Introduction
Head and neck cancer is the eighth most common form
of neoplasia and the fifteenth most common cause of cancer-related
deaths in the UK (1). Oral cancer
is the most common form of head and neck cancer and most frequently
is of squamous histology (oral squamous cell carcinomas-OSCC). OSCC
includes cancers of the oral cavity including tongue throat, lips
and gums (2). Oral cancers are
often diagnosed in the advance stages of the disease, consequently,
decreasing the probability of curative treatment (3). A frequent precursor to oral cancer is
oral dysplasia (OD), which presents as white or red lesions
(leukoplakia or erythroplakia, respectively). However, ODs do not
always undergo malignant transformation (MT) and can remain benign.
Leukoplakia is the most prevalent and the rate of MT is 5–17%
(4,5). Tobacco and alcohol use are well known
risk factors of oropharyngeal squamous cell carcinoma (OPSCC)
(6–9), and are also primary risk factors of
developing OD (5,10).
Currently, the major challenge in the clinical
management of ODs is the accurate prediction of MT. The risk of MT
is graded as high/low through clinical observation. However, this
is subjective and often results in misdiagnosis (11). Surgical removal of precancerous
lesions can be performed as a preventative treatment, but it cannot
guarantee a lack of recurrence and can cause long-term morbidity
for patients, such as dysarthria and dysphagia (11,12).
The Liverpool Management Algorithm, provides OD management advice
based on the available evidence (13). However, accurate prediction of MT
remains elusive, pointing to the potential application of
chemoprevention in OD patients.
Histone acetylation
Cancer development is associated with genetic
mutations (14), as well as
epigenetic changes, which can alter chromatin structure (15). Histone tail acetylation is an
important epigenetic change, which is involved in the regulation of
gene expression (14,16). This is controlled by two enzymatic
groups; histone acetyltransferases (HATs) and histone deacetylases
(HDACs) (17,18). HATs transfer an acetyl group to the
lysine residue of the N-terminal of histones (19). This results in a relaxed chromatin
structure and expression activation. HDACs catalyse the hydrolytic
removal of acetyl, causing chromatin condensation and
transcriptional silencing (14,19).
HDACs also deacetylate non-histone proteins involved in the
regulation of cell-cycle progression, differentiation and apoptosis
(14). An imbalance between HATs
and HDACs activity is implicated in a number of human diseases,
such as neurodegenerative (20) and
cardiovascular diseases (21), and
cancer (22,23).
HDACs are divided into four classes: Class I (HDAC1,
HDAC2, HDAC3, HDAC8); Class II, which is subdivided into Class IIa
(HDAC4, HDAC5, HDAC7, HDAC9) and Class IIb (HDAC6, HDAC10); and
Class IV (HDAC11) (Table I)
(16). Class I, II and IV share a
common mechanism that requires zinc for their enzymatic activity.
Class III (sirtuins, SIRT1-7) are dependent on NAD+
rather than zinc. HDACs demonstrate a remarkable variability
regarding the processed RNA transcript splice variants and
consequent protein isoforms (Table
I) (https://www.ncbi.nlm.nih.gov/ and
http://www.rcsb.org/). This diversity creates
complex substrate specificity of HDACs and, therefore, produces a
diverse range of functions (18).
Furthermore, in addition to acetylation, HDACs can undergo
alternative post-translational modifications including,
methylation, phosphorylation and ubiquitination, which can alter
the enzymatic activity of HDACs in different ways. For example,
phosphorylation of HDAC1 increases its activity and phosphorylation
of Class IIa HDACs determines their cellular localisation (16). Overall, the different variable
factors mentioned, produce huge functional variability of HDACs
and, therefore, allow many possible opportunities for interference
with human diseases.
 | Table I.HDAC classification highlighting the
high variability of HDACs due to splice variance. |
Table I.
HDAC classification highlighting the
high variability of HDACs due to splice variance.
