Introduction
Breast cancer (BC) remains a leading cause of
cancer-related mortality in female patients, reflecting profound
disease heterogeneity, metastasis and therapeutic resistance
(1). The heterogeneity of this
tumour is determined mainly by the expression of the estrogen
receptor/progesterone receptor (ER/PR), human epidermal growth
factor receptor 2 (HER2) and the proliferative index of the Ki-67
antigen, which are considered the basis for the molecular
classification of BC and selecting appropriate treatment approach
(2–10). The relationship between the
receptors expressed on BC cells in terms of C-X-C motif chemokine
ligand 8 (CXCL8) and C-X-C chemokine receptor (CXCR)1/2 is the
subject of numerous studies and controversy (11–13).
For this reason, it seems reasonable to better understand the role
of this system in the network of interactions shaping the tumour
microenvironment (TME), which may be used in the development of
potential diagnostic or prognostic markers, but also potentially
become the target of therapeutic intervention.
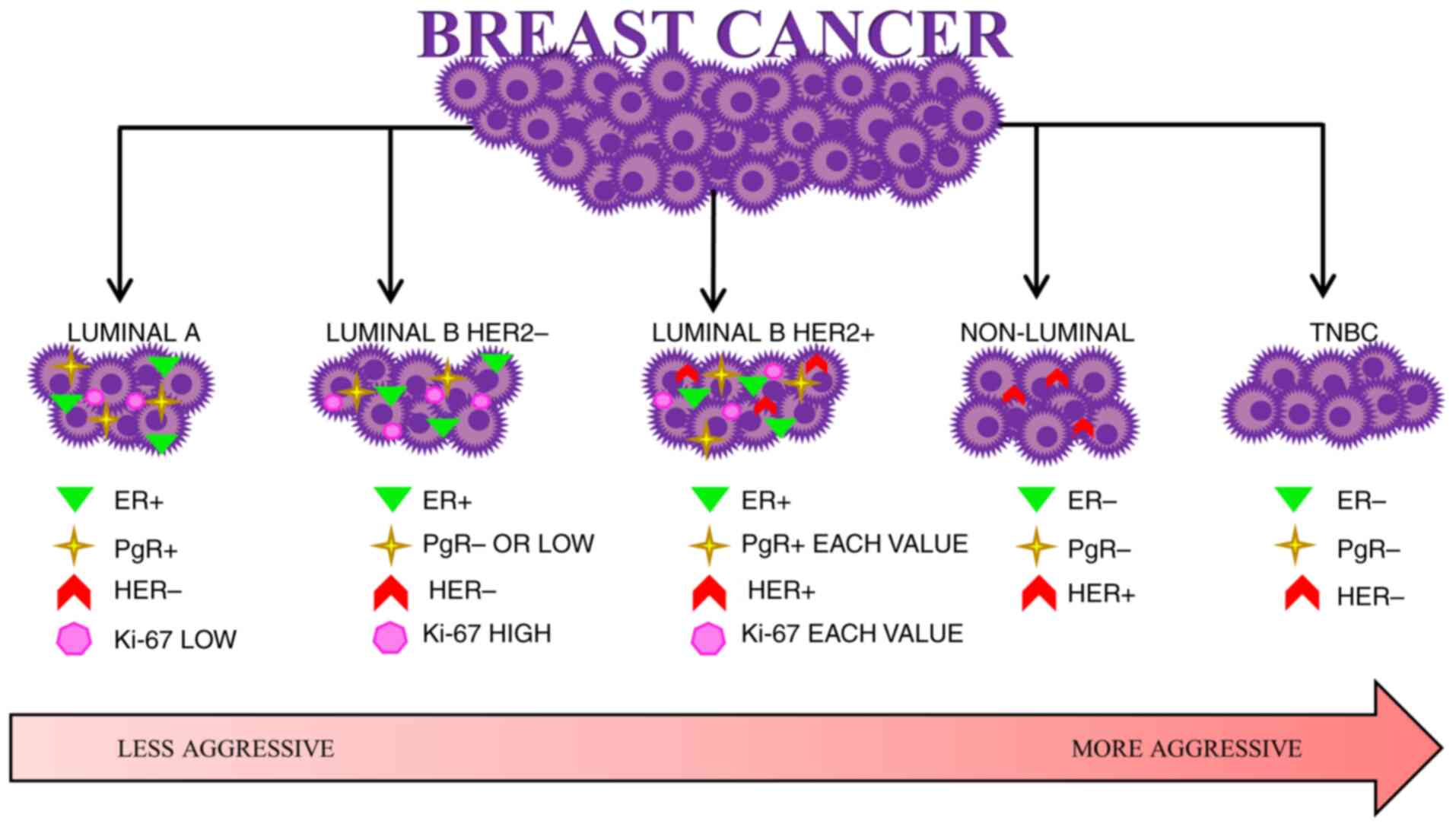
Due to the determination of the presence or absence
of expression of the aforementioned receptors, the following
molecular subtypes of BC can be distinguished: Luminal A, luminal
B, non-luminal and basal triple-negative BC (TNBC). Luminal A
cancer is ER+ and PR+ and is characterised by
a low level of Ki-67 (Ki-67<14%) and lack of HER2 expression
(HER2−). Luminal B cell cancer is also divided by either
the presence or absence of HER2. HER2− luminal B cancer
is ER+, can be PR− or characterized by low PR
expression (PR<20%) and high Ki-67 expression (>20%), while
HER2+ luminal B cancer is ER+, and the
expression of PR and Ki-67 is variable. Non-luminal cancers are
HER2+ and ER− and PR−. TNBC is
ER−, PR− and HER2− (14–19).
The BC classification is shown in Fig.
1.
The CXCL8 chemokine, also known as interleukin
(IL)-8, belongs to the group of chemokines that participate in the
activation of neutrophils and the recruitment of granulocytes at
the site of inflammation (12,20–23).
It is secreted by monocytes/macrophages, lymphocytes, neutrophils,
fibroblasts, endothelial and epithelial cells. CXCL8 synthesis
occurs under the influence of tumour necrosis factor-α (TNF-α),
IL-1, IL-6 and environmental and chemical stressors such as hypoxia
and reactive oxygen species (12,22,24).
CXCL8 may increase the immunoregulatory capacity to
defend against cancer and may also modify the TME thus facilitating
tumour development (20,25). This chemokine can attract
neutrophils, myeloid-derived suppressor cells and tumour-associated
macrophages, cancer-associated fibroblasts to the TME, which are
the source of both pro-cancer and anti-cancer factors. It has been
proven that the presence of tumour infiltrating neutrophils has a
strong relationship with disease progression and the lack of
effects in the implemented treatment. It was recently suggested
that neutrophil extracellular traps activate cancer cells,
influence cancer growth and development, and promote metastasis
processes. For this reason, tumour cells produce CXCL8 and
consequently attract cells expressing CXCR1 and CXCR2, resulting in
a reduced ability to prevent tumour growth (25–33).
CXCL8 is expressed at high levels in ER−
BC and increases the invasiveness and metastatic potential of both
ER− and ER+ BC cells. It is also expressed at
high levels in HER2+ BC (34). The elevated serum CXCL8 level is
associated with advanced clinical status, high tumour burden and
earlier presence of distant metastases (20,35).
