). Finally, seven prospective studies were included that met
all the selection criteria. A flow diagram detailing the present
procedure is shown in Fig. 1
(19–25).
A total of seven prospective clinical trials with a
combined total of 407 participants were included (382 patients in
the safety analysis and 395 in the efficacy analysis). All studies
were published between 2020–2023; details of each study are
provided in Table I. The studies by
Xuhong et al (22) and Shi
et al (25), were reports
from the same clinical trial (ChiCTR Identifier: ChiCTR1900022293)
but presented different data. Patients in this clinical trial
received a sequential anthracycline-taxane regimen, while patients
in the five other studies received paclitaxel-based chemotherapy
(23,24).
 | Table I.Basic characteristics and treatment
schedules of eligible studies. |
Table I.
Basic characteristics and treatment
schedules of eligible studies.
| Author(s),
year | Design | Number of
patients | Median age
(range) | Pyrotinib | Trastuzumab | Chemotherapy | Cycles | Median duration of
therapy (range) | (Refs.) |
|---|
| Xuhong et
al, 2020 | Single arm,
prospective study | 20 | 47.5 (30–66) | 400 mg once
daily | 8 mg/kg first load
followed by 6 mg/kg on day 1, for cycles 5 to 8 | Epirubicin: 100
mg/m2 on day 1, for cycles 1 to 4; cyclophosphamide: 600
mg/m2 on day 1, for cycles 1 to 4; docetaxel: 100
mg/m2 on day 1, for cycles 5 to 8 | Eight 21-day
cycles | 5.7 (5.3–6.1)
months | (22) |
| Zhong et al,
2022 | Single arm,
prospective study | 21 | 48 (28–57) | 400 mg once
daily | 4 mg/kg loading
dose, followed by 2 mg/kg once a week | Nab-paclitaxel: 125
mg/m2 on days 1, 8 and 15 | Four 21-day
cycles | 2.7 (2.6–3.1)
months | (19) |
| Liu et al,
2022 | Single arm,
prospective study | 74 | 50 (31–64) | 400 mg once
daily | 8 mg/kg loading
dose and 6 mg/kg maintenance dose on day 1 | Docetaxel: 75
mg/m2 on day 1; carboplatin: 6 mg/ml/min on day 1 | Six 21-day
cycles | NR | (21) |
| Yin et al,
2022 | Single arm,
prospective study | 53 | 47 (26–66) | 400 mg once
daily | 4 mg/kg loading
dose and 2 mg/kg maintenance once a week | Paclitaxel: 80
mg/m2 on days 1, 8, 15 and 22; cisplatin: 25
mg/m2 on days 1, 8 and 15 | Four 28-day
cycle | NR | (20) |
| Shi et al,
2023 | Single arm,
prospective study | 45 | 48 (NR) | 400 mg once
daily | 8 mg/kg first load
followed by 6 mg/kg on day 1, for cycles 5 to 8 | Epirubicin: 100
mg/m2 on day 1, for cycles 1 to 4; cyclophosphamide: 600
mg/m2 on day 1, for cycles 1 to 4; docetaxel: 100
mg/m2 on day 1, for cycles 5 to 8 | Eight 21-day
cycles | NR | (25) |
| Wu et al,
2022 | Randomized,
prospective study | 178 | 50 (43–55) | 400 mg once
daily | 8 mg/kg loading
dose and 6 mg/kg maintenance dose on day 1 | Docetaxel: 100
mg/m2 on day 1 | Four 21-day
cycles | NR | (24) |
| Ding et al,
2023 | Randomized,
prospective study | 36 | 53 (31–69) | 400 mg once
daily | 8 mg/kg first load
followed by 6 mg/kg on day 1, for cycles 5 to 8 | Docetaxel: 75
mg/m2 on day 1; carboplatin: 6 mg/ml/min on day 1 | Six 21-day
cycles | NR | (23) |
TRAEs
Overall, 382 patients across six studies (19–24)
were included in the safety analysis. Details of TRAEs associated
with pyrotinib combination therapy are shown in Table II. The pooled incidences of TRAEs
(occurring in ≥40% patients) were: diarrhea [98%; 95% confidence
interval (CI): 92–100%, P<0.01]; anemia (71%; 95% CI: 50–89%;
P<0.01); vomiting (69%; 95% CI: 55–82%; P<0.01); leucopenia
(66%; 95% CI: 35–91%; P<0.01); neutropenia (59%; 95% CI: 33–82%;
P<0.01); nausea (59%; 95% CI: 38–77%; P<0.01); fatigue (58%;
95% CI: 34–81%; P<0.01); alanine transaminase (ALT) increased
(42%; 95% CI: 31–54%; P<0.01); rash (42%; 95% CI: 21–64%;
P<0.01). The aggregated incidence of Grade ≥3 TRAEs is displayed
in Table III. Diarrhea,
neutropenia and leucopenia were the most frequently reported Grade
≥3 TRAEs, with incidences of 44% (95% CI: 39–49%; P=0.82), 23% (95%
CI: 10–39%; P<0.01) and 20% (95% CI: 8–36%; P<0.01),
respectively. No treatment-related deaths were reported.
 | Table II.Treatment-related adverse events (any
grades) occurred in patients who received neoadjuvant therapy. |
Table II.