| HDAC class | Co-factor | No. of exons | No. of transcript
variants | Chromosome
location |
|---|
| Class I |
Zn2+ |
|
|
|
|
HDAC1 |
| 14 | 1 | 1p35.2-p35.1 |
|
HDAC2 |
| 14 | 3 | 6q21 |
|
HDAC3 |
| 15 | 10 | 5q31.3 |
|
HDAC8 |
| 11 | 7 | Xq13.1 |
| Class IIa |
Zn2+ |
|
|
|
|
HDAC4 |
| 26 | 5 | 2q37.3 |
|
HDAC5 |
| 26 | 3 | 17q21.31 |
|
HDAC7 |
| 25 | 6 | 12q13.11 |
|
HDAC9 |
| 10 | 40 | 7p21.1 |
| Class IIb |
Zn2+ |
|
|
|
|
HDAC6 |
| 28 | 11 | Xp11.23 |
|
HDAC10 |
| 20 | 2 | 22q13.33 |
| Class III |
NAD+ |
|
|
|
|
SIRT1 |
| 9 | 3 | 10q21.3 |
|
SIRT2 |
| 14 | 5 | 19q13.2 |
|
SIRT3 |
| 6 | 33 | 11p15.5 |
|
SIRT4 |
| 3 | 4 |
12q24.23-q24.31 |
|
SIRT5 |
| 8 | 26 | 6p23 |
|
SIRT6 |
| 7 | 9 | 19p13.3 |
|
SIRT7 |
| 10 | 1 | 17q25.3 |
| Class IV |
Zn2+ |
|
|
|
|
HDAC11 |
| 10 | 3 | 3p25.1 |
HDAC function
Class I HDACs are ubiquitously expressed and are
involved in cell proliferation and survival (24). HDAC1, HDAC2 and HDAC3 have
repressive functions, for example, HDAC1 and HDAC2 repress p21 and
p57, which are involved in the progression of the cell cycle
(25). Class II have more
tissue-specific functions than other HDACs (26). They freely shuttle between the
nucleus and cytoplasm, suggesting their interaction with
non-histone proteins. Localisation is determined by
phosphorylation, which also regulates transcriptional repression
capacity (24). For example, HDAC9
represses myocyte enhancer factor-2 until the enzyme receives a
signal to be transported to the cytoplasm. Class IIb HDACs are
structurally different to Class IIa, due to a second catalytic
domain (16). HDAC6 has a role in
the clearance of misfolded proteins, which makes it an important
target for Alzheimer's disease (20). Currently, little is known about the
function of Class IV HDACs.
HDAC inhibition and cancer
Acetylation is involved in the regulation of
important oncogenic mechanisms (24). Therefore, due to frequent increased
HDAC expression and activity in cancer, tumour formation is
promoted (14). However, the
expression pattern can differ between tumour types; high HDAC8
expression has been associated with poor prognosis of neuroblastoma
patients and HDAC1, HDAC2 and HDAC6 have been shown to be
upregulated in HNSCC (27,28). HDACs play a role in the silencing of
tumour suppressor genes, therefore, an increase in their activity
would exaggerate this function. Ultimately, this will result in
effects, such as cell-cycle persistence and apoptosis
reduction.
HDACs are promising targets for anti-cancer therapy,
specifically through utilisation of HDAC inhibitors (HDACis)
(24). Heterogeneity of HDAC
expression in tumour types, however, poses a challenge (29). HNSCC cells, specifically, have been
shown to have low levels of histone acetylation, suggesting that
HDACis may produce beneficial effects in patients (23,28).
There are five classes of HDACi; hydroxamic acids, short-chain
fatty acids, benzamides, cyclic tetrapeptides and sirtuin
inhibitors (24). Among these are
pan-HDACis, which inhibit all HDAC classes, while others exert
specificity against certain HDAC classes (29). HDACis that are currently clinically
approved include, Vorinostat (SAHA), Belinostat (PXD101),
Panobinostat (LBH589), Romidepsin (FK228), Chidamide
(CS055/HBI-8000), while there are more currently in clinical trials
(18).
Valproic acid and oral cancer therapy
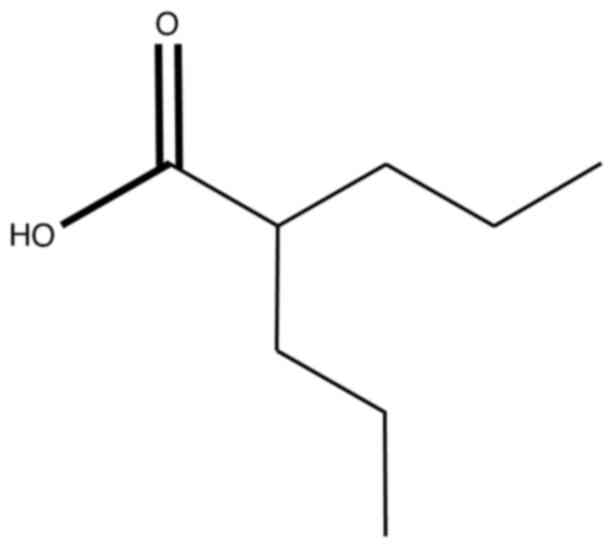
The short-chain fatty acid, valproic acid (VPA), is
currently under investigation in the treatment of cancer (Fig. 1) (23,30–32).