CXCL8 can bind to two membrane receptors, CXCR1 and CXCR2,
initiating the activation of multiple intracellular signalling
pathways. Moreover, CXCR1 is specific to the CXCL8 chemokine,
unlike CXCR2 which may also bind to other ILs (21,25,36).
These receptors are present on the surface of various cells,
including normal and neoplastic cells (21,37).
The CXCL8-CXCR1/2 signalling axis may play a notable
role in the process of carcinogenesis and formation of secondary
neoplastic foci by controlling the process of proliferation and
self-renewal of cancer stem cells (CSCs) (12,21,31).
The CXCL8-CXCR1 signalling pathway enhances tumour cell
proliferation, while the CXCL8-CXCR2 pathway affects angiogenesis
(20).
The aim of the current study was to analyse the
concentration of CXCL8 and its receptors, CXCR1 and CXCR2, in the
serum of female patients with invasive BC and to evaluate the
expression of these parameters at the mRNA level, taking into
account the molecular subtypes and grades of cancer, and
considering the fact that so far these parameters have not been
assessed in a single study and in the same patients at the protein
and mRNA level.
Materials and methods
Study group
The study group of the present study consisted of 62
female patients aged 39–83 (mean age ± SD, 65.35±12.67 years) with
histopathologically confirmed invasive BC. The patients were
diagnosed at the Oncology Outpatient Clinic of the Regional
Specialist Hospital No. 3 in Rybnik due to a solid breast lump
detected using imaging, specifically breast ultrasound and
mammography. Patients were referred for laboratory tests and a
thick-needle biopsy of the breast nodule. If axillary lymph node
metastasis was suspected in ultrasound findings and detection of
enlarged lymph nodes on physical examination, a fine-needle biopsy
of the suspected lymph nodes was also recommended. All patients
underwent imaging, specifically chest X-ray, abdominal ultrasound
and a CT scan in some situations to investigate the presence of
distant metastases.
Patients with other chronic diseases, including
cancer and autoimmune diseases, were excluded. Patients who were
not on drug treatment were included in the present study. The
results of the histopathological examination confirmed invasive BC
and additionally included the information on histological type,
degree of malignancy (G1, G2 and G3), where G1, G2 and G3 referred
to highly, moderately and poorly differentiated BC, respectively,
and receptor status (expression of ER, PR and HER2) as well as
expression of the Ki67 proliferation index. Based on clinical data,
tumour staging according to the TNM classification was assessed
(38). Molecular features included
in the histopathological protocol allowed patients to be classified
into one of the following types of BC: Luminal A (n=21),
HER− luminal B (n=25), HER+ luminal B (n=5),
HER+ non-luminal (n=4) and basal TNBC (n=7).
Histological examination was based on microscopic
evaluation of material stained with haematoxylin and eosin.
Briefly, 4% aqueous formaldehyde solution was used as a fixative
for 24–48 h at room temperature. The clinical material was then
sliced on a semi-automatic microtome into 4 µm thick slices.
Material was stained with Mayer's Hematoxylin (5 min), water eosin
(2 min) at room temperature. The material was evaluated under an
Olympus BX43 light microscope using 20×, 40× and/or 60×
magnification. Then, immunohistochemical tests were performed to
determine the expression of estrogen, progesterone and the HER2
receptors, as well as Ki 67, p63 and E-cadherin. When HER2
expression was ambiguous, CISH or FISH testing was ordered.
VENTANA® HER2 Dual ISH DNA Probe Cocktail was used with
the Ventana Benchmark Ultra automatic stainer. At the end of each
incubation step, the BenchMark IHC/ISH instrument washes the
sections to remove unbound material and applies a liquid coverslip
which minimizes the evaporation of the aqueous reagents from the
slide. Results are interpreted using a light microscope using 20×,
40×, and/or 60×.
Control group
The control group consisted of 18 female patients
aged 28–76 (mean age ± SD, 46.50±13.09 years) with
histopathologically confirmed fibroadenoma, a benign breast nodule.
Patients with other chronic diseases, including cancer, were
excluded. The material analyzed was serum and whole blood. The tube
obtained for clotting after 30 min was centrifuged at 1,500 × g for
15 min at room temperature, and the serum obtained was dissected
and frozen at −80°C. Similarly, whole blood was stored at the same
temperature. Thick-needle biopsy of the tumour was performed under
ultrasound guidance, after prior local anaesthesia of the tumour
area with 2% lignocaine. Laboratory tests, imaging and
histopathological examinations were performed at the Diagnostic
Centre of the Regional Specialist Hospital in Rybnik. The
biological material used in the present study was collected between
September 2021 and January 2023.
The present study was conducted according to the
guidelines of the Declaration of Helsinki and approved by the
Ethics Committee of Medical University of Silesia in Katowice,
Poland (protocol code PCN/CBN/0022/KB1/75/21).
ELISA tests
Serum CXCL8 (IL-8) concentration was determined
using a sandwich ELISA immunoenzymatic assay using the CLOUD-CLONE
Human Interleukin-8 ELISA kit from Cloud-Clone Corp. The kit allows
in vitro quantification of CXCL8 in human serum,
anticoagulants EDTA, heparin and citrate in plasma, and saliva. The
sensitivity of the assay was 5.9 pg/ml. The concentration of CXCR1
(IL-8 Ra) and CXCR2 (IL-8 Rb) was determined using a sandwich ELISA
immunoenzymatic assay with the CLOUD-CLONE ELISA kit from
Cloud-Clone Corp. The kit allows in vitro quantification of
the α and β receptor for IL-8 in human tissue homogenates, cell
lysates and other human biological fluids. The sensitivity of the
assay for CXCR1 was 0.054 ng/ml, while that for CXCR2 was 0.33
ng/ml.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA extraction was performed using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and assessed prior to analysis with the use of MaestroNano
MN-913 (MaestroGen, Inc.). The quantitative analysis of CXCL8,
CXCR1 and CXCR2 transcripts was carried out using
GoTaq® 1-Step RT-qPCR System (Promega Corporation),
KiCqStart SYBR Green primers (Sigma-Aldrich; KGaA) as follows:
CXCL8, forward (F) 5′-TACTCCAAACCTTTCCACC-3′, reverse (R)
5′-CTCAGCCCTCTTCAAAAAC-3′; CXCR1, F
5′-TTAAGTCACTCTGATCTCTGAC-3′, R 5′-TGGTTTGATCTAACTGAAGC-3′;
CXCR2, F 5′-GTGATAGCTGAGAATATGCAG-3′, R
5′-ACTTAAATCCTGACTGGGTC-3′; β-actin, F
5′-GACGACATGGAGAAAATCTG-3′, R 5′-ATGATCTGGGTCATCTTCTC-3′ and
LightCycler® 480 System (Roche Diagnostics). All steps
were performed according to the manufacturers' instructions.
Reaction specificity was confirmed by the melting curve analysis
and agarose gel electrophoresis. Relative expression levels of the
studied genes were calculated using the 2−ΔΔCq method
and β-actin as an internal control (39).
Statistical analysis
The obtained results were statistically analysed
using Statistica (version 13.3, StatSoft Polska Sp. z o.o.). The
normality of distribution of the studied variables was assessed
using the Shapiro-Wilk test. The median and interquartile range
were determined for the tested parameters, and the obtained results
were compared using the Mann-Whitney test. Correlation was
investigated using Spearman's rank correlation and presented as a
correlation coefficient (r). P<0.05 was considered to indicate a
statistically significant difference.