Treatment-related adverse events (any
grades) occurred in patients who received neoadjuvant therapy.
| Adverse events | Number of
studies | Incidence, % | 95% CI, % | P-value |
|---|
| Diarrhea | 6 | 98 | 92-100 | <0.01 |
| Leucopenia | 5 | 66 | 35-91 | <0.01 |
| Vomiting | 6 | 69 | 55-82 | <0.01 |
| Anemia | 5 | 71 | 50-89 | <0.01 |
| Neutropenia | 5 | 59 | 33-82 | <0.01 |
| Fatigue | 6 | 58 | 34-81 | <0.01 |
| Nausea | 6 | 59 | 38-77 | <0.01 |
| ALT increased | 6 | 42 | 31-54 | <0.01 |
| Rash | 4 | 42 | 21-64 | <0.01 |
| AST increased | 6 | 35 | 23-48 | <0.01 |
| Creatinine
increased | 4 | 26 | 17-38 | 0.05 |
 | Table III.Treatment-related adverse events (≥3
grades) occurred in patients who received neoadjuvant therapy. |
Table III.
Treatment-related adverse events (≥3
grades) occurred in patients who received neoadjuvant therapy.
| Adverse events | Number of
studies | Incidence, % | 95% CI, % | P-value |
|---|
| Diarrhea | 6 | 44 | 39-49 | 0.82 |
| Leucopenia | 5 | 20 | 8-36 | <0.01 |
| Vomiting | 6 | 5 | 0-12 | <0.01 |
| Anemia | 5 | 6 | 0-21 | <0.01 |
| Neutropenia | 5 | 23 | 10-39 | <0.01 |
| Fatigue | 6 | 1 | 0-3 | 0.05 |
| Nausea | 6 | 0 | 0-1 | 0.91 |
| ALT increased | 6 | 2 | 1-4 | 0.23 |
| Rash | 4 | 0 | 0-1 | 0.94 |
| AST increased | 6 | 1 | 0-2 | 0.81 |
| Creatinine
increased | 4 | 1 | 0-3 | 0.66 |
PCR rate
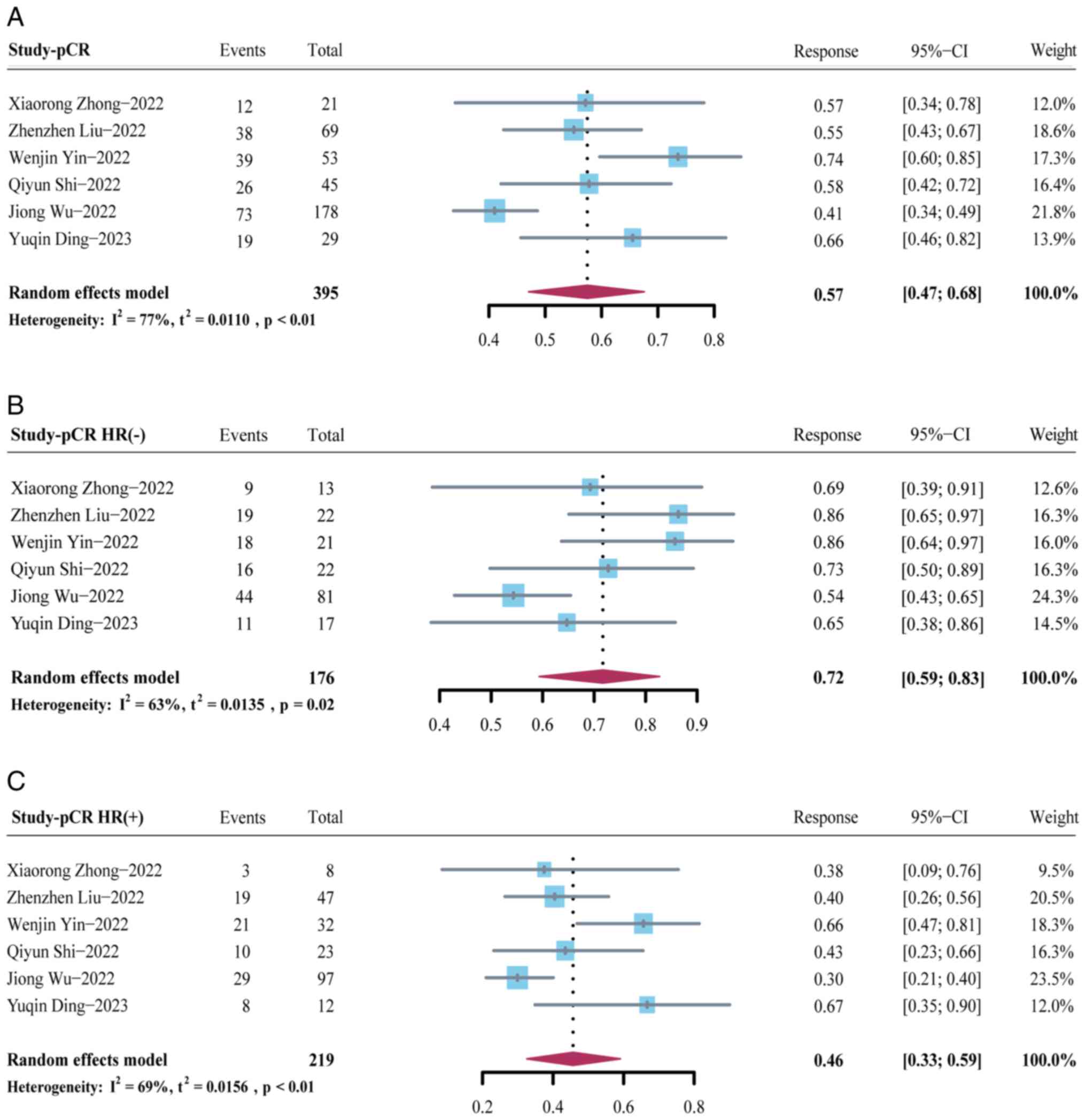
To calculate the overall pCR rate for pyrotinib in
neoadjuvant settings, the pCR values for 395 patients across six
studies (19–21,23–25)
were pooled. The proportion of participants who achieved pCR was
57% (95% CI: 47–68%; P<0.01) (Fig.
2A). Moreover, the association between pCR rate and hormone
