Introduction
Esculetin (also termed 6,7-dihydroxy-2-chromenoe or
aesculetin; Esc) is a naturally occurring coumarin derivative
(1). A notable source of Esc is
Cortex Fraxini (2), a Traditional
Chinese Medicine (TCM) herb recognized for its wide range of health
benefits. Esc can also be derived from a variety of other plants,
including Artemisia capillaris (3), Citrus limonia (4), Viola yedoensis (5) and Cichorium intybus (6).
Despite notable medical breakthroughs, cancer
progression is still among the foremost causes of global morbidity
and mortality (7). In 2023, cancer
was the second leading cause of death globally, after heart
disease. Cancer has some notable features: Self-sufficiency in
growth signals, insensitivity to antigrowth signals, evasion of
apoptosis, limitless replicative potential and sustained
angiogenesis (8).
Esc is an active ingredient with significant
medicinal value. Esc has gained attention for its therapeutic
implications as an antioxidant and in diabetes, anti-inflammation
and oncology (9). Studies on TCM
primarily focus on the anti-inflammation and analgesic effects of
Esc (10,11). However, as an increasing number of
anticancer effects of Esc have been reported, future clinical
research is essential. The role of Esc in modulating various cancer
cell pathways and inducing apoptosis offers potential as a natural
remedy against cancer. In the present review, the role of Esc in
cancer is summarized.
In vitro anticancer properties of Esc
IC50 of Esc in cancer cell
lines
The IC50 of Esc shows some differences in
various cancer types. In laryngeal cancer, the IC50 of
Esc treatment for 72 h was 1.958 µM in Hep-2 cells (a HeLa
contaminated cell line) (12). In
addition, in colorectal cancer, the IC50 for 48 h in
HT-29 cells was 55 µM (13), and
the IC50 for 24 h in HCT116 cells was 100 µM (13). Furthermore, the IC50 of
the designed compound in breast cancer, Esc-NO-DEAC ternary hybrid
A11, was 8 nM for 48 h in MDA-MB-231 cells (14). Table
I shows the different IC50 values for Esc treatment
in various cell lines.
 | Table I.Different IC50 values for
Esc treatment in various cell lines. |
Table I.
Different IC50 values for
Esc treatment in various cell lines.
| First author/s,
year | Compound/agent | Cancer type | Cell line | IC50 for
24 h | IC50 for
48 h | IC50 for
72 h | (Refs.) |
|---|
| Wen et al,
2023 | Esc-NO-DEAC ternary
hybrid A11 | Breast cancer | MDA-MB-231 | - | 8.000 nM | - | (14) |
| Park et al,
2011 | Esc | Colon cancer | HT-29 | - | 55.000 µΜ | - | (13) |
| Park et al,
2011 | Esc | Colon cancer | HCT116 | 100.000 µM | - | - | (13) |
| Yin et al,
2023 | Esc | Ovarian cancer | SKOV3 | 223.810 µM | 86.900 µM | - | (35) |
| Yin et al,
2023 | Esc | Ovarian cancer | ID8 | 192.740 µM | 86.870 µM | - | (35) |
| Jiang et al,
2021 | Esc | Endometrial
cancer | Ishikawa | - | 95.000 µM | - | (36) |
| Jiang et al,
2021 | Esc | Endometrial
cancer | HEC-1B | - | 142.500 µM | - | (36) |
| Yang et al,
2010 | Esc | Cervical
cancer | HeLa | - | - | 37.800 µM | (37) |
| Zhang et al,
2019 | Esc | Laryngeal
cancer | Hep-2 (a HeLa
contaminated cell line) | - | - | 1.958 µΜ | (12) |
| Arora et al,
2016 | Esc | Pancreatic
cancer | PANC-1 | - | 100.000 µM | - | (28) |
Colorectal cancer (CRC)
In the world, CRC ranks as the third most lethal
malignancy, following breast and lung cancer in mortality rates
(7). Esc has shown notable
anticancer properties against CRC cells, as evidenced by its
ability to inhibit cell proliferation, induce apoptosis, arrest the
cell cycle in the G1 phase, reduce metastatic potential
and decrease reactive oxygen species (ROS) production (15–17).
Furthermore, in Esc-treated cell lines such as HCT116, HT-29 and
DLD-1, inhibition of proliferation in these cell lines in a dose-
and time-dependent manner was consistently observed (9–14).
Choi et al (16) reported
that Esc selectively targets cancer cells, sparing normal colonic
epithelial cells. The results also showed that Esc notably
suppressed anchorage-independent cell proliferation in a
dose-dependent manner (16). Choi
et al (16) and Lee et
al (18) performed colony
formation assays to visually demonstrate the inhibition of cell
proliferation. In a study conducted by Li et al (19), the BrdU test was used, which
revealed that the Esc-treated group exhibited a significantly lower
number of BrdU+ cells than the control group.
Additionally, the induction of cellular apoptosis was also
evidenced by the formation of apoptotic bodies, an increase in the
percentage of cells in the sub-G1 phase and DNA
fragmentation. Esc induces ROS formation; however, treatment with
an antioxidant reduced Esc-induced apoptotic cell death (19). Moreover, Esc increases mitochondrial
membrane depolarization, resulting in the release of cytochrome c
into the cytoplasm (17). In the
study by Choi et al (16),
annexin V/PI staining revealed that Esc promoted apoptosis in the
LoVo cell line in a dose-dependent manner. Further supporting these
findings, western blotting analyses indicated elevated levels of
pro-apoptotic proteins, including Bax, cleaved caspase-3, −7 and
−9, and poly (ADP-ribose) polymerase (PARP) in Esc-treated cells,
whereas the levels of anti-apoptotic protein, Bcl-2, was decreased
(16,17). Concurrently, findings such as
endoplasmic reticulum (ER) staining, mitochondrial calcium overload
and the upregulation of ER stress-related proteins, as mentioned in
the study by Kim et al (20), suggest that the ER stress response
is instrumental in targeting human colon cancer cells.
In addition, the antimetastatic effects of Esc on
HCT116 cells have been assessed using wound healing and invasion
assays. Increased E-cadherin promoter activity by Esc has been
linked to reduced cell migration and invasion, with
concentration-dependent inhibition of wound closure and cell
invasion (15).
Using PI-stained flow cytometry, in HCT116, HT-29
and LoVo cells, Esc was shown to increase the population of cells
in the G1 phase compared with the control, indicating
G1 phase cell cycle arrest (13,16,20).
Park et al (13) emphasized
the non-altering effects of Esc on p21WAF1 and p53 levels in HCT116
cells, but a marked increase in p27KIP expression was observed,
which led to the inhibition of CDK/cyclin complexes, resulting in
G1 phase cell cycle arrest. This study pioneered the
discovery of crucial roles of p27KIP and cyclin/CDK in Esc-induced
cell cycle arrest in cancer cell lines. Western blotting results
also demonstrated increased levels of p53, p27 and p21 and
decreased levels of cyclin D1 and specificity protein 1 (Sp1),
which is the transcription factor of p53, p27, p21, cyclin D1 and
Sp1 proteins, in a dose-dependent manner, suggesting that Esc
modulates cell cycle-related proteins (13). Cell cycle arrest has also been
reported in HT-29 and LoVo cell lines (HT-29 cells were treated
with 55 µM Esc for 24 h; LoVo cells were treated with 300 and 600
µM Esc for 24 h) (16,20).
Gastric cancer
Gastric cancer ranks fifth for incidence and fourth
for mortality among all types of cancer worldwide (7). Esc inhibits tumor cell proliferation,
and only a small percentage of cell death was found in normal
gastric cells, GES-1 cells, indicating its potential as a selective
and effective anticancer agent (21). Triciribine (an inhibitor of Akt) and
LY294002 (an inhibitor of PI3K), enhanced the pro-apoptotic effects
of Esc, indicating the involvement of the PI3K/Akt pathway in
Esc-induced proliferation inhibition and pro-apoptosis (22). This was consistent with the results
of a previous study that observed the inhibitory effects of Esc on
PI3K/Akt activation (23). Esc was
also shown to exhibit dose- and time-dependent antiproliferative
effects on SGC-7901 gastric cancer cells as the clonogenic
potential of SGC-7901 cells was significantly reduced following
exposure to Esc. The apoptosis induced by Esc treatment at
concentrations of 0, 12, 48 and 96 µM for 24 h in SGC-7901 cells
was confirmed using AO/EB and Annexin V/PI staining assays.
Moreover, it was also demonstrated that Esc induced cell cycle
arrest specifically in the G2/M phase (21).
Liver and pancreatic cancer
Liver cancer was the third leading cause of
cancer-related death worldwide in 2020, while pancreatic cancer has
a poor prognosis and is the seventh leading cause of cancer-related
death in both sexes (7).
Corroborating research has demonstrated that
oxidative stress is closely related to ferroptosis, and ferroptosis
is characterized by detrimental lipid peroxidation. Ferritinophagy
increases intracellular Fe2+ levels, which initiates the
Fenton reaction to produce ROS (H2O2),
leading to an increase in oxidative stress and glutathione (GSH)
levels, resulting in ferroptosis (24–26).