VPA is a well-established treatment for epilepsy and other
neurological diseases (33). VPA is
described to have various mechanisms of action contributing to its
anti-epileptic effects, however, these pathways are yet to be fully
understood. Suggested mechanisms include; inhibition of
voltage-gated sodium channels resulting in blockade of abnormal
electrical impulses responsible for seizures and interference with
gamma-aminobutyric acid (GABA) signalling through inhibition of
GABA transaminase or promotion of GABA synthesis, again, preventing
occurrence of seizures. More recently, it was reported that VPA is
a Class I and IIa HDACi, therefore, may be useful in anti-cancer
therapy (34,35). Binding studies suggest that VPA
exerts its HDAC inhibitory function through blockade of substrate
binding to the catalytic centre of HDAC enzymes (36). It is thought that this is via
interaction of the carboxyl group of VPA with Zn and other residues
of HDAC active sites (Fig. 1)
(37). A large-scale study
investigating long-term VPA treatment for psychiatric diseases in
US veterans, reported a significant association of VPA with a
reduced risk of HNSCC (38). This
same result was not observed for other tumour types, suggesting
that VPA may not be useful for all cancers. Consequently, VPA
presents as an encouraging treatment for HNSCC specifically.
Potent in vitro and in vivo growth
inhibition has been reported following VPA treatment (30,39,40).
VPA can inhibit the growth of HNSCC cells through upregulation of
p21 and induction of G0/G1 arrest (30), while similar results were found in
breast cancer cells (31). VPA
interferes with the self-renewal of HNSCC cancer stem cells and
suppresses expression of stem cell markers (39).
In addition to VPA use as a single agent, favourable
results are shown for its use in combination treatment of HNSCC
patients (32,38–40).
VPA is shown to potentiate the antitumour effect of cisplatin and
cetuximab in HNSCC xenografts (41). VPA may, therefore, sensitise cancer
cells to chemotherapeutics, improving their efficacy and
subsequently reducing the necessary dose, resulting in lower
toxicity and resistance.
The ongoing SAVER clinical trial investigates VPA as
a chemo-preventive epigenetic agent in individuals with high-risk
OD (42). This randomised,
double-blind, placebo-controlled trial measures the histological
and clinical response rate of OD to VPA. Therefore, determining its
use as a preventative treatment for MT of high-risk OD. A previous
study has reported HDAC2 upregulation in pre-cancerous ODs
(43), further supporting this
hypothesis. A mechanistic study is conducted in parallel to SAVER
to define the mechanism of action of VPA in HNSCC cells.
Questions surrounding the cellular responses and how
pathways are affected by HDAC inhibition remain unanswered. In
particular, the way HDACis influences the expression of their
target genes is not fully elucidated. It is possible that by-pass
and feedback loops may be in play, so that when cells are exposed
to HDACis, changes in expression levels of HDACs may be triggered
(44). In addition, the expression
levels of HDACs could potentially be used as markers of response to
HDACis in patients (45).
Therefore, understanding the specific expression patterns of HDACs
in cancers before and after HDACi treatment is important.
Valproic acid (VPA) has been considered a good
candidate for anticancer therapy. A reasonable option may be to
employ it as monotherapy (46) or
in combination (32,47) with other chemotherapeutic agents in
recurrent and/or metastatic squamous cell carcinoma of Head and
Neck (SCCHN) trials. Two studies reported changes in HDAC
expression with VPA in combination treatment (48,49). A
reduction in HDAC4 protein levels was found in a head and neck
cancer cell line when cells were treated with VPA in combination
with the tumour necrosis factor (TNF)-related apoptosis-inducing
ligand (TRAIL), compared to TRAIL treatment alone (49).
HDAC1 mRNA downregulation was reported in a human
cholangiocarcinoma cell line when VPA was used in combination with
gemcitabine (GEM), compared to GEM as a single agent (48). These findings indicate that VPA may
sensitise cells to other treatments, therefore, may be useful for
combination therapy.
HNSCC prognosis
In recent years, the prognosis and survival of HNSCC
have seen a minor improvement, however, the 5-year overall survival
rate remains low, at approximately 40–60% (5,50).
Early diagnosis of HNSCC is key to ensuring the best possible
outcome for patients and improves survival to 80% (28,51).
However, currently, there is a lack of prognostic and predictive
markers of HNSCC, which restricts early diagnosis. Therefore, the
majority of HNSCC cases are diagnosed in the later stages of the
disease and more aggressive treatment is necessary (52).
ODs are a common precursor to oral HNSCCs (13). However, the occurrence of these ODs
does not necessarily equate to cancer. There is a potential for the
lesions to undergo MT, with factors, such as tobacco use,
increasing the probability (5).
Therefore, prediction and prevention of the transformation of
precancerous ODs are extremely important to increase the survival
of HNSCC. Currently, the methods to do this are surgery or the
prediction of cancerous lesions by observation. However, surgery
often leads to long-term issues for patients and misdiagnosis is
common with observation (53).