receptor (HR) status was evaluated. The results of the present
study revealed that the pooled pCR rate for patients with HR
negative status (estrogen receptor and progesterone receptor
negative) and HR positive status (estrogen receptor and/or
progesterone receptor positive) was 72% (95% CI: 59–83%; P=0.02)
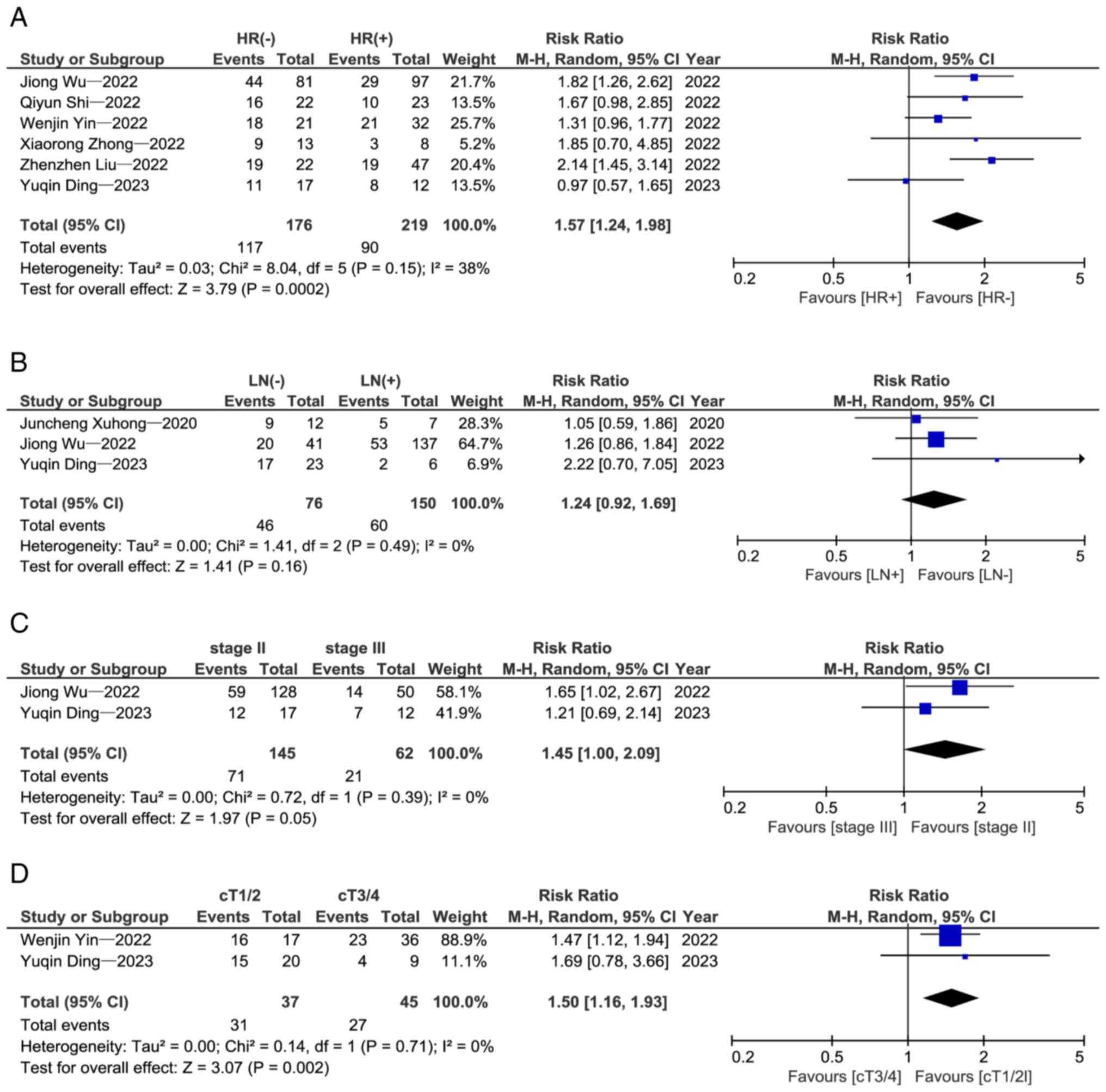
and 46% (95% CI: 33–59%, P<0.01), respectively (Fig. 2B and C). HR negative status was
associated with a significantly higher pCR rate than HR positive
status [relative risk (RR)=1.57; 95% CI: 1.24–1.98; P=0.0002]
(Fig. 3A).
In addition to hormonal status, there are other
factors that may influence patient outcomes. Therefore, a subgroup
analysis was performed which revealed that early nodal stage
(RR=1.24; 95% CI: 0.92–1.69; P=0.16; Fig. 3B), early clinical stage (RR=1.45;
95% CI: 1.00–2.09; P=0.05; Fig. 3C)
and early clinical tumor stage (RR=1.50; 95% CI: 1.16–1.93,
P=0.002; Fig. 3D) were associated
with a higher pCR rate.
Sensitivity analysis
The sensitivity analysis of the present study
revealed that the arbitrary deletion of a study had little effect
on the final pooled outcome, indicating that the results of this
data analysis are reliable (Fig.
S2A-F).
Risk of publication bias. As demonstrated in
Fig. S2, Fig. S3, Fig.
S4 and Table SII, the presence
of asymmetric funnel plots indicated a potential publication bias.
However, the asymmetry in the funnel plots could also be attributed
to other factors, such as genuine heterogeneity among the studies.
Furthermore, no significant publication bias was detected with
Egger's test for both the incidence of TRAEs and the pooled pCR
rate (P>0.05).
Discussion
Monoclonal antibodies (mABs), small molecule TKIs,
