Introduction
Colorectal cancer (CRC) is a prevalent malignant
tumor worldwide, with significant mortality rates (1,2).
Unfortunately, multiple patients are diagnosed with locally
advanced CRC at the time of initial diagnosis (3,4). In
such cases, neoadjuvant therapy plays a crucial role in providing
patients with more opportunities for subsequent surgical resection
and improving their long-term survival outcomes (5–7).
Currently, the primary neoadjuvant regimen for patients with
locally advanced CRC involves platinum-based chemotherapy, such as
capecitabine plus oxaliplatin (XELOX) and fluorouracil (8,9).
Nonetheless, the effectiveness of these treatment protocols is
deemed unsatisfactory (10).
Consequently, it is imperative to devise alternative neoadjuvant
regimens to manage patients with locally advanced CRC.
As an oral inhibitor of vascular endothelial growth
factor receptor-2 (VEGFR2), apatinib possesses anti-angiogenic
properties that are considered to regulate angiogenesis and
β-catenin signaling, thereby inhibiting CRC cell proliferation,
migration and invasion (11).
Previous studies have established the efficacy and safety of
combining apatinib with chemotherapy for the therapy of patients
with advanced CRC (12,13). For instance, a meta-analysis has
demonstrated that the combination of apatinib and chemotherapy
yields a favorable objective response rate (ORR), disease control
rate (DCR), and survival rate with manageable adverse reactions
among patients with advanced CRC (12). Furthermore, another study has
indicated that the combination of apatinib and chemotherapy
enhances progression-free survival and exhibits an acceptable
tolerance in patients with refractory metastatic CRC (13). Nevertheless, there is a dearth of
pertinent evidence concerning neoadjuvant apatinib in combination
with XELOX in patients with locally advanced CRC.
The purpose of the present study was to investigate
radiological response, pathological response, survival outcomes and
adverse events in patients diagnosed with locally advanced CRC who
underwent neoadjuvant treatment with apatinib and XELOX.
Patients and methods
Patients
A retrospective analysis was conducted on a total of
100 patients with locally advanced CRC who received treatment at
The Affiliated Hospital of Hebei University (Baoding, China)
between January 2017 and January 2019. The inclusion criteria
contained: i) Patients who were histologically or cytologically
confirmed to have CRC; ii) had a clinical stage of cT3-4b/N + /M0
for patients with rectal cancer or cT4b/N + /M0 for patients with
colon cancer, which was appraised by computed tomography (CT) or
magnetic resonance imaging; iii) >18 years old; iv) the eastern
cooperative oncology group performance status (ECOG PS) score of
0–1; v) received surgical resection; vi) had accessible and
available clinical data for study analysis. The exclusion criteria
contained: i) Had severe infections; ii) had severe dysfunctions of
the liver or kidney; iii) had coagulation disorders; iv) had severe
heart failures; v) had uncontrollable hypertensive diseases.
Clinical characteristics of patients (including sex and age
distribution) are included in Table
I. The present study was approved by (approval no.
ChiECRCT20210395) by the Ethics Committee of The Affiliated
Hospital of Hebei University (Baoding, China). Written informed
consent was provided by each patient or their guardian (if the
patient died).
 | Table I.Clinical characteristics. |
Table I.
Clinical characteristics.
| Clinical
characteristics | XELOX group
(n=50) | Apatinib plus XELOX
group (n=50) | P-value |
|---|
| Age, years (%) |
|
| 0.110 |
|
<65 | 21 (42.0) | 29 (58.0) |
|
| ≥65 | 29 (58.0) | 21 (42.0) |
|
| Sex, n (%) |
|
| 0.414 |
|
Female | 22 (44.0) | 18 (36.0) |
|
| Male | 28 (56.0) | 32 (64.0) |
|
| BMI, n (%) |
|
| 0.841 |
| <24
kg/m2 | 25 (50.0) | 24 (48.0) |
|
| ≥24
kg/m2 | 25 (50.0) | 26 (52.0) |
|
| ECOG PS score, n
(%) |
|
| 0.841 |
| 0 | 22 (44.0) | 23 (46.0) |
|
| 1 | 28 (56.0) | 27 (54.0) |
|
| Diagnosis, n
(%) |
|
| 0.275 |
| Colon
cancer | 6 (12.0) | 10 (20.0) |
|
| Rectal
cancer | 44 (88.0) | 40 (80.0) |
|
| Lesion site, n
(%) |
|
| 0.204 |
|
Left | 49 (98.0) | 45 (90.0) |
|
|
Right | 1 (2.0) | 5 (10.0) |
|
| Differentiation, n
(%) |
|
| 0.086 |
|
Well | 6 (12.0) | 10 (20.0) |
|
|
Moderate | 36 (72.0) | 37 (74.0) |
|
|
Poor | 8 (16.0) | 3 (6.0) |
|
| Distance of tumor
from anus, n (%) |
|
| 0.509 |
| ≤5
cm | 16 (32.0) | 13 (26.0) |
|
| >5
cm | 34 (68.0) | 37 (74.0) |
|
| Vascular invasion,
n (%) |
|
| 0.298 |
| No | 39 (78.0) | 43 (86.0) |
|
|
Yes | 11 (22.0) | 7 (14.0) |
|
| Perineural
invasion, n (%) |
|
| 1.000 |
| No | 44 (88.0) | 44 (88.0) |
|
|
Yes | 6 (12.0) | 6 (12.0) |
|
| cTNM stage, n
(%) |
|
| 0.812 |
|
IIIB | 39 (78.0) | 38 (76.0) |
|
|
IIIC | 11 (22.0) | 12 (24.0) |
|
| PDC, n (%) |
|
| 0.221 |
| Low
(0–4) | 17 (34.0) | 23 (46.0) |
|
| High
(≥5) | 33 (66.0) | 27 (54.0) |
|
| TB, n (%) |
|
| 0.028 |
| Low
(0–4) | 18 (36.0) | 29 (58.0) |
|
| High
(≥5) | 32 (64.0) | 21 (42.0) |
|
Data collection and treatment
Patient clinical characteristics, biochemical
indices and treatment data were collected, along with poorly
differentiated clusters (PDC) and tumor budding (TB) measurements.