Results
Concentration of CXCL8, CXCR1 and
CXCR2
The serum levels of CXCL8, CXCR1 and CXCR2 were
determined in female patients in the control group and female
patients with BC. As the obtained results did not follow a normal
distribution, they were presented as a median with a lower and
upper interquartile range (Q1 and Q3). The
analysis of the results showed a significantly higher concentration
of CXCL8 in the serum of female patients with invasive BC compared
with in controls (P<0.05). No statistically significant
differences were observed with regards to the other parameters
(Tables I and II).
 | Table I.Serum concentrations of CXCL8 and its
receptors in female patients with invasive BC (n=62) and in the
control group (n=18). |
Table I.
Serum concentrations of CXCL8 and its
receptors in female patients with invasive BC (n=62) and in the
control group (n=18).
| Characteristic | Invasive BC
group | Control group | P-value |
|---|
| Age, years | 65.35±12.67 | 46.50±13.09 | P<0.05 |
| Serum CXCL8,
pg/ml | 16.68
(11.70–21.20) | 11.04
(7.29–16.79) | P<0.05 |
| Serum CXCR1,
ng/ml | 0.06
(0.04–0.07) | 0.06
(0.03–0.07) | NS |
| Serum CXCR2,
ng/ml | 0.81
(0.47–1.35) | 0.59
(0.38–0.80) | NS |
 | Table II.Serum concentrations of parameters in
female patients with BC considering molecular subtypes of BC and in
the control group. |
Table II.
Serum concentrations of parameters in
female patients with BC considering molecular subtypes of BC and in
the control group.
|
|
| BC subtype |
|
|---|
|
|
|
|
|
|---|
| Studied
parameters | Statistical
parameters | Luminal A
(n=21) | Luminal B
HER2− (n=25) | Luminal B
HER2+ (n=5) | Non-luminal
(n=4) | TNBC (n=7) | Control (n=18) |
|---|
| CXCL8, ng/ml | Me | 17.23 | 17.67 | 13.25 | 9.72 | 11.93 | 11.04 |
|
|
Q1-Q3 | 12.37–21.65 | 12.37–20.76 | 10.60–20.10 | 4.53–29.82 | 8.39–29.82 | 7.29–16.79 |
|
| P-value |
<0.05a |
<0.05a | >0.05 | >0.05 | >0.05 |
|
| CXCR1, pg/ml | Me | 0.05 | 0.06 | 0.07 | 0.07 | 0.07 | 0.06 |
|
|
Q1-Q3 | 0.04–0.07 | 0.04–0.08 | 0.05–0.07 | 0.06–0.08 | 0.05–0.23 | 0.03–0.07 |
|
| P-value | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
|
| CXCR2, pg/ml | Me | 0.92 | 0.67 | 1.35 | 0.81 | 0.67 | 0.60 |
|
|
Q1-Q3 | 0.64–1.30 | 0.44–1.35 | 0.97–1.35 | 0.56–1.10 | 0.47–0.97 | 0.38–0.80 |
|
| P-value | >0.05 | >0.05 |
<0.01b | >0.05 | >0.05 |
|
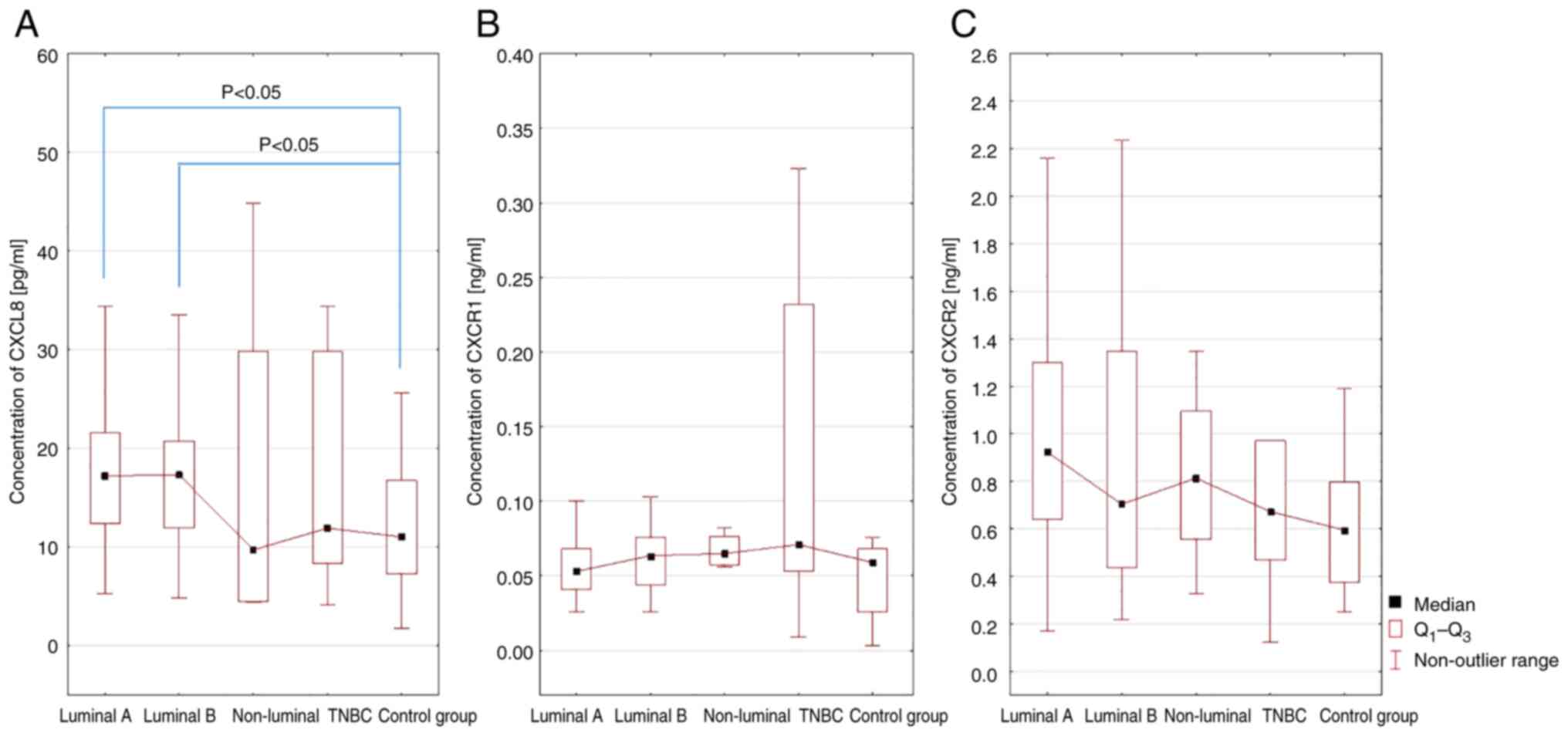
Next, the serum concentrations of CXCL8, CXCR1 and
CXCR2 in female patients with luminal A, luminal B, non-luminal and
TNBC were investigated compared with those in the control group. A
statistically significant difference was shown only for CXCL8 serum
levels in female patients with luminal A and luminal B BC compared
with the control group (P<0.05; Fig.
2).
