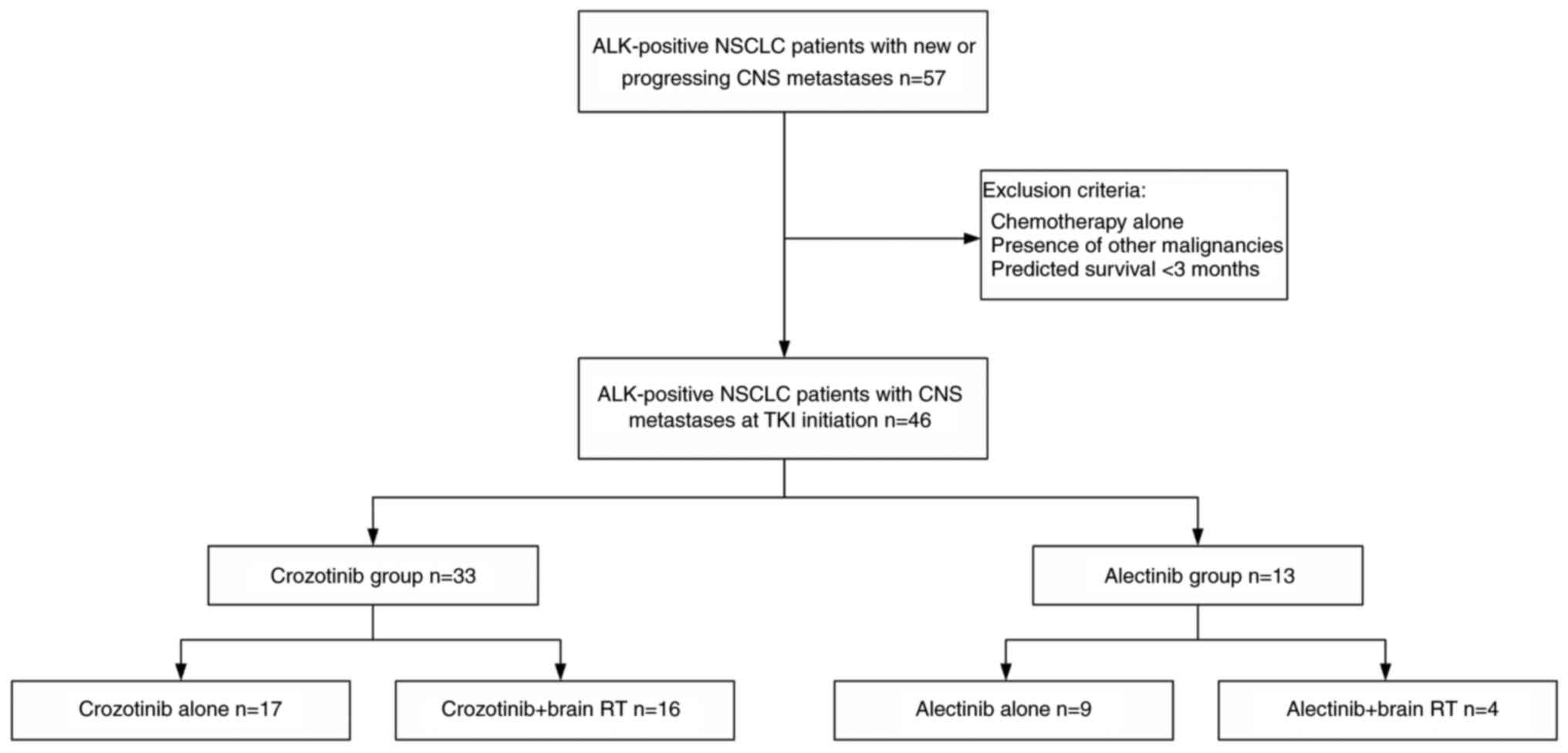
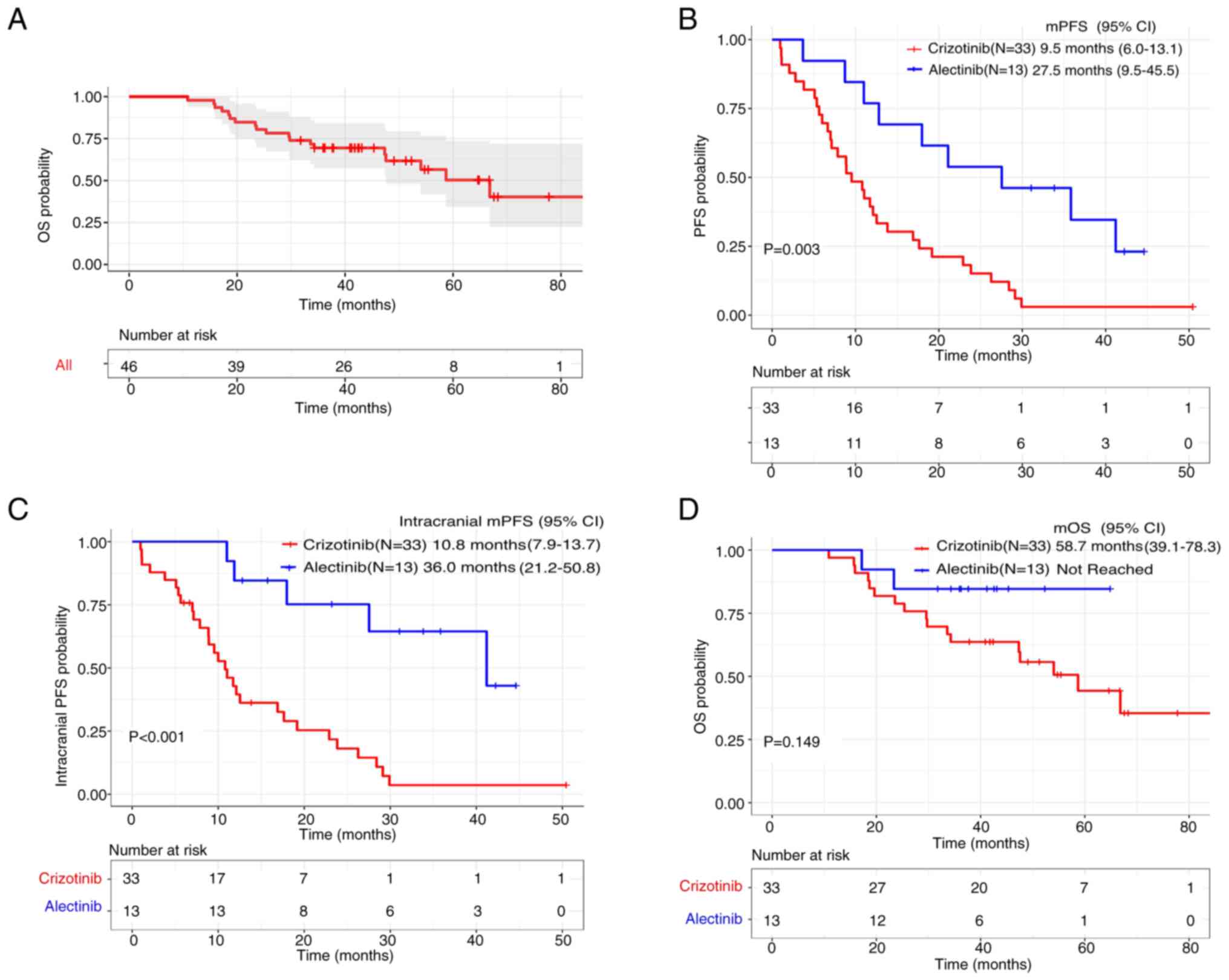
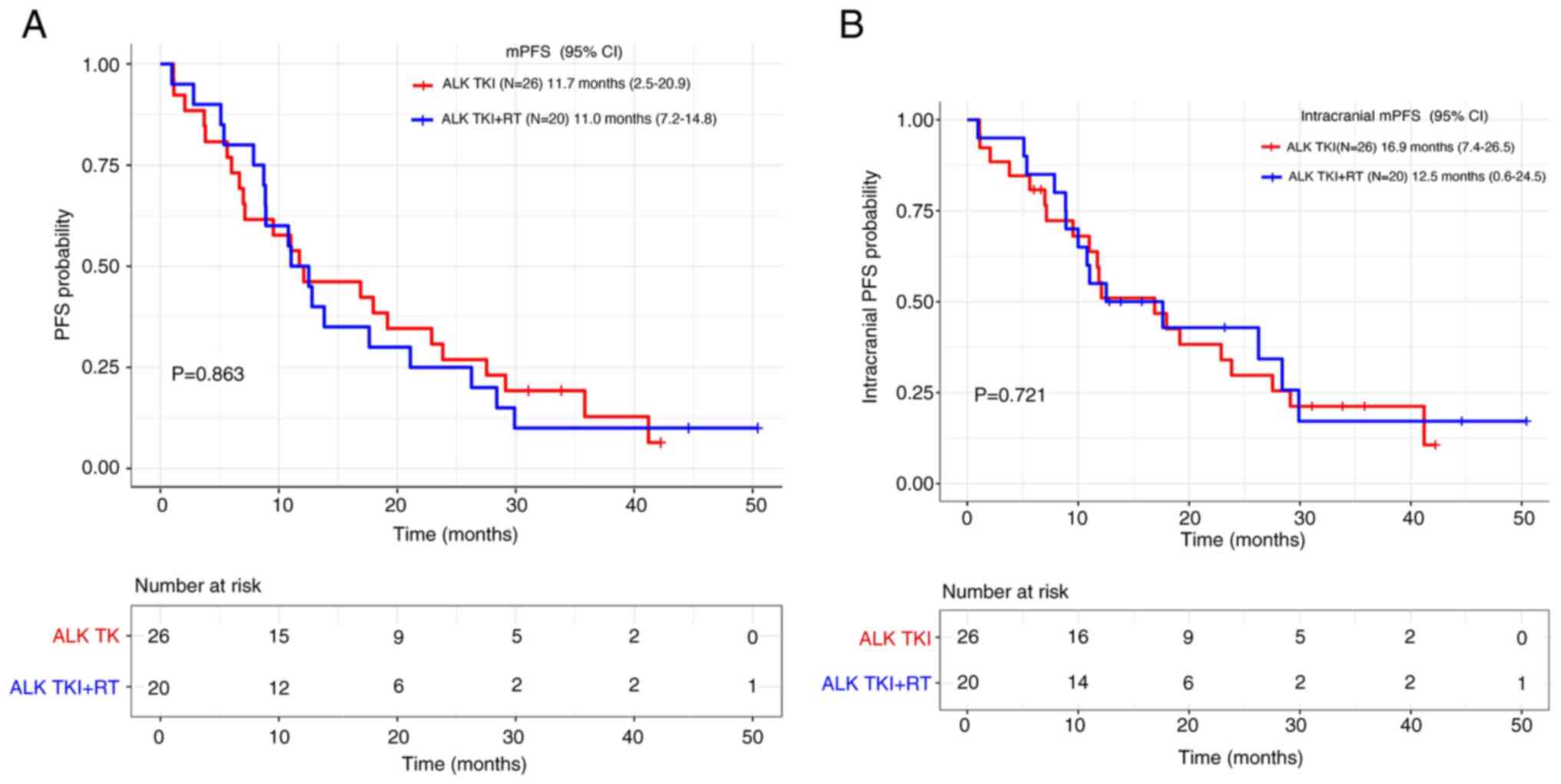
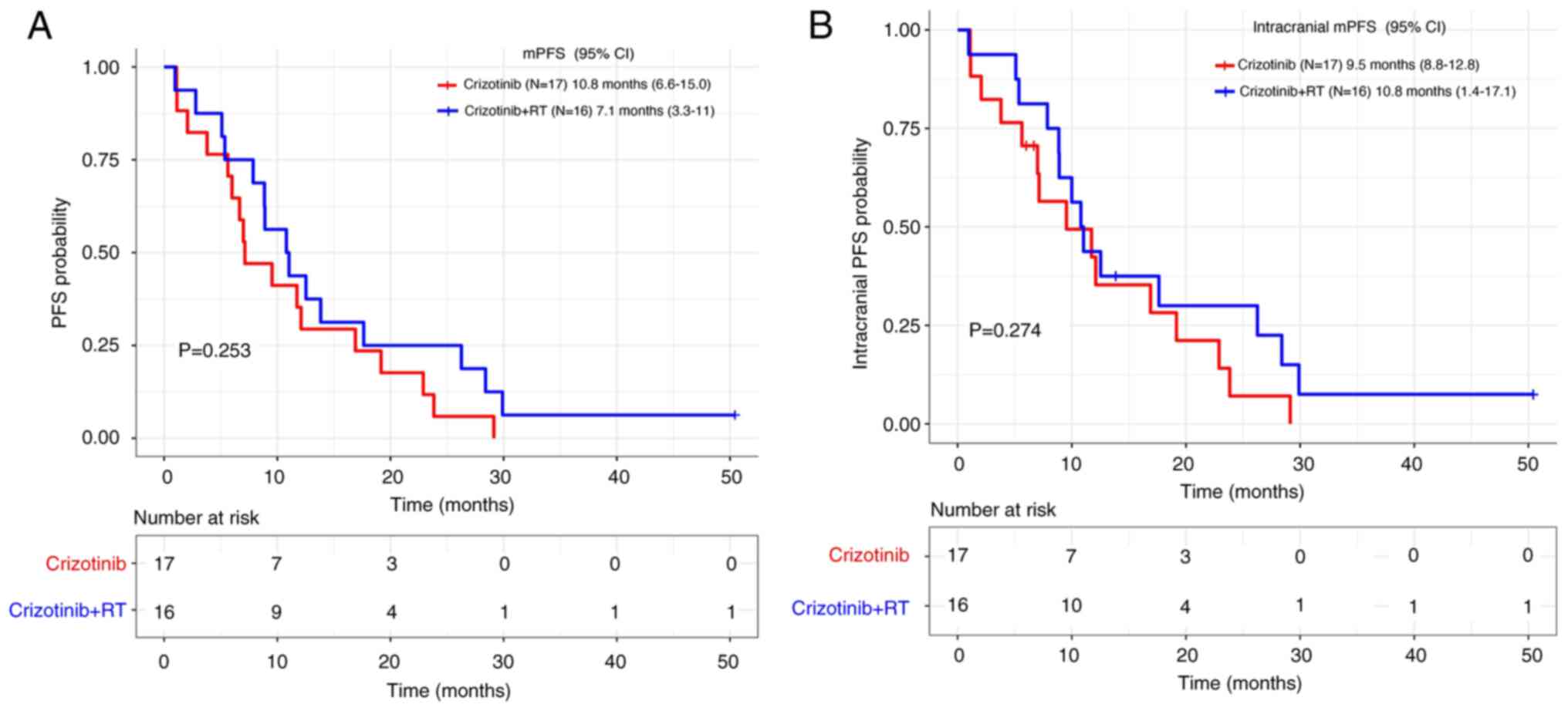
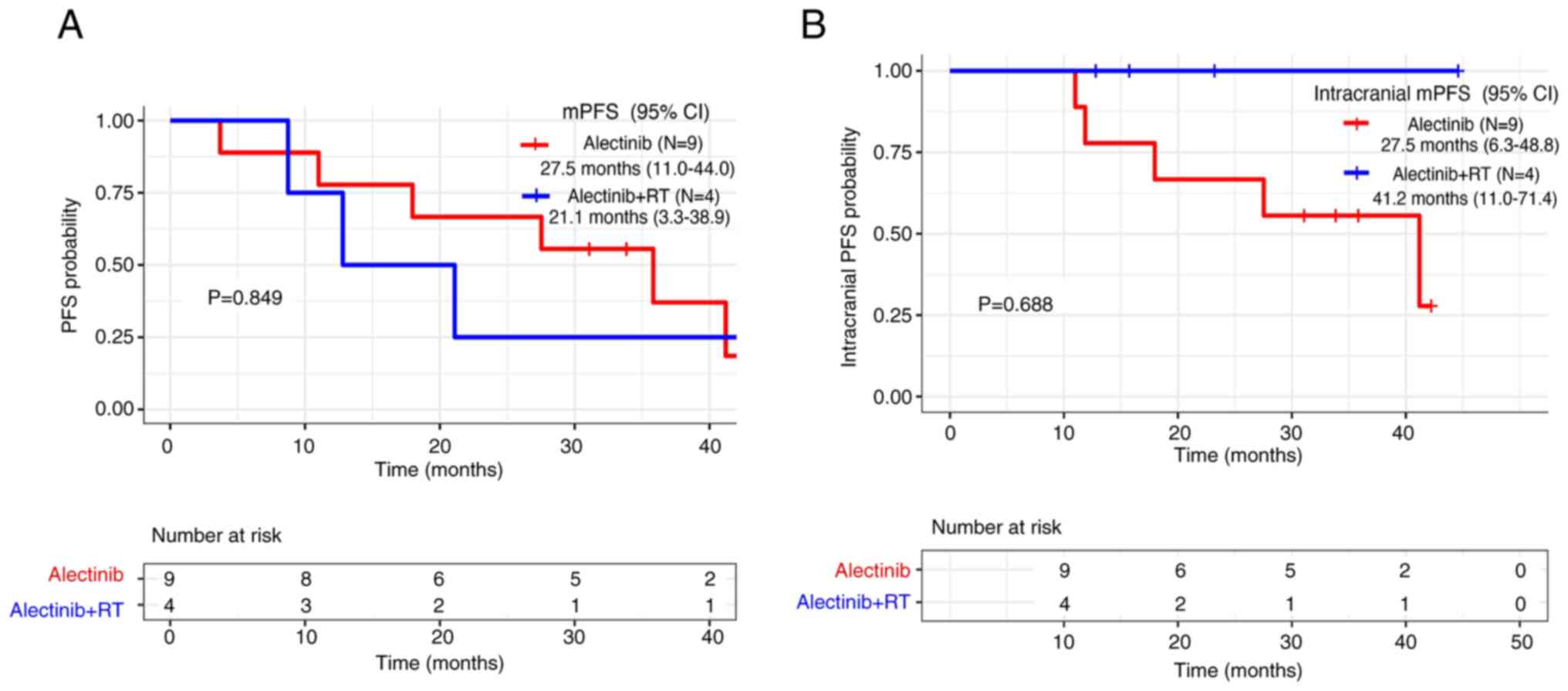
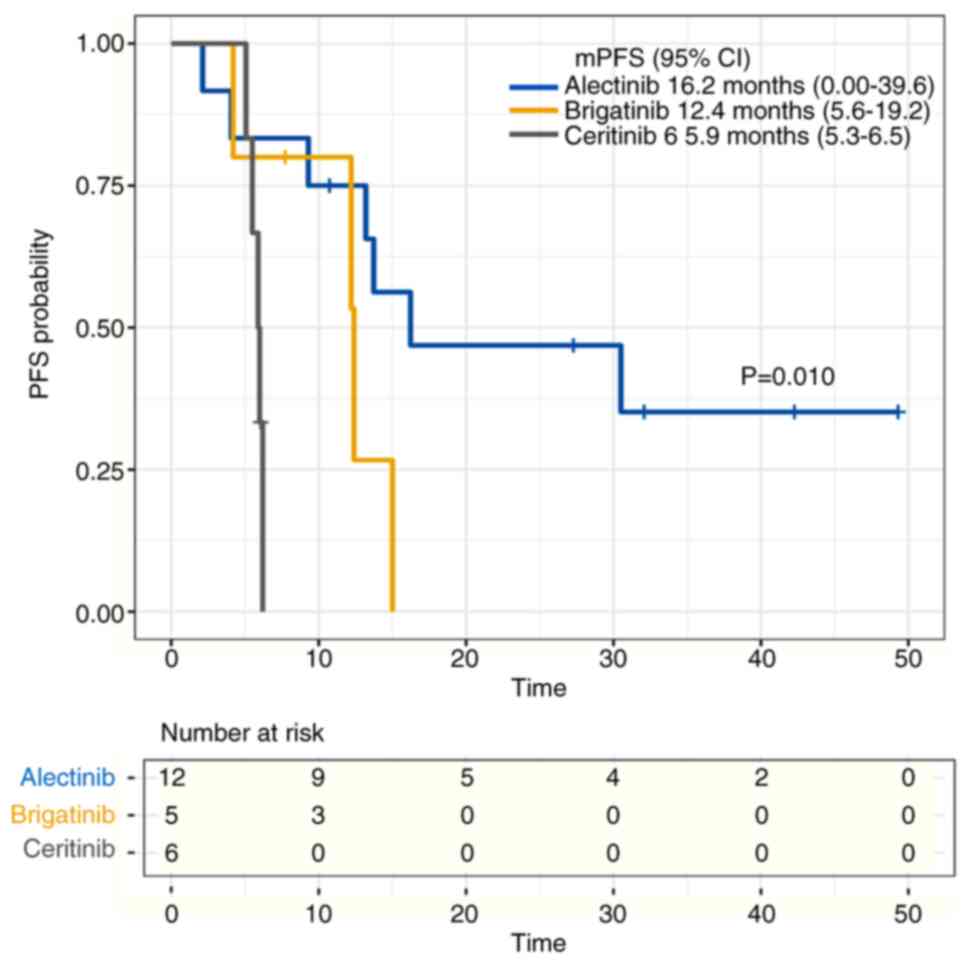
|
1
|
Page S, Milner-Watts C, Perna M, Janzic U,
Vidal N, Kaudeer N, Ahmed M, McDonald F, Locke I, Minchom A, et al:
Systemic treatment of brain metastases in non-small cell lung
cancer. Eur J Cancer. 132:187–198. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Costa DB, Shaw AT, Ou SH, Solomon BJ,
