|
1
|
Nadile M, Kornel A, Sze NSK and Tsiani E:
A comprehensive review of Genistein's effects in preclinical models
of cervical cancer. Cancers (Basel). 16:352023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cervical Cancer Treatment, . Patient
version. PDQ Cancer Information Summaries. National Cancer
Institute; Bethesda, MD: 2002
|
|
3
|
Ikeda S, Ueda Y, Hara M, Yagi A, Kitamura
T, Kitamura Y, Konishi H, Kakizoe T, Sekine M, Enomoto T and Sobue
T: Human papillomavirus vaccine to prevent cervical intraepithelial
neoplasia in Japan: A nationwide case-control study. Cancer Sci.
112:839–846. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marth C, Landoni F, Mahner S, McCormack M,
Gonzalez-Martin A, Colombo N and Committee EG: Cervical cancer:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 28 (Suppl 4):iv72–iv83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meijer CJLM, Berkhof J, Castle PE,
Hesselink AT, Franco EL, Ronco G, Arbyn M, Bosch FX, Cuzick J,
Dillner J, et al: Guidelines for human papillomavirus DNA test
requirements for primary cervical cancer screening in women 30
years and older. Int J Cancer. 124:516–520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolf JL, Billingsley CC, Kendler A and
Jackson AL: Cervical stratified mucin-producing intraepithelial
lesion: A systematic review of diagnosis and management. J Low
Genit Tract Dis. 24:259–264. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fischer M, Uxa S, Stanko C, Magin TM and
Engeland K: Human papilloma virus E7 oncoprotein abrogates the
p53-p21-DREAM pathway. Sci Rep. 7:26032017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rebolj M, Rimmer J, Denton K, Tidy J,
Mathews C, Ellis K, Smith J, Evans C, Giles T, Frew V, et al:
Primary cervical screening with high risk human papillomavirus
testing: observational study. BMJ. 364:l2402019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Argyri E, Tsimplaki E, Daskalopoulou D,