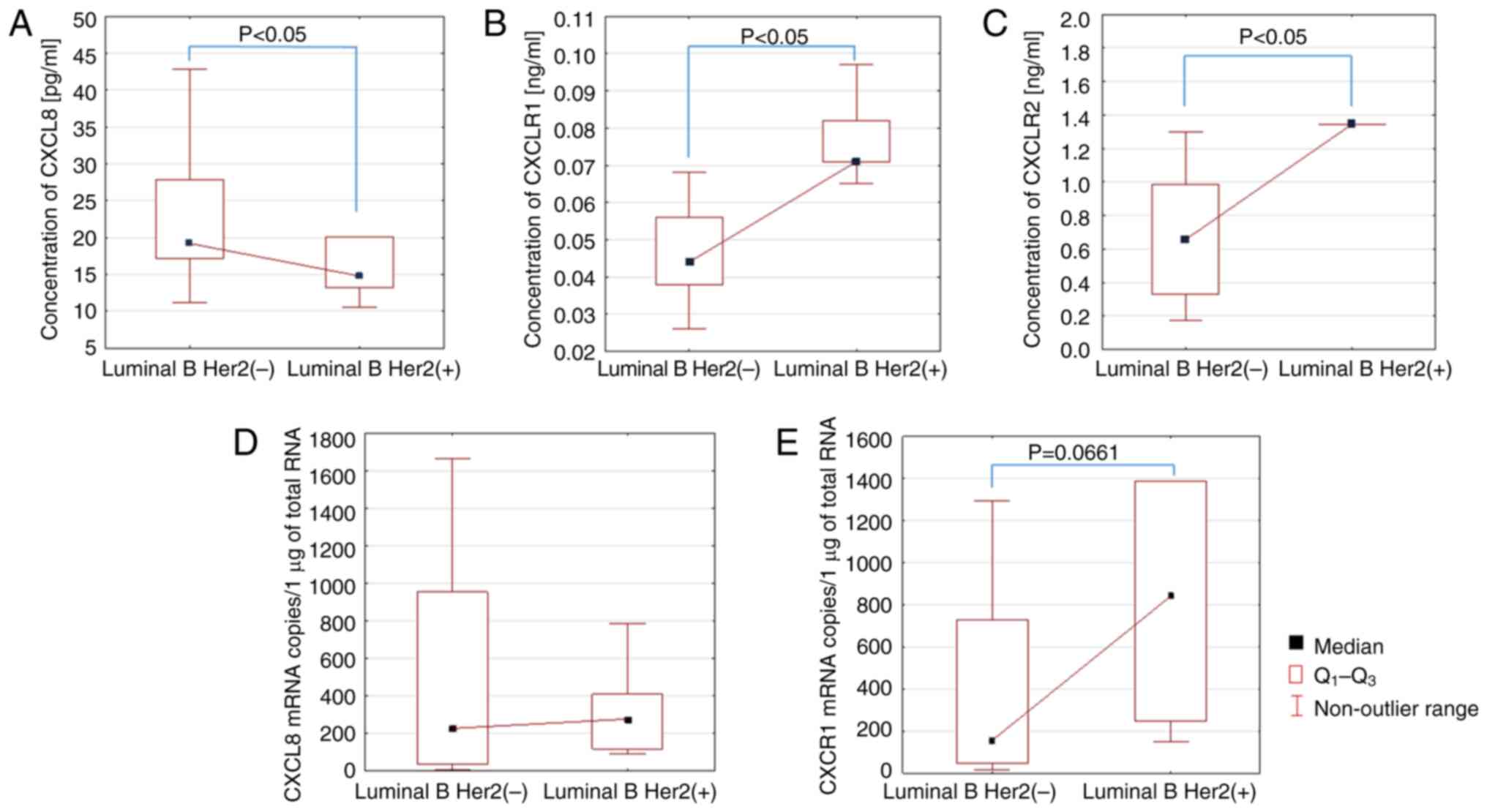
After that, serum CXCL8 levels were assessed in
patients with luminal B HER2− and luminal B
HER2+ BC. The analysis performed showed a statistically
significant reduction in serum CXCL8 levels in female patients with
luminal B HER2+ BC compared with luminal B
HER2− BC (P<0.05; Fig.
3A). On the other hand, the analysis of CXCR1 and CXCR2 levels
showed a significant increase in serum levels of female patients
with luminal B HER2+ BC compared with luminal B
HER2− BC (P<0.05; Fig. 3B
and C).
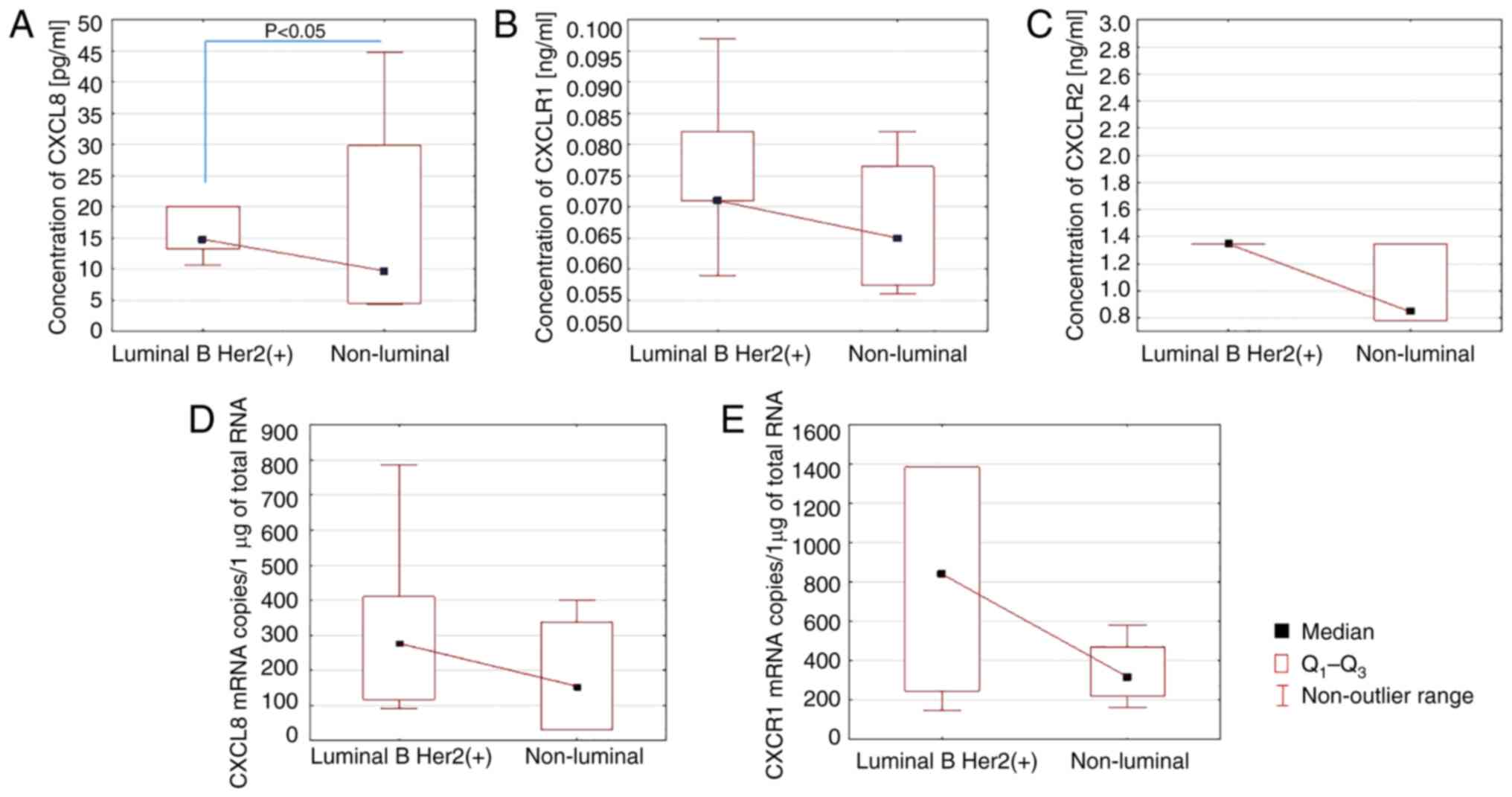
The analysis of the serum levels of the parameters
studied in patients with luminal B HER2+ and non-luminal
BC showed a significant reduction in CXCL8 levels in the serum of
patients with non-luminal cancer (P<0.05; Fig. 4A). There was no statistical
correlation between the serum levels of CXCR1 and CXCR2 in the
studied patients with luminal B HER2+ and non-luminal
cancer.