and antibody-drug conjugates are being increasingly adopted in
clinical practice, which has enriched the treatment options for
patients and helped to overcome the problem of therapeutic
resistance. Studies have confirmed that small-molecule TKIs, such
as lapatinib, can also be an effective neoadjuvant treatment
strategy (26). A network
meta-analysis of neoadjuvant therapy for HER2-positive breast
cancer reported that the combination of dual-targeted therapy
(trastuzumab plus pertuzumab) and neoadjuvant chemotherapy showed
the highest efficacy (27). The pCR
rates for patients who received this neoadjuvant therapy ranged
from 39.3–66.2% (28–31). However, pyrotinib was not included
in the analyses because it had just been approved at the time, and
there were no available RCTs. In the present study, a meta-analysis
to explore the potential of pyrotinib as neoadjuvant therapy for
HER2-positive breast cancer was performed, the results of which may
provide useful, informative data for treatment decision-making in
clinical practice. Currently, most prospective studies on the
combination of pyrotinib and trastuzumab in neoadjuvant therapy are
either single-arm studies or RCTs comparing it with a placebo. In
the present study, the efficacy of pyrotinib was assessed by
analyzing the pooled pCR rate. The pCR rates for patients who
received neoadjuvant chemotherapy with the dual-target treatment
based on pyrotinib ranged from 41 to 73.58%. While a direct
comparison with trastuzumab plus pertuzumab is not feasible, the
data suggested that the efficacy of pyrotinib-based dual-targeted
therapy is comparable to the current standard treatment
(trastuzumab plus pertuzumab).