Riely GJ, Ahn MJ, Zhou C, Shreeve SM, Selaru P, Polli A, et al:
Clinical experience with crizotinib in patients with advanced
ALK-rearranged non-small-cell lung cancer and brain metastases. J
Clin Oncol. 33:1881–1888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaw AT, Yeap BY, Mino-Kenudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, et al: Clinical features and outcome of patients with
non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol.
27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JK, Park HS, Kim DW, Kulig K, Kim TM,
Lee SH, Jeon YK, Chung DH, Heo DS, Kim WH and Bang YJ: Comparative
analyses of overall survival in patients with anaplastic lymphoma
kinase-positive and matched wild-type advanced non-small cell lung
cancer. Cancer. 118:3579–3586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang HJ, Lim HJ, Park JS, Cho YJ, Yoon HI,
Chung JH, Lee JH and Lee CT: Comparison of clinical characteristics
between patients with ALK-positive and EGFR-positive lung
adenocarcinoma. Respir Med. 108:388–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rangachari D, Yamaguchi N, VanderLaan PA,
Folch E, Mahadevan A, Floyd SR, Uhlmann EJ, Wong ET, Dahlberg SE,
Huberman MS and Costa DB: Brain metastases in patients with
EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung
Cancer. 88:108–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sperduto PW, Kased N, Roberge D, Xu Z,
Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al: Summary
report on the graded prognostic assessment: An accurate and facile
diagnosis-specific tool to estimate survival for patients with
brain metastases. J Clin Oncol. 30:419–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D'Antonio C, Passaro A, Gori B, Del
Signore E, Migliorino MR, Ricciardi S, Fulvi A and de Marinis F:
Bone and brain metastasis in lung cancer: Recent advances in
therapeutic strategies. Ther Adv Med Oncol. 6:101–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Solomon BJ, Cappuzzo F, Felip E, Blackhall
FH, Costa DB, Kim DW, Nakagawa K, Wu YL, Mekhail T, Paolini J, et
al: Intracranial efficacy of crizotinib versus chemotherapy in
patients with advanced ALK-positive non-small-cell lung cancer:
Results from PROFILE 1014. J Clin Oncol. 34:2858–2865. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kodama T, Tsukaguchi T, Yoshida M, Kondoh
O and Sakamoto H: Selective ALK inhibitor alectinib with potent
antitumor activity in models of crizotinib resistance. Cancer Lett.
351:215–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gettinger SN, Bazhenova LA, Langer CJ,
Salgia R, Gold KA, Rosell R, Shaw AT, Weiss GJ, Tugnait M,
Narasimhan NI, et al: Activity and safety of brigatinib in
ALK-rearranged non-small-cell lung cancer and other malignancies: A
single-arm, open-label, phase 1/2 trial. Lancet Oncol.