In addition, further analysis assessed the way the
serum concentrations of the studied parameters developed in female
patients with BC at the successive stages of the disease.
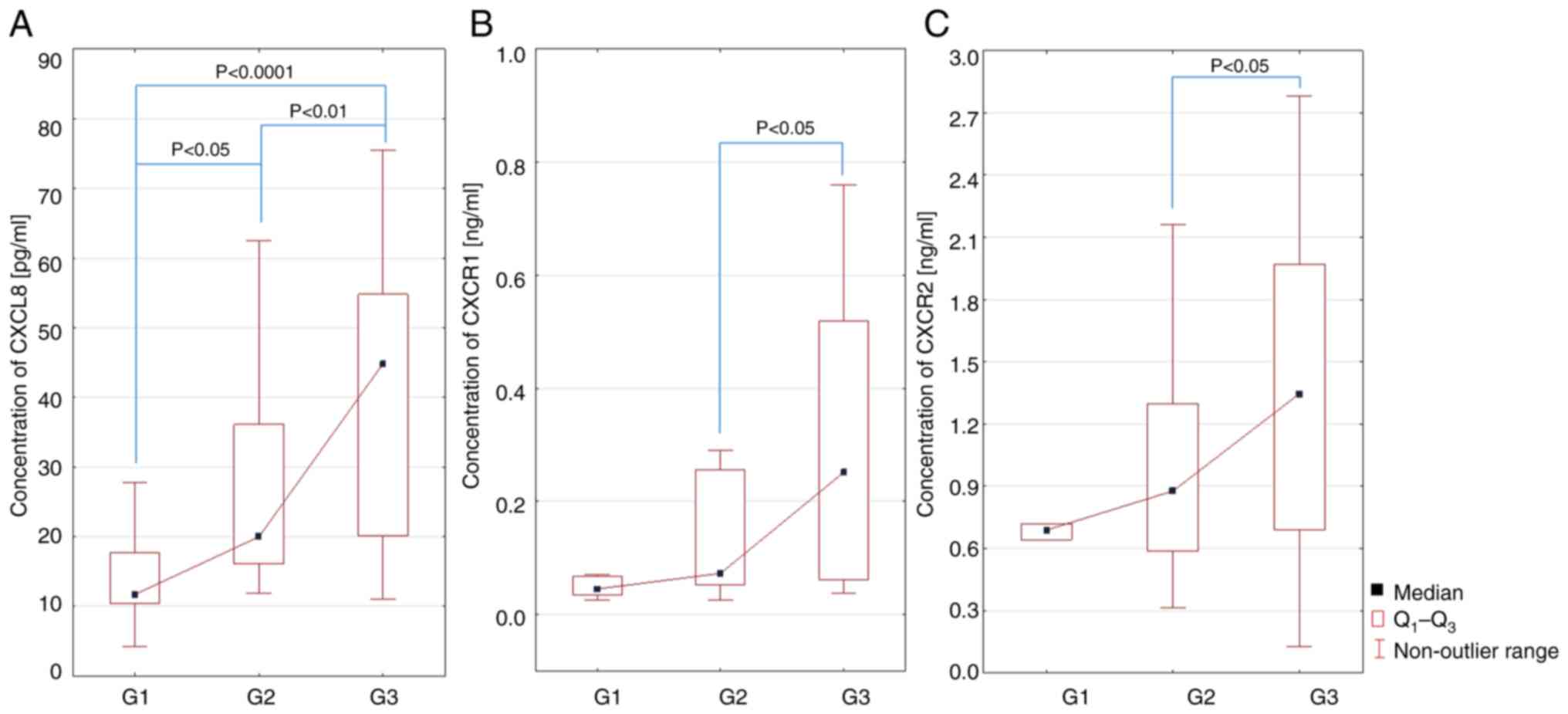
The analysis of CXCL8 serum levels in female
patients with BC showed a statistically significant difference
between the clinical stage G1 and G2 (P<0.05; Fig. 5A), G2 and G3 (P<0.01; Fig. 5A) and G1 and G3 (P<0.0001;
Fig. 5A). On the other hand, the
analysis of CXCR1 and CXCR2 serum levels in the studied patients
showed a statistically significant difference between G2 and G3
(P<0.05, Fig. 5B and C).
mRNA expression levels of CXCL8 and
its receptors CXCR1 and CXCR2
The assays at the transcript level showed an
increase in the mRNA copy number of the CXCR1 gene in the group of
female patients with luminal B HER2+ BC compared with
luminal B HER2− BC. However, this was not a
statistically significant difference, yet there was a trend towards
a statistical significance (P=0.0661; Fig. 3E). For the CXCL8 and CXCR2 genes, no
differences in the transcript copy number were observed.
Furthermore, there were no differences in mRNA copy number of the
analysed genes between luminal B HER2+ and non-luminal
HER2+ cancers. There were also no differences in the
mRNA copy number of the analysed genes depending on the stage of
the disease.
Discussion
The CXCL8-CXCR1/2 signalling axis is one of the
numerous mechanisms stimulating the immune system against cancer
development and possibly affecting the TME, promoting its
development. This pathway plays an important role in the formation
of a number of cancer types including breast, ovarian, prostate,
lung, colorectal, gastric and melanoma cancer (20).
A number of studies are available on the role of the
signalling pathway involving CXCL8 and its receptors CXCR1 and
CXCR2 in BC (13,20,27,40–42).
The current study presented a new aspect in the study of the
pathway, with both the expression and the serum levels of the
CXCL8-CXCR1/R2 axis being determined for the first time in the same
patient, allowing for a deeper analysis of the correlation involved
and indicating the clinical aspect.
The studies conducted so far have shown that the
chemokine CXCL8 in BC affects the process of tumour formation
because all BC cells express CXCR1 and CXCR2 (13,20,27,40–42).
CXCL8 synthesised by cancer cells initiates the neovascularization
process by stimulating vascular endothelial growth factor. The
emerging new blood vessels initiate the process of BC development,
but also provide distant metastases with nutrients supplied with
the blood (20). CXCL8 acts
directly on cancer cells in TNBC, making them more invasive and
aggressive. Based on a mouse TNBC model, Liubomirski et al
(27) showed that CXCL8 regulated
by CXCR2 and C-C motif chemokine ligand 2 (CCL2) regulated by the
receptor for chemokine CCL2 (CCR2) affect tumour-associated
neutrophils and macrophages and influence their migration to the
tumour site.