The results of a study indicated that Esc elevated ROS
(H2O2) production in HUH7 and HCCLM3 liver
cancer cells in a dose-dependent manner, simultaneously diminishing
intracellular free radical scavenging and lowering antioxidant
activity (27). Esc treatment also
led to significant proliferation inhibition, G1-phase
cell cycle arrest and mitochondrial-dependent apoptosis activation
in PANC-1, MIA PaCa-2 and AsPC-1 pancreatic cancer cell lines via
caspase 3, 8, and 9. Additionally, Esc treatment resulted in
decreased intracellular ROS and p65-nuclear factor-κB (NF-κB)
protein levels in PANC-1 cells (28). Esc also inhibits hepatitis B virus
(HBV) both in vitro and in vivo (29). Therefore, Esc has been hypothesized
to suppress hepatocellular carcinoma by affecting HBV DNA
replication, consequently leading to cell death.
In studies on liver and pancreatic cancer, the
changes in ROS levels in cancer cells treated with Esc showed
pronounced contrasts. This suggests that there are two distinct
modes of apoptosis in these cell types. In a study conducted by
Arora et al (28), Esc
treatment decreased ROS levels, leading to mitochondrial-dependent
apoptosis. Conversely, in a study by Xiu et al (27), Esc inflicted mitochondrial damage,
which subsequently accounted for elevated ROS levels.
Breast cancer
Breast cancer is the most commonly diagnosed cancer
and the leading cause of cancer-related mortality in women
worldwide (7,30). The results of two studies showed
that Esc and Esc-Furoxan-DEAC ternary hybrids could induce
mitochondrial apoptosis through the calcium and ROS accumulation
pathways, respectively, thus promoting the apoptosis of breast
cancer cells (14,31). In a study by Chang et al
(31), the first to explore the
effects of Esc on Ca2+ in ZR-75-1 human breast cancer
cells, Esc was found to induce a dose-dependent Ca2+
increase in these cells. The increase in Ca2+ was
attributed to both Ca2+ entry and Ca2+
release, which were partially inhibited by the removal of
extracellular Ca2+. However, Esc did not induce
Ca2+ increase in MCF-7 and MDA-MB-231 breast cancer
cells. In ZR-75-1 cells, Esc decreased the mitochondrial membrane
potential, increased the release of cytochrome c and activated
caspase-9 and −3, indicating its involvement in the
Ca2+-associated mitochondrial apoptosis pathway. Esc
also induced G2/M cell cycle arrest, potentially through
the upregulation of p53 and p21 and the downregulation of CDK1 and
cyclin B1 proteins in ZR-75-1 cells (31).
A total of 12 new hybrid Esc compounds have been
synthesized, which involved the integration of a furoxan-based NO
donor and/or a mitochondria-targeting group onto the 6,7-dihydroxy
structure of Esc (14). In
vitro findings demonstrated that Esc-Furoxan-DEAC ternary
hybrids can induce MDA-MB-231 cell apoptosis and halt mitosis at
the G2/M phase, with its effectiveness varying in
relation to the dose. This outcome suggested that Esc-Furoxan-DEAC
ternary hybrids triggers cell apoptosis via the mitochondrial
pathway and that Esc has a crucial role in the Esc-Furoxan-DEAC
ternary hybrid-driven enhancement of CypD levels (14).
Ovarian, endometrial and cervical
cancer
Ovarian cancer ranks seventh in terms of incidence
of malignant tumors and eighth in terms of the cause of
cancer-related death in women worldwide (32,33),
while endometrial cancer is the sixth most common cancer in women
(34), and cervical cancer is the
fourth most frequently diagnosed cancer and the fourth leading
cause of cancer-related death in women globally (7). Despite advancements in screening and
vaccination, these cancers collectively account for a substantial
number of cancer-related deaths among women worldwide, highlighting
the need for urgent research on prevention, early detection and
therapeutic strategies.
Studies conducted by Yin et al (35), Jiang et al (36) and Yang et al (37) revealed that Esc inhibited the
proliferation of SKOV3 ovarian cancer cells, Ishikawa and HEC-1B
endometrial cancer cells, and HeLa cervical adenocarcinoma cells in
a time- and dose-dependent manner, but Esc showed low cytotoxicity
towards human L02 normal hepatocyte cells (35). In addition to inhibiting cell
proliferation, a single cell colony formation assay showed that Esc
exerted an inhibiting effect on the colony forming ability of SKOV3
ovarian cancer cells. Esc also delayed wound healing in these
cells, indicating its migration-inhibitory properties. Transwell
assays also demonstrated that Esc suppressed the migration and
invasion of SKOV3 cells (35,37).
Furthermore, flow cytometry and western blotting analysis revealed
that Esc treatment decreased the expression of cyclin D1, CDK4,
CDK2 and cyclin E, suggesting its role in regulating the cell cycle
in SKOV3 and HeLa cells (35). As
also shown in HeLa cells with Esc treatment at 0 mM (control), or
50 mM for 12, 24 or 48 h in flow cytometry analysis, the
subG1 population, which indicates apoptotic cells,
increased in a time-dependent manner. These results indicated that
Esc induced cell cycle arrest in the G2/M phase and the
apoptosis of HeLa cells in a time-dependent manner (37). Esc also exhibits antimitogenic
effects on ovarian, endometrial and cervical cancer cells.
Treatment with Esc led to an increase in the ratio of red to green
fluorescence, indicating a reduction in the mitochondrial membrane
potential by Mitochondrial Membrane Potential Detection Kit (JC-10)
(35). Western blotting analysis
confirmed that Esc induced apoptosis, as concentration-dependent
increase of apoptosis-related proteins and a decrease of
anti-apoptosis proteins was induced in SKOV3 ovarian cancer cells,
Ishikawa and HEC-1B endometrial cancer cells by causing
mitochondrial membrane potential collapse. Additionally, Esc
induced ROS (O2−) generation as demonstrated
by flow cytometry and fluorescence microscopy (35,36).
This resulted in an early increase in mitochondrial ROS
(O2−) production, leading to the opening of
the mitochondrial permeability transition pore and a subsequent
drop in mitochondrial membrane potential (35,37).
These observations demonstrated that the overproduction of ROS
(O2−) triggers mitochondrial permeability
transition pore opening, leading to decreased membrane potential
and a subsequent release of cytochrome c from the intermembrane
space into the cytosol, resulting in activation of the caspase
cascade and apoptotic cell death. These findings suggest that Esc
has potential anticancer effects and may be used as a treatment for
cancer.
Lung cancer
Lung cancer is the leading cause of cancer-related
mortality worldwide for both sexes and is frequently diagnosed in
the advanced stages when treatment alternatives are limited
(7). In total, two pivotal research
articles on lung cancer have studied the antiproliferative effects
of Esc and its role in attenuating cell invasion and
epithelial-mesenchymal transition (EMT) using the cell lines, Lewis
lung carcinoma (LLC) and A549 (a non-small cell lung cancer line)
(38,39). These studies observed that Esc
suppressed the viability of LLC and A549 cells in a dose- and time-
dependent manner. In addition, 40 and 80 µM Esc led to reduced
colony formation of LLC cells in soft agar (38). In the study by Li et al
(39), the treatment of A549 cells
with Esc significantly and dose-dependently reduced cell invasive
capabilities. Analyses using reverse transcription-quantitative
(RT-qPCR and western blotting) in Esc (5 and 20 µM)-treated A549
cells incubated for 24 h indicated an upregulation of E-cadherin
mRNA and protein levels, along with a downregulation of vimentin
and Snail, which regulate the EMT of A549 cells. However, Zhu et
al (38) did not report any
changes in the invasion capabilities of LLC cells treated with Esc.
This may be due to the differential effects of Esc in different
cell lines. Further research is still required to elucidate the
detailed mechanisms.
Oral and laryngeal cancer
Oral squamous cell carcinoma (OSCC) is ranked
eighteenth in terms of cancer incidence and is the most common head
and neck squamous cell carcinoma worldwide (7). Laryngeal cancer is the most common
malignancy in otolaryngology and comprises 30–40% of head and neck
malignancies worldwide (40).
In studies by Cho et al (41) and Kok et al (42), the viabilities of both the HN22 and
HSC4 OSCC cell lines and the SAS human oral cancer cell line were
inhibited following Esc treatment in a time- and dose-dependent
manner, as demonstrated by MTT assay. Furthermore,
fluorescence-activated cell sorting analysis and
4′,6-diamidino-2-phenylindole staining of HN22 and HSC4 cells
treated with Esc at concentrations of 5, 10 and 20 µM indicated
that Esc hindered cell proliferation by inducing cell cycle arrest
in the G0/G1 phase and promoting apoptosis.
Additionally, an Annexin V assay suggested early apoptosis was
induced by Esc in these cell lines (41). Western blotting and enzyme-linked
immunosorbent assays were performed to examine the apoptotic
pathways activated by Esc, which revealed that Esc did not trigger
the mitochondria-mediated apoptotic pathway in SAS cells, but
instead triggered the death receptor (DR) pathway (42).
In a cell proliferation assay, as a positive
control, the IC50 of cisplatin against laryngeal cancer
cells was 2.15 µM for Hep-2 cells (a HeLa contaminated cell line),
while Esc was 1.958 µM. In Hep-2 laryngeal cancer cells, 72 h Esc
treatment inhibited cell proliferation, migration and invasion,
alongside ROS accumulation and G1/S cell cycle arrest
(12).
