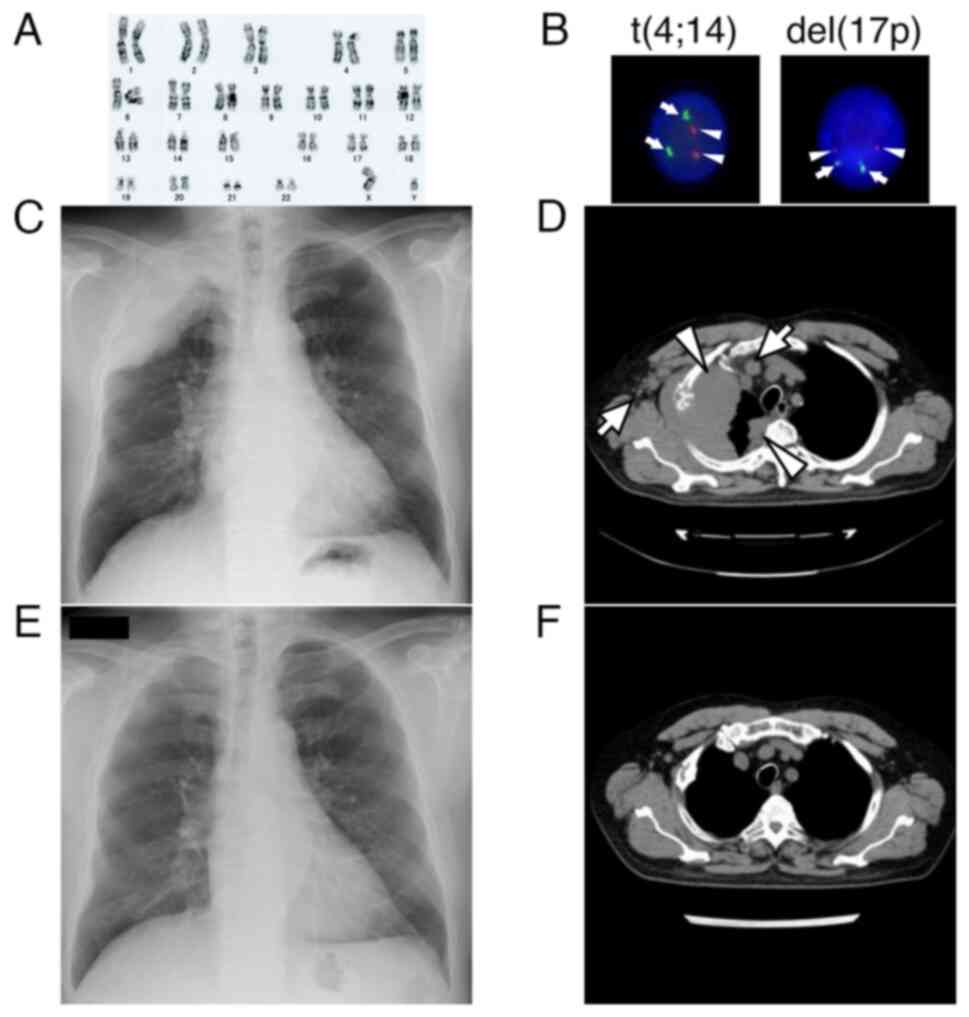
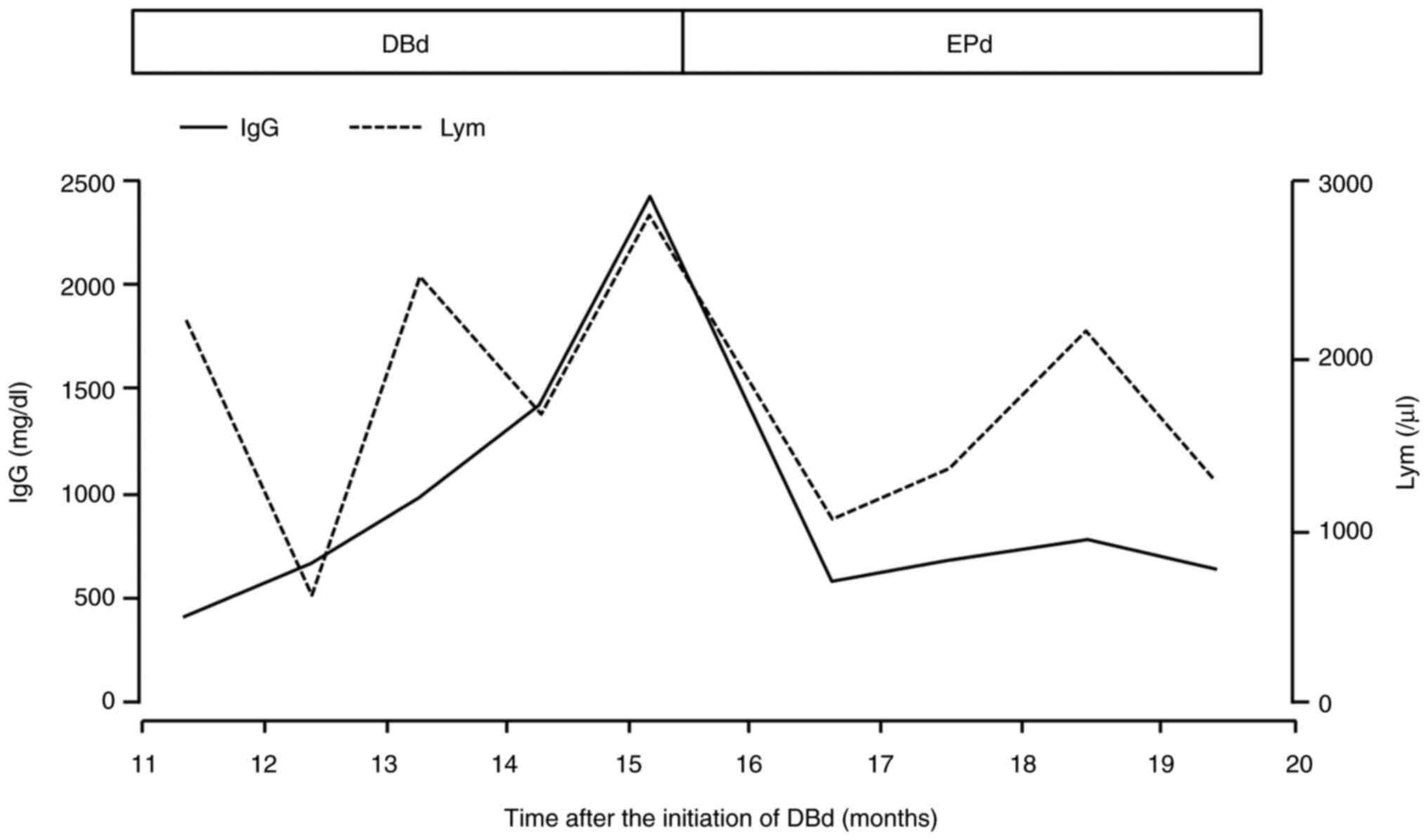
|
1
|
Bladé J, Beksac M, Caers J, Jurczyszyn A,
von Lilienfeld-Toal M, Moreau P, Rasche L, Rosiñol L, Usmani SZ,
Zamagni E and Richardson P: Extramedullary disease in multiple
myeloma: A systematic literature review. Blood Cancer J. 12:452022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laubach JP, van de Donk N, Davies FE and
Mikhael J: Practical considerations for antibodies in myeloma. Am
Soc Clin Oncol Educ Book. 38:667–674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Casneuf T, Xu XS, Adams HC III, Axel AE,
Chiu C, Khan I, Ahmadi T, Yan X, Lonial S, Plesner T, et al:
Effects of daratumumab on natural killer cells and impact on
clinical outcomes in relapsed or refractory multiple myeloma. Blood
Adv. 24:2105–2114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
His ED, Steinle R, Balasa B, Szmania S,
Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y,
et al: CS1, a potential new therapeutic antibody target for the
treatment of multiple myeloma. Clin Cancer Res. 14:2775–2784. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tai YT, Dillon M, Song W, Leiba M, Li XF,
Burger P, Lee AI, Podar K, Hideshima T, Rice AG, et al: Anti-CS1
humanized monoclonal antibody HuLuc63 inhibits myeloma cell
adhesion and induces antibody-dependent cellular cytotoxicity in
the bone marrow milieu. Blood. 112:1329–1337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoylman E, Brown A, Perissinotti AJ,
Marini BL, Pianko M, Ye JC, Campagnaro E and Nachar VR: Optimal
sequence of daratumumab and elotuzumab in relapsed and refractory