Stravopodis DJ, Kouikoglou O, Terzakis E and Panotopoulou E: E6/E7
mRNA expression of high-risk HPV types in 849 Greek women.
Anticancer Res. 33:4007–4011. 2013.PubMed/NCBI
|
|
10
|
Hoppe-Seyler K, Bossler F, Braun JA,
Herrmann AL and Hoppe-Seyler F: The HPV E6/E7 oncogenes: Key
factors for viral carcinogenesis and therapeutic targets. Trends
Microbiol. 26:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang R, Pan W, Jin L, Huang W, Li Y, Wu D,
Gao C, Ma D and Liao S: Human papillomavirus vaccine against
cervical cancer: Opportunity and challenge. Cancer Lett.
471:88–102. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Derbie A, Mekonnen D, Woldeamanuel Y, Van
Ostade X and Abebe T: HPV E6/E7 mRNA test for the detection of high
grade cervical intraepithelial neoplasia (CIN2+): A systematic
review. Infect Agent Cancer. 15:92020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryan D and Giele H: The scratch collapse
test: A QUADAS-2 assessment of a systematic review. J Plast
Reconstr Aesthet Surg. 72:1418–1833. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
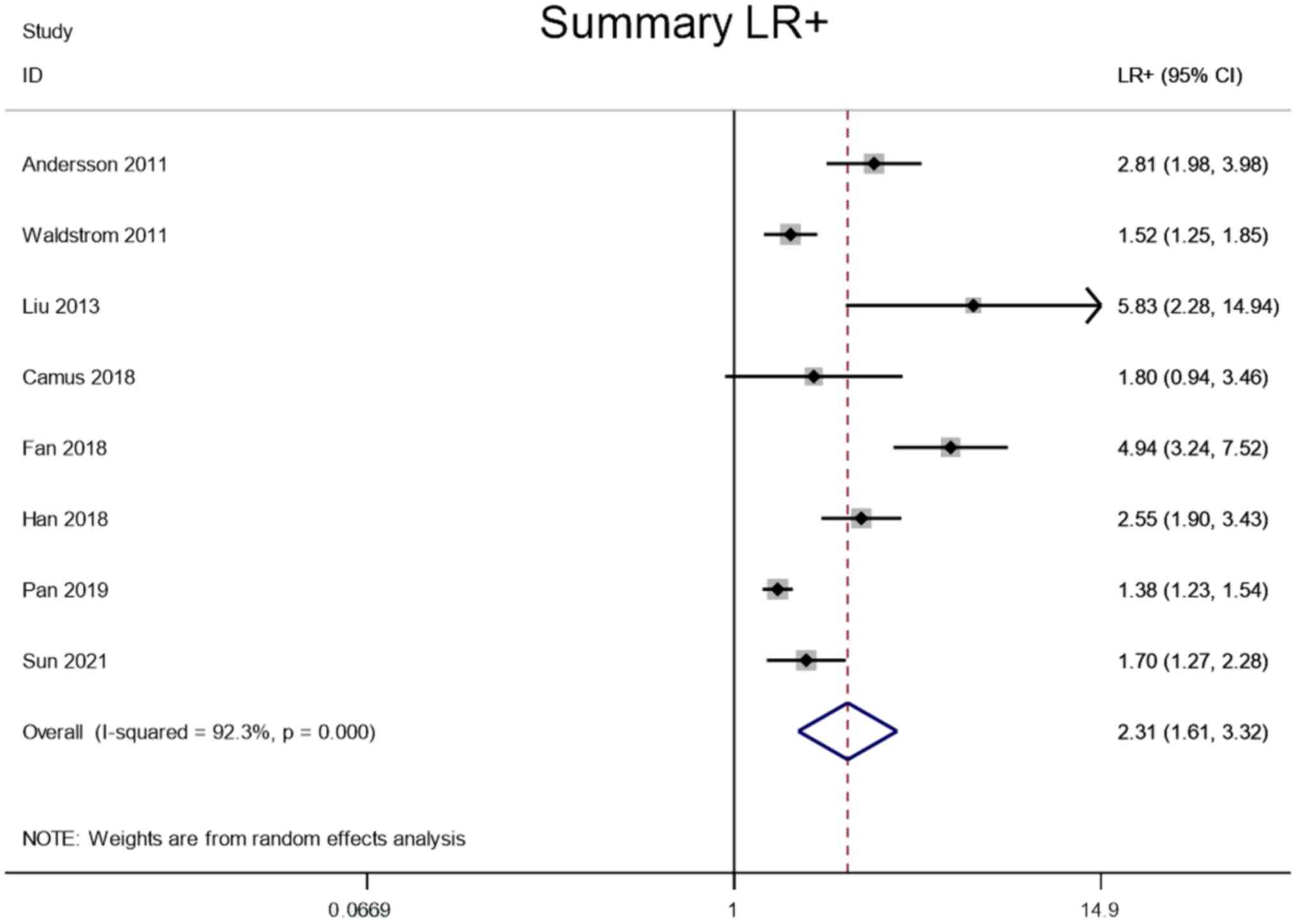
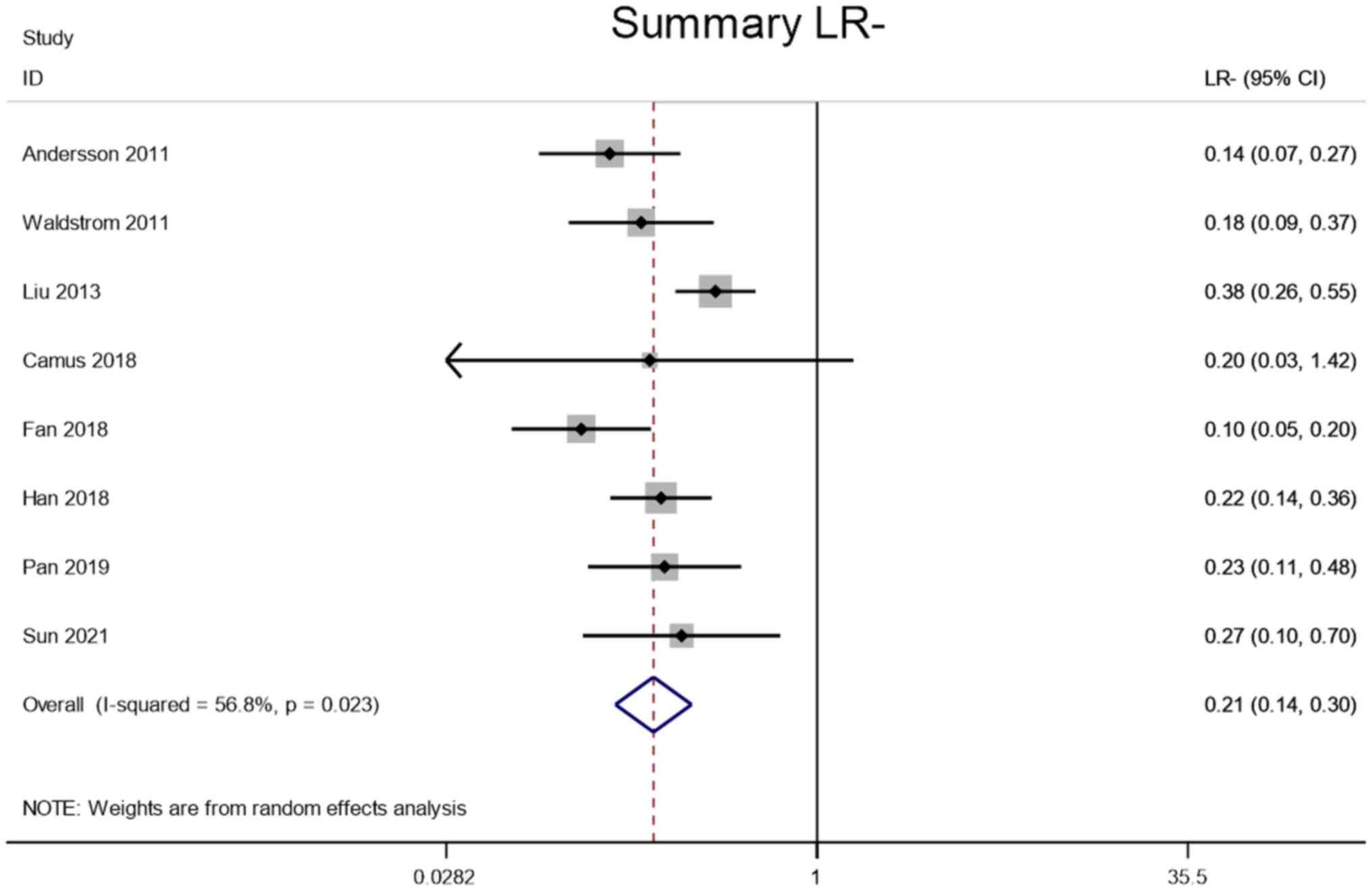
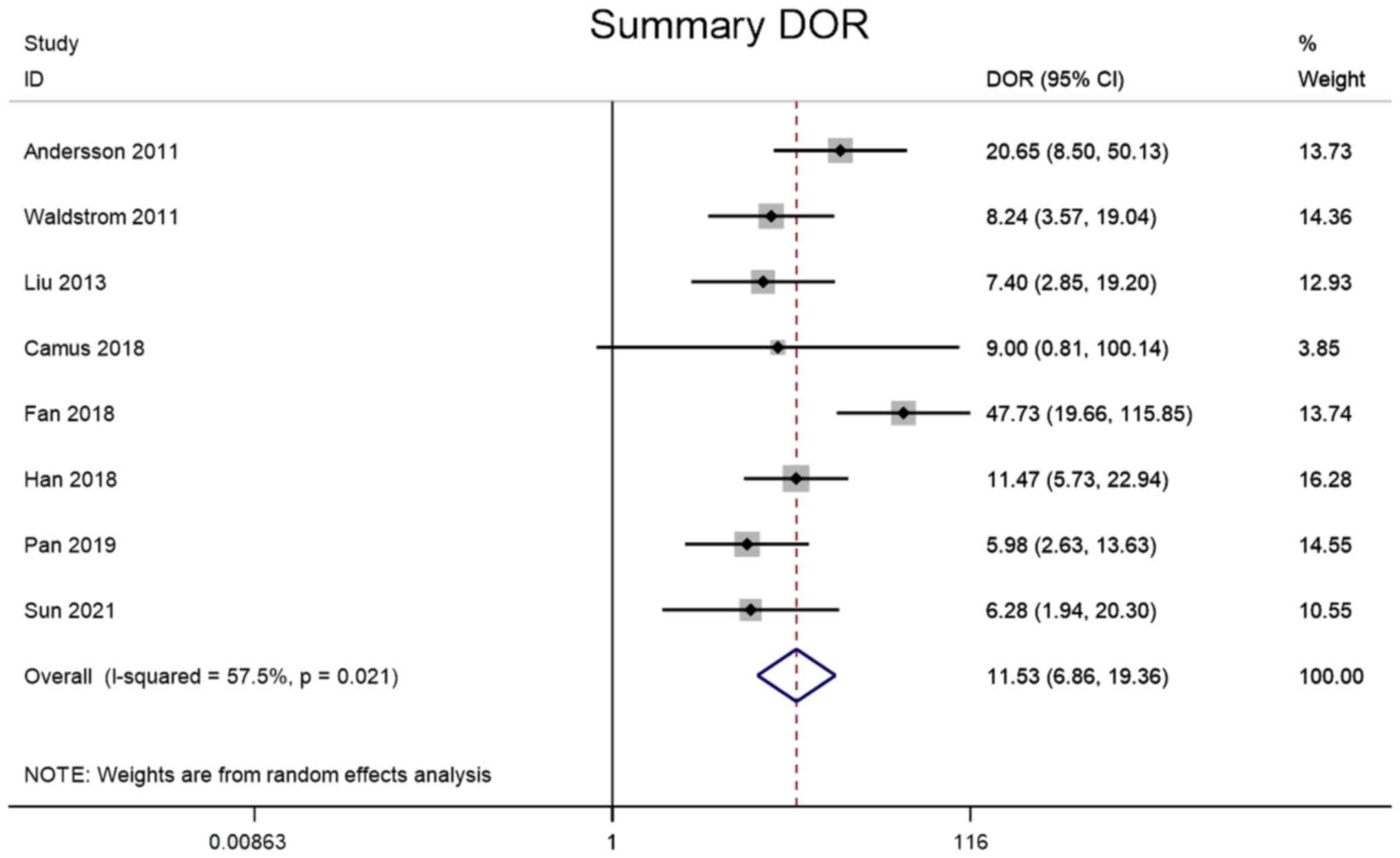
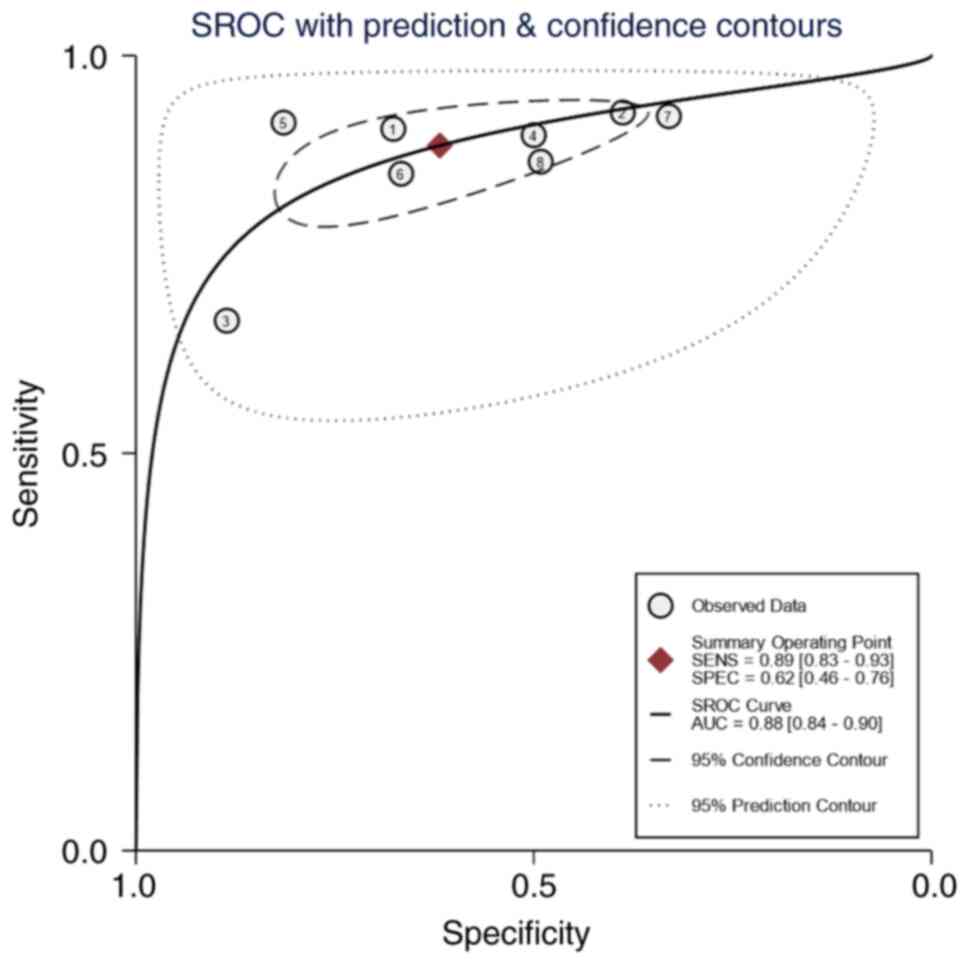
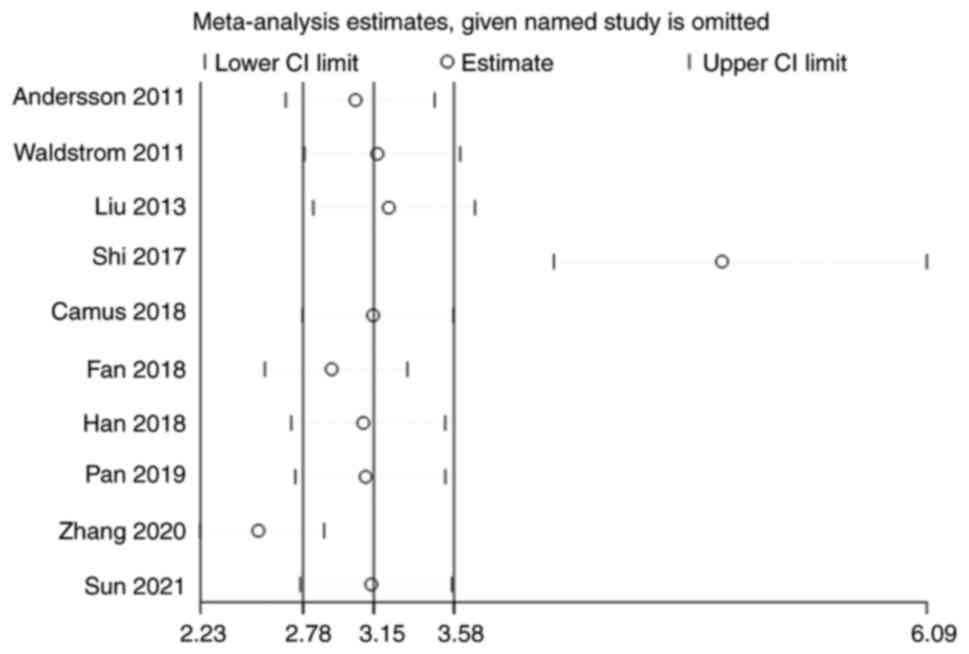
Andersson E, Kärrberg C, Rådberg T,
Blomqvist L, Zetterqvist BM, Ryd W, Lindh M and Horal P:
Type-specific human papillomavirus E6/E7 mRNA detection by
real-time PCR improves identification of cervical neoplasia. J Clin
Microbiol. 49:3794–3799. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Waldstrom M and Ornskov D: Clinical
performance of a human papillomavirus messenger RNA test (Aptima
HPV Assay) on residual material from archived 3-year-old PreservCyt
samples with low-grade squamous intraepithelial lesion. Arch Pathol
Lab Med. 135:1052–1056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu TY, Xie R, Luo L, Reilly KH, He C, Lin
YZ, Chen G, Zheng XW, Zhang LL and Wang HB: Diagnostic validity of
human papillomavirus E6/E7 mRNA test in cervical cytological
samples. J Virol Methods. 196:120–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi WJ, Liu H, Wu D, Tang ZH, Shen YC and
Guo L: E6/E7 proteins are potential markers for the screening and
diagnosis of cervical pre-cancerous lesions and cervical cancer in
a Chinese population. Oncol Lett. 14:6251–6258. 2017.PubMed/NCBI
|
|
18
|
Camus C, Vitale S, Loubatier C, Pénaranda
G, Khiri H, Plauzolles A, Carcopino X, Halfon P and Giordanengo V:
Quantification of HPV16 E6/E7 mRNA spliced isoforms viral load as a
novel diagnostic tool for improving cervical cancer screening. J
Clin Med. 7:5302018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan Y and Shen Z: The clinical value of
HPV E6/E7 and STAT3 mRNA detection in cervical cancer screening.