Prostate and bladder cancer
Prostate cancer is the fourth most common cancer
worldwide, while bladder cancer is the tenth most commonly
diagnosed cancer worldwide (7). In
studies by Turkekul et al (43) and Han et al (44), it was demonstrated that cancer cell
survival was inhibited after 48 h of treatment with Esc at
concentrations of 0, 100 and 200 µM. In addition, the induction of
apoptosis, G1 phase cell cycle arrest and metastasis can
be summarized from the experimental results. The detailed molecular
mechanisms underlying Esc-induced apoptosis in PC3 cells were
investigated using RT-qPCR. Post-Esc treatment resulted in a
significant increase in the mRNA levels of TNF-receptor 1,
caspase-8, caspase-3, Bax and cytochrome c, alongside a notable
reduction in Bcl-2 mRNA. Additionally, western blotting analysis
demonstrated a dose-dependent increase in cytochrome c protein
levels in PC3 cells treated with Esc (43). Consistently, Han et al
(44) confirmed that Esc induced
the mitochondria-dependent apoptosis of 5637 cells. Esc-treatment
caused a loss of mitochondrial membrane potential, the release of
cytochrome c and the activation of caspase-9 and −3.
Human acute myeloid leukemia
Human acute myeloid leukemia remains a rare
malignancy, but it occupies close to one-third of all diagnosed
leukemia (45). Although a number
of treatment modalities are currently available for leukemia,
numerous malignancies lack efficient treatment owing to multidrug
resistance (46). Gong et al
(47) investigated the anticancer
properties of Esc in human acute myeloid leukemia cancer cells,
including peripheral blood mononuclear cells as a normal cell line.
Transmission electron microscopy and western blotting demonstrated
the increase of apoptosis induction, the reduction of the Bcl-2/Bax
ratio and the suppression of cancer cell migration after Esc
treatment.
In vivo anticancer properties of Esc
To the best of our knowledge, 8 articles pertaining
to in vivo cancer experiments involving Esc, covering 7
types of cancer have been published (12,15,18,22,27,35,36,38).
Table II shows the function of Esc
in in vivo experiments. All articles found an effective
tumorigenesis and metastasis inhibition by Esc with minimal
toxicity to normal cells, which is a hallmark advantage of TCM. In
addition, the combination of Esc with paclitaxel demonstrated
potent anticancer properties. Among the 8 studies, only 1 focused
on orthotopic mouse tumors in xenograft models (15).
 | Table II.Function of Esc in in vivo
experiments. |
Table II.
Function of Esc in in vivo
experiments.
| First author/s,
year | Cancer type | Targets/regulators
and signaling pathways | Cell line | Animal model | Routes of
administration | Outcome | (Refs.) |
|---|
| Lee et al,
2013 | Colorectal
cancer |
Wnt-β/catenin/Tcf | HCT-116 | Xenograft in Female
athymic nude mice (6 to 7-week-old) |
Intraperitoneally | ↓Ki-67, cyclin D1
and c-Myc | (18) |
|
|
|
| - | Embryo of
Xenopus | - | Confirmed the
Wnt-β-catenin-Tcf pathway-not tumor related |
|
| Kim et al,
2018 | Colorectal
cancer |
Axin2/Snail1/E-cadherin pathway | HCT-116 | Orthotopically
inoculated in Male nude mice (BALB/c-nu), 4–6 weeks old, |
Intraperitoneally | ↓Axin2, c-Myc and
cyclin D1; ↑E-cadherin; ↓Ki-67. | (15) |
| Wang et al,
2017 | Gastric cancer | IGF-1/PI3K/Akt | MGC-803 | Xenograft in nude
mice model | Gavage | ↑caspase-3; ↓Bcl-2
and Ki-67; ↓IGF-1, p-PI3K and p-Akt | (22) |
| Xiu et al,
2023 | Liver cancer | NCOA4
pathway-mediation ferritinophagy | HUHT | Xenograft in nude
mice (BALB/c) | Unspecified | ↑Fe2+
and MDA level in serum; ↓Ki67; ↓NFE2L2, GPX4 and HO-1; ↑LC3II and
NCOA4; ↓FTH1 | (27) |
| Yin et al,
2023 | Ovarian cancer | JAK2/STAT3 | ID8 | Xenograft in BALB/c
nude mice | Unspecified | ↓Ki67 | (35) |
| Jiang et al,
2021 | Endometrial
cancer | via hnRNPA1 to
downregulate Bcl-xl and XIAP | HEC-1B | Xenograft in BALB/c
nude mice | Intra-tumoral | ↓hnRNPA1, Bcl-xl
and XIAP | (36) |
| Zhu et al,
2018 | Lung cancer | Downregulating
Wnt-targeted genes and suppressing NF-κB | LLC | Xenograft in BALB/c
nude mice (6 to 8-week-old female) | Subcutaneously | No significant
change in body weight was observed between the two groups. | (38) |
| Zhang et al,
2019 | Laryngeal
cancer | JAK/STAT3
activation | Hep-2 (a HeLa
contaminated cell line) | 0.2 ml Hep-2 cell
(concentration: 6×105/ml) Suspension was subcutaneously
injected into the axilla of BALB/c male nude mice | Oral | ↓p-JAK1, p-JAK2 and
p-STAT3 | (12) |
A total of two in vivo experiments (15,19)
with distinct designs examined Esc in colon cancer but noted the
effects on different molecular mechanisms: The Wnt/β-catenin/T-cell
factor (Tcf) and Axin2/Snail1/E-cadherin pathways. In one study,
HCT-116 cell xenografts in female athymic nude mice were used,
while the other study involved orthotopic transplantation into the
mouse ceca. Following surgical resection, isolation and
homogenization of the primary culture, L-2 cells were reintroduced
orthotopically. Treatments were administered intraperitoneally and
the mice were euthanized after 1 week for tumor analysis.
Biochemical analysis showed a decrease in Axin2, c-Myc, cyclin D1
and Ki-67 expression and an increase in E-cadherin expression,
indicating the antitumor and antimetastatic properties of Esc
(15).
In a study by Lee et al (18), Xenopus embryos were used to
demonstrate that Esc inhibits the β-catenin/Tcf-dependent signaling
pathway, which was supported by a previous study. In an LLC lung
cancer investigation using 80 µM Esc treatment fro 24 h, it was
demonstrated that the mechanism involved downregulating Wnt
targeted genes and suppressing NF-κB. Although in vivo
experiments did not show changes in related molecules, Esc was
found to reduce lung cancer tumor size and weight (38).
Furthermore, during the examination of laryngeal
cancer, Esc not only significantly inhibited tumor proliferation by
80%, but also impeded the JAK/STAT3 signaling pathway in
vivo, as evidenced by western blotting analysis showing reduced
levels of phosphorylated (p-)JAK1, p-JAK2 and p-STAT3.
An animal model experiment using an MGC-803 cell (a
HeLa contaminated cell line) xenograft nude mouse model for gastric
cancer research showed increased caspase-3 expression and decreased
Bcl-2, Ki-67, IGF-1, p-PI3K and p-Akt expression following
treatment with Esc, suggesting the induction of apoptosis in
gastric cancer cells via the IGF-1/PI3K/Akt signaling pathway.
Furthermore, Esc induces lipid peroxidation and iron accumulation
in tumor tissue. In hepatocellular carcinoma cells, Esc suppresses
tumor proliferation, reduces antioxidant levels, increases
ferritinophagy-related protein levels and activates ferritinophagy
to promote ferroptosis. Esc treatment downregulates the export of
Bcl-xl and X-linked inhibitor of apoptosis protein (XIAP) mRNA from
the nucleus to the cytoplasm, as confirmed by nuclear RNA and
cytoplasmic RNA isolation experiments. Esc and paclitaxel together
inhibit endometrial cancer cell proliferation in vivo and
have potential as a clinical treatment (22).
Mechanism of Esc in different cancer
types
Since Esc is a plant-derived compound, its apoptotic
effects on cancer cell lines have been extensively studied. Studies
have verified its role in mitochondrial apoptosis across multiple
cancer types including colon (17),
gastric (22,48), ovarian (35), cervical (37) and bladder cancers (44), and its capacity to induce ER stress
(20) and ferritinophagy (27).
The role of Esc in cancer cells was revealed to be
related to apoptosis (Fig. 1).
Apoptosis is a form of programmed cell death that is triggered by
DRs and the mitochondrial pathway (49). Table
III shows the different apoptotic pathway changes following Esc
treatment in cancer cells. A total of 6 studies (17,22,35,37,44,48) on
mitochondrial apoptosis have all reported increased levels of
pro-apoptotic proteins (including Bax and Bak) and decreased levels
of anti-apoptotic proteins (including Bcl-2 and Bcl-xl) in
Esc-treated cancer cells. In addition, transfer of cytochrome c
from the mitochondria to the cytoplasm, upregulation of related
proteases, such as caspase-3, caspase-9 and PARP, and increased ROS
production were observed. These results indicate that Esc-induced
apoptosis is mediated by the mitochondrial pathway in various cell
lines (17,22,35,37,44,48).