Therefore, there is an unmet clinical need for better prevention or
prediction of MT to reduce oral cancer cases.
Cancer chemoprevention by HDAC
inhibition
Due to developments in research, it is now known
that cancer development not only arises due to genetic alterations
but can also arise from changes in epigenetic mechanisms as well
(29,54). Acetylation is a crucial histone
modification that has an important role in chromatin remodelling.
Interruptions to the balance of HATs and HDACs, leading to hyper or
hypoacetylation of histone and non-histone proteins, has been shown
to be implicated in a number of human diseases (21,27).
In particular, HDACs involvement with cancer has been highlighted
in a number of studies, with results indicating that HDAC
expression is increased in certain cancers (19,55,56).
This is a significant alteration due to multiple functions of HDACs
implicating tumour progression mechanisms. For example, Class I
HDACs repress the transcription of the cell-cycle inhibitor, p21
(24). Consequently, if HDACs are
overexpressed, this may contribute to the uncontrolled
proliferation of cells. Moreover, Chang et al reported that
HDAC2 expression is upregulated in oral pre-malignant lesions,
suggesting that HDACis could be used for chemoprevention in oral
cancers (43).
VPA modulates HDACs in HNSCC
The recent discovery of HDAC inhibition for cancer
treatment has seen the approval of five HDACis for clinical use
(24). Compared with traditional
anti-cancer therapies, HDACis offer a much-improved toxicity
profile, due to minimal effects on normal cells (16). Although clinically manageable, there
are still toxicities associated with HDACis, including
thrombocytopenia, nausea and vomiting. However, VPA, which is in
phase II trials, does not exhibit these side effects and it is
known that long-term use is tolerable for patients due to its
well-established use as an anti-epileptic (32). Therefore, in addition to the
encouraging in vitro and in vivo evidence, VPA
appears to be an attractive anti-cancer agent (30,31,41,57).
Furthermore, the epidemiological study by Kang et al
suggests that VPA treatment is associated with a lower risk of
HNSCC development and, therefore, may be a suitable candidate for
treatment and/or chemoprevention (38).
Due to the plethora of targets and functions exerted
by HDACs, HDACis can act in multiple different ways (44). In addition, the mechanism of action
by which HDACis act differs according to the cancer being treated
and the inhibitor being used. Therefore, there is still much
unknown about the biological mechanisms of HDACis (22,58).
Understanding the precise mechanisms of action is key to
elucidating which cancers are best treated by HDACis and the
specificity of which inhibitor for which tumour type. For example,
VPA was found to be effective in reducing the incidence of HNSCC,
but not the incidence of lung cancer (38). Therefore, understanding why this
happens will allow improved treatment strategies.
The present review aimed to explicate if HDACis
alter the expression pattern of HDACs and, in particular, whether
VPA could alters the expression of HDACs in oral cancer. From the
studies presented, it is clear that HDACis can regulate the
expression levels of HDACs both at the mRNA and protein level.
However, the reported changes vary between studies. This is likely
due to the involvement of different HDACs per cancer, which may
alter the outcomes produced by each HDACi (29). The HDACi, apicidin, has been
investigated in three different cancers across three studies
(59–61). The variation in results demonstrates
the theory that different HDACs are involved between cancers,
which, therefore, alters the inhibitory effects of individual
HDACs. In addition to differing levels of upregulation, HDACs can
also harbour mutations, which vary greatly in frequency between
cancers (62). For example, lung
cancer and melanoma have a high percentage of mutations in all
HDACs, whereas, ovarian and glioblastoma have very few. These
factors, in addition to the different targets of HDACis, may
explain the variety of results found here.
The majority of studies reviewed herein report a
decrease in HDAC expression following VPA treatment. However, in
some cases, an upregulation of HDACs is seen. This may be due to a
compensatory mechanism against HDAC inhibition that has been
previously described (63). This
has been suggested for HDAC1 and HDAC2 where one HDAC is
downregulated to enable the upregulation of the other. However,
some of the studies reviewed only investigated one HDAC, making it
difficult to determine if a compensatory mechanism is in place. In
the studies that investigated multiple HDACs, fluctuations of
different HDACs expression are observed. In 19i-treated UC cells, a
decrease in HDAC7 is reported, whilst there is an increase in HDAC4
(64). This is due to 19i
demonstrating preferential inhibition against HDAC4 but not HDAC7.
Therefore, the decrease in HDAC7 allows increases in HDAC4
expression to combat the loss of function. Not only does this
suggest a compensatory mechanism, but it also implies that HDACs
can regulate the expression of one another. In addition, this
indicates that HDAC inhibition may alter the de novo
synthesis of HDACs to counteract the loss of function. These
results support the hypothesis that HDAC inhibition triggers
feedback for the expression control of HDAC genes.