Pathol Res Pract. 214:767–775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han L, Husaiyin S, Zhao F, Rezhake R and
Niyazi M: Clinical value of human papillomavirus E6/E7 mRNA
detection in screening for cervical cancer in women positive for
human papillomavirus DNA or. Clin Lab. 64:1363–1371. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan D, Zhang CQ, Liang QL and Hong XC: An
efficient method that combines the ThinPrep cytologic test with
E6/E7 mRNA testing for cervical cancer screening. Cancer Manag Res.
11:4773–4780. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang SK, Guo Z, Wang P, Kang LN, Jia MM,
Wu ZN, Chen Q, Cao XQ, Zhao DM, Guo PP, et al: The potential
benefits of HPV E6/E7 mRNA test in cervical cancer screening in
China. Front Oncol. 10:5332532020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun J, Yue Y, Li R, Sun Q, Hu C, Ge X and
Guan Q: Detection of HPV E6/E7 mRNA in the diagnosis of cervical
cancer and precancerous lesions after kidney transplantation. Am J
Transl Res. 13:7312–7317. 2021.PubMed/NCBI
|
|
24
|
Qian S, Zhang S, Lu M, Chen S, Liu L, Liu
S, Jiang F and Zhang J: The accuracy of screening tools for
sarcopenia in older Chinese adults: A systematic review and
meta-analysis. Front Public Health. 12:13103832024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Molden T, Kraus I, Skomedal H, Nordstrom T
and Karlsen F: PreTect HPV-proofer: Real-time detection and typing
of E6/E7 mRNA from carcinogenic human papillomaviruses. J Virol
Methods. 142:204–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Basu P, Banerjee D, Mittal S, Dutta S,
Ghosh I, Chowdhury N, Abraham P, Chandna P and Ratnam S:
Sensitivity of APTIMA HPV E6/E7 mRNA test in comparison with hybrid
capture 2 HPV DNA test for detection of high risk oncogenic human
papillomavirus in 396 biopsy confirmed cervical cancers. J Med
Virol. 88:1271–1278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song Y, Zhang M, Zhang C, Du S and Zhai F:
HPV E6/E7 mRNA combined with thin-prep cytology test for the
diagnosis of residual/recurrence after loop electrosurgical
excision procedure in patients with cervical intraepithelial
neoplasia. Diagn Microbiol Infect Dis. 108:1161192024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duvlis S, Popovska-Jankovic K, Arsova ZS,
Memeti S, Popeska Z and Plaseska-Karanfilska D: HPV E6/E7 mRNA
versus HPV DNA biomarker in cervical cancer screening of a group of
Macedonian women. J Med Virol. 87:1578–1586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Wang J, Zhang R, Lei F and Lai S:
Application value of detection of high-risk HPV infection in early
cervical cancer patients in disease diagnosis and prognosis
evaluation. Clin Lab. 66:2020. View Article : Google Scholar
|