PDC was categorized as low (0–4) or high (≥5), while TB was
classified as low (0–4 buds) or high (≥5 buds) based on the
International Consortium on TB Recommendations (14,15).
Patients were stratified into two groups based on their neoadjuvant
regimens: The XELOX group and the apatinib plus XELOX group.
Neoadjuvant therapy was administered for three cycles, with each
cycle lasting 21 days. The standard regimens for the XELOX group
were as follows: For the XELOX group, 130 mg/m2 XELOX
was administered intravenously on day 1, 1.0 g/m2
capecitabine was given orally 2 times/day for 14 days with a
7-day-off; for the apatinib plus XELOX group, apatinib was given
orally at 0.25 g/day on the basis of XELOX. The dose of apatinib
was determined referring to the instruction, and the dose of
neoadjuvant XELOX was determined referring to the clinical
guidelines (16). Moreover,
surgical information (laparoscopic radical resection or radical
resection) was collected based on an assessment at 4–5 weeks after
discontinuing neoadjuvant therapy.
Assessment
The imaging data obtained from patients after
neoadjuvant treatment were utilized to assess clinical response
based on Response Evaluation Criteria in Solid Tumors version 1.1
(RECIST v.1.1) and evaluated post-neoadjuvant pathologic
tumor-node-metastasis (ypTNM) (17). Furthermore, the Becker's grading
system was employed to evaluate tumor regression grade (TRG) based
on surgery information, which was graded as 0, 1, 2, and 3
(18). Major pathological response
was defined as grade 0–1 of TRG. Additionally, follow-up
information was collected to appraise disease-free survival (DFS)
and overall survival (OS) of patients. Additionally, adverse events
were detected. The primary outcome of the present study was
DFS.
Statistical analysis
SPSS v20.0 (IBM Corp.) was utilized for analyses.
GraphPad Prism v7.02 (Dotmatics) was utilized for plotting.
Comparison analyses were conducted using the Chi-square test and
Wilcoxon rank sum test. Factors related to ORR and major
pathological response were screened using forward-stepwise
multivariate logistic regression models. Survival information was
shown using Kaplan-Meier curves with a log-rank test. Factors
linked with DFS and OS were determined using forward-stepwise and
enter method multivariate Cox's proportional hazard regression
models. P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline characteristics
The baseline traits of the present study population
were analyzed. The apatinib plus XELOX group comprised 18 (36.0%)
female and 32 (64.0%) male patients, with 29 (58.0%) patients
<65 and 21 (42.0%) patients aged ≥65 years. The XELOX group
consisted of 22 (44.0%) female and 28 (56.0%) male patients, with
21 (42.0%) patients <65 and 29 (58.0%) patients ≥65 years. In
the apatinib plus XELOX group, there were 45 (90.0%) patients with
left lesions and 5 (10.0%) patients with right lesions. In the
XELOX group, there were 49 (98.0%) patients with left lesions and 1
(2.0%) patient with right lesions. The majority of clinical
characteristics were similar between groups (all P>0.05), except
that the proportion of patients with high TB in the apatinib plus
XELOX group was lower than the proportion of patients with high TB
in the XELOX group (42.0 vs. 64.0%) (P=0.028). A more detailed
description of the two groups is presented in Table I.
Comparison of neutrophil-to-lymphocyte
ratio (NLR), platelet-to-lymphocyte ratio (PLR), and
carcinoembryonic antigen (CEA) levels between cohorts
Prior to neoadjuvant therapy, no significant
difference was illustrated between groups regarding abnormal NLR
(14.0 vs. 6.0%) (P=0.182) or abnormal PLR (60.0 vs. 46.0%)
(P=0.161), but the proportion of patients with abnormal CEA was
found to be higher in the apatinib plus XELOX group vs. the XELOX
group (58.0 vs. 36.0%) (P=0.028). Following neoadjuvant therapy, no
significant discrepancy was observed between groups in abnormal NLR
(16.0 vs. 30.0%) (P=0.096), abnormal PLR (48.0 vs. 44.0%)
(P=0.688), and abnormal CEA (38.0 vs. 24.0%) (P=0.130) between
cohorts (Table II).
 | Table II.Levels of NLR, PLR and CEA. |
Table II.
