Introduction
Gastrointestinal cancers account for 26% of the
global cancer burden and 35% of all cancer-related deaths. In 2018,
there were an estimated 4.8 million new cases and 3.4 million
deaths related to gastric cancer worldwide (1). Peritoneal carcinomatosis is one of the
leading causes of death in patients with gastrointestinal cancers.
For example, up to 30% of colorectal cancer patients will develop
peritoneal metastases in the course of their disease (2). Therapeutic options include
cytoreductive surgery of all peritoneal disease combined with or
without hyperthermic intraperitoneal chemotherapy (HIPEC) (3). While cytoreductive surgery is a
potentially curative option, only about a fraction of patients
qualifies for resection due to extensive peritoneal disease and/or
synchronous metastases at additional sites which cannot be
completely resected. Pressurized intraperitoneal aerosol
chemotherapy (PIPAC) is another recent therapy proposed for
patients with peritoneal carcinomatosis (4–10).
During the PIPAC procedure, laparoscopy is performed, biopsies are
taken, and chemotherapeutic drugs are delivered into the abdominal
cavity as an aerosol under pressure (11,12).
The PIPAC procedure is usually repeated every four to eight weeks.
It is a minimally invasive procedure which can usually be performed
via two small incisions, so recovery time and hospital stay are
short and patients can often continue with their next chemotherapy
cycle without any delay caused by the operation. As long as a
minimally invasive approach is feasible and the patient can undergo
anesthesia safely, PIPAC can be performed. Initially, PIPAC was
introduced for very advanced peritoneal carcinomatosis (13–16)
and was recently proposed for the use in a neoadjuvant and adjuvant
setting (17,18) to allow for the regional treatment of
the peritoneal surfaces. Studies have shown that PIPAC is safe,
well tolerated, and can improve the quality of life in patients
with peritoneal carcinomatosis (19–25).
Two methods can be employed to assess treatment response in
patients. The Peritoneal Cancer Index (PCI) according to Sugarbaker
(26) is determined during
laparoscopy to quantify the extent of the disease macroscopically.
The presence and size of peritoneal cancer nodules in 13 defined
abdominal regions is assessed and a score is assigned (27,28).
PCI ranges from 0 (indicating no visible peritoneal implants) to 39
(peritoneal cancer everywhere) and a change over time is associated
with treatment response (28).
Another classification method is the histological assessment of
peritoneal biopsies taken during laparoscopy. In 2016, Solass et
al (29) introduced the
four-tiered peritoneal regression grading score (PRGS) to assess
the response to chemotherapy in peritoneal metastasis by histology
(30). The PRGS is based on the
presence of residual tumor cells and/or the extent of regressive
features such as fibrosis, accumulation of macrophages, necrosis,
or presence of mucin with or without tumor cells. To determine the
score, the proportion between both is assessed (31). PRGS distinguishes between four
scenarios: complete response (absence of tumor cells), major
response (predominant regressive changes), minor response (mainly
viable tumor cells) and no response (no regressive changes)
(29). Here, we present a new
variation of the PRGS scoring system based on quantitative
assessment of histological regression in peritoneal carcinomatosis
(QARP) after PIPAC treatment. QARP combines elements of the PRGS
with a semiquantitative assessment of tumor cells versus regressive
changes. A semiquantitative scoring system is in line with the
standard practice at our institute for the assessment of cancer
specimens and is being used in established regression scores for
various cancer types.
Materials and methods
Peritoneal biopsies
Following approval by Institutional Ethics Committee
of Ärztekammer Westfalen-Lippe (2022-850-f-S), we examined samples
from 27 patients with gastrointestinal cancers and peritoneal
metastasis of various origins including esophageal, gastric,
pancreatic, biliary, appendiceal, and colorectal who had undergone
two to six PIPAC procedures (a total of 72 PIPACs) at the
University Hospital Muenster Department of Surgery between May 2020
and August 2022. Median age was 62 years ranging from 41 to 82.
Patients with colorectal primaries received PIPAC with oxaliplatin
120 mg/m2 BSA (body surface area), patients with all
other primary cancers received PIPAC with doxorubicin 2.1
mg/m2 BSA and cisplatin 10.5 mg/m2 BSA.
During the laparoscopic procedure, PCI was determined according to
the Sugarbaker classification (26)
and peritoneal biopsies were taken from cancerous lesions in
different abdominal quadrants, embedded in formalin, and processed
in standardized fashion by the Gerhard-Domagk Institute of
Pathology at the University Hospital Muenster. Briefly, fresh
biopsies were fixated in 10% buffered formalin for 24 to 48 h,
embedded in paraffin, under controlled temperature, cut in 3 to 5
µm sections and stained with hematoxylin and eosin (H&E) and
Periodic Acid-Schiff (PAS) according to standardized protocols.