Pyrotinib acts by competitively binding to the HER2
intracellular kinase domain, effectively inhibiting the activation
of downstream signaling pathways. However, EGFR and HER2 are also
expressed in healthy cells. Consequently, up to 96% of patients
with diarrhea treated with second-generation TKIs are assumed to
have direct mucosal atrophy and injury caused by the inhibition of
ErbB signaling within the intestinal epithelia (32,33).
The results from the analysis of the present study revealed that
gastrointestinal reactions, as well as myelosuppression, are the
most common adverse events of any Grade and also Grade ≥3, which
was consistent with other retrospective studies (34–37).
It is worth noting that nearly half of the participants in the
present analysis experienced Grade 3 diarrhea, with a significantly
higher incidence compared with capecitabine combination therapy in
advanced or metastatic breast cancer. However, diarrhea (any Grade
or Grade ≥3) mainly occurred during cycles 1–2 of treatment, and
was generally reversible with appropriate drugs and dose reduction.
It is likely that the severity and incidence of diarrhea will
increase when TKIs are used in combination with chemotherapy;
therefore, clinicians should pay attention to published guidelines
on the treatment of diarrhea when managing patients in practice
(38).
Of note, it was discovered that the incidence of ALT
increased, aspartate transaminase increased, leukopenia and
neutropenia were similar between the pyrotinib group and the
placebo group in the studies by Wu et al (24) and Ding et al (23), suggesting that these TRAEs are not
significantly related to the addition of pyrotinib, and could be
reversed in most patients after symptomatic and prophylactic
therapy. It was suggested that drug-related cardiotoxicity should
also be closely monitored in clinical practice since anthracycline
is associated with cardiotoxicity, especially when given in
combination with trastuzumab (39).
Small-molecule TKIs are less cardiotoxic compared with mABs
(40). It was confirmed that no
increased risk of cardiac insufficiency with concomitant pyrotinib
and trastuzumab or anthracycline in previous studies (20,22–24).
The incidence of other TRAEs caused by pyrotinib-contained
neoadjuvant therapy, such as anemia, vomiting, fatigue and
creatinine increase was <10%.
Overall, 207 out of 395 patients who received
pyrotinib-containing neoadjuvant therapy achieved pCR (defined as
the proportion of patients who achieved a complete response or
partial response), and the objective response rate was close to
100% across all five studies. Real-world studies have confirmed the
activity of pyrotinib in the neoadjuvant setting (34–37).
Owing to discrepancies in inclusion criteria, drug dosage and
duration of therapy, optimal dosing of pyrotinib in combination
with chemotherapy remains unknown and must be further explored in
future research. However, several trails published to date have
demonstrated that standard neoadjuvant chemotherapy with
anthracyclines or paclitaxel plus pyrotinib was well tolerated and
effective.
Of note, patients with HR-negative status were more
likely to achieve pCR than HR-positive positive (72 vs. 46%,
respectively). This is likely due to the high dependence of
HR-negative tumors on the HER2 gene for growth and proliferation.
Tumors with HR-positive status also rely on the estrogen receptor
pathway, and blocking HER2 alone is not sufficient to achieve a
potent antitumor effect (36).
Despite this, it was identified that pCR was positively associated
with long-term outcomes regardless of HR status. PIK3CA mutations
are common in breast cancer, and ~20–25% of patients with
HER2-positive breast cancer have this mutation. PIK3CA has emerged
as a major cause of resistance to HER2-targeted therapy and is
associated with a lower pCR rate and poor prognosis (41–43).
In the NeoATP trial, ~24% (n=13) of patients with HER2-positive
breast cancer had PIK3CA mutations, and their pCR rate after
neoadjuvant therapy was not significantly different from that of
wild-type patients (76.92 vs. 72.50%, respectively; P=0.753).
However, this is in contradiction with the results reported in a
number of studies (25,44).
In the past, numerous research analyses on pyrotinib
in patients with advanced HER2-positive breast cancer have been
published (14,45). However, the present study represents
the first investigation into the safety and efficacy of pyrotinib
in neoadjuvant therapy for HER2-positive breast cancer patients, to
the best of the authors' knowledge. Additionally, in the present
research, the relationships between tumor staging, hormone status,
PIK3CA mutations and treatment efficacy were explored. Certainly,
there are several limitations to the analysis of the present study.