Levels of NLR, PLR and CEA.
|
| Before neoadjuvant
therapy | After neoadjuvant
therapy |
|---|
|
|
|
|
|---|
| Items | XELOX group
(n=50) | Apatinib plus XELOX
group (n=50) | P-value | XELOX group
(n=50) | Apatinib plus XELOX
group (n=50) | P-value |
|---|
| NLR, n (%) |
|
| 0.182 |
|
| 0.096 |
|
Normal | 47 (94.0) | 43 (86.0) |
| 35 (70.0) | 42 (84.0) |
|
|
Abnormal | 3 (6.0) | 7 (14.0) |
| 15 (30.0) | 8 (16.0) |
|
| PLR, n (%) |
|
| 0.161 |
|
| 0.688 |
|
Normal | 27 (54.0) | 20 (40.0) |
| 28 (56.0) | 26 (52.0) |
|
|
Abnormal | 23 (46.0) | 30 (60.0) |
| 22 (44.0) | 24 (48.0) |
|
| CEA, n (%) |
|
| 0.028 |
|
| 0.130 |
|
Normal | 32 (64.0) | 21 (42.0) |
| 38 (76.0) | 31 (62.0) |
|
|
Abnormal | 18 (36.0) | 29 (58.0) |
| 12 (24.0) | 19 (38.0) |
|
Radiological and pathological
comparisons between cohorts
The findings of the present study indicated a
significant discrepancy in radiological response between the
apatinib plus XELOX group and the XELOX group (P=0.012), with the
former exhibiting a more favorable outcome. The proportion of
patients achieving ORR was also revealed to be higher in the
apatinib plus XELOX group vs. the XELOX group (86.0 vs. 68.0%)
(P=0.032). However, the DCR was not different between groups (98.0
vs. 86.0%) (P=0.059).
As a whole, based on Dworak's scale, tumor
regression was staged as follows: TRG 0 received 5.0% of the cases,
TRG 1 received 29.0%, TRG 2 received 29.0%, and TRG 3 received
37.0%. The TRG demonstrated superiority in the apatinib plus XELOX
group as compared with the XELOX group (P<0.001). The apatinib
plus XELOX group exhibited an increased rate of major pathological
response compared with the XELOX group (46.0 vs. 22.0%) (P=0.011).
With regards to the TNM stage following neoadjuvant therapy, no
discernible difference was illustrated in ypTNM stage (P=0.200) or
TNM stage decline (60.0 vs. 46.0%) (P=0.161) between groups
(Table III).
 | Table III.Radiological and pathological
response. |
Table III.
Radiological and pathological
response.
| A, Radiological
response |
|---|
|
|---|
| Items | XELOX group
(n=50) | Apatinib plus XELOX
group (n=50) | P-value |
|---|
| Radiological
response, n (%) |
|
| 0.012 |
| CR | 1 (2.0) | 4 (8.0) |
|
| R | 33 (66.0) | 39 (78.0) |
|
| SD | 9 (18.0) | 6 (12.0) |
|
| D | 7 (14.0) | 1 (2.0) |
|
| ORR, n (%) |
|
| 0.032 |
|
Yes | 34 (68.0) | 43 (86.0) |
|
| No | 16 (32.0) | 7 (14.0) |
|
| DCR, n (%) |
|
| 0.059 |
|
Yes | 43 (86.0) | 49 (98.0) |
|
| No | 7 (14.0) | 1 (2.0) |
|
|
| B, Pathological
response |
|
| Items | XELOX group
(n=50) | Apatinib plus
XELOX group (n=50) | P-value |
|
| TRG, n (%) |
|
| <0.001 |
| Grade
0 | 1 (2.0) | 4 (8.0) |
|
| Grade
1 | 10 (20.0) | 19 (38.0) |
|
| Grade
2 | 10 (20.0) | 19 (38.0) |
|
| Grade
3 | 29 (58.0) | 8 (16.0) |
|
| Major pathological
response, n (%) | 11 (22.0) | 23 (46.0) | 0.011 |
|
| C, TNM stage
after neoadjuvant therapy |
|
| Items | XELOX group
(n=50) | Apatinib plus
XELOX group (n=50) | P-value |
|
| ypTNM stage, n
(%) |
|
| 0.200 |
| 0 | 1 (2.0) | 4 (8.0) |
|
| I | 11 (22.0) | 10 (20.0) |
|
| II | 11 (22.0) | 16 (32.0) |
|
|
III | 27 (54.0) | 20 (40.0) |
|
| TNM stage decline,
n (%) | 23 (46.0) | 30 (60.0) | 0.161 |
Associated factors with ORR and major
pathological responses
A forward-stepwise multivariate logistic regression
model was applied to recognize factors associated with ORR and
major pathological response. The results indicated that treatment
with apatinib plus XELOX, as opposed to XELOX alone, was
independently associated with higher ORR rates in patients with
locally advanced CRC [odds ratio (OR)=2.891, P=0.037], as depicted
in Fig. 1A. Furthermore, treatment
with apatinib plus XELOX was independently linked with higher rates
of major pathological response (OR=3.431, P=0.008), while the
distance of the tumor from the anus (>5 cm vs. ≤5 cm) was
independently related to lower rates of major pathological response
(OR=0.354, P=0.032) in patients with locally advanced CRC, as
demonstrated in Fig. 1B.