Role of VPA in HNSCC treatment
The limited number of studies available reflects our
largely incomplete understanding of the different HDAC expression
changes and their functional consequences in the wider spectrum of
tumour types. Among the studies reviewed, only five cancer types
were investigated more than once, making it difficult to validate
results. Therefore, there is a critical need for more research to
determine the differential expression of HDACs and how inhibitors
affect them in individual cancers, especially in those that lack
well-defined prognostic factors that lead to poorer therapeutic
management, such as HNSCC (52).
Conclusion
HDACs expression has significant clinical impact in
oral cancer (65). Although the
search conducted here reviewing several studies reported the
therapeutic role of VPA in treating oral cancers, only one study
has clearly reported that lowering HDAC expression following VPA
treatment has induced cellular death in oral cancer (49). In addition, we reviewed few studies
that reported its use in combination treatment of HNSCC rather than
alone. Nevertheless, the studies have not investigated the
inhibitory changes in HDAC expression after VPA was involved in the
therapeutic regimen. Another study demonstrated reduced HDAC7
expression in oral cancer after HDACi treatment (59), providing further evidence that HDAC
inhibition may have an effect on HDAC expression in oral cancers.
Clearly, there is a crucial need for further research into HDAC
expression changes in oral cancers treated with HDACis, especially,
since reports of the specific efficacy of VPA in this tumour type
(38). Therefore, due to the
evidence of HDAC upregulation in pre-cancerous lesions and oral
cancers, additional efforts should be given to further clarify the
changes and the epigenetic landscape caused by VPA treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ASKAK made substantial contributions to the
conception and design of the review and drafted the work. LMW
contributed to the acquisition, analysis and interpretation of
literature data, and revised the manuscript. HHA contributed to the
acquisition of literature data, interpreted the data and revised
the manuscript. TL participated in the acquisition and
interpretation of literature data and revised the manuscript. All
the authors have read and approved the final version of the
manuscript for publication. Data authentication is not
required.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Authors' information
Dr Ahmed S. K. Al-Khafaji, ORCID ID:
0000-0002-6802-5816; Miss Lydia M. Wang, ORCID ID:
0009-0004-8072-0418; Dr Haidar H. Alabdei, ORCID ID:
0000-0003-1960-7331; Dr Triantafillos Liloglou, ORCID ID:
0000-0003-0460-1404.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
OD
|
oral dysplasia
|
|
MT
|
malignant transformation
|
|
HAT
|
histone acetyltransferase
|
|
HDAC
|
histone deacetylase
|
|
HDACi
|
histone deacetylase inhibitor
|
|
VPA
|
valproic acid
|
|
TRAIL
|
TNF-related apoptosis-inducing
ligand
|
|
GEM
|
gemcitabine
|
|
UC
|
urothelial carcinoma
|
References
|
1
|
Cancer Research UK, . Head and neck
cancers statistics. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers#heading-OneFebruary
16–2024
|
|
2
|
National Cancer Institute, . Head and Neck
Cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheetFebruary
16–2024
|
|
3
|
Ranganathan K and Kavitha L: Oral
epithelial dysplasia: Classifications and clinical relevance in
risk assessment of oral potentially malignant disorders. J Oral
Maxillofac Pathol. 23:19–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mello FW, Miguel AFP, Dutra KL, Porporatti
AL, Warnakulasuriya S, Guerra ENS and Rivero ERC: Prevalence of
oral potentially malignant disorders: A systematic review and
meta-analysis. J Oral Pathol Med. 47:633–640. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rhodus NL, Kerr AR and Patel K: Oral
Cancer: Leukoplakia, premalignancy, and squamous cell carcinoma.
Dent Clin North Am. 58:315–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen TC, Wu CT, Ko JY, Yang TL, Lou PJ,
Wang CP and Chang YL: Clinical characteristics and treatment
outcome of oropharyngeal squamous cell carcinoma in an endemic
betel quid region. Sci Rep. 10:5262020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee KW, Kuo WR, Tsai SM, Wu DC, Wang WM,
Fang FM, Chiang FY, Ho KY, Wang LF, Tai CF, et al: Different impact
from betel quid, alcohol and cigarette: Risk factors for pharyngeal
and laryngeal cancer. Int J Cancer. 117:831–836. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saito Y, Ebihara Y, Ushiku T, Omura G,
Kobayashi K, Ando M, Sakamoto T, Fukayama M, Yamasoba T and Asakage
T: Negative human papillomavirus status and excessive alcohol
consumption are significant risk factors for second primary
malignancies in Japanese patients with oropharyngeal carcinoma. Jpn
J Clin Oncol. 44:564–569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Khafaji ASK, Pantazi P, Acha-Sagredo A,
Schache A, Risk JM, Shaw RJ and Liloglou T: Overexpression of HURP
mRNA in head and neck carcinoma and association with in
vitro response to vinorelbine. Oncol Lett. 19:2502–2507.