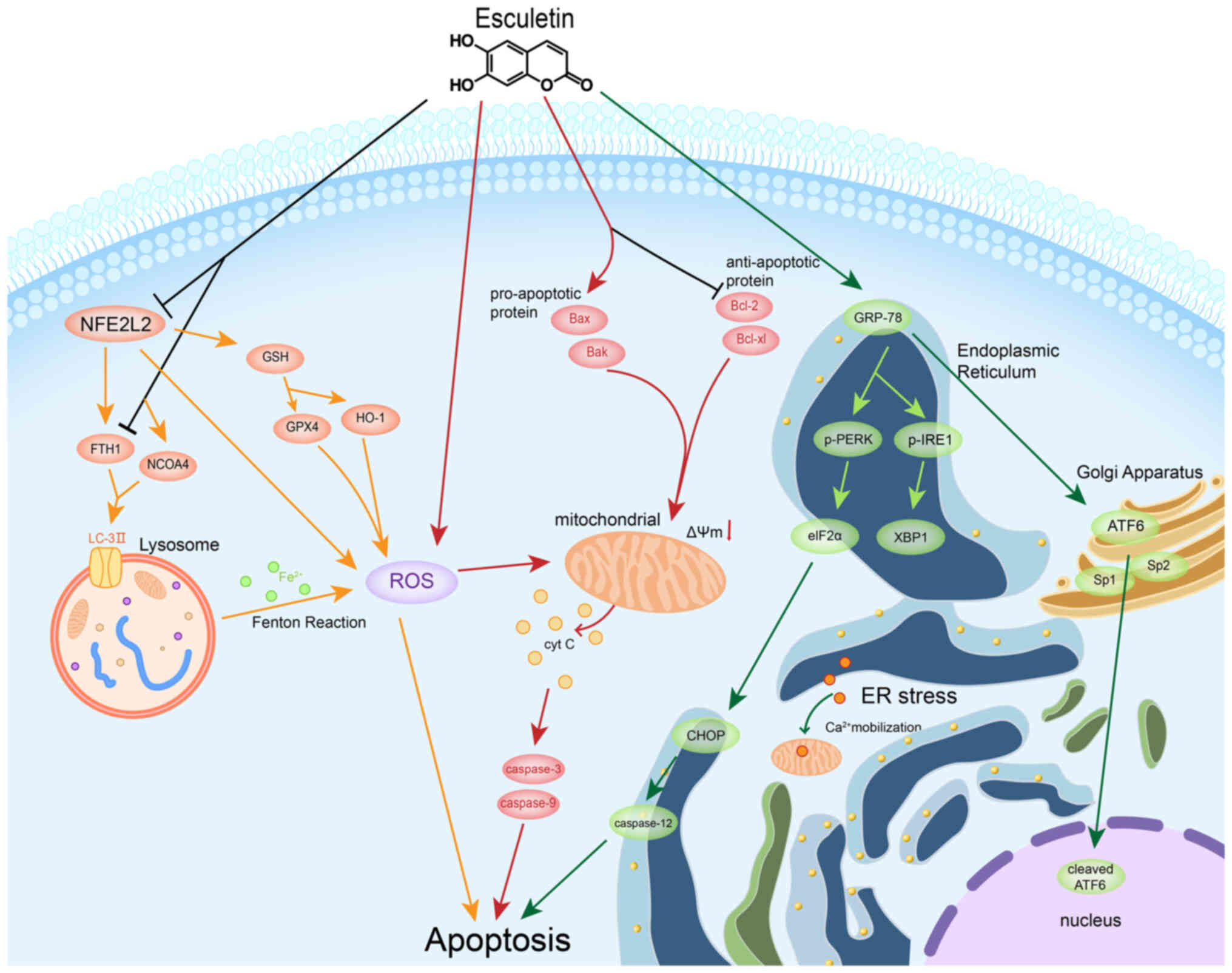 | Figure 1.The role of Esc in cancer cells was
revealed to be related to apoptosis, which is governed by the
mitochondrial pathway, ER stress and ferritinophagy. Esc induces
mitochondrial apoptosis by stimulating cyt C release, activating
caspase-3 and caspase-9 and enhancing ROS generation across various
cell lines. ROS further modulate the mitochondrial membrane
potential and amplify pro-apoptotic molecules in the cytosol. ER
stress, which is vital for cellular processes, responds to Esc by
increasing mitochondrial Ca2+ levels in cancer cells. ER
stress activates the UPR, activating signaling molecules (such as
PERK, IRE1 and ATF6) and transcription factors (such as XBP1 and
CHOP) that regulate UPR genes and trigger ER stress-induced
apoptosis, particularly caspase-12 activation. Esc elevates ROS
levels, suppresses antioxidants and downregulates NFE2L2, fostering
ferroptosis in liver cancer cells by promoting
NCOA4/LC3II/FTH1-mediated ferritinophagy. Esc-induced
ferritinophagy enhances intracellular iron levels and inhibits
liver cancer cell proliferation. Esc, Esculetin; ER, endoplasmic
reticulum; ROS, reactive oxygen species; UPR, unfolded protein
response; IRE1, inositol-requiring enzyme 1; ATF6, activating
transcription factor 6; NCOA4, nuclear receptor coactivator 4;
FTH1, ferritin heavy chain 1; GSH, glutathione; GPX4, glutathione
peroxidase 4; HO-1, heme oxygenase-1; GRP-78, glucose regulatory
protein 78; XBP1, X-box binding protein 1; p-, phosphorylated; cyt
C, cytochrome c; NFE2L2, NFE2-like BZIP transcription factor 2;
Sp1/2, specificity protein 1/2. |
 | Table III.Different apoptotic pathway changes
following Esc treatment in cancer cells. |
Table III.
Different apoptotic pathway changes
following Esc treatment in cancer cells.
| First author/s,
year | Type of
apoptosis | Cancer | Cell line | Molecular
change | (Refs.) |
|---|
| Kim et al,
2015 | Mitochondrial
apoptosis | Colon cancer | HT-29 | ↑pro-apoptotic
protein, Bax; ↓anti-apoptotic protein, Bcl-2 ↑ROS; ↑active
(cleaved) caspase-9, caspase-3 and cleaved PARP (a target protein
of caspase-3) | (17) |
| Pan et al,
2015 | Mitochondrial
apoptosis | Gastric cancer | MGC-803 | ↑caspase-9 and
caspase-3; ↑cytochrome c in the cytosol; ↑Bax and Bak
(pro-apoptotic regulatory proteins); ↓Bcl-2 and Bcl-xl
(anti-apoptotic regulatory proteins); ↑ROS | (48) |
| Wang et al,
2017 | Mitochondrial
apoptosis | Gastric cancer | MGC-803 | ↑The release of
cytochrome c from mitochondria into cytoplasm; ↓the ratio of
Bcl-2/Bax, caspase-9 and caspase-3 activity; ↓IGF-1, p-PI3K and
p-Akt; ↓cell apoptotic ratio and caspase-3 and caspase-9 activity;
↑Bcl-2/Bax ratio; ↓cytochrome c from mitochondria to cytoplasm | (22) |
| Yin et al,
2023 | Mitochondrial
apoptosis | Ovarian cancer | SKOV3 | ↓Mitochondrial
membrane potential; ↑Bax, cytochrome c, cleaved caspase 9 and
cleaved caspase 3; ↓Bcl-2; ↑Bax/Bcl-2 ratio; ↑ROS | (35) |
| Yang et al,
2010 | Mitochondrial
apoptosis | Cervical
cancer | HeLa | The loss of
mitochondrial membrane potential; ↑caspase-3/7/9; ↑the cytosolic
level of cytochrome c; ↑ROS | (37) |
| Han et al,
2023 | Mitochondrial
apoptosis | Bladder cancer | 5637 | ↓In the
mitochondrial membrane potential; ↑cytochrome c release to the
cytoplasm and the activation of caspase-9 and caspase-3; ↑the ratio
of BAX/Bcl-2 proteins | (44) |
| Kim et al,
2015 | Endoplasmic
reticulum stress | Colon cancer | HT-29 | ↑GRP-78, p-PERK and
p-IRE1 and cleaved ATF6; PERK → ↑p-eIF2α; IRE1α→ ↑ XBP1; ↑cleaved
ATF6; ↑spliced XBP1 mRNA; ↑CHOP and cleaved caspase-12 | (20) |
| Xiu et al,
2023 | Ferritinophagy | Liver cancer | HUH7 and
HCCLM3 | ↓Antioxidant
proteins (NFE2L2, GPX4 and HO-1); ↑ROS; ferrostatin-1, a
ferroptosis inhibitor, bafilomycin and 3-MA could inhibit
esculetin-mediated cell death; ↑Fe2+; ↑NCOA4 and LC3-II
in HUH7 and HCCLM3 cells, but ↓FTH1 ↓ p62 expression; Bafilomycin
(Baf) and Esc co-treatment, ↑ p62 expression; ↓LC3-II expression,
suggested Esc can promote autophagy flux | (27) |
The ER is essential for protein synthesis and
folding, and calcium homeostasis (50). Esc treatment resulted in
mitochondrial Ca2+ accumulation in HT-29 cells,
suggesting that Esc triggered Ca2+ mobilization from the
ER. Malfunctioning ER leads to the accumulation of unfolded
proteins, triggering the unfolded protein response (UPR). During
this process, glucose regulatory protein 78 (GRP-78) activates
three key transmembrane signaling molecules: PERK,
inositol-requiring enzyme 1 (IRE1) and activating transcription
factor 6 (ATF6). As demonstrated through western blotting and qPCR
analyses, with the Esc treatment at a concentration of 55 µM,
GRP-78, p-PERK, p-IRE1 and cleaved ATF6 were upregulated in HT-29
cells (17). Prolonged ER stress
leads to ATF6 relocation to the Golgi apparatus for processing by
Sp1 and Sp2 proteases. Cleaved ATF6 then migrates to the nucleus,
enhancing the expression of UPR target genes (51). Furthermore, X-box binding protein 1
(XBP1) is spliced by IRE1α, removing a 26-nucleotide intron from
XBP1 mRNA, which results in a potent transcription activator that
boosts the expression of UPR genes and activates the ER-associated
degradation pathway (52). During
UPR, the continued synthesis of proteins becomes unsustainable;
hence, protein kinase R phosphorylates eIF2α to inhibit protein
translation. In HT-29 cells, 55 µM Esc treatment has been shown to
cause a time-dependent increase in the expression of spliced eIF2α,
XBP1 and cleaved ATF6. The induction of IRE1, PERK and ATF6 during
ER stress upregulates expression of the transcription factor, CHOP
(53). In addition, caspase-12 is
the key initiator of ER stress-induced apoptosis (54). Esc-treatment of colon cancer cells
increases the expression of both CHOP and caspase-12 (20).