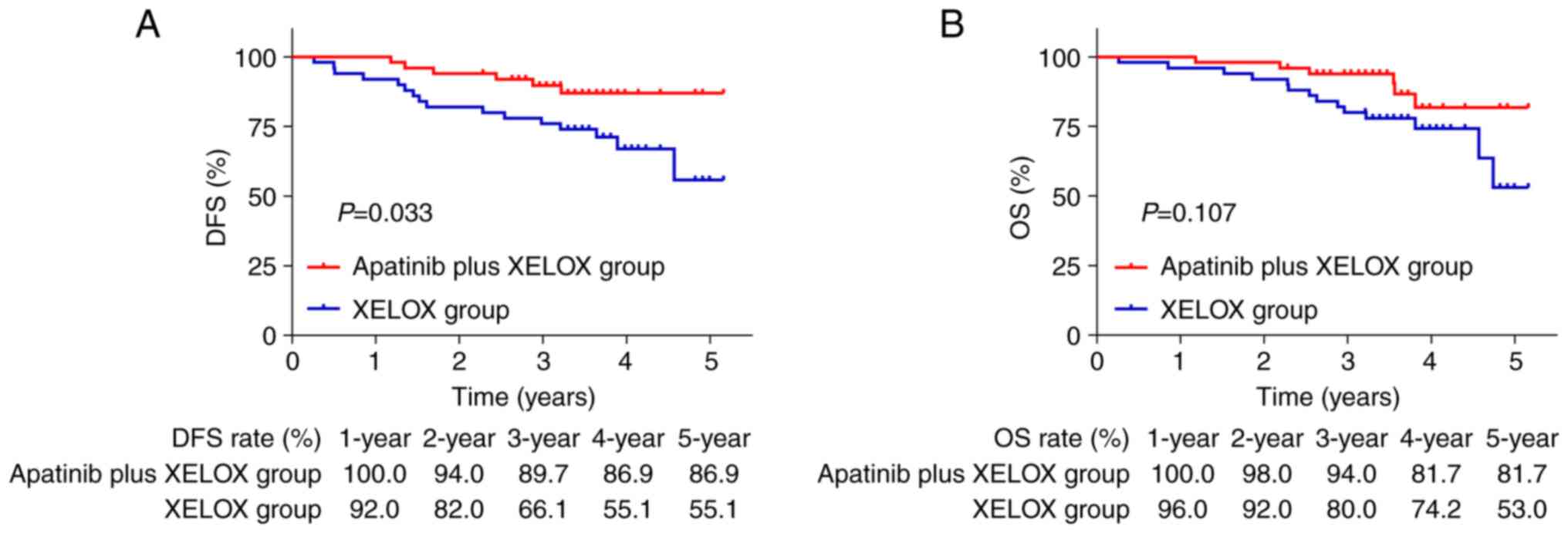
DFS and OS between cohorts
The DFS was found to be significantly higher in the
apatinib plus XELOX group compared with the XELOX group (P=0.033;
Fig. 2A). Nevertheless, no
significant difference was revealed in OS between the two groups
(P=0.107; Fig. 2B). The
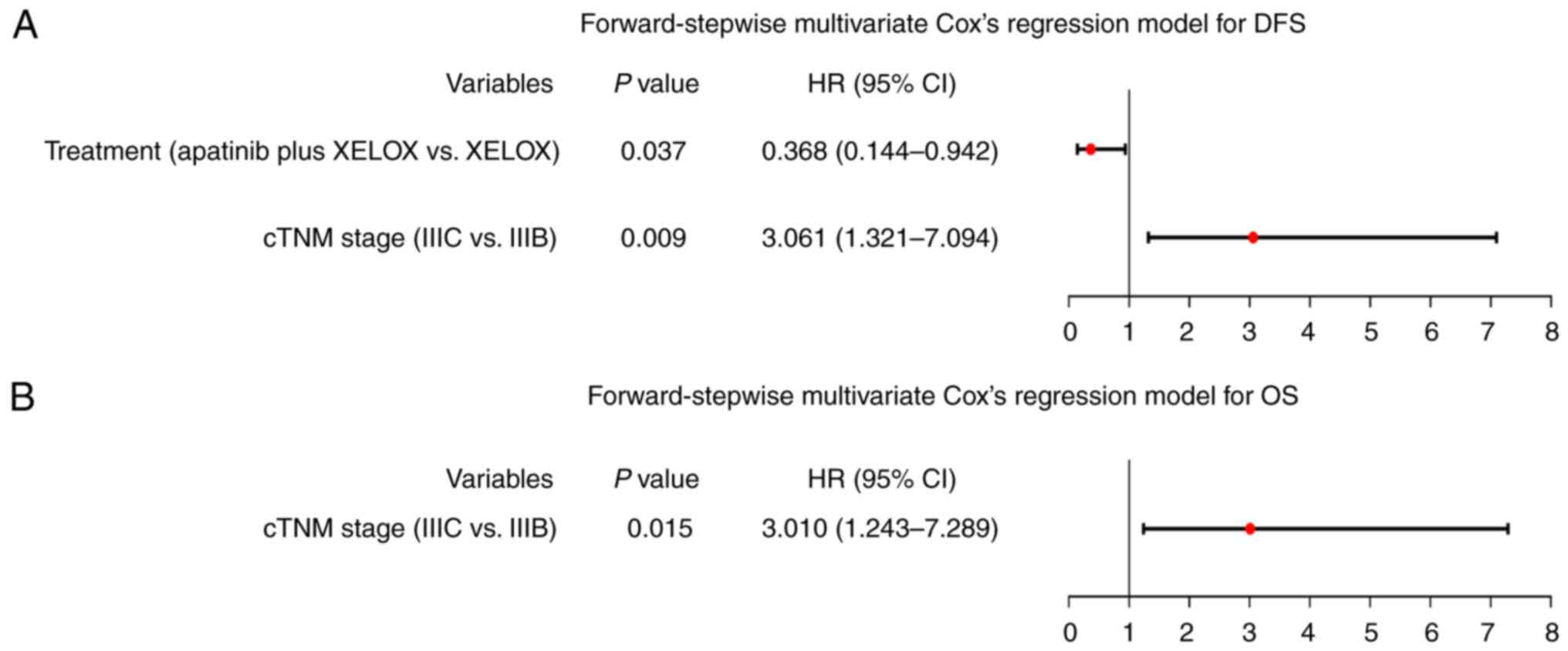
forward-stepwise multivariate Cox's regression model revealed that
treatment (apatinib plus XELOX vs. XELOX) was independently linked
with prolonged DFS [hazard ratio (HR)=0.368, P=0.037].
Additionally, cTNM stage (IIIC vs. IIIB) was found to be
independently associated with shorter DFS (HR=3.061, P=0.009) in
patients with locally advanced CRC, as illustrated in Fig. 3A. Furthermore, cTNM stage (IIIC vs.
IIIB) was independently related to shorter OS in patients with
locally advanced CRC (HR=3.010, P=0.015), as revealed in Fig. 3B. Meanwhile, independent factors
linked with DFS and OS in patients with locally advanced CRC by
multivariate Cox's regression model with the enter method are
presented in Table SI.
Comparison of adverse events between
cohorts
The present study revealed that the apatinib plus
XELOX group exhibited a higher incidence of leukopenia (72.0 vs.
52.0%; P=0.039), neutropenia (48.0 vs. 12.0%; P<0.001) and
anorexia (46.0 vs. 26.0%; P=0.037) compared with the XELOX group.
No difference was disclosed in the incidences of other adverse
events, including nausea and vomiting, thrombocytopenia,
hypertension, proteinuria and hemoglobinopenia (all P>0.05).
Notably, all adverse events were grade 1–2, and there was no grade
3–4 adverse event in both groups (Table IV).
 | Table IV.Adverse events. |
Table IV.
Adverse events.
| Adverse events | XELOX group, n=50
(%) | Apatinib plus XELOX
group, n=50 (%) | P-value |