Firstly, some of the included studies were single-arm, phase II
trials with small patient populations and no control arm. Secondly,
each trial used different regimens and doses of neoadjuvant
chemotherapy, and it was not possible to estimate the impact of
different chemotherapy strategies on the incidence and severity of
adverse events, which may have led to bias in the results of the
present study. Finally, included clinical trials were carried out
in recent years and had a short follow-up time; therefore, time
followed-up, mature survival data were not available. In spite of
these limitations, both pooled data and individual data from each
trial demonstrated the efficacy and safety of pyrotinib for
neoadjuvant therapy in patients with HER-2 positive breast
cancer.
In conclusion, the results of the present
meta-analysis, affirmed that pyrotinib plus trastuzumab is a
relatively tolerable and effective dual-HER2 blockade regimen for
patients with HER2-positive breast cancer in the neoadjuvant
setting, whether in combination with paclitaxel- or
anthracycline-based chemotherapies. However, given the notable
incidence of adverse events in the analysis of the present study,
proactive management of toxicities and regular laboratory
examination are essential for patients on combination therapy, with
particular vigilance required for the development of severe
diarrhea, leukopenia, and neutropenia. Importantly, most adverse
events are reversible with drug reduction or symptomatic treatment.
In the future, more relevant clinical RCTs will be required to
verify the conclusions of the analysis of the present study. In
addition, additional studies are needed to identify the optimal
combination therapies, patient population, dosage and treatment
cycles with pyrotinib-containing neoadjuvant therapy in clinical
practice.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QM conceptualized the present study. BCW and BW
developed methodology. QM and BCW extracted data. QM, BCW, BW, GW,
XZ and YW performed formal analysis. QM wrote the original draft.
BCW and BW wrote, reviewed and edited the manuscript. QM, BW and
BCW confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loibl S and Gianni L: HER2-positive breast
cancer. Lancet. 389:2415–2429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh JC, Jhaveri K and Esteva FJ:
HER2-positive advanced breast cancer: Optimizing patient outcomes
and opportunities for drug development. Br J Cancer. 111:1888–1898.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J and Xu B: Targeted therapeutic
options and future perspectives for HER2-positive breast cancer.
Signal Transduct Target Ther. 4:342019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loibl S, Poortmans P, Morrow M, Denkert C
and Curigliano G: Breast cancer. Lancet. 397:1750–1769. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Korde LA, Somerfield MR, Carey LA, Crews
JR, Denduluri N, Hwang ES, Khan SA, Loibl S, Morris EA, Perez A, et
al: Neoadjuvant chemotherapy, endocrine therapy, and targeted
therapy for breast cancer: ASCO guideline. J Clin Oncol.
39:1485–1505. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spring LM, Fell G, Arfe A, Sharma C,
Greenup R, Reynolds KL, Smith BL, Alexander B, Moy B, Isakoff SJ,
et al: Pathologic complete response after neoadjuvant chemotherapy
and impact on breast cancer recurrence and survival: A
comprehensive meta-analysis. Clin Cancer Res. 26:2838–2848. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Yang C, Wan H, Zhang G, Feng J and
Zhang L, Chen X, Zhong D, Lou L, Tao W and Zhang L: Discovery and
development of pyrotinib: A novel irreversible EGFR/HER2 dual
tyrosine kinase inhibitor with favorable safety profiles for the
treatment of breast cancer. Eur J Pharm Sci. 110:51–61. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu
Y, Li H, Yu S, Feng J, Wang S, et al: Pyrotinib or lapatinib
combined with capecitabine in HER2-positive metastatic breast
cancer with prior taxanes, anthracyclines, and/or trastuzumab: A
randomized, phase II study. J Clin Oncol. 37:2610–2619. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin S, Chi Y, Du Y, Wang J, Shan C, Yi W,
Shang M, Man X, Tan Q and Li H: Efficacy and safety of
pyrotinib-containing regimen in the patients with HER2-positive
metastatic breast cancer: A multicenter real-world study. Cancer
Med. 12:2333–2344. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan M, Bian L, Hu X, Zhang Q, Ouyang Q,
Feng J, Yin Y, Sun T, Tong Z, Wang X, et al: Pyrotinib plus
capecitabine for human epidermal factor receptor 2-positive
metastatic breast cancer after trastuzumab and taxanes (PHENIX): A
randomized, double-blind, placebo-controlled phase 3 study. Transl
Breast Cancer Res. 1:132020. View Article : Google Scholar
|
|
12
|
Yan M, Ouyang Q, Sun T, Niu L, Yang J, Li
L, Song Y, Hao C, Chen Z, Orlandi A, et al: Pyrotinib plus
capecitabine for patients with human epidermal growth factor
receptor 2-positive breast cancer and brain metastases (PERMEATE):
A multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol.