multiple myeloma. Leuk Lymphoma. 61:691–698. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimazu Y, Kanda J, Kosugi S, Ito T,
Kaneko H, Imada K, Shimura Y, Fuchida SI, Fukushima K, Tanaka H, et
al: Efficacy of elotuzumab for multiple myeloma in reference to
lymphocyte counts and kappa/lambda ratio or B2 microglobulin. Sci
Rep. 13:51592023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised International Staging System
for multiple myeloma: A report from International Myeloma Working
Group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar S, Paiva B, Anderson KC, Durie B,
Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, et
al: International Myeloma Working Group consensus criteria for
response and minimal residual disease assessment in multiple
myeloma. Lancet Oncol. 17:e328–e346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palumbo A, Chanan-Khan A, Weisel K, Nooka
AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV,
et al: Daratumumab, bortezomib, and dexamethasone for multiple
myeloma. N Engl J Med. 375:754–766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dimopoulos MA, Dytfeld D, Grosicki S,
Moreau P, Takezako N, Hori M, Leleu X, LeBlanc R, Suzuki K, Raab
MS, et al: Elotuzumab plus pomalidomide and dexamethasone for
multiple myeloma. N Engl J Med. 379:1811–1822. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Badar T, Srour S, Bashir Q, Shah N,
Al-Atrash G, Hosing C, Popat U, Nieto Y, Orlowski RZ, Champlin R
and Qazilbash MH: Predictors of inferior clinical outcome in
patients with standard-risk multiple myeloma. Eur J Haematol.