|---|
| Leukopenia, n
(%) | 26 (52.0) | 36 (72.0) | 0.039 |
| Neutropenia, n
(%) | 6 (12.0) | 24 (48.0) | <0.001 |
| Nausea and
vomiting, n (%) | 19 (38.0) | 20 (40.0) | 0.838 |
| Anorexia, n
(%) | 13 (26.0) | 23 (46.0) | 0.037 |
| Thrombocytopenia, n
(%) | 7 (14.0) | 15 (30.0) | 0.053 |
| Hypertension, n
(%) | 8 (16.0) | 14 (28.0) | 0.148 |
| Proteinuria, n
(%) | 8 (16.0) | 14 (28.0) | 0.148 |
| Hemoglobinopenia, n
(%) | 5 (10.0) | 8 (16.0) | 0.372 |
Discussion
The VEGF pathway-mediated angiogenesis plays a
crucial role in providing nutrients for tumor growth, thereby
contributing to the progression of CRC (19). Apatinib, an oral antiangiogenic
agent, has been shown to inhibit tumor angiogenesis by restraining
VEGFR-2, which presents a promising treatment strategy for CRC
(11,20). The present study demonstrated that
neoadjuvant apatinib in combination with XELOX significantly
increased the ORR and major pathological response compared with
XELOX alone. In addition, the results of the present study revealed
that apatinib in combination with XELOX improved radiological
response compared with XELOX alone. This was attributed to
apatinib's enhancement of conventional chemotherapy. This effect
could be attributed to the ability of apatinib to restrain
angiogenesis and the VEGFR2-β-catenin pathway, leading to tumor
regression in patients with locally advanced CRC (11). Furthermore, apatinib promoted
ferroptosis by targeting the elongation of very long chain fatty
acids protein 6/acyl-CoA synthetase long-chain family member 4
signaling in CRC cells, which eliminated CRC cells and inhibited
CRC growth (21). Therefore,
neoadjuvant apatinib in combination with XELOX improved ORR and
major pathological response in patients with locally advanced
CRC.