2020.PubMed/NCBI
|
|
10
|
Lewin F, Norell SE, Johansson H,
Gustavsson P, Wennerberg J, Biörklund A and Rutqvist LE: Smoking
tobacco, oral snuff, and alcohol in the etiology of squamous cell
carcinoma of the head and neck. Cancer. 82:1367–1375. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balasundaram I, Payne KFB, Al-Hadad I,
Alibhai M, Thomas S and Bhandari R: Is there any benefit in surgery
for potentially malignant disorders of the oral cavity? J Oral
Pathol Med. 43:239–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van der Waal I: Potentially malignant
disorders of the oral and oropharyngeal mucosa; terminology,
classification and present concepts of management. Oral Oncol.
45:317–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Field EA, McCarthy CE, Ho MW, Rajlawat BP,
Holt D, Rogers SN, Triantafyllou A, Field JK and Shaw RJ: The
management of oral epithelial dysplasia: The Liverpool algorithm.
Oral Oncol. 51:883–887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ropero S and Esteller M: The role of
histone deacetylases (HDACs) in human cancer. Mol Oncol. 1:19–25.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perri F, Longo F, Giuliano M, Sabbatino F,
Favia G, Ionna F, Addeo R, Della Vittoria Scarpati G, Di Lorenzo G
and Pisconti S: Epigenetic control of gene expression: Potential
implications for cancer treatment. Crit Rev Oncol Hematol.
111:166–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seto E and Yoshida M: Erasers of histone
acetylation: The histone deacetylase enzymes. Cold Spring Harb
Perspect Biol. 6:a0187132014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Delcuve GP, Khan DH and Davie JR: Roles of
histone deacetylases in epigenetic regulation: Emerging paradigms
from studies with inhibitors. Clin Epigenetics. 4:52021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sixto-López Y, Bello M and Correa-Basurto
J: Exploring the inhibitory activity of valproic acid against the
HDAC family using an MMGBSA approach. J Comput Aided Mol Des.
34:857–878. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parbin S, Kar S, Shilpi A, Sengupta D, Deb
M, Rath SK and Patra SK: Histone Deacetylases: A saga of perturbed
acetylation homeostasis in cancer. J Histochem Cytochem. 62:11–33.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu X, Wang L, Yu C, Yu D and Yu G: Histone
acetylation modifiers in the pathogenesis of Alzheimer's Disease.
Front Cell Neurosci. 9:2262015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Miao X, Liu Y, Li F, Liu Q, Sun J
and Cai L: Dysregulation of histone acetyltransferases and
deacetylases in cardiovascular diseases. Oxid Med Cell Longev.
2014:6419792014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SY and Kim JS: A short guide to
histone deacetylases including recent progress on class II enzymes.
Exp Mol Med. 52:204–212. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He L, Gao L, Shay C, Lang L, Lv F and Teng
Y: Histone deacetylase inhibitors suppress aggressiveness of head
and neck squamous cell carcinoma via histone
acetylation-independent blockade of the EGFR-Arf1 axis. J Exp Clin
Cancer Res. 38:842019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verza FA, Das U, Fachin AL, Dimmock JR and
Marins M: Roles of histone deacetylases and inhibitors in
anticancer therapy. Cancers (Basel). 12:16642020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi T, Cubizolles F, Zhang Y,
Reichert N, Kohler H, Seiser C and Matthias P: Histone deacetylases
1 and 2 act in concert to promote the G1-to-S progression. Genes
Dev. 24:455–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haberland M, Montgomery RL and Olson EN:
The many roles of histone deacetylases in development and
physiology: Implications for disease and therapy. Nat Rev Genet.
10:32–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oehme I, Deubzer HE, Wegener D, Pickert D,
Linke JP, Hero B, Kopp-Schneider A, Westermann F, Ulrich SM, von
Deimling A, et al: Histone deacetylase 8 in neuroblastoma
tumorigenesis. Clin Cancer Res. 15:91–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar B, Yadav A, Lang J, Teknos T and
Kumar P: Suberoylanilide hydroxamic acid (SAHA) reverses
chemoresistance in head and neck cancer cells by targeting cancer
stem cells via the downregulation of nanog. Genes Cancer.