98:263–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rasche L, Bernard C, Topp MS, Kapp M,
Duell J, Wesemeier C, Haralambieva E, Maeder U, Einsele H and Knop
S: Features of extramedullary myeloma relapse: High proliferation,
minimal marrow involvement, adverse cytogenetics: A retrospective
single-center study of 24 cases. Ann Hematol. 91:1031–1037. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Danhof S, Rasche L, Mottok A, Steinmüller
T, Zhou X, Schreder M, Kilian T, Strifler S, Rosenwald A, Hudecek
M, et al: Elotuzumab for the treatment of extramedullary myeloma: A
retrospective analysis of clinical efficacy and SLAMF7 expression
patterns. Ann Hematol. 100:1537–1546. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krejcik J, Casneuf T, Nijhof IS, Verbist
B, Bald J, Plesner T, Syed K, Liu K, van de Donk NW, Weiss BM, et
al: Daratumumab depletes CD38+ immune regulatory cells, promotes
T-cell expansion, and skews T-cell repertoire in multiple myeloma.
Blood. 128:384–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dimopoulos MA, Oriol A, Nahi H, San-Miguel
J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki
K, et al: Daratumumab, lenalidomide, and dexamethasone for multiple
myeloma. N Engl J Med. 375:1319–1331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dimopoulos MA, Oriol A, Nahi H, San-Miguel
J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Suzuki K, Plesner T,
et al: Overall survival with daratumumab, lenalidomide, and
dexamethasone in previously treated multiple myeloma (POLLUX): A
randomized, open-label, phase III trial. J Clin Oncol.
41:1590–1599. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sonneveld P, Chanan-Khan A, Weisel K,
Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos
MV, et al: Overall survival with daratumumab, bortezomib, and
dexamethasone in previously treated multiple myeloma (CASTOR): A
randomized, open-label, phase III trial. J Clin Oncol.
41:1600–1609. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mateos MV, Dimopoulos MA, Cavo M, Suzuki
K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Kaplan P, et al:
Daratumumab plus bortezomib, melphalan, and prednisone for
untreated myeloma. N Engl J Med. 378:518–528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mateos MV, Cavo M, Blade J, Dimopoulos MA,
Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, et al:
Overall survival with daratumumab, bortezomib, melphalan, and
prednisone in newly diagnosed multiple myeloma (ALCYONE): A
randomised, open-label, phase 3 trial. Lancet. 395:132–141. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Facon T, Kumar S, Plesner T, Orlowski RZ,
Moreau P, Bahlis N, Basu S, Nahi H, Hulin C, Quach H, et al:
Daratumumab plus lenalidomide and dexamethasone for untreated
myeloma. N Engl J Med. 380:2104–2115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Facon T, Kumar SK, Plesner T, Orlowski RZ,
Moreau P, Bahlis N, Basu S, Nahi H, Hulin C, Quach H, et al:
Daratumumab, lenalidomide, and dexamethasone versus lenalidomide
and dexamethasone alone in newly diagnosed multiple myeloma (MAIA):
Overall survival results from a randomised, open-label, phase 3
trial. Lancet Oncol. 22:1582–1596. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danhof S, Strifler S, Hose D, Kortüm M,
Bittrich M, Hefner J, Einsele H, Knop S and Schreder M: Clinical
and biological characteristics of myeloma patients influence
response to elotuzumab combination therapy. J Cancer Res Clin
Oncol. 145:561–571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thangaraj JL, Ahn SY, Jung SH, Vo MC, Chu
TH, Thi Phan MT, Kwon M, Lee KH, Kim M, Song GY, et al: Expanded
natural killer cells augment the antimyeloma effect of daratumumab,
bortezomib, and dexamethasone in a mouse model. Cell Mol Immunol.
18:1652–1661. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kurdi AT, Glavey SV, Bezman NA, Jhatakia
A, Guerriero JL, Manier S, Moschetta M, Mishima Y, Roccaro A,
Detappe A, et al: Antibody-dependent cellular phagocytosis by
macrophages is a novel mechanism of action of elotuzumab. Mol
Cancer Ther. 17:1454–1463. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dimopoulos MA, Dytfeld D, Grosicki S,
Moreau P, Takezako N, Hori M, Leleu X, LeBlanc R, Suzuki K, Raab
MS, et al: Elotuzumab plus pomalidomide and dexamethasone for
relapsed/refractory multiple myeloma: Final overall survival
analysis from the randomized phase II ELOQUENT-3 trial. J Clin
Oncol. 41:568–578. 2023. View Article : Google Scholar : PubMed/NCBI
|