In terms of ferritinophagy, it was demonstrated in
a study by Xiu et al (27)
that Esc enhanced the production of ROS
(H2O2) in HUH7 and HCCLM3 cells in a
dose-related manner, while simultaneously reducing intracellular
free radical scavenging and antioxidant activities. NFE2-like BZIP
transcription factor 2 (NFE2L2), a known regulator of ferroptosis
through its regulation of glutathione peroxidase 4 (GPX4), ferritin
heavy chain 1 (FTH1) and heme oxygenase-1 (HO-1), was found to be
downregulated by Esc both in vivo and in vitro, which
led to suppressed levels of HO-1, GPX4 and GSH, and the ability to
scavenge hydroxyl radicals in serum, thereby altering the
antioxidant equilibrium in tumor tissues (55). There is an increase in
Fe2+ levels, lipid peroxidation and iron deposition in
tumor tissues, which are indicative of ferroptosis. Nuclear
receptor coactivator 4 (NCOA4), known to bind to FTH1 and associate
with LC3 protein on the autophagosome membrane, targets ferritin
complexes for autolysis, a key process in ferroptosis. The
inhibition of NCOA4 expression or autophagy prevents ferritinophagy
and ferroptosis (56). A previous
study indicated that Esc increases NCOA4, LC3-II and lysosome
levels, while decreasing FTH1 expression, thus promoting
ferritinophagy (57). Furthermore,
using gene silencing and overexpression techniques, it was
demonstrated that Esc suppressed FTH1 expression, and increased
NCOA4 and LC3II expression post-silencing. Esc also enhanced the
co-expression of NCOA4 and LC3II, facilitating ferritinophagy.
Therefore, by activating ferritinophagy, Esc increases
intracellular free iron levels, suggesting its potential to
suppress liver cancer cell proliferation by inducing ferritinophagy
via the NCOA4/LC3II/FTH1 signaling pathway (57). Although experiments have shown that
Esc activates ferritinophagy to inhibit the proliferation of HUH7
and HCCLM3 cells, the potential of Esc beyond liver cancer cells
and the involvement of other pathways in promoting ferritinophagy
remain uncertain. Further research is needed to determine whether
Esc can induce ferritinophagy through alternative pathways, and
whether it has inhibitory effects on other types of cancer.
Table IV shows
different signaling pathway changes following Esc treatment in
cancer cells. Esc, a compound with potential anticancer properties,
promotes apoptosis and inhibits the proliferation of tumor cells
(Fig. 2). The Wnt/β-catenin
signaling pathway, frequently cited in studies regarding Esc,
stands out due to its crucial role in the mechanisms affected by
Esc. Finding small compounds that block Wnt signaling is therefore
a possible cancer prevention or therapy approach. In the colon
cancer cell lines, HCT116 and SW480, as well as in the LLC lung
cancer cell line, post-treatment with Esc led to a downregulation
of proteins such as c-Myc and cyclin D1. Specifically, in the
HCT116 cell line, Esc showed a direct binding affinity with
β-catenin (18), and in both HCT116
and SW480 cells, a downregulation of the β-catenin/Tcf complex was
observed (18,19). Notably, in LLC lung cancer cells, in
addition to the downregulation of cell cycle proteins, the
downregulation of NF-κB was also regulated by Esc (38). In a study by Kim et al
(15), it was revealed that Esc
impeded the transcriptional activity of β-catenin by preventing its
nuclear translocation. In CRC cells, Esc regulates E-cadherin
expression by attenuating Snail1 levels, and also modulates the
expression of Snail1 via a nuclear GSK3β-dependent degradation
pathway, thereby influencing EMT processes. This modulation is
achieved by altering Axin2 and miRNA-34a levels (15).
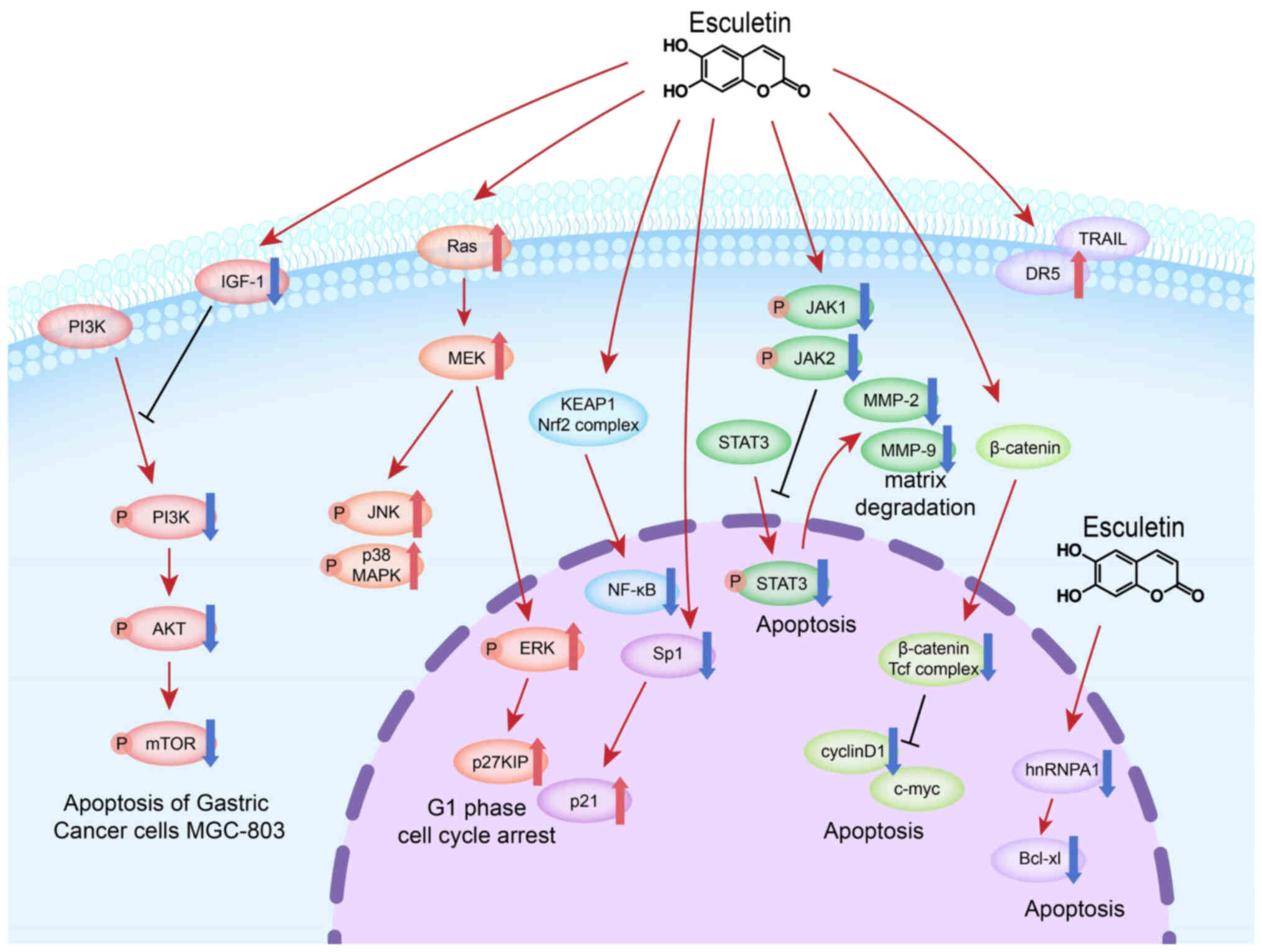 | Figure 2.Esc, a compound with potential
anticancer properties, promotes apoptosis and inhibits the
proliferation of tumor cells. These effects are mediated by various
molecular mechanisms involving different signaling pathways. In the
PI3K/Akt pathway, Esc modulates the upstream molecule, IGF-1, to
regulate the PI3K/Akt pathway in gastric cancer cells. Esc also
targets downstream mTOR molecules to induce apoptosis and cell
cycle arrest. Esc induces G1 phase cell cycle arrest by
upregulating Ras, ERK1/2 and p27 KIP8, and activates MAPK signaling
to induce apoptosis in colon and bladder cancer cells. Esc also
regulates NF-κB in lung cancer cells. Esc activates the ARE pathway
in pancreatic cancer cells by disrupting the Nrf2-KEAP1
interaction. Esc decreased Sp1 expression in oral squamous cell
carcinoma cells and enhances TRAIL-induced apoptosis in oral cancer
cells by upregulating DR5. Esc regulates the JAK/STAT3 pathway in
ovarian and laryngeal cancers, leading to decreased expression of
MMP2 and MMP9. In the Wnt/β-catenin pathway, Esc downregulates
proteins such as c-Myc and cyclin D1, binds directly to β-catenin
and inhibits the β-catenin/Tcf complex. Esc binds to hnRNPA1 in
endometrial cancer, affecting Bcl-xl expression. These pathways
highlight the diverse mechanisms through which Esc inhibits cancer
proliferation and suggest its potential as a targeted therapy for
various cancer types. Esc, esculetin; ERK, extracellular
signal-regulated kinase; JAK, janus kinase; STAT3, signal
transducer and activator of transcription-3; Nrf2, nuclear factor
erythroid 2-related factor 2; MMP, matrix metalloproteinase; NF-κB,
nuclear factor-κB; Sp1, specificity protein 1; DR, death receptor;
MEK, mitogen-activated protein kinase kinase; hnRNPA1,
heterogeneous nuclear ribonucleoprotein A1. |
 | Table IV.Different signaling pathway changes
following Esc treatment in cancer cells. |
Table IV.
