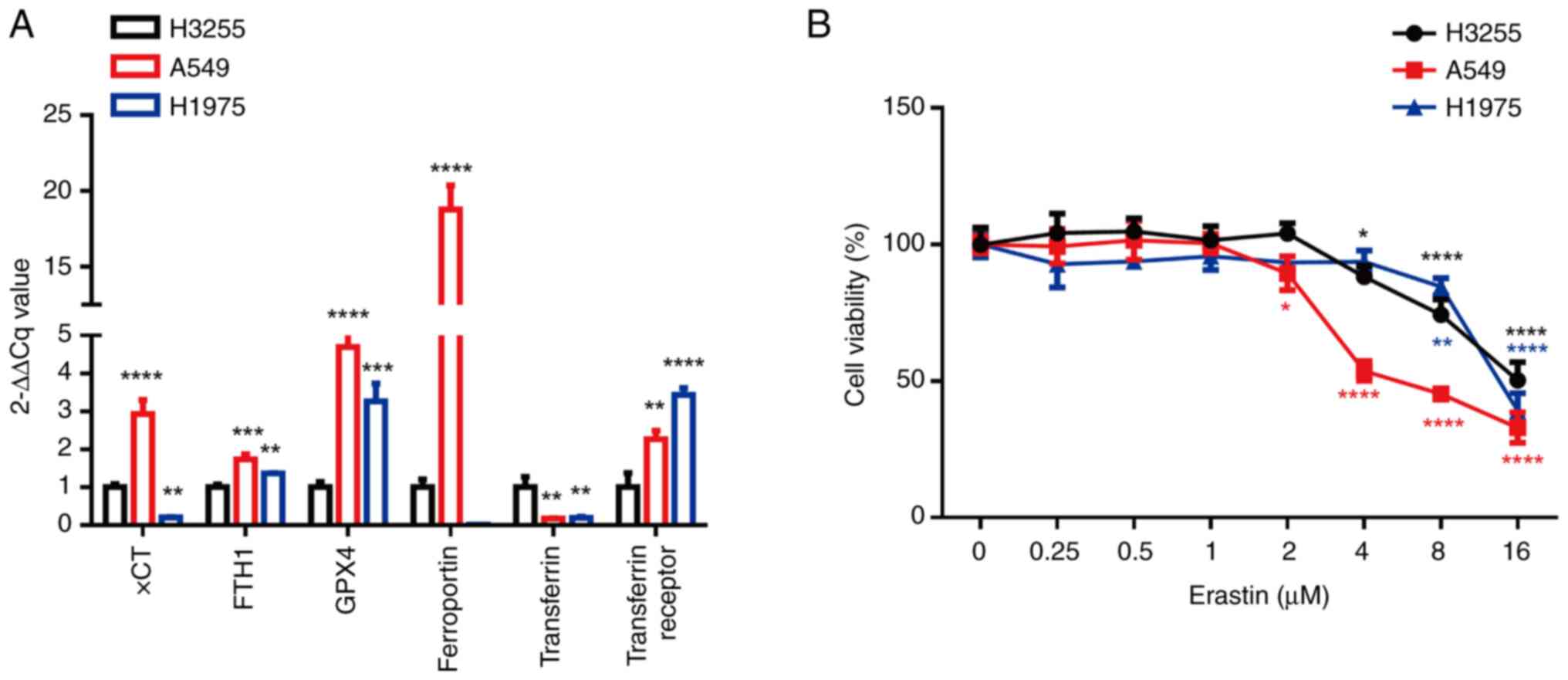
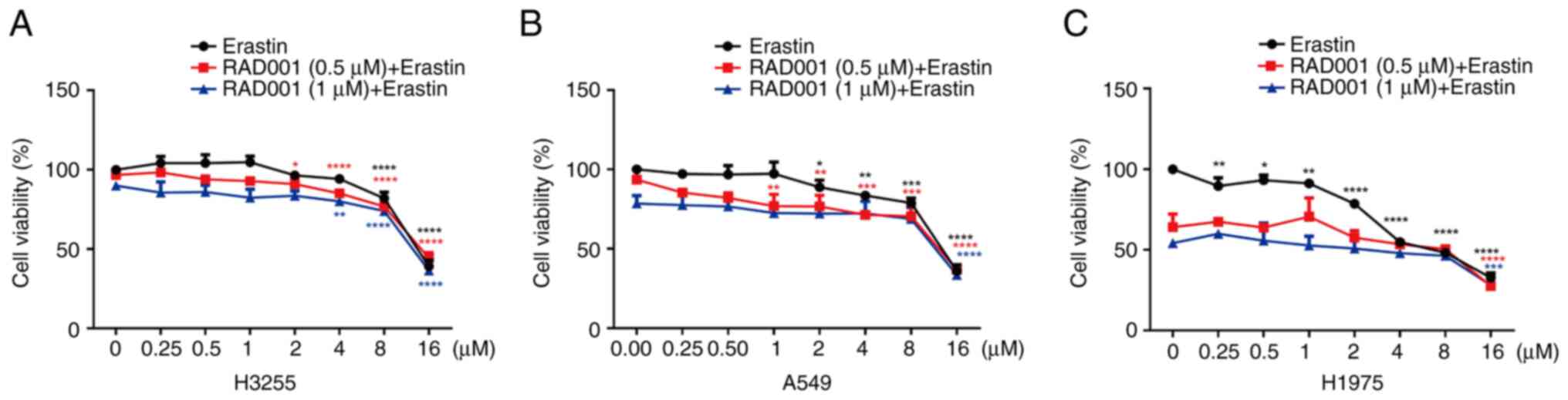
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ochi N, Takeyama M, Miyake N, Fuchigami M,
Yamane H, Fukazawa T, Nagasaki Y, Kawahara T, Nakanishi H and
Takigawa N: The complexity of EGFR exon 19 deletion and L858R
mutant cells as assessed by proteomics, transcriptomics, and
metabolomics. Exp Cell Res. 424:1135032023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamaguchi R, Okamoto T, Sato M, Hasegawa M
and Wada H: Effects of an alkaline diet on EGFR-TKI therapy in EGFR
mutation-positive NSCLC. Anticancer Res. 37:5141–5145.
2017.PubMed/NCBI
|
|
4
|
Lim SH, Lee JY, Sun JM, Ahn JS, Park K and
Ahn MJ: Comparison of clinical outcomes following gefitinib and
erlotinib treatment in non-small-cell lung cancer patients
harboring an epidermal growth factor receptor mutation in either
exon 19 or 21. J Thorac Oncol. 9:506–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang H, Pan Z, Wang W, Guo C, Chen D,
Zhang J, Zhang Y, Tang S, He J and Liang W; written on behalf of
AME Lung Cancer Cooperative Group, : The alteration of T790M
between 19 del and L858R in NSCLC in the course of EGFR-TKIs
therapy: A literature-based pooled analysis. J Thorac Dis.
10:2311–2320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller VA, Hirsh V, Cadranel J, Chen YM,
Park K, Kim SW, Zhou C, Su WC, Wang M, Sun Y, et al: Afatinib
versus placebo for patients with advanced, metastatic
non-small-cell lung cancer after failure of erlotinib, gefitinib,
or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase
2b/3 randomised trial. Lancet Oncol. 13:528–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walter AO, Sjin RT, Haringsma HJ, Ohashi
K, Sun J, Lee K, Dubrovskiy A, Labenski M, Zhu Z, Wang Z, et al:
Discovery of a mutant-selective covalent inhibitor of EGFR that
overcomes T790M-mediated resistance in NSCLC. Cancer Discov.
3:1404–1415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ward RA, Anderton MJ, Ashton S, Bethel PA,
Box M, Butterworth S, Colclough N, Chorley CG, Chuaqui C, Cross DA,
et al: Structure- and reactivity-based development of covalent
inhibitors of the activating and gatekeeper mutant forms of the
epidermal growth factor receptor (EGFR). J Med Chem. 56:7025–7048.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thress KS, Paweletz CP, Felip E, Cho BC,
Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y, et
al: Acquired EGFR C797S mutation mediates resistance to AZD9291 in
non-small cell lung cancer harboring EGFR T790M. Nat Med.
21:560–562. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou F and Zhou CC: Targeted therapies for
patients with advanced NSCLC harboring wild-type EGFR: What's new
and what's enough. Chin J Cancer. 34:310–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JK, Hahn S, Kim DW, Suh KJ, Keam B,
Kim TM, Lee SH and Heo DS: Epidermal growth factor receptor
tyrosine kinase inhibitors vs conventional chemotherapy in
non-small cell lung cancer harboring wild-type epidermal growth
factor receptor: A meta-analysis. JAMA. 311:1430–1437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JY, Kim WK, Bae KH, Lee SC and Lee EW:
Lipid metabolism and ferroptosis. Biology (Basel).