17:1683–1696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marsilje TH, Pei W, Chen B, Lu W, Uno T,
Jin Y, Jiang T, Kim S, Li N, Warmuth M, et al: Synthesis,
structure-activity relationships, and in vivo efficacy of the novel
potent and selective anaplastic lymphoma kinase (ALK) inhibitor
5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine
(LDK378) currently in phase 1 and phase 2 clinical trials. J Med
Chem. 56:5675–5690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Camidge DR, Dziadziuszko R, Peters S, Mok
T, Noe J, Nowicka M, Gadgeel SM, Cheema P, Pavlakis N, de Marinis
F, et al: Updated efficacy and safety data and impact of the
EML4-ALK fusion variant on the efficacy of alectinib in untreated
ALK-positive advanced non-small cell lung cancer in the global
phase III ALEX study. J Thorac Oncol. 14:1233–1243. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou C, Kim SW, Reungwetwattana T, Zhou J,
Zhang Y, He J, Yang JJ, Cheng Y, Lee SH, Bu L, et al: Alectinib
versus crizotinib in untreated Asian patients with anaplastic
lymphoma kinase-positive non-small-cell lung cancer (ALESIA): A
randomised phase 3 study. Lancet Respir Med. 7:437–446. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng YD, Zhang L, Liao H, Liang Y, Xu F,
Liu JL, Dinglin XX and Chen LK: Gefitinib alone or with concomitant
whole brain radiotherapy for patients with brain metastasis from
non-small-cell lung cancer: A retrospective study. Asian Pac J
Cancer Prev. 13:909–914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
An N, Wang H, Li J, Zhai X, Jing W, Jia W,
Kong L, Zhu H and Yu J: Therapeutic effect of first-line EGFR-TKIs
combined with concurrent cranial radiotherapy on NSCLC patients
with EGFR activating mutation and brain metastasis: A retrospective
study. Onco Targets Ther. 12:8311–8378. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Yang J, Li X, Hao D, Wu X, Yang Y,
He C, Wang W and Wang J: First-line epidermal growth factor
receptor (EGFR)-tyrosine kinase inhibitor alone or with whole-brain
radiotherapy for brain metastases in patients with EGFR-mutated
lung adenocarcinoma. Cancer Sci. 107:1800–1805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: Non-small cell lung cancer, version 3.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, Lin JJ, Pal N, Polito L, Trinh H,
Hilton M, Smoljanović V, Kurtsikidze N, Archer V, Krebs MG, et al:
Real-world comparative effectiveness of first-line alectinib versus
crizotinib in patients with advanced ALK-positive NSCLC with or
without baseline central nervous system metastases. JTO Clin Res
Rep. 4:1004832023.PubMed/NCBI
|
|
20
|
Mok T, Camidge DR, Gadgeel SM, Rosell R,
Dziadziuszko R, Kim DW, Pérol M, Ou SI, Ahn JS, Shaw AT, et al:
Updated overall survival and final progression-free survival data
for patients with treatment-naive advanced ALK-positive
non-small-cell lung cancer in the ALEX study. Ann Oncol.
31:1056–1064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito K, Yamanaka T, Hayashi H, Hattori Y,
Nishino K, Kobayashi H, Oya Y, Yokoyama T, Seto T, Azuma K, et al:
Sequential therapy of crizotinib followed by alectinib for
non-small cell lung cancer harbouring anaplastic lymphoma kinase
rearrangement (WJOG9516L): A multicenter retrospective cohort
study. Eur J Cancer. 145:183–193. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hotta K, Hida T, Nokihara H, Morise M, Kim
YH, Azuma K, Seto T, Takiguchi Y, Nishio M, Yoshioka H, et al:
Final overall survival analysis from the phase III J-ALEX study of
alectinib versus crizotinib in ALK inhibitor-naïve Japanese
patients with ALK-positive non-small-cell lung cancer. ESMO Open.
7:1005272022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou Z, Gu Y, Liang L, Hao X, Fan C, Xin T,
Zhao S, Liu Z, Guo Y, Ma K, et al: Alectinib as first-line
treatment for advanced ALK-positive non-small cell lung cancer in
the real-world setting: Preliminary analysis in a Chinese cohort.
Transl Lung Cancer Res. 11:2495–2506. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Costa DB, Kobayashi S, Pandya SS, Yeo WL,
Shen Z, Tan W and Wilner KD: CSF concentration of the anaplastic
lymphoma kinase inhibitor crizotinib. J Clin Oncol. 29:e443–e445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chinnaiyan P, Huang S, Vallabhaneni G,
Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM and Harari PM:
Mechanisms of enhanced radiation response following epidermal
growth factor receptor signaling inhibition by erlotinib (Tarceva).