Different signaling pathway changes
following Esc treatment in cancer cells.
| First author/s,
year | Cancer type | Cell line | Signaling
Pathways | Molecules
altered | (Refs.) |
|---|
| Park et al,
2011 | Colorectal
cancer | HCT116 | Ras/ERK1/2 | ↑Ras, ERK1/2,
p27KIP | (13) |
| Lee et al,
2013 | Colorectal
cancer | HCT116, HCT15 and
DLD-1 | β-catenin/Tcf | Esc directly binds
to β-catenin; ↓β-catenin/Tcf complex, c-Myc and cyclinD1 | (18) |
| Kim et al,
2015 | Colorectal
cancer | HT-29 | JNK, p38 MAPK, and
ERK | ↑p-JNK, p-p38 MAPK
and p-ERK | (17) |
| Li et al,
2018 | Colorectal
cancer | SW480 | Wnt/β-catenin | ↓β-catenin/Tcf
complex, c-Myc and cyclin D1 | (19) |
| Kim et al,
2018 | Colorectal
cancer | HEK293, CT116,
SW480, LS174T and HCT15 |
Axin2/E-cadherin | Formation of
E-cadherin/β-catenin complexes; ↑E-cadherin; ↓Snail1 (a
transcriptional repressor of the E-cadherin promoter);
↑microRNA-34a expression (a known regulator of EMT and negative
regulator of Axin2 post-transcription) | (15) |
| Zhang et al,
2021 | Gastric cancer | MGC-803 | PI3K/Akt/mTOR | Activity of mTOR
and Akt remained almost unchanged; ↓p-mTOR, p-AKT, p-PI3K and
PI3K | (21) |
| Wang et al,
2017 | Gastric cancer | MGC-803 | IGF-1/PI3K/Akt | ↓IGF-1, p-PI3K and
p-Akt; ↓cell apoptotic ratio and caspase-3 and caspase-9 activity;
↑Bcl-2/Bax ratio; ↓cytochrome c from mitochondria to cytoplasm;
combination treatment with Esc and triciribine or LY294002 could
further enhance the apoptotic effect of Esc | (22) |
| Xiu et al,
2023 | Liver cancer | HUH7 and
HCCLM3 |
NCOA4/LC3II/FTH1 | ↓Antioxidant
proteins (NFE2L2, GPX4 and HO-1); ↑ROS; ferrostatin-1, a
ferroptosis inhibitor, bafilomycin and 3-MA could inhibit
Esc-mediated cell death; ↑Fe2+; ↑NCOA4 and LC3-II in
HUH7 and HCCLM3 cells, but ↓FTH1 ↓ p62 expression; Bafilomycin
(Baf) and ESCM co-treatment, notably ↑ p62 expression; ↓LC3-II
expression, suggested Esc could promote autophagy flux | (27) |
| Arora et al,
2016 | Pancreatic
cancer | PANC-1 | KEAP1/Nrf2 | ↑Nuclear
accumulation of Nrf2; ↑NQO1, a direct target of Nrf2 | (28) |
| Yin et al,
2023 | Ovarian cancer | SKOV3 | JAK2/STAT3 | ↓p-JAK2 and STAT3;
↓MMP2 and MMP9 | (35) |
| Jiang et al,
2021 | Endometrial
cancer | Ishikawa | hnRNPA1/Bcl-xl and
XIAP | ↓cellular
concentration of hnRNPA1; ↓p-Akt, and anti-apoptotic proteins,
Bcl-xl and XIAP; ↑nucleoplasmic RNA ratio of Bcl-xl and XIAP mRNA
after si-hnRNPA1 transfection | (36) |
| Zhu et al,
2018 | Lung cancer | Murine LLC | Wnt and NF-κB | ↓c-Myc and cyclin
D1; ↓NF-κB | (38) |
| Cho et al,
2015 | Oral squamous cell
carcinoma | HN22 and HSC4 | Sp1 | ↓Sp1; ↑cell cycle
arrest-related proteins such as p27 and p21; ↓cyclin D1, Mcl-1 and
survivin (proteins related to cell proliferation and survival);
↓apoptosis-related proteins, BID and PARP; ↑Bax, cleaved caspase-3
and cleaved PARP; ↓anti-apoptotic protein, Bcl-xl | (41) |
| Kok et al,
2009 | Oral oncology | SAS | DR5 | ↑DR5 | (42) |
| Zhang et al,
2019 | Laryngeal
cancer | Hep-2 (a HeLa
contaminated cell line) | JAK/STAT3 | ↓p-STAT3, pJAK1 and
pJAK2 | (12) |
| Han et al,
2023 | Bladder cancer | 5637 | MEK/ERK | ↓p-ERK and p-MEK;
no change in the ERK expression | (44) |
The MAPK family, which includes p38 MAPK,
extracellular-regulated protein kinase (ERK) and c-Jun-N-terminal
kinase (JNK), are essential mediators of signal transmission from
the cell membrane to the nucleus and are triggered by a variety of
external stimuli. Many physiological processes, such as cell
proliferation, differentiation and apoptosis, are regulated by
MAPKs (58). In HCT116 cells, Esc
achieves G1 phase cell cycle arrest by upregulating Ras,
ERK1/2 and p27KIP8 (13), while in
HT-29 and 5637 cell lines, involvement of the MAPK signaling
pathway is evident (17,44). In colon cancer, apoptosis is induced
by Esc through MAPK activation (17), whereas in bladder cancer, its
antitumor effects depend on the mitogen-activated protein kinase
kinase (MEK)/ERK pathway (44). In
contrast to the HT-29 cell line, where Esc treatment activates JNK,
p38 MAPK and ERK, leading to the upregulation of p-ERK, the
response of bladder cancer cells to Esc exposure is characterized
by the downregulation of p-MEK and p-ERK.
Separate studies have highlighted the regulatory
effect of Esc on the JAK/STAT3 signaling pathway in ovarian and
laryngeal cancers. Yin et al (35) observed a reduction in p-JAK2 and
p-STAT3 by Esc, leading to decreased matrix metalloproteinase
(MMP)2 and MMP9 levels and the promotion of matrix breakdown in
SKOV3 cells. While in the Hep-2 cells (a HeLa contaminated cell
line), Zhang et al (12)
showed that Esc decreased the expression of p-STAT3, p-JAK, and
p-JAK2.
Moreover, other studies have investigated other
signaling pathways that are influenced by Esc. In an article by
Arora et al (28), it was
demonstrated that Esc activated the antioxidant responsive element
(ARE) pathway in pancreatic cancer cells by disrupting the nuclear
factor erythroid 2-related factor 2 (Nrf2)-kelch-like
ECH-associated protein 1 (KEAP1) interaction. Esc activates the
Nrf2/ARE pathway by binding to the KEAP1 protein, leading to the
suppression of cancer cell proliferation, which is mediated through
the ROS-sensitive transcription factor, NF-κB, emphasizing the
potential efficacy of Esc in the targeted treatment of pancreatic
cancer. Additionally, Jiang et al (36) showed that Esc directly binds to
heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1). This
interaction is crucial for modulating key cellular components,
including the expression of p-Akt and anti-apoptotic proteins such
as BCL-xl and XIAP. In endometrial cancer (36), Esc has been shown to increase the
nucleocytoplasmic RNA of BCL-xl and XIAP mRNA through binding to
hnRNPA1, as observed both in vitro and in vivo. In
HN22 and HSC4 OSCC cell lines, Esc treatment decreased Sp1
expression, leading to proliferation arrest and apoptosis of these
cells (41). By contrast, within
the context of oral cancer, Esc increased TRAIL-induced apoptosis
in the SAS human oral cancer cell line. This enhancement was
achieved through the upregulation of DR5 (42), highlighting the differential
mechanisms of action across the various cellular environments
related to oral cancer.