23:353–361. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q,
Tong Z, Li H, Zhang Q, Sun T, et al: Pyrotinib plus capecitabine
versus lapatinib plus capecitabine for the treatment of
HER2-positive metastatic breast cancer (PHOEBE): A multicentre,
open-label, randomised, controlled, phase 3 trial. Lancet Oncol.
22:351–360. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan Y, Liu X, Cai Y and Li W: Pyrotinib
versus lapatinib therapy for HER2 positive metastatic breast cancer
patients after first-line treatment failure: A meta-analysis and
systematic review. PLoS One. 18:e02797752023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blair HA: Pyrotinib: First global
approval. Drugs. 78:1751–1755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higgins JPT, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong X, He P, Chen J, Yan X, Wei B, Zhang
Z, Bu H, Li J, Tian T, Lv Q, et al: Neoadjuvant pyrotinib plus
trastuzumab and nab-paclitaxel for HER2-positive early or locally
advanced breast cancer: An exploratory phase II trial. Gland Surg.
11:216–225. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin W, Wang Y, Wu Z, Ye Y, Zhou L, Xu S,
Lin Y, Du Y, Yan T, Yang F, et al: Neoadjuvant trastuzumab and
pyrotinib for locally advanced HER2-positive breast cancer
(NeoATP): Primary analysis of a phase II study. Clin Cancer Res.
28:3677–3685. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Z, Wang C, Chen X, Zhu J, Sun X, Xia
Q, Lu Z, Qiao J, Zhou Y, Wang H, et al: Pathological response and
predictive role of tumour-infiltrating lymphocytes in HER2-positive
early breast cancer treated with neoadjuvant pyrotinib plus
trastuzumab and chemotherapy (Panphila): A multicentre phase 2
trial. Eur J Cancer. 165:157–168. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xuhong J, Qi X, Tang P, Fan L, Chen L,
Zhang F, Tan X, Yan W, Zhong L, He C, et al: Neoadjuvant pyrotinib
plus trastuzumab and chemotherapy for stage I–III HER2-positive
breast cancer: A phase II clinical trial. Oncologist.
25:e1909–e1920. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding Y, Mo W, Xie X, Wang O, He X, Zhao S,
Gu X, Liang C, Qin C, Ding K, et al: Neoadjuvant pyrotinib plus
trastuzumab, docetaxel, and carboplatin in early or locally
advanced human epidermal receptor 2-positive breast cancer in
China: A multicenter, randomized, double-blind, placebo-controlled
phase 2 trial. Oncol Res Treat. 46:303–311. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu J, Jiang Z, Liu Z, Yang B, Yang H, Tang
J, Wang K, Liu Y, Wang H, Fu P, et al: Neoadjuvant pyrotinib,
trastuzumab, and docetaxel for HER2-positive breast cancer
(PHEDRA): A double-blind, randomized phase 3 trial. BMC Med.
20:4982022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi Q, Xuhong J, Luo T, Ge J, Liu F, Lan
Y, Chen Q, Tang P, Fan L, Chen L, et al: PIK3CA mutations are
associated with pathologic complete response rate to neoadjuvant
pyrotinib and trastuzumab plus chemotherapy for HER2-positive
breast cancer. Br J Cancer. 128:121–129. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guarneri V, Griguolo G, Miglietta F, Conte
PF, Dieci MV and Girardi F: Survival after neoadjuvant therapy with
trastuzumab-lapatinib and chemotherapy in patients with
HER2-positive early breast cancer: A meta-analysis of randomized
trials. ESMO Open. 7:1004332022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Yu Y, Lin Y, Kang S, Lv X, Liu Y,
Lin J, Wang J and Song C: P079-Efficacy and safety of neoadjuvant
therapy for HER2-positive early breast cancer: A network
meta-analysis. Breast. 56 (Suppl 1):S49–S50. 2021. View Article : Google Scholar
|
|
28
|
Gianni L, Pienkowski T, Im YH, Roman L,
Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J,
Im SA, et al: Efficacy and safety of neoadjuvant pertuzumab and
trastuzumab in women with locally advanced, inflammatory, or early
HER2-positive breast cancer (NeoSphere): A randomised multicentre,
open-label, phase 2 trial. Lancet Oncol. 13:25–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shao Z, Pang D, Yang H, Li W, Wang S, Cui
S, Liao N, Wang Y, Wang C, Chang YC, et al: Efficacy, safety, and
tolerability of pertuzumab, trastuzumab, and docetaxel for patients
with early or locally advanced ERBB2-positive breast cancer in
Asia: The PEONY phase 3 randomized clinical trial. JAMA Oncol.