The aim of the current study was to assess the
expression of the chemokine CXCL8 and its receptors CXCR1 and CXCR2
in patients with invasive BC and additionally to assess the
concentration of these parameters in the serum, considering the
molecular subtypes and clinical stages. In the present analysis, a
significantly increased concentration of this chemokine was
observed in the group of patients with confirmed invasive BC
compared with the group of female patients diagnosed with benign
tumours (P<0.05), which confirms the involvement of CXCL8 in the
development of BC. The results of the current study are consistent
with the observations of Ma et al (13), Zare Moaiedi et al (40), Snoussi et al (41) and Motyka et al (42) who showed an increased concentration
of CXCL8 in patients with BC compared with healthy female
individuals. In addition, a statistical significance was observed
between the clinical stages G1 and G2, G2 and G3, and G1 and G3
(P<0.05, P<0.01 and P<0.0001, respectively).
Similar studies were conducted by Wang et al
(43), who analysed the
concentration of selected chemokines and their receptors, including
the CXCL8 chemokine in patients with BC. Their results showed that
during BC, the concentration of CXCL8 was markedly different in all
examined cases, ranging from a benign lesion to invasive cancer. In
addition, the authors found that tumour size was associated with
CXCL8 concentration. Moreover, Ma et al (13) showed that the concentration of CXCL8
is not only associated with the stage of clinical advancement but
is also associated with the occurrence of secondary neoplastic
foci.
Chemokines, including CXCL8 and its receptors CXCR1
and CXCR2, are involved in the autocrine proliferation and
metastasis of cancer cells by supporting tumour signalling
pathways, epithelial-mesenchymal transition or also by acquiring
resistance to chemotherapy treatment (44). Moreover, it is assumed that the
proliferation of CSCs may affect the process of cancer cell
migration (12). However,
Todorović-Raković and Milovanović (34) suggested that CXCL8 may promote the
formation of secondary neoplastic foci also in a paracrine manner
by accumulating neutrophils and tumour suppressor cells at the site
of tumour development, resulting in the creation of a highly
immunogenic and pro-cancer tumour environment (44). The TME is an important element not
only in the process of angiogenesis, but also in the process of
growth, survival of cancer cells, signalling between cells in the
tumour environment and infiltration of a number of cells to the
tumour site, thus contributing to the increase in the invasive
nature of cancer. As pointed out by Messeha et al (45), especially in BC, CXCL8 and CCL2 play
an important pro-cancer role.
Motyka et al (42) showed markedly increased CXCL8
concentration in the luminal BC subtype compared with that in group
of patients with benign lesions and healthy female individuals. The
obtained results are consistent with those of the present study
which showed a statistical significance between the concentration
of CXCL8 in patients with luminal BC compared with that in patients
with benign lesions (P<0.05). A similar study was conducted by
Wang et al (43), who
analysed selected chemokines at various stages of BC. The authors
showed there was a notable difference between CXCL8, CXCL12 and
CXCR4 concentration and BC stage. In addition, they also showed
that the concentration of the chemokine CXCL8 was associated with
the size of the tumour. Todorović-Raković and Milovanović (34) indicated a high expression of CXCL8
in ER− BC. According to the authors, this chemokine
increases the invasiveness and metastatic potential of both
ER− and ER+ BC cells and is also highly
expressed in HER2+ BC.
Erlichman et al (46) indicated that chemokines play an
important role in programmed death-ligand 1 signalling in TNBC
cells by autocrine signalling through chemokine receptors,
especially CCR2 and CCR5, and to a lesser extent also CXCR1/2,
which results in an increased secretion or increased synthesis of
CCL2, CCL5 and CXCL8. The authors suggested that these chemokines
activate specific receptors through a feedback mechanism.
The biological activity of chemokines is determined
by the existence of specific, intrinsic receptors (12,21,47).
There are numerous studies on the role of CXCR1/2 receptors in
carcinogenesis. Xue et al (48) analysed the expression of CXCR1 in
physiological breast tissue, breast fibroadenoma and invasive BC
using immunohistochemistry. They showed that in physiological
breast tissue only a few cells expressed CXCR1, while in
fibroadenoma the percentage of cells expressing this receptor was
higher. In BC, almost all cells expressed CXCR1, which, according
to the authors, suggests the involvement of CXCR1 in the
pathogenesis of BC. A similar study was conducted by Snoussi et
al (41), who showed that the
occurrence of polymorphisms in the CXCL8 and CXCR2 genes
contributes to an increased risk of BC development and increases
the aggressiveness of the course of the disease.
In the present study, no difference between serum
CXCR1 concentration was identified during luminal, non-luminal and
TNBC compared with that in the control group. However, a
significantly increased concentration of CXCR1 was observed in
luminal B HER2+ BC compared with that in luminal B
HER2− BC (P<0.05), which may indicate the involvement
of this receptor in the process of BC carcinogenesis.
The studies available so far have shown that the
expression of CXCR2 is higher in cancerous tissue characterised by
a high degree of malignancy compared with benign lesions and normal
breast tissue (49–51). According to Liu et al
(11), CXCR2 is an important factor
that may facilitate the process of metastasis, where the main
location of secondary tumour foci are bones. CXCR2 promotes BC
metastasis by blocking AKT1 and stimulating COX2. According to
Vazquez et al (52), the
expression of CXCR1 and CXCR2 may vary depending on the subtype of
BC. The authors found that CXCR1 expression was notably lower in
TNBC compared with HER2+ luminal A and luminal B BC. On
the other hand, lower expression of CXCR2 was found in luminal B
HER2+ carcinoma compared with luminal A carcinoma. In
the present study, a statistically significant difference between
increased concentration of CXCR1 and CXCR2 in the serum of patients
with luminal B HER2+ BC compared with the group of
patients with luminal B HER2− BC was observed. A
significant correlation between the concentration of CXCR1 and
CXCR2 in luminal B HER2+ carcinoma compared with
non-luminal BC was not observed.
Previous studies have shown that changes in gene
expression at the mRNA level assessed in blood samples of patients
with BC may constitute potential diagnostic markers differentiating
patients from healthy ones (53,54).
However, there are still no studies evaluating these parameters in
the ‘clinical approach’ (53). The
molecular analysis of the present study showed no relationship
between the number of mRNA copies of genes in HER2+
luminal B and non-luminal HER2+ BC. Moreover, the number
of transcript copies was not shown to be dependent on the stage of
the disease. The assays at the mRNA level indicated that the
expression of the genes of the immune system studied circulating in
the blood is likely not the source of the protein, which may
indicate that they come from the TME. However, expression at the
mRNA level is not always associated with expression at the protein
level due to the complicated regulation mechanisms of this process.
Furthermore, there is regulation of release of soluble protein,
which may possibly be altered in cancers. Regardless of the
mechanism, the results of the present study clearly indicate that
in the case of CXCL8 as well as CXCR1 and CXCR2, it is reasonable
to measure the serum concentration of these proteins. However, the
usefulness of the evaluated expression at the mRNA level in blood
requires further research. The present study showed an increase in
the number of CXCR1 mRNA copies in the group of female patients
with luminal B HER2+ BC compared with the luminal B
HER2− BC group with a trend towards statistical
significance.