Discussion and future perspectives
Unlike widely used chemotherapeutic agents, Esc is
a TCM compound extracted from natural agents and possesses numerous
advantages, including low cytotoxicity, the capability to affect
various oncogenic pathways and novel bioactive structures. In some
studies, chemotherapeutic drugs such as cisplatin, 5-fluorouracil
and irinotecan, were used as positive controls. Studies involving
in vivo experiments have suggested that administration of
Esc has no significant effect on the weight of mice and does not
exhibit hepatotoxicity or nephrotoxicity (15,18,38).
The present review summarized 10 articles that
determined the IC50 values of Esc in cancer cells. A
novel compound, Esc-NO-DEAC ternary hybrid A11, designed for
triple-negative breast cancer has an IC50 as low as 8
nM. However, the effects of Esc on other cancer cells were in the
micromolar range, demonstrating its efficacy. Additionally, Esc
showed its best effect in Hep-2 laryngeal cancer cells (a HeLa
contaminated cell line) with a 72-h IC50 value as low as
1.958 µM, which is notably different from other cancer types,
indicating the need for more research on the ability of Esc to
inhibit laryngeal cancer cells effectively. Kim et al
(15) found that the
IC50 values of Esc and cisplatin differed by ~20 times
in a number of colon cancer cell lines, suggesting that Esc, as a
compound, is still far from clinical application and requires more
experimental studies to explore its mechanisms in cancer cells or
in combination with paclitaxel in endometrial cancer (36). Therefore, further studies on the
resistance, sensitivity and toxicity of Esc in combination with
existing chemotherapy drugs are crucial. Xenograft animal models
are mainly used for in vivo experiments, but one relevant
study utilized orthotopic transplantation to better simulate the
in vivo tumor environment (15). In the HCT116 colon cancer cell line,
tumor inhibition reached 64%, whereas in the Hep-2 laryngeal cancer
cell line (a HeLa contaminated cell line), the inhibition rate was
as high as 80% (12,18). Due to the different administration
methods (subcutaneous injection and gavage) used in the two
experiments, the comparison was not sufficiently rigorous,
reflecting differences between the various cancer types, similar to
the reported IC50 values. Further in vivo
experiments are required to explore the antitumor properties of
Esc.
In the reviewed studies, assessment of the
biological behavior focused on the ability of Esc to inhibit cancer
cell proliferation and promote cancer cell apoptosis, with some
studies mentioning G1 phase cell cycle arrest. Only one
study has examined the role of Esc in promoting ferroptosis in
liver cancer cells (27).
Meanwhile, Esc has been shown to affect multiple signaling pathways
in cancer cells, the most common being the Wnt/β-catenin, PI3K/Akt,
MAPK and JAK/STAT3 pathways. Furthermore, topics such as the tumor
microenvironment, cellular autophagy and pyroptosis still have
research potential in Esc-related studies. In the field of
oncology, research on the mechanisms of Esc through micro (mi)RNA
or exosomes remains unexplored. Therefore, further exploration of
the role of ESC in these areas is warranted.
Although Esc has shown promising anticancer effects
in many tumor studies, its absence as a frontline cancer drug
worldwide raises questions. There are concerns regarding issues
such as dosage and the fact that its positive effects have only
been observed in cell experiments and xenograft models, with only
one study involving orthotopically transplanted tumors (15). To the best of our knowledge, no
studies have used a patient-derived xenograft (PDX) model to study
Esc, and ethical concerns have hindered clinical trials. Therefore,
while Esc has demonstrated potential in in vitro and in
vivo experiments using cancer cell lines, further research is
needed to prove its efficacy in studies closer to human tumors.
Acknowledgements
Not applicable.
Funding
This study was supported by the program for Natural Science
Foundation of Henan Province (grant no. 222300420533), Commercial
research of 2021 (grant no. 20210106A),
Technology-Benefiting-People Program of Zhengzhou (grant no.
2022KJHM0031) and Key Research Project of Higher Education of Henan
Province (grant no. 24A310026).
Availability of data and materials
Not applicable.
Authors' contributions
ML, JW, YH, XY and MW were responsible for writing
the original draft. YS and FG helped to write the manuscript and
were responsible for visualization of mechanism diagrams. SZ
reviewed and edited the manuscript. PL conceptualized the
manuscript and was responsible for supervision. Data authentication
is not applicable. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Esc
|
esculetin
|
|
IC50
|
half-maximal inhibitory
concentration
|
|
ROS
|
reactive oxygen species
|
|
CypD
|
cyclophilin D
|
|
ER
|
endoplasmic reticulum
|
|
IRE1
|
inositol-requiring enzyme 1
|
|
GRP-78
|
glucose regulatory protein 78
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JAK
|
janus kinase
|
|
STAT3
|
signal transducer and activator of
transcription-3
|
|
NFE2L2
|
NFE2-like BZIP transcription factor
2
|
|
GPX4
|
glutathione peroxidase 4
|
|
HO-1
|
heme oxygenase-1
|
|
LC3II
|
light chain 3
phosphatidylethanolamine conjugate
|
|
NCOA4
|
nuclear receptor coactivator 4
|
|
FTH1
|
ferritin heavy chain 1
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
MMP
|
matrix metalloproteinase
|
|
NF-κB
|
nuclear factor-κB
|
|
Sp1
|
specificity protein 1
|
|
DR
|
death receptor
|
|
MEK
|
mitogen-activated protein kinase
kinase
|
|
Tcf
|
T-cell factor
|
References
|
1
|
Sekiya K, Okuda H and Arichi S: Selective
inhibition of platelet lipoxygenase by esculetin. Biochim Biophys
Acta. 713:68–72. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang H, Li Q, Shi Z, Hu Z and Wang R:
Analysis of aesculin and aesculotin in Cortex fraxini by capillary
zone electrophoresis. Talanta. 52:607–621. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bora KS and Sharma A: The genus artemisia:
A comprehensive review. Pharm Biol. 49:101–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang SH: Flavonoids, coumarins and
acridone alkaloids from the root bark of Citrus limonia.
Phytochemistry. 29:351–353. 1990. View Article : Google Scholar
|
|
5
|
Oshima N, Narukawa Y, Takeda T and Kiuchi
F: Collagenase inhibitors from Viola yedoensis. J Nat Med.
67:240–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Street RA, Sidana J and Prinsloo G:
Cichorium intybus: Traditional uses, phytochemistry, pharmacology,
and toxicology. Evid Based Complement Alternat Med.
2013:5793192013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kadakol A, Sharma N, Kulkarni YA and
Gaikwad AB: Esculetin: A phytochemical endeavor fortifying effect
against non-communicable diseases. Biomed Pharmacother.
84:1442–1448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rzodkiewicz P, Gasinska E, Maslinski S and
Bujalska-Zadrozny M: Antinociceptive properties of esculetin in
non-inflammatory and inflammatory models of pain in rats. Clin Exp
Pharmacol Physiol. 42:213–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fylaktakidou KC, Hadjipavlou-Litina DJ,
Litinas KE and Nicolaides DN: Natural and synthetic coumarin
derivatives with anti-inflammatory/antioxidant activities. Curr
Pharm Des. 10:3813–3833. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang G, Xu Y and Zhou HF: Esculetin
inhibits proliferation, invasion, and migration of laryngeal cancer
in vitro and in vivo by inhibiting janus kinas (JAK)-signal
transducer and activator of transcription-3 (STAT3) activation. Med
Sci Monit. 25:7853–7863. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SS, Park SK, Lim JH, Choi YH, Kim WJ
and Moon SK: Esculetin inhibits cell proliferation through the
Ras/ERK1/2 pathway in human colon cancer cells. Oncol Rep.
25:223–230. 2011.PubMed/NCBI
|
|
14
|
Wen M, Sun J, Yang M, Zhang X, Wang Y,
Zhou W, Shi Y, Huang Y, Li N and Chen L: Design, synthesis, and
biological evaluation of Esculetin-Furoxan-DEAC ternary hybrids for
anti-triple negative breast cancer. J Med Chem. 66:12446–12458.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim WK, Byun WS, Chung HJ, Oh J, Park HJ,
Choi JS and Lee SK: Esculetin suppresses tumor growth and
metastasis by targeting Axin2/E-cadherin axis in colorectal cancer.
Biochem Pharmacol. 152:71–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi YJ, Lee CM, Park SH and Nam MJ:
Esculetin induces cell cycle arrest and apoptosis in human colon
cancer LoVo cells. Environ Toxicol. 34:1129–1136. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim AD, Han X, Piao MJ, Hewage SRKM, Hyun
CL, Cho SJ and Hyun JW: Esculetin induces death of human colon
cancer cells via the reactive oxygen species-mediated mitochondrial
apoptosis pathway. Environ Toxicol Pharmacol. 39:982–989. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SY, Lim TG, Chen H, Jung SK, Lee HJ,
Lee MH, Kim DJ, Shin A, Lee KW, Bode AM, et al: Esculetin
suppresses proliferation of human colon cancer cells by directly
targeting β-catenin. Cancer Prev Res (Phila). 6:1356–1364. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li T, Zhang L and Huo X: Inhibitory
effects of aesculetin on the proliferation of colon cancer cells by
the Wnt/β-catenin signaling pathway. Oncol Lett. 15:7118–7122.