The efficacy of current neoadjuvant chemotherapy for
patients with CRC remains suboptimal, as evidenced by previous
research (22–24). Specifically, studies have
illustrated a 5-year OS rate of 67–76% in patients with locally
advanced CRC who undergo neoadjuvant chemotherapy (23,24).
By contrast, the investigation of the present study demonstrated
that patients with locally advanced CRC who received neoadjuvant
apatinib in combination with XELOX had a 5-year DFS rate of 86.9%
and a 5-year OS rate of 81.7%, which surpassed the outcomes of
neoadjuvant XELOX alone in the present study and neoadjuvant
chemotherapy in previous studies (23,24).
Additionally, it was identified that cTNM stage (IIIC vs. IIIB) was
independently associated with shorter DFS and OS in patients with
locally advanced CRC. This superiority might be attributed to the
inclusion of apatinib in the treatment regimen. The potential
rationales were as follows: Firstly, apatinib was found to inhibit
angiogenesis and induce ferroptosis, thereby impeding the
progression and recurrence of CRC (11,21,25).
Secondly, apatinib was linked to a more favorable pathological
response, leading to an extension of DFS in patients with CRC
(26). Consequently, the
administration of neoadjuvant apatinib in combination with XELOX
resulted in an improved DFS in patients with locally advanced CRC.
Additionally, no significant difference was suggested in OS between
the apatinib plus XELOX group and the XELOX group. This could be
attributed to the relatively low mortality rate during the
follow-up period, which resulted in a small effect. The impact of
neoadjuvant chemotherapy on patients' OS depended on numerous
factors, such as the effectiveness of surgery and the selection of
postoperative treatment methods. Therefore, the OS between groups
did not differ statistically significantly.
In addition to efficacy, the safety of neoadjuvant
apatinib in combination with XELOX in patients with locally
advanced CRC is also a noteworthy issue. In the present study,
neoadjuvant apatinib in combination with XELOX increased the
incidences of leukopenia, neutropenia and anorexia compared with
neoadjuvant XELOX alone. The possible reasons were as follows: i)
Apatinib restrained the colony formation of bone marrow by
inhibiting VEGFR-2, causing myelosuppression, thus decreasing
leukocytes and neutrophils (27).
ii) Apatinib inhibited VEGFR-2, which might lead to
gastrointestinal mucosal injury and gastritis, thus increasing
anorexia (28,29). Interestingly, hypertension and
proteinuria are considered common adverse events associated with
apatinib (30). A previous study
reported that the incidence of hypertension and proteinuria in
patients with advanced CRC who receive apatinib is 25.9 and 22.2%,
respectively (31). Similar to the
aforementioned study, the incidences of hypertension and
proteinuria in the present study were both 28% in the apatinib plus
XELOX group. Additionally, there was no new adverse event occurring
in the apatinib plus XELOX group. These results supported the
favorable tolerance of neoadjuvant apatinib in combination with
XELOX in patients with locally advanced CRC. The findings of the
present study indicated that clinicians needed to pay attention to
adverse events caused by apatinib and provide timely treatment.
Notably, the present study did not intervene in the
neoadjuvant treatment regimens of patients with locally advanced
CRC, and all regimens were selected based on the physician's
recommendations or patients' wishes. In the present study, the
majority of clinical characteristics of patients in both groups
were non-differential, while there was a lower proportion of
patients with high TB in the apatinib plus XELOX group vs. the
XELOX group. In detail, TB is a histological characteristic of
tumor cells that represents the dissociation of a single cancer
cell or clusters of up to four cancer cells from the invasive tumor
front (32,33). The 2016 International TB Consensus
Conference (ITBCC) has indicated that TB is a well-established
independent factor for predicting the prognosis of CRC patients
(15). Thus, the difference in TB
between the two groups in the present study represented that
patients in the apatinib plus XELOX group might have improved
prognosis vs. the XELOX group, which might influence the results to
some extent. However, the current study used forward-stepwise
multivariate Cox's proportional hazard regression models to correct
confounding factors, which found that apatinib in combination with
XELOX treatment was independently linked with prolonged DFS in
patients with locally advanced CRC.