The analysis performed revealed statistically
significantly elevated concentration of CXCR1 only in luminal B
HER2(+) BC compared to the control group, which may indicate the
contribution of this receptor to the process of carcinogenesis in
this type of BC, which is probably related to the fact that the
CXCR1 receptor has a higher specificity to the chemokine CXCL8 in
contrast to the CXCR2 receptor.
Moreover, our study also showed that the increase in
CXCR1 gene mRNA copy number in the group of female patients with
luminal B HER2(+) BC compared to luminal B HER2(−) BC showed a
trend toward statistical significance.
The lack of statistically significant differences in
CXCR1/R2 concentration in other types of BC may indicate the
absence of CXCL8-mediated signalling involving these receptors in
the patients studied. The analysis of the levels of CXCL8 and its
receptors CXCR1 and CXCR2 in the serum of female patients with BC
with respect to the degrees of malignancy (G1, G2, G3) also
provided interesting observations. The obtained data indicate the
existence of a relationship between CXCL8 secretion and the degree
of malignancy of G1, G2 and G3 cancers, which indicates the
involvement of the studied chemokine in the pathomechanism of BC
development, probably influencing the increased invasiveness and
aggressiveness of cancer cells. Moreover, the demonstration of a
correlation also between the concentration of CXCR1 and CXCR2
receptor in the serum of the studied patients and the degree of G2
and G3 malignancy proves their important role in the process of
tumorigenesis, which may find a potential application in diagnosis,
but this requires further research.
Furthermore, the results obtained provide a
rationale for further studies, which we intend to conduct in the
future on a larger group of patients, particularly including a
larger study group with triple-negative BC (TNBC), which may allow
us to demonstrate that measuring CXCR1 and CXCR2 levels will
distinguish luminal BC from TNBC.
The abnormalities of the immune response involving
the CXCL8-CXCR1/2 signalling axis in patients with invasive BC
indicate a significant contribution of the studied parameters to
the development of these cancers. Moreover, the observed severity
of changes occurring at the protein level may suggest the possible
usefulness of their determination as potential diagnostic
markers.
Acknowledgements
Not applicable.
Funding
The present study was funded by Medical University of Silesia in
Katowice, Poland (grant no. PCN-1-185/K/2/O).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AMP conceptualized the study. SS, MSK, JMG, PO, CKR
and JK developed methodology and carried out formal analysis. JK
completed data curation. AMP and SS prepared the original draft of
the manuscript. JMG, PO, JK and AMP reviewed and edited the
manuscript. AMP, JMG and PO supervised the project. SS and CKR
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The current study was conducted according to the
guidelines of the Declaration of Helsinki and approved by the
Ethics Committee of Medical University of Silesia in Katowice,
Poland (protocol code PCN/CBN/0022/KB1/75/21).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nolan E, Lindeman GJ and Visvader JE:
Deciphering breast cancer: From Biology to the Clinic. Cell.
186:1708–1728. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeo SK and Guan JL: Breast cancer:
Multiple subtypes within a tumor? Trends Cancer. 3:753–760. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Z, Wei H, Li S, Wu P and Mao X: The
role of progesterone receptors in breast cancer. Drug Des Devel
Ther. 16:305–314. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang L, Chen W, Liu S and Chen C:
Targeting breast cancer stem cells. Int J Biol Sci. 19:552–570.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slepicka PF, Cyrill SL and Dos Santos CO:
Pregnancy and breast cancer: Pathways to understand risk and
prevention. Trends Mol Med. 25:866–881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Houghton SC and Hankinson SE: Cancer
progress and priorities: Breast cancer. Cancer Epidemiol Biomarkers
Prev. 30:822–844. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garrido-Castro AC, Lin NU and Polyak K:
Insights into molecular classifications of triple-negative breast
cancer: Improving patient selection for treatment. Cancer Discov.
9:176–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cocco S, Piezzo M, Calabrese A, Cianniello
D, Caputo R, Lauro VD, Fusco G, Gioia GD, Licenziato M and De
Laurentiis M: Biomarkers in triple-negative breast cancer:
State-of-the-art and future perspectives. Int J Mol Sci.
21:45792020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Faria SS, Costantini S, De Lima VCC, De
Andrade VP, Rialland M, Cedric R, Budillon A and Magalhães KG:
NLRP3 inflammasome-mediated cytokine production and pyroptosis cell
death in breast cancer. J Biomed Sci. 28:262021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jenkins S, Kachur ME, Rechache K, Wells JM
and Lipkowitz S: Rare breast cancer subtypes. Curr Oncol Rep.
23:542021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, Yang Z, Lu W, Chen Z, Chen L, Han
S, Wu X, Cai T and Cai Y: Chemokines and chemokine receptors: A new
strategy for breast cancer therapy. Cancer Med. 9:3786–3799. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ha H, Debnath B and Neamati N: Role of the
CXCL8-CXCR1/2 axis in cancer and inflammatory diseases.
Theranostics. 7:1543–1588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Y, Ren Y, Dai ZJ, Wu CJ, Ji YH and Xu
J: IL-6, IL-8 and TNF-α Levels Correlate with Disease Stage in
Breast Cancer Patients. Adv Clin Exp Med. 26:421–426. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao JJ and Swain SM: Luminal A breast
cancer and molecular assays: A review. Oncologist. 23:556–565.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kudela E, Samec M, Koklesova L, Liskova A,
Kubatka P, Kozubik E, Rokos T, Pribulova T, Gabonova E, Smolar M
and Biringer K: MiRNA expression profiles in luminal A breast
cancer-implications in biology, prognosis, and prediction of
response to hormonal treatment. Int J Mol Sci. 21:76912020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wełnicka-Jaśkiewicz M: Zalecenia Dotyczące
Uzupełniającego Leczenia Chorych Na Wczesnego Raka Piersi
Sprawozdanie z 13. Międzynarodowej Konferencji w St. Gallen.
Nowotwory. J Oncol. 63:432–435. 2013.
|
|
17
|
Melitto AS, Arias VEA, Shida JY, Gebrim LH
and Silveira L Jr: Diagnosing molecular subtypes of breast cancer
by means of raman spectroscopy. Lasers Surg Med. 54:1143–1156.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mueller C, Haymond A, Davis JB, Williams A
and Espina V: Protein biomarkers for subtyping breast cancer and
implications for future research. Expert Rev Proteomics.
15:131–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin L, Duan JJ, Bian XW and Yu S:
Triple-Negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22:612020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T,
Chen Y, Han X and Wu K: The CXCL8-CXCR1/2 pathways in cancer.
Cytokine Growth Factor Rev. 31:61–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiong X, Liao X, Qiu S, Xu H, Zhang S,
Wang S, Ai J and Yang L: CXCL8 in tumor biology and its
implications for clinical translation. Front Mol Biosci.
9:7238462022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bie Y, Ge W, Yang Z, Cheng X, Zhao Z, Li
S, Wang W, Wang Y, Zhao X, Yin Z and Li Y: The crucial role of
CXCL8 and its receptors in colorectal liver metastasis. Dis
Markers. 2019:80234602019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joseph PRB, Sawant KV and Rajarathnam K:
Heparin-Bound chemokine CXCL8 monomer and dimer are impaired for
CXCR1 and CXCR2 Activation: Implications for gradients and
neutrophil trafficking. Open Biol. 7:1701682017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Waugh DJJ and Wilson C: The Interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han ZJ, Li YB, Yang LX, Cheng HJ, Liu X
and Chen H: Roles of the CXCL8-CXCR1/2 axis in the tumor
microenvironment and immunotherapy. Molecules. 27:1372021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zha C, Meng X, Li L, Mi S, Qian D, Li Z,
Wu P, Hu S, Zhao S, Cai J and Liu Y: Neutrophil extracellular traps
mediate the crosstalk between glioma progression and the tumor
microenvironment via the HMGB1/RAGE/IL-8 Axis. Cancer Biol Med.