Peritoneal cancer regression
score
Histological assessment of the peritoneal biopsies
was performed by two board certified pathologists (MA, EW).
Microscopic features of regression, previously published by Solass
et al, included mucin without cells, fibrosis,
infarct-like-necrosis, inflammatory changes with giant cells, and
lipid-laden foamy macrophages (Fig.
S1) (29). The samples were
scored according to our grading system by quantitative assessment
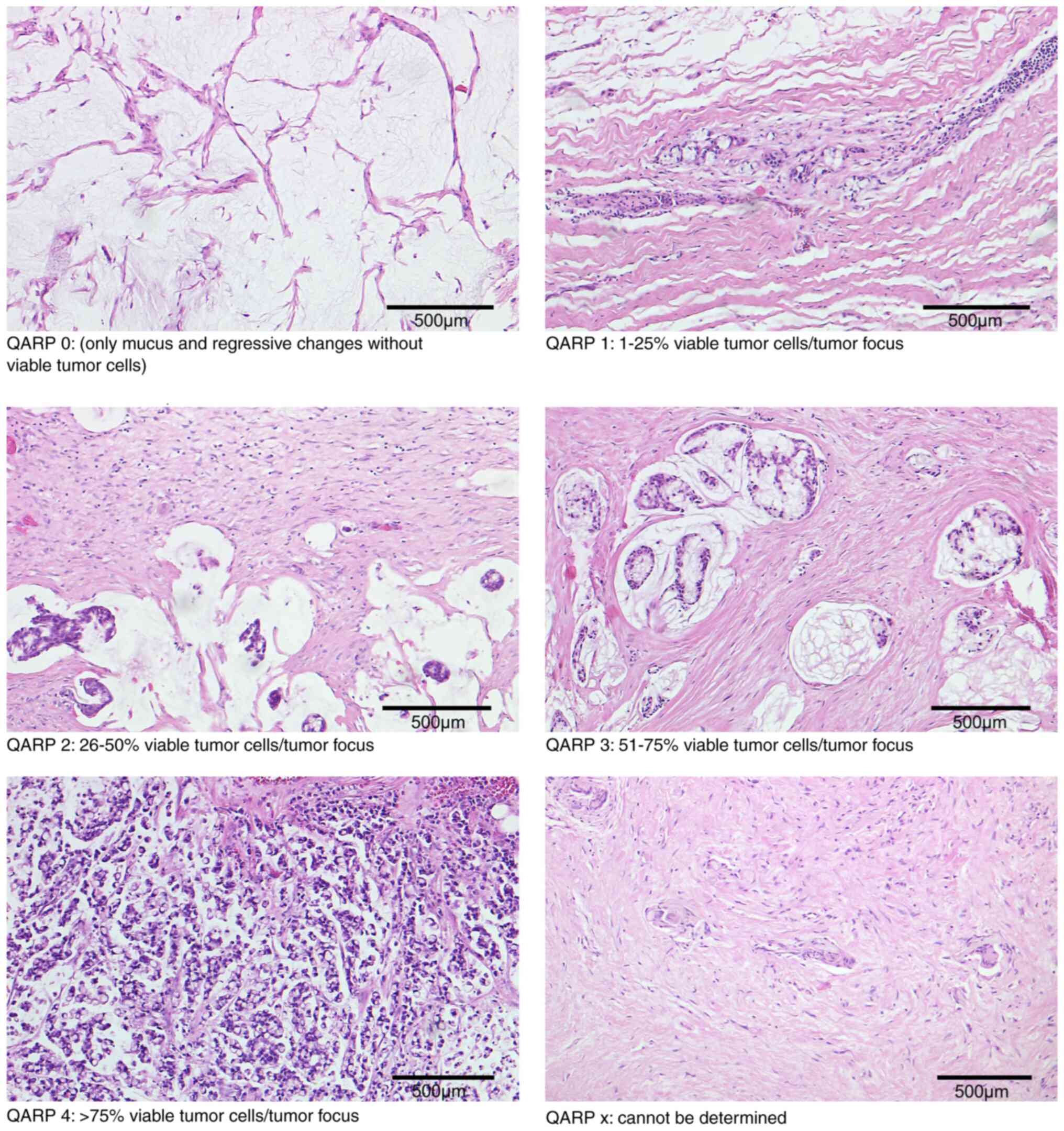
of viable tumor cells in relation to the tumor focus (Table I). The five-tiered system was graded
as follows: Grade 0-no residual tumor cells with regressive changes
present; grade 1–1 to 25% viable tumor cells per tumor focus with
regressive changes present; grade 2–26 to 50% viable tumor cells
per tumor focus with regressive changes present, grade 3–51 to 75%
viable tumor cells per tumor focus with few regressive changes;
grade 4-more than 75% viable tumor cells per tumor focus with
minimal or no regressive changes (Fig.