The present study involved several limitations worth
noting: i) The present study reviewed as numerous patients as
possible who met the inclusion criteria and did not meet the
exclusion criteria. However, there was a small sample size, and
further studies should consider including a large sample size to
verify the efficacy and safety of neoadjuvant apatinib in
combination with XELOX in patients with locally advanced CRC; ii)
the present study was retrospective, which might lead to bias to
some extent. Thus, future randomized, controlled studies are
required for further verification; iii) in the present study,
neither neoadjuvant apatinib in combination with XELOX nor
neoadjuvant XELOX alone were evaluated for quality of life; and iv)
the conventional doses of apatinib used in patients with CRC are
0.25 g/day or 0.5 g/day (34),
while the present study only used 0.25 g/day doses of apatinib, and
future studies should consider evaluating the clinical efficacy and
safety of 0.5 g/day doses of apatinib used for neoadjuvant therapy
in patients with locally advanced CRC.
In conclusion, the administration of neoadjuvant
apatinib in combination with XELOX has been found to enhance
radiological and pathological responses, as well as improve DFS
with acceptable tolerance in patients diagnosed with locally
advanced CRC. The primary outcome measured neoadjuvant apatinib in
combination with XELOX is effectiveness and safety for treating
locally advanced CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZ, XP, GL, LY, AZ and XJ contributed to the study
conception and design. TZ, XP, GL and LY prepared material,
collected data and performed analysis. TZ and XP wrote the first
draft of the manuscript, and all authors commented on previous
versions of the manuscript. AZ and XJ confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
ChiECRCT20210395) by the Ethics Committee of the Affiliated
Hospital of Hebei University (Baoding, China). Written informed
consent was obtained from each patient or guardian.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh
Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M and Saad A:
Colorectal cancer epidemiology: Recent trends and impact on
outcomes. Curr Drug Targets. 22:998–1009. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Yang R, Wu T, Cai X, Li G, Yu K,
Li Y, Ding R, Dong C, Li J, et al: Efficacy and safety of
neoadjuvant monoimmunotherapy with PD-1 inhibitor for dMMR/MSI-H
locally advanced colorectal cancer: A single-center real-world
study. Front Immunol. 13:9134832022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Wu T, Cai X, Dong J, Xia C, Zhou
Y, Ding R, Yang R, Tan J, Zhang L, et al: Neoadjuvant immunotherapy
for MSI-H/dMMR locally advanced colorectal cancer: New strategies
and unveiled opportunities. Front Immunol. 13:7959722022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chuang JP, Tsai HL, Chen PJ, Chang TK, Su
WC, Yeh YS, Huang CW and Wang JY: Comprehensive review of
biomarkers for the treatment of locally advanced colon cancer.
Cells. 11:37442022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vassantachart A, Marietta M, Mehta S, Lin
E and Bian SX: Racial disparities and standard treatment in locally
advanced rectal cancer: A national cancer database study. J
Gastrointest Oncol. 13:2922–2937. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Body A, Prenen H, Latham S, Lam M,
Tipping-Smith S, Raghunath A and Segelov E: The role of neoadjuvant
chemotherapy in locally advanced colon cancer. Cancer Manag Res.
13:2567–2579. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Xiao Q, Venkatachalam N, Hofheinz
RD, Veldwijk MR, Herskind C, Ebert MP and Zhan T: Predicting
response to neoadjuvant chemoradiotherapy in rectal cancer: From
biomarkers to tumor models. Ther Adv Med Oncol.
14:175883592210779722022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gosavi R, Chia C, Michael M, Heriot AG,
Warrier SK and Kong JC: Neoadjuvant chemotherapy in locally
advanced colon cancer: A systematic review and meta-analysis. Int J
Colorectal Dis. 36:2063–2070. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang C, Bliggenstorfer JT, Liu J, Shearer
J, Dreher P, Bingmer K, Stein SL and Steinhagen E: Not all patients
with locally advanced rectal cancer benefit from neoadjuvant
therapy. Am Surg. 89:4327–4333. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai X, Wei B, Li L, Chen X, Yang J, Li X,
Jiang X, Lv M, Li M, Lin Y, et al: Therapeutic potential of
apatinib against colorectal cancer by inhibiting VEGFR2-mediated
angiogenesis and β-catenin signaling. Onco Targets Ther.
13:11031–11044. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen D, Zhong X, Lin L, Xie J, Lian Y and
Xu L: Comparative efficacy and adverse reactions of
apatinib-chemotherapy combinations versus chemotherapy alone for
treatment of advanced colorectal cancer: A meta-analysis of
randomized controlled trials. Am J Transl Res. 14:6703–6711.