17:154–168. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liubomirski Y, Lerrer S, Meshel T,
Rubinstein-Achiasaf L, Morein D, Wiemann S, Körner C and Ben-Baruch
A: Tumor-Stroma-Inflammation networks promote pro-metastatic
chemokines and aggressiveness characteristics in triple-negative
breast cancer. Front Immunol. 10:7572019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng X, Ji Z and Yang G: ASS1 Regulates
Immune Microenvironment via CXCL8 Signaling in Ovarian Cancer.
Biochem Biophys Res Commun. 631:86–92. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gonzalez-Aparicio M and Alfaro C:
Influence of interleukin-8 and neutrophil extracellular trap (NET)
formation in the tumor microenvironment: Is there a pathogenic
role? J Immunol Res. 2019:62521382019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ospina-Muñoz N and Vernot JP: Partial
acquisition of stemness properties in tumorspheres obtained from
interleukin-8-treated MCF-7 cells. Tumour Biol.
42:10104283209794382020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mishra A, Suman KH, Nair N, Majeed J and
Tripathi V: An updated review on the role of the CXCL8-CXCR1/2 axis
in the progression and metastasis of breast cancer. Mol Biol Rep.
48:6551–6561. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nie G, Cao X, Mao Y, Lv Z, Lv M, Wang Y,
Wang H and Liu C: Tumor-Associated Macrophages-Mediated CXCL8
infiltration enhances breast cancer metastasis: Suppression by
danirixin. Int Immunopharmacol. 95:1071532021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai Z, Zhang M, Boafo Kwantwi L, Bi X,
Zhang C, Cheng Z, Ding X, Su T, Wang H and Wu Q: Breast cancer
cells promote self-migration by secreting interleukin 8 to induce
NET Formation. Gene. 754:1449022020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Todorović-Raković N and Milovanović J:
Interleukin-8 in breast cancer progression. J Interferon Cytokine
Res. 33:563–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Benoy IH, Salgado R, Van Dam P, Geboers K,
Van Marck E, Scharpé S, Vermeulen PB and Dirix LY: Increased serum
interleukin-8 in patients with early and metastatic breast cancer
correlates with early dissemination and survival. Clin Cancer Res.
10:7157–7162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Antonosante A, Brandolini L, d'Angelo M,
Benedetti E, Castelli V, Maestro MD, Luzzi S, Giordano A, Cimini A
and Allegretti M: Autocrine CXCL8-Dependent invasiveness triggers
modulation of actin cytoskeletal network and cell dynamics. Aging
(Albany NY). 12:1928–1951. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gales D, Clark C, Manne U and Samuel T:
The chemokine CXCL8 in carcinogenesis and drug response. ISRN
Oncol. 2013:8591542013.PubMed/NCBI
|
|
38
|
American Joint Committee on Cancer (AJCC),
. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017
|
|
39
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zare Moaiedi M, Ahmadpoor F, Rashidi M,
Ahmadzadeh A, Salmasi AA and Mohammadzadeh G: The Association
between MRNA Expression of Resistin, TNF-α, IL-6, IL-8, and ER-α in
peripheral blood mononuclear cells and breast cancer. Turk J Med
Sci. 51:1345–1353. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Snoussi K, Mahfoudh W, Bouaouina N, Fekih
M, Khairi H, Helal AN and Chouchane L: Combined Effects of IL-8 and
CXCR2gene polymorphisms on breast cancer susceptibility and
aggressiveness. BMC Cancer. 10:2832010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Motyka J, Gacuta E, Kicman A, Kulesza M,
Ławicki P and Ławicki S: Plasma levels of CXC motif chemokine 1
(CXCL1) and chemokine 8 (CXCL8) as diagnostic biomarkers in Luminal
A and B breast cancer. J Clin Med. 11:66942022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang J, He Q, Shao YG and Ji M: Chemokines
fluctuate in the progression of primary breast cancer. Eur Rev Med
Pharmacol Sci. 17:596–608. 2013.PubMed/NCBI
|
|
44
|
Amante RJ, Auf Der Maur P, Richina V,
Sethi A, Iesmantavicius V, Bonenfant D, Aceto N and Bentires-Alj M:
Protein tyrosine phosphatase shp2 controls interleukin-8 expression
in breast cancer cells. J Mammary Gland Biol Neoplasia. 27:145–153.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Messeha SS, Zarmouh NO, Mendonca P, Cotton
C and Soliman KFA: Molecular mechanism of gossypol mediating CCL2
and IL-8 attenuation in triple-negative breast cancer cells. Mol
Med Rep. 22:1213–1226. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Erlichman N, Baram T, Meshel T, Morein D,
Da'adoosh B and Ben-Baruch A: Tumor cell-autonomous pro-metastatic
activities of PD-L1 in human breast cancer are mediated by
PD-L1-S283 and chemokine axes. Cancers (Basel). 14:10422022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Molczyk C and Singh RK: CXCR1: A cancer
stem cell marker and therapeutic target in solid tumors.
Biomedicines. 11:5762023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xue MQ, Liu J, Sang JF, Su L and Yao YZ:
Expression characteristic of CXCR1 in different breast tissues and
the relevance between its expression and efficacy of neo-adjuvant
chemotherapy in breast cancer. Oncotarget. 8:48930–48937. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo F, Long L, Wang J, Wang Y, Liu Y, Wang
L and Luo F: Insights on CXC chemokine receptor 2 in breast cancer:
An emerging target for oncotherapy. Oncol Lett. 18:5699–5708.
2019.PubMed/NCBI
|
|
50
|
Romero-Moreno R, Curtis KJ, Coughlin TR,
Miranda-Vergara MC, Dutta S, Natarajan A, Facchine BA, Jackson KM,
Nystrom L, Li J, et al: The CXCL5/CXCR2 Axis is sufficient to
promote breast cancer colonization during bone metastasis. Nat
Commun. 10:44042019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Boissière-Michot F, Jacot W, Fraisse J,
Gourgou S, Timaxian C and Lazennec G: Prognostic value of CXCR2 in
breast cancer. Cancers (Basel). 12:20762020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vazquez ED, Fang X, Levesque LA, Huynh M,
Venegas C, Lu N and Salazar N: Chemokine receptors differentially
expressed by race category and molecular subtype in the breast
cancer TCGA Cohort. Sci Rep. 12:108252022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Olsen KS, Holden M, Thalabard JC,
Rasmussen Busund LT, Lund E and Holden L: Global blood gene
expression profiles following a breast cancer diagnosis-clinical
follow-up in the NOWAC post-genome cohort. PLoS One.
16:e02466502021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Delgado AB, Tylden ES, Lukic M, Moi L,
Busund LR, Lund E and Olsen KS: Cohort profile: The clinical and
multi-omic (CAMO) cohort, part of the norwegian women and cancer
(NOWAC) study. PLoS One. 18:e02812182023. View Article : Google Scholar : PubMed/NCBI
|