1). If there was no clear evidence for regressive changes in
the absence of tumor cells, QARP was graded as ‘x’-cannot be
determined. The histopathological features of viable tumor cells
were characterized by hyperchromatic nuclei, eosinophilic
cytoplasm, mitotic figures, and/or apoptosis. As mentioned above,
regressive changes were associated with fibrosis, mucin without
cells, necrosis, and inflammatory changes with giant cells.
Surrounding tissue without any sign of previously present tumor
cells was excluded from the quantitative assessment. Patient data
was extracted by chart review of the electronical medical record.
The median follow-up time was 10.2 months.
 | Table I.QARP. |
Table I.
QARP.
| Grade | Percentage of
viable tumor cells and presence of regressive changes in relation
to the tumor focus | Interpretation |
|---|
| QARP 0 | No tumor cells,
regressive changes present | Complete
response |
| QARP 1 | 1-25% viable tumor
cells/tumor focus, regressive changes present | Major response |
| QARP 2 | 26-50% viable tumor
cells/tumor focus, regressive changes present | Moderate
response |
| QARP 3 | 51-75% viable tumor
cells/tumor focus, few regressive changes present | Minor response |
| QARP 4 | >75% viable
tumor cells/tumor focus, +/-minimal regressive changes | No/minimal
response |
| QARP x | No clear sign of
tumor cells or regressive changes in the biopsy | Cannot be
determined |
Statistical analysis
Data was analyzed using Datatab Online Statistics
Calculator (https://datatab.net/statistics-calculator/descriptive-statistics).
Spearman's rank correlation coefficient was calculated to examine
the relationship between QARP and PCI. Survival data was plotted on
Kaplan Meier curves and compared using log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The median age of patients included in this study
was 62 years at the time of the first PIPAC. There were 13 male
(48.1%) and 14 female (51.9%) patients. The primary cancer origins
were as follows, esophagogastric in 8 patients (29.6%), colorectal
in 10 patients (37.0%), pancreatic in five patients (18.5%),
biliary in three patients (11.1%) and appendiceal in two patients
(7.4%) (Table II). Patients with
colorectal primaries received PIPAC with oxaliplatin 120
mg/m2 BSA, patients with all other primary cancers
received PIPAC with doxorubicin 2.1 mg/m2 BSA and
cisplatin 10.5 mg/m2 BSA. The one patient with two
primaries (pancreatic and colorectal) was treated with
doxorubicin/cisplatin. PIPAC procedure was performed according to a
standardized protocol.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| A, Patient
characteristics |
|---|
|
|---|
| Characteristic | Value |
|---|
| Median age at first
PIPAC (range), years | 62 (41–82) |
| Sex, n (%) |
|
|
Male | 13 (48.1%) |
|
Female | 14 (51.9%) |
| ECOG, n (%) |
|
| 0 | 10 (37.0%) |
| 1 | 17 (63.0%) |
| PIPAC drugs, n
(%) |
|
|
Doxorubicin/cisplatin | 16 (59.3%) |
|
Oxaliplatin | 11 (40.7%) |
|
| B, Pathological
characteristics |
|
| Tumor entities (one
patient with two primaries), n (%) |
|
|
Esophagogastric | 8 (29.6%) |
|
Signet ring cell
adenocarcinoma | 5 |
|
Poorly cohesive
adenocarcinoma | 2 |
|
Mucinous
adenocarcinoma | 1 |
| Colorectal | 10 (37.0%) |
|
Mucinous
adenocarcinoma | 5 |
|
Signet ring cell
adenocarcinoma | 3 |
|
Adenocarcinoma,
NOS | 2 |
| Appendiceal | 2 (7.4%) |
|
Signet ring cell
adenocarcinoma | 1 |
|
Mucinous
adenocarcinoma | 1 |
| Pancreatic | 5 (18.5%) |
|
Adenocarcinoma,
NOS | 5 |
| Biliary | 3 (11.1%) |
|
Adenocarcinoma,
NOS | 3 |
Regressive changes of peritoneal
carcinomatosis determined by QARP