6:169–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eckschlager T, Plch J, Stiborova M and
Hrabeta J: Histone deacetylase inhibitors as anticancer drugs. Int
J Mol Sci. 18:14142017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gan CP, Hamid S, Hor SY, Zain RB, Ismail
SM, Wan Mustafa WM, Teo SH, Saunders N and Cheong SC: Valproic
acid: Growth inhibition of head and neck cancer by induction of
terminal differentiation and senescence. Head Neck. 34:344–353.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma XJ, Wang YS, Gu WP and Zhao X: The role
and possible molecular mechanism of valproic acid in the growth of
MCF-7 breast cancer cells. Croat Med J. 58:349–357. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Caponigro F, Di Gennaro E, Ionna F, Longo
F, Aversa C, Pavone E, Maglione MG, Di Marzo M, Muto P, Cavalcanti
E, et al: Phase II clinical study of valproic acid plus cisplatin
and cetuximab in recurrent and/or metastatic squamous cell
carcinoma of Head and Neck-V-CHANCE trial. BMC Cancer. 16:9182016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blaheta RA and Cinatl J Jr: Anti-tumor
mechanisms of valproate: A novel role for an old drug. Med Res Rev.
22:492–511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Phiel CJ, Zhang F, Huang EY, Guenther MG,
Lazar MA and Klein PS: Histone deacetylase is a direct target of
valproic acid, a potent anticonvulsant, mood stabilizer, and
teratogen. J Biol Chem. 276:36734–36741. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gurvich N, Tsygankova OM, Meinkoth JL and
Klein PS: Histone deacetylase is a target of valproic acid-mediated
cellular differentiation. Cancer Res. 64:1079–1086. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Göttlicher M, Minucci S, Zhu P, Krämer OH,
Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG
and Heinzel T: Valproic acid defines a novel class of HDAC
inhibitors inducing differentiation of transformed cells. EMBO J.
20:6969–6978. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun J, Piao J, Li N, Yang Y, Kim KY and
Lin Z: Valproic acid targets HDAC1/2 and HDAC1/PTEN/Akt signalling
to inhibit cell proliferation via the induction of autophagy in
gastric cancer. FEBS J. 287:2118–2133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang H, Gillespie TW, Goodman M, Brodie
SA, Brandes M, Ribeiro M, Ramalingam SS, Shin DM, Khuri FR and
Brandes JC: Long-term use of valproic acid in US veterans is
associated with a reduced risk of smoking-related cases of head and
neck cancer. Cancer. 120:1394–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee SH, Nam HJ, Kang HJ, Samuels TL,
Johnston N and Lim YC: Valproic acid suppresses the self-renewal
and proliferation of head and neck cancer stem cells. Oncol Rep.
34:2065–2071. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Erlich RB, Rickwood D, Coman WB, Saunders
NA and Guminski A: Valproic acid as a therapeutic agent for head
and neck squamous cell carcinomas. Cancer Chemother Pharmacol.
63:381–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Iannelli F, Zotti AI, Roca MS, Grumetti L,
Lombardi R, Moccia T, Vitagliano C, Milone MR, Ciardiello C,
Bruzzese F, et al: Valproic acid synergizes with cisplatin and
cetuximab in vitro and in vivo in head and neck cancer by targeting
the mechanisms of resistance. Front Cell Dev Biol. 8:7322020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liverpool Clinical Trials Centre (LCTC), .
SAVER: Sodium Valproate for the Epigenetic Reprogramming of
High-Risk Oral Epithelial Dysplasia. 2019.https://www.lctc.org.uk/research/saver/
|
|
43
|
Chang HH, Chiang CP, Hung HC, Lin CY, Deng
YT and Kuo MY: Histone deacetylase 2 expression predicts poorer
prognosis in oral cancer patients. Oral Oncol. 45:610–614. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hull EE, Montgomery MR and Leyva KJ: HDAC
inhibitors as epigenetic regulators of the immune system: Impacts
on cancer therapy and inflammatory diseases. Biomed Res Int.
2016:87972062016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dokmanovic M, Perez G, Xu W, Ngo L, Clarke
C, Parmigiani RB and Marks PA: Histone deacetylase inhibitors
selectively suppress expression of HDAC7. Mol Cancer Ther.
6:2525–2534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McCarthy C, Sacco J, Fedele S, Ho M,
Porter S, Liloglou T, Greenhalf B, Robinson M, Young B, Cicconi S,
et al: SAVER: Sodium valproate for the epigenetic reprogramming of
high-risk oral epithelial dysplasia-a phase II randomised control
trial study protocol. Trials. 22:4282021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stauber RH, Knauer SK, Habtemichael N,
Bier C, Unruhe B, Weisheit S, Spange S, Nonnenmacher F, Fetz V,
Ginter T, et al: A combination of a ribonucleotide reductase
inhibitor and histone deacetylase inhibitors downregulates EGFR and
triggers BIM-dependent apoptosis in head and neck cancer.