2022.PubMed/NCBI
|
|
13
|
Dai Y, Sun L, Zhuang L, Zhang M, Zou Y,
Yuan X and Qiu H: Efficacy and safety of low-dose apatinib plus S-1
versus regorafenib and fruquintinib for refractory metastatic
colorectal cancer: A retrospective cohort study. J Gastrointest
Oncol. 13:722–731. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueno H, Hase K, Hashiguchi Y, Shimazaki H,
Tanaka M, Miyake O, Masaki T, Shimada Y, Kinugasa Y, Mori Y, et al:
Site-specific tumor grading system in colorectal cancer:
Multicenter pathologic review of the value of quantifying poorly
differentiated clusters. Am J Surg Pathol. 38:197–204. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lugli A, Kirsch R, Ajioka Y, Bosman F,
Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann
A, et al: Recommendations for reporting tumor budding in colorectal
cancer based on the international tumor budding consensus
conference (ITBCC) 2016. Mod Pathol. 30:1299–1311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chinese Society Of Clinical Oncology Csco
Diagnosis and Treatment Guidelines For Colorectal Cancer Working
Group, . Chinese society of clinical oncology (CSCO) diagnosis and
treatment guidelines for colorectal cancer 2018 (english version).
Chin J Cancer Res. 31:117–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dworak O, Keilholz L and Hoffmann A:
Pathological features of rectal cancer after preoperative
radiochemotherapy. Int J Colorectal Dis. 12:19–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang D, Xu F, Lai X, Li Y, Hou T, Wu L, Ma
D and Li Z: Identifying predictive biomarkers of apatinib in
third-line treatment of advanced colorectal cancer through
comprehensive genomic profiling. Anticancer Drugs. 34:431–438.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian X, Li S and Ge G: Apatinib promotes
ferroptosis in colorectal cancer cells by targeting ELOVL6/ACSL4
signaling. Cancer Manag Res. 13:1333–1342. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu J, Lian J, Xu B, Pang X, Ji S, Zhao Y
and Lu H: Neoadjuvant immunotherapy for colorectal cancer: Right
regimens, right patients, right directions? Front Immunol.
14:11206842023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sauer R, Becker H, Hohenberger W, Rödel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
et al: Preoperative versus postoperative chemoradiotherapy for
rectal cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Gooyer JM, Verstegen MG, 't Lam-Boer J,
Radema SA, Verhoeven RHA, Verhoef C, Schreinemakers JMJ and de Wilt
JHW: Neoadjuvant chemotherapy for locally advanced T4 colon cancer:
A nationwide propensity-score matched cohort analysis. Dig Surg.
37:292–301. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng X, Feng H, Wu H, Jin Z, Shen X,
Kuang J, Huo Z, Chen X, Gao H, Ye F, et al: Targeting autophagy
enhances apatinib-induced apoptosis via endoplasmic reticulum
stress for human colorectal cancer. Cancer Lett. 431:105–114. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rödel C, Martus P, Papadoupolos T, Füzesi
L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R,
Sauer R and Wittekind C: Prognostic significance of tumor
regression after preoperative chemoradiotherapy for rectal cancer.
J Clin Oncol. 23:8688–8696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar R, Crouthamel MC, Rominger DH,
Gontarek RR, Tummino PJ, Levin RA and King AG: Myelosuppression and
kinase selectivity of multikinase angiogenesis inhibitors. Br J
Cancer. 101:1717–1723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pollom EL, Deng L, Pai RK, Brown JM,
Giaccia A, Loo BW Jr, Shultz DB, Le QT, Koong AC and Chang DT:
Gastrointestinal toxicities with combined antiangiogenic and
stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys.
92:568–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spiller RC: ABC of the upper
gastrointestinal tract: Anorexia, nausea, vomiting, and pain. BMJ.
323:1354–1357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang Z, Wang Y, Yu Y, Cui Y, Liang L, Xu
C, Shen Z, Shen K, Wang X, Liu T and Sun Y: Neoadjuvant apatinib
combined with oxaliplatin and capecitabine in patients with locally
advanced adenocarcinoma of stomach or gastroesophageal junction: A
single-arm, open-label, phase 2 trial. BMC Med. 20:1072022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liao X, Li H, Liu Z, Liao S, Li Q, Liang
C, Huang Y, Xie M, Wei J and Li Y: Clinical efficacy and safety of
apatinib in patients with advanced colorectal cancer as the
late-line treatment. Medicine (Baltimore). 97:e136352018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lugli A, Zlobec I, Berger MD, Kirsch R and
Nagtegaal ID: Tumour budding in solid cancers. Nat Rev Clin Oncol.
18:101–115. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen K, Collins G, Wang H and Toh JWT:
Pathological features and prognostication in colorectal cancer.
Curr Oncol. 28:5356–5383. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao L, Yu Q, Gao C, Xiang J, Zheng B,
Feng Y, Li R, Zhang W, Hong X, Zhan YY, et al: Studies of the
efficacy of low-dose apatinib monotherapy as third-line treatment
in patients with metastatic colorectal cancer and apatinib's novel
anticancer effect by inhibiting tumor-derived exosome secretion.
Cancers (Basel). 14:24922022. View Article : Google Scholar : PubMed/NCBI
|