2018.PubMed/NCBI
|
|
20
|
Kim AD, Hewage SRK, Piao MJ, Kang KA, Cho
SJ and Hyun JW: Esculetin induces apoptosis in human colon cancer
cells by inducing endoplasmic reticulum stress. Cell Biochem Funct.
33:487–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Feng M and Guan W: Naturally
occurring aesculetin coumarin exerts antiproliferative effects in
gastric cancer cells mediated via apoptotic cell death, cell cycle
arrest and targeting PI3K/AKT/M-TOR signalling pathway. Acta
Biochim Pol. 68:109–113. 2021.PubMed/NCBI
|
|
22
|
Wang G, Lu M, Yao Y, Wang J and Li J:
Esculetin exerts antitumor effect on human gastric cancer cells
through IGF-1/PI3K/Akt signaling pathway. Eur J Pharmacol.
814:207–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saito Y, Okamoto H, Mizusaki S and Yoshida
D: Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced
induction of Epstein-Barr virus early antigen in Raji cells by some
inhibitors of tumor promotion. Cancer Lett. 32:137–144. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei S, Qiu T, Yao X, Wang N, Jiang L, Jia
X, Tao Y, Wang Z, Pei P, Zhang J, et al: Arsenic induces pancreatic
dysfunction and ferroptosis via mitochondrial
ROS-autophagy-lysosomal pathway. J Hazard Mater. 384:1213902020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park E and Chung SW: ROS-mediated
autophagy increases intracellular iron levels and ferroptosis by
ferritin and transferrin receptor regulation. Cell Death Dis.
10:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qian X, Zhang J, Gu Z and Chen Y:
Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic
tumor therapy. Biomaterials. 211:1–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiu Z, Li Y, Fang J, Han J, Li S, Li Y,
Yang X, Song G, Li Y, Jin N, et al: Inhibitory effects of esculetin
on liver cancer through triggering NCOA4 pathway-mediation
Ferritinophagy in vivo and in vitro. J Hepatocell Carcinoma.
10:611–629. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arora R, Sawney S, Saini V, Steffi C,
Tiwari M and Saluja D: Esculetin induces antiproliferative and
apoptotic response in pancreatic cancer cells by directly binding
to KEAP1. Mol Cancer. 15:642016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang SX, Mou JF, Luo Q, Mo QH, Zhou XL,
Huang X, Xu Q, Tan XD, Chen X and Liang CQ: Anti-Hepatitis B virus
activity of esculetin from Microsorium fortunei in vitro and in
vivo. Molecules. 24:34752019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilkinson L and Gathani T: Understanding
breast cancer as a global health concern. Br J Radiol.
95:202110332022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang HT, Chou CT, Lin YS, Shieh P, Kuo
DH, Jan CR and Liang WZ: Esculetin, a natural coumarin compound,
evokes Ca(2+) movement and activation of Ca(2+)-associated
mitochondrial apoptotic pathways that involved cell cycle arrest in
ZR-75-1 human breast cancer cells. Tumour Biol. 37:4665–4678. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gaona-Luviano P, Medina-Gaona LA and
Magaña-Pérez K: Epidemiology of ovarian cancer. Chin Clin Oncol.
9:472020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Momenimovahed Z, Tiznobaik A, Taheri S and
Salehiniya H: Ovarian cancer in the world: Epidemiology and risk
factors. Int J Womens Health. 11:287–299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crosbie EJ, Kitson SJ, McAlpine JN,
Mukhopadhyay A, Powell ME and Singh N: Endometrial cancer. Lancet.
399:1412–1428. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin W, Fu X, Chang W, Han L, Meng J, Cao
A, Ren X, Fan Z and Zhou S: Antiovarian cancer mechanism of
esculetin: Inducing G0/G1 arrest and apoptosis via JAK2/STAT3
signalling pathway. J Pharm Pharmacol. 75:87–97. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang R, Su G, Chen X, Chen S, Li Q, Xie B
and Zhao Y: Esculetin inhibits endometrial cancer proliferation and
promotes apoptosis via hnRNPA1 to downregulate BCLXL and XIAP.
Cancer Lett. 521:308–321. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang J, Xiao YL, He XR, Qiu GF and Hu XM:
Aesculetin-induced apoptosis through a ROS-mediated mitochondrial
dysfunction pathway in human cervical cancer cells. J Asian Nat
Prod Res. 12:185–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu X, Gu J and Qian H: Esculetin
attenuates the growth of lung cancer by downregulating Wnt targeted
genes and suppressing NF-κB. Arch Bronconeumol (Engl Ed).
54:128–133. 2018.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li H, Wang Q, Wang Y, Xu Z and Han Z:
Esculetin inhibits the proliferation of human lung cancer cells by
targeting epithelial-to-mesenchymal transition of the cells. Cell
Mol Biol (Noisy-le-grand). 65:95–98. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Igissin N, Zatonskikh V, Telmanova Z,
Tulebaev R and Moore M: Laryngeal cancer: Epidemiology, etiology,
and prevention: A narrative review. Iran J Public Health.
52:2248–2259. 2023.PubMed/NCBI
|
|
41
|
Cho JH, Shin JC, Cho JJ, Choi YH, Shim JH
and Chae JI: Esculetin (6,7-dihydroxycoumarin): A potential cancer
chemopreventive agent through suppression of Sp1 in oral squamous
cancer cells. Int J Oncol. 46:265–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kok SH, Yeh CC, Chen ML and Kuo MYP:
Esculetin enhances TRAIL-induced apoptosis through DR5 upregulation
in human oral cancer SAS cells. Oral Oncol. 45:1067–1072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Turkekul K, Colpan RD, Baykul T, Ozdemir
MD and Erdogan S: Esculetin inhibits the survival of human prostate
cancer cells by inducing apoptosis and arresting the cell cycle. J
Cancer Prev. 23:10–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han L, Li P, Fu X, Huang Z and Yin W:
Aesculetin inhibits proliferation and induces mitochondrial
apoptosis in bladder cancer cells by suppressing the MEK/ERK
signaling pathway. Anticancer Agents Med Chem. 23:478–487. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pelcovits A and Niroula R: Acute myeloid
leukemia: A review. R I Med J (2013). 103:38–40. 2020.PubMed/NCBI
|
|
46
|
Lucas DM, Still PC, Pérez LB, Grever MR
and Kinghorn AD: Potential of plant-derived natural products in the
treatment of leukemia and lymphoma. Curr Drug Targets. 11:812–822.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gong J, Zhang WG, Feng XF, Shao MJ and
Xing C: Aesculetin (6,7-dihydroxycoumarin) exhibits potent and
selective antitumor activity in human acute myeloid leukemia cells
(THP-1) via induction of mitochondrial mediated apoptosis and
cancer cell migration inhibition. J BUON. 22:1563–1569.
2017.PubMed/NCBI
|
|
48
|
Pan H, Wang BH, Lv W, Jiang Y and He L:
Esculetin induces apoptosis in human gastric cancer cells through a
cyclophilin D-mediated mitochondrial permeability transition pore
associated with ROS. Chem Biol Interact. 242:51–60. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang G, Mao J, Ji Z and Ailati A:
Stachyose-induced apoptosis of Caco-2 cells via the
caspase-dependent mitochondrial pathway. Food Funct. 6:765–771.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Anelli T and Sitia R: Protein quality
control in the early secretory pathway. EMBO J. 27:315–327. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ye J, Rawson RB, Komuro R, Chen X, Davé
UP, Prywes R, Brown MS and Goldstein JL: ER stress induces cleavage
of membrane-bound ATF6 by the same proteases that process SREBPs.
Mol Cell. 6:1355–1364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee AH, Iwakoshi NN and Glimcher LH: XBP-1
regulates a subset of endoplasmic reticulum resident chaperone
genes in the unfolded protein response. Mol Cell Biol.
23:7448–7459. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Harding HP, Zhang Y, Bertolotti A, Zeng H
and Ron D: Perk is essential for translational regulation and cell
survival during the unfolded protein response. Mol Cell. 5:897–904.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi
F, Katayama T and Tohyama M: Activation of caspase-12, an
endoplastic reticulum (ER) resident caspase, through tumor necrosis
factor receptor-associated factor 2-dependent mechanism in response
to the ER stress. J Biol Chem. 276:13935–13940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mancias JD, Vaites LP, Nissim S, Biancur
DE, Kim AJ, Wang X, Liu Y, Goessling W, Kimmelman AC, Harper JW, et
al: Ferritinophagy via NCOA4 is required for erythropoiesis and is
regulated by iron dependent HERC2-mediated proteolysis. Elife.
4:e103082015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gao M, Monian P, Pan Q, Zhang W, Xiang J
and Jiang X: Ferroptosis is an autophagic cell death process. Cell
Res. 26:1021–1032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ahmed-Choudhury J, Williams KT, Young LS,
Adams DH and Afford SC: CD40 mediated human cholangiocyte apoptosis
requires JAK2 dependent activation of STAT3 in addition to
activation of JNK1/2 and ERK1/2. Cell Signal. 18:456–468. 2006.
View Article : Google Scholar : PubMed/NCBI
|
