10:1842021.PubMed/NCBI
|
|
13
|
Seibt TM, Proneth B and Conrad M: Role of
GPX4 in ferroptosis and its pharmacological implication. Free Radic
Biol Med. 133:144–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Badgley MA, Kremer DM, Maurer HC,
DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J,
Firl CEM, et al: Cysteine depletion induces pancreatic tumor
ferroptosis in mice. Science. 368:85–89. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wish JB: Assessing iron status: Beyond
serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 1
(Suppl 1):S4–S8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh
HJ III, Kang R and Tang D: Autophagy promotes ferroptosis by
degradation of ferritin. Autophagy. 12:1425–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gkouvatsos K, Papanikolaou G and
Pantopoulos K: Regulation of iron transport and the role of
transferrin. Biochim Biophys Acta. 1820:188–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang X, Wang H, Han D, Xie E, Yang X, Wei
J, Gu S, Gao F, Zhu N, Yin X, et al: Ferroptosis as a target for
protection against cardiomyopathy. Proc Natl Acad Sci USA.
116:2672–2680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang C, Lu X, Liu X, Xu J, Li J, Qu T,
Dai J and Guo R: Carbonic anhydrase IX controls vulnerability to
ferroptosis in gefitinib-resistant lung cancer. Oxid Med Cell
Longev. 2023:13679382023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu C, Jiang ZB, Shao L, Zhao ZM, Fan XX,
Sui X, Yu LL, Wang XR, Zhang RN, Wang WJ, et al: β-Elemene enhances
erlotinib sensitivity through induction of ferroptosis by
upregulating lncRNA H19 in EGFR-mutant non-small cell lung cancer.
Pharmacol Res. 191:1067392023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vasan N, Razavi P, Johnson JL, Shao H,
Shah H, Antoine A, Ladewig E, Gorelick A, Lin TY, Toska E, et al:
Double PIK3CA mutations in cis increase oncogenicity and
sensitivity to PI3Kα inhibitors. Science. 366:714–723. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aylett CH, Sauer E, Imseng S, Boehringer
D, Hall MN, Ban N and Maier T: Architecture of human mTOR complex
1. Science. 351:48–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hasskarl J: Everolimus. Recent Results
Cancer Res. 211:101–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujiwara R, Taniguchi Y, Rai S, Iwata Y,
Fujii A, Fujimoto K, Kumode T, Serizawa K, Morita Y, Espinoza JL,
et al: Chlorpromazine cooperatively induces apoptosis with tyrosine
kinase inhibitors in EGFR-mutated lung cancer cell lines and
restores the sensitivity to gefitinib in T790M-harboring resistant
cells. Biochem Biophys Res Commun. 626:156–166. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ni J, Chen K, Zhang J and Zhang X:
Inhibition of GPX4 or mTOR overcomes resistance to Lapatinib via
promoting ferroptosis in NSCLC cells. Biochem Biophys Res Commun.
567:154–160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lou JS, Zhao LP, Huang ZH, Chen XY, Xu JT,
Tai WC, Tsim KWK, Chen YT and Xie T: Ginkgetin derived from Ginkgo
biloba leaves enhances the therapeutic effect of cisplatin via
ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR
wild-type non-small-cell lung cancer. Phytomedicine. 80:1533702021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan WY, Cai J, Wang JN, Gong YS and Ding
XB: Co-treatment of betulin and gefitinib is effective against EGFR
wild-type/KRAS-mutant non-small cell lung cancer by inducing
ferroptosis. Neoplasma. 69:648–656. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang Y, Chen Y, Mei Q, Chen Y, Yu S and
Xia S: Combined inhibition of the EGFR and mTOR pathways in EGFR
wild-type non-small cell lung cancer cell lines with different
genetic backgrounds. Oncol Rep. 29:2486–2492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh S, Sadhukhan S and Sonawane A: 20
Years since the approval of first EGFR-TKI, gefitinib: Insight and
foresight. Biochim Biophys Acta Rev Cancer. 1878:1889672023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morimoto K, Yamada T, Takeda T, Shiotsu S,
Date K, Tamiya N, Goto Y, Kanda H, Chihara Y, Kunimatsu Y, et al:
Clinical efficacy and safety of first- or second-generation
EGFR-TKIs after osimertinib resistance for EGFR mutated lung
cancer: A prospective exploratory study. Target Oncol. 18:657–665.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reck M, Carbone DP, Garassino M and
Barlesi F: Targeting KRAS in non-small-cell lung cancer: Recent
progress and new approaches. Ann Oncol. 32:1101–1110. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakajima EC, Drezner N, Li X,
Mishra-Kalyani PS, Liu Y, Zhao H, Bi Y, Liu J, Rahman A, Wearne E,
et al: FDA approval summary: Sotorasib for KRAS G12C-mutated
metastatic NSCLC. Clin Cancer Res. 28:1482–1486. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hofmann MH, Gerlach D, Misale S,
Petronczki M and Kraut N: Expanding the reach of precision oncology
by drugging all KRAS mutants. Cancer Discov. 12:924–937. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pao W, Wang TY, Riely GJ, Miller VA, Pan
Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG and Varmus HE: KRAS
mutations and primary resistance of lung adenocarcinomas to
gefitinib or erlotinib. PLoS Med. 2:e172005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rodenhuis S, Slebos RJ, Boot AJ, Evers SG,
Mooi WJ, Wagenaar SS, van Bodegom PC and Bos JL: Incidence and
possible clinical significance of K-ras oncogene activation in
adenocarcinoma of the human lung. Cancer Res. 48:5738–5741.