Oncotarget. 3:31–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Iwahashi S, Shimada M, Utsunomiya T,
Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J and Saito Y:
Histone deacetylase inhibitor enhances the anti-tumor effect of
gemcitabine: A special reference to gene-expression microarray
analysis. Oncol Rep. 26:1057–1062. 2011.PubMed/NCBI
|
|
49
|
Lee BS, Kim YS, Kim HJ, Kim DH, Won HR,
Kim YS and Kim CH: HDAC4 degradation by combined TRAIL and valproic
acid treatment induces apoptotic cell death of TRAIL-resistant head
and neck cancer cells. Sci Rep. 8:125202018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
WHO, . HEAD AND NECK CANCER: 2014 Review
of Cancer Medicines on the WHO List of Essential Medicines. Union
for International Cancer Control; 2014
|
|
51
|
Ho MW, Field EA, Field JK, Risk JM,
Rajlawat BP, Rogers SN, Steele JC, Triantafyllou A, Woolgar JA,
Lowe D and Shaw RJ: Outcomes of oral squamous cell carcinoma
arising from oral epithelial dysplasia: Rationale for monitoring
premalignant oral lesions in a multidisciplinary clinic. Br J Oral
Maxillofac Surg. 51:594–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cadoni G, Giraldi L, Petrelli L,
Pandolfini M, Giuliani M, Paludetti G, Pastorino R, Leoncini E,
Arzani D, Almadori G and Boccia S: Prognostic factors in head and
neck cancer: A 10-year retrospective analysis in a
single-institution in Italy. Acta Otorhinolaryngol Ital.
37:458–466. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dost F, Lê Cao K, Ford PJ, Ades C and
Farah CS: Malignant transformation of oral epithelial dysplasia: A
real-world evaluation of histopathologic grading. Oral Surg Oral
Med Oral Pathol Oral Radiol. 117:343–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Spurling CC, Godman CA, Noonan EJ,
Rasmussen TP, Rosenberg DW and Giardina C: HDAC3 overexpression and
colon cancer cell proliferation and differentiation. Mol Carcinog.
47:137–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hayashi A, Horiuchi A, Kikuchi N, Hayashi
T, Fuseya C, Suzuki A, Konishi I and Shiozawa T: Type-specific
roles of histone deacetylase (HDAC) overexpression in ovarian
carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates
cell migration with downregulation of E-cadherin. Int J Cancer.
127:1332–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sang Z, Sun Y, Ruan H, Cheng Y, Ding X and
Yu Y: Anticancer effects of valproic acid on oral squamous cell
carcinoma via SUMOylation in vivo and in vitro. Exp
Ther Med. 12:3979–3987. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Marks PA: Histone deacetylase inhibitors:
A chemical genetics approach to understanding cellular functions.
Biochim Biophys Acta. 1799:717–725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ahn MY and Yoon JH: Histone deacetylase 7
silencing induces apoptosis and autophagy in salivary
mucoepidermoid carcinoma cells. J Oral Pathol Med. 46:276–283.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ahn MY, Chung HY, Choi WS, Lee BM, Yoon S
and Kim HS: Anti-tumor effect of apicidin on Ishikawa human
endometrial cancer cells both in vitro and in vivo by
blocking histone deacetylase 3 and 4. Int J Oncol. 36:125–131.
2010.PubMed/NCBI
|
|
61
|
Ahn MY, Kang DO, Na YJ, Yoon S, Choi WS,
Kang KW, Chung HY, Jung JH, Min do S and Kim HS: Histone
deacetylase inhibitor, apicidin, inhibits human ovarian cancer cell
migration via class II histone deacetylase 4 silencing. Cancer
Lett. 325:189–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ceccacci E and Minucci S: Inhibition of
histone deacetylases in cancer therapy: Lessons from leukaemia. Br
J Cancer. 114:605–611. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jurkin J, Zupkovitz G, Lagger S,
Grausenburger R, Hagelkruys A, Kenner L and Seiser C: Distinct and
redundant functions of histone deacetylases HDAC1 and HDAC2 in
proliferation and tumorigenesis. Cell Cycle. 10:406–412. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kaletsch A, Pinkerneil M, Hoffmann MJ,
Jaguva Vasudevan AA, Wang C, Hansen FK, Wiek C, Hanenberg H,
Gertzen C, Gohlke H, et al: Effects of novel HDAC inhibitors on
urothelial carcinoma cells. Clin Epigenetics. 10:1002018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pouloudi D, Manou M, Sarantis P, Tsoukalas
N, Tsourouflis G, Dana E, Karamouzis MV, Klijanienko J and
Theocharis S: Clinical significance of histone deacetylase
(HDAC)-1, −2, −4 and −6 Expression in salivary gland tumors.
Diagnostics (Basel). 11:5172021. View Article : Google Scholar : PubMed/NCBI
|