6:e1936922020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hurvitz SA, Martin M, Symmans WF, Jung KH,
Huang CS, Thompson AM, Harbeck N, Valero V, Stroyakovskiy D,
Wildiers H, et al: Neoadjuvant trastuzumab, pertuzumab, and
chemotherapy versus trastuzumab emtansine plus pertuzumab in
patients with HER2-positive breast cancer (KRISTINE): A randomised,
open-label, multicentre, phase 3 trial. Lancet Oncol. 19:115–126.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schneeweiss A, Chia S, Hickish T, Harvey
V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai YF, Ratnayake J, et al:
Pertuzumab plus trastuzumab in combination with standard
neoadjuvant anthracycline-containing and anthracycline-free
chemotherapy regimens in patients with HER2-positive early breast
cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann
Oncol. 24:2278–2284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Sebille YZA, Gibson RJ, Wardill HR and
Bowen JM: ErbB small molecule tyrosine kinase inhibitor (TKI)
induced diarrhoea: Chloride secretion as a mechanistic hypothesis.
Cancer Treat Rev. 41:646–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yusta B, Holland D, Koehler JA, Maziarz M,
Estall JL, Higgins R and Drucker DJ: ErbB signaling is required for
the proliferative actions of GLP-2 in the murine gut.
Gastroenterology. 137:986–996. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian C, Wang M, Liu H, Liu J, Xu M and Ma
L: Efficacy and safety of neoadjuvant pyrotinib plus
docetaxel/liposomal doxorubicin/cyclophosphamide for HER2-positive
breast cancer. Ir J Med Sci. 192:1041–1049. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mao X, Lv P, Gong Y, Wu X, Tang P, Wang S,
Zhang D, You W, Wang O, Zhou J, et al: Pyrotinib-containing
neoadjuvant therapy in patients with HER2-positive breast cancer: A
multicenter retrospective analysis. Front Oncol. 12:8555122022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Q, Wang Y, Zhu M, Gu Y and Tang Y:
Clinical observation of neoadjuvant chemotherapy with pyrotinib
plus trastuzumab in HER2-positive breast cancer: A cohort study.
Gland Surg. 10:3389–3402. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao DS, Wang W, Chang JY, Zhang Y, Zhang
HW, Xu JX and Cai HF: Neoadjuvant pyrotinib plus nab-paclitaxel,
doxorubicin, and cyclophosphamide for HER2-positive locally
advanced breast cancer: A retrospective case-series study. Gland
Surg. 10:3362–3368. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Califano R, Tariq N, Compton S, Fitzgerald
DA, Harwood CA, Lal R, Lester J, McPhelim J, Mulatero C,
Subramanian S, et al: Expert consensus on the management of adverse
events from EGFR tyrosine kinase inhibitors in the UK. Drugs.
75:1335–1348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Swain SM, Shastry M and Hamilton E:
Targeting HER2-positive breast cancer: Advances and future
directions. Nat Rev Drug Discov. 22:101–126. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roy V and Perez EA: Beyond trastuzumab:
Small molecule tyrosine kinase inhibitors in HER-2-positive breast
cancer. Oncologist. 14:1061–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim JW, Lim AR, You JY, Lee JH, Song SE,
Lee NK, Jung SP, Cho KR, Kim CY and Park KH: PIK3CA mutation is
associated with poor response to HER2-targeted therapy in breast
cancer patients. Cancer Res Treat. 55:531–541. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Loibl S, Majewski I, Guarneri V,
Nekljudova V, Holmes E, Bria E, Denkert C, Schem C, Sotiriou C, Loi
S, et al: PIK3CA mutations are associated with reduced pathological
complete response rates in primary HER2-positive breast cancer:
Pooled analysis of 967 patients from five prospective trials
investigating lapatinib and trastuzumab. Ann Oncol. 27:1519–1525.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Loibl S, von Minckwitz G, Schneeweiss A,
Paepke S, Lehmann A, Rezai M, Zahm DM, Sinn P, Khandan F, Eidtmann
H, et al: PIK3CA mutations are associated with lower rates of
pathologic complete response to anti-human epidermal growth factor
receptor 2 (her2) therapy in primary HER2-overexpressing breast
cancer. J Clin Oncol. 32:3212–3220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J,
Luo Y, Xing P, Lan B, Li M, et al: Phase I study and biomarker
analysis of pyrotinib, a novel irreversible pan-erbb receptor
tyrosine kinase inhibitor, in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer. J Clin Oncol.
35:3105–3112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu W, Yang J, Zhang Z, Xu D and Li N:
Pyrotinib for HER2-positive metastatic breast cancer: A systematic
review and meta-analysis. Transl Cancer Res. 12:247–256. 2023.
View Article : Google Scholar : PubMed/NCBI
|