1988.PubMed/NCBI
|
|
38
|
Saxton RA and Sabatini DM: mTOR signaling
in growth, metabolism, and disease. Cell. 168:960–976. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu GY and Sabatini DM: mTOR at the nexus
of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol.
21:183–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lei G, Zhuang L and Gan B: mTORC1 and
ferroptosis: Regulatory mechanisms and therapeutic potential.
Bioessays. 43:e21000932021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feng Z, Zhang H, Levine AJ and Jin S: The
coordinate regulation of the p53 and mTOR pathways in cells. Proc
Natl Acad Sci USA. 102:8204–8209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shaw RJ, Bardeesy N, Manning BD, Lopez L,
Kosmatka M, DePinho RA and Cantley LC: The LKB1 tumor suppressor
negatively regulates mTOR signaling. Cancer Cell. 6:91–99. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Byun JK, Park M, Yun JW, Lee J, Kim JS,
Cho SJ, Lee YM, Lee IK, Choi YK and Park KG: Oncogenic KRAS
signaling activates mTORC1 through COUP-TFII-mediated lactate
production. EMBO Rep. 20:e474512019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Prabowo AS, Iyer AM, Veersema TJ, Anink
JJ, Schouten-van Meeteren AY, Spliet WG, van Rijen PC, Ferrier CH,
Capper D, Thom M and Aronica E: BRAF V600E mutation is associated
with mTOR signaling activation in glioneuronal tumors. Brain
Pathol. 24:52–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yi J, Zhu J, Wu J, Thompson CB and Jiang
X: Oncogenic activation of PI3K-AKT-mTOR signaling suppresses
ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA.
117:31189–31197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hua H, Kong Q, Zhang H, Wang J, Luo T and
Jiang Y: Targeting mTOR for cancer therapy. J Hematol Oncol.
12:712019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Swanda RV, Nie L, Liu X, Wang C,
Lee H, Lei G, Mao C, Koppula P, Cheng W, et al: mTORC1 couples
cyst(e)ine availability with GPX4 protein synthesis and ferroptosis
regulation. Nat Commun. 12:15892021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu Y, Wang Y, Liu J, Kang R and Tang D:
Interplay between MTOR and GPX4 signaling modulates
autophagy-dependent ferroptotic cancer cell death. Cancer Gene
Ther. 28:55–63. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Muhoberac BB and Vidal R: Iron, Ferritin,
hereditary ferritinopathy, and neurodegeneration. Front Neurosci.
13:11952019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jung KH, Kim SE, Go HG, Lee YJ, Park MS,
Ko S, Han BS, Yoon YC, Cho YJ, Lee P, et al: Synergistic
renoprotective effect of melatonin and zileuton by inhibition of
ferroptosis via the AKT/mTOR/NRF2 signaling in kidney injury and
fibrosis. Biomol Ther (Seoul). 31:599–610. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hu Z and Li L, Li M, Zhang X, Zhang Y, Ran
J and Li L: miR-21-5p inhibits ferroptosis in hepatocellular
carcinoma cells by regulating the AKT/mTOR signaling pathway
through MELK. J Immunol Res. 2023:89295252023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yamaguchi H, Hsu JL, Chen CT, Wang YN, Hsu
MC, Chang SS, Du Y, Ko HW, Herbst R and Hung MC:
Caspase-independent cell death is involved in the negative effect
of EGF receptor inhibitors on cisplatin in non-small cell lung
cancer cells. Clin Cancer Res. 19:845–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yagoda N, von Rechenberg M, Zaganjor E,
Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM,
Boniface JJ, et al: RAS-RAF-MEK-dependent oxidative cell death
involving voltage-dependent anion channels. Nature. 447:864–868.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bebber CM, Thomas ES, Stroh J, Chen Z,
Androulidaki A, Schmitt A, Höhne MN, Stüker L, de Pádua Alves C,
Khonsari A, et al: Ferroptosis response segregates small cell lung
cancer (SCLC) neuroendocrine subtypes. Nat Commun. 12:20482021.
View Article : Google Scholar : PubMed/NCBI
|