Cancer Res. 65:3328–3335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Metro G, Lunardi G, Floridi P, Pascali JP,
Marcomigni L, Chiari R, Ludovini V, Crinò L and Gori S: CSF
concentration of crizotinib in two ALK-positive non-small-cell lung
cancer patients with CNS metastases deriving clinical benefit from
treatment. J Thorac Oncol. 10:e26–e27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas NJ, Myall NJ, Sun F, Patil T,
Mushtaq R, Yu C, Sinha S, Pollom EL, Nagpal S, Camidge DR, et al:
Brain metastases in EGFR- and ALK-positive NSCLC: Outcomes of
central nervous system-penetrant tyrosine kinase inhibitors alone
versus in combination with radiation. J Thorac Oncol. 17:116–129.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh R, Lehrer EJ, Ko S, Peterson J, Lou
Y, Porter AB, Kotecha R, Brown PD, Zaorsky NG and Trifiletti DM:
Brain metastases from non-small cell lung cancer with EGFR or ALK
mutations: A systematic review and meta-analysis of
multidisciplinary approaches. Radiother Oncol. 144:165–179. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ni J, Li G, Yang X, Chu L, Wang J, Li Y,
Zou L, Li Y, Xie C and Zhu Z: Optimal timing and clinical value of
radiotherapy in advanced ALK-rearranged non-small cell lung cancer
with or without baseline brain metastases: Implications from
pattern of failure analyses. Radiat Oncol. 14:442019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doebele RC, Pilling AB, Aisner DL,
Kutateladze TG, Le AT, Weickhardt AJ, Kondo KL, Linderman DJ,
Heasley LE, Franklin WA, et al: Mechanisms of resistance to
crizotinib in patients with ALK gene rearranged non-small cell lung
cancer. Clin Cancer Res. 18:1472–1482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sakamoto H, Tsukaguchi T, Hiroshima S,
Kodama T, Kobayashi T, Fukami TA, Oikawa N, Tsukuda T, Ishii N and
Aoki Y: CH5424802, a selective ALK inhibitor capable of blocking
the resistant gatekeeper mutant. Cancer Cell. 19:679–690. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gainor JF, Dardaei L, Yoda S, Friboulet L,
Leshchiner I, Katayama R, Dagogo-Jack I, Gadgeel S, Schultz K,
Singh M, et al: Molecular mechanisms of resistance to first- and
second-generation ALK inhibitors in ALK-rearranged lung cancer.
Cancer Discov. 6:1118–1133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Friboulet L, Li N, Katayama R, Lee CC,
Gainor JF, Crystal AS, Michellys PY, Awad MM, Yanagitani N, Kim S,
et al: The ALK inhibitor ceritinib overcomes crizotinib resistance
in non-small cell lung cancer. Cancer Discov. 4:662–673. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang J, Zhao C, Zhang F, Liu Z, Zhou K,
Ren X and Wan Y: ALK inhibitors in ALK-rearranged non-small cell
lung cancer with and without brain metastases: Systematic review
and network meta-analysis. BMJ Open. 12:e0607822022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Horn L, Whisenant JG, Wakelee H, Reckamp
KL, Qiao H, Leal TA, Du L, Hernandez J, Huang V, Blumenschein GR,
et al: Monitoring therapeutic response and resistance: Analysis of
circulating tumor DNA in patients with ALK+ lung cancer. J Thorac
Oncol. 14:1901–1911. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naito T, Shiraishi H and Fujiwara Y:
Brigatinib and lorlatinib: Their effect on ALK inhibitors in NSCLC
focusing on resistant mutations and central nervous system
metastases. Jpn J Clin Oncol. 51:37–44. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shaw AT, Bauer TM, de Marinis F, Felip E,
Goto Y, Liu G, Mazieres J, Kim DW, Mok T, Polli A, et al:
First-line lorlatinib or crizotinib in advanced ALK-positive lung
cancer. N Engl J Med. 383:2018–2029. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baba K and Goto Y: Lorlatinib as a
treatment for ALK-positive lung cancer. Future Oncol. 18:2745–2766.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuang S and Leighl NB: Lorlatinib in
ALK-rearranged lung cancer. Cancer Cell. 39:25–27. 2021. View Article : Google Scholar : PubMed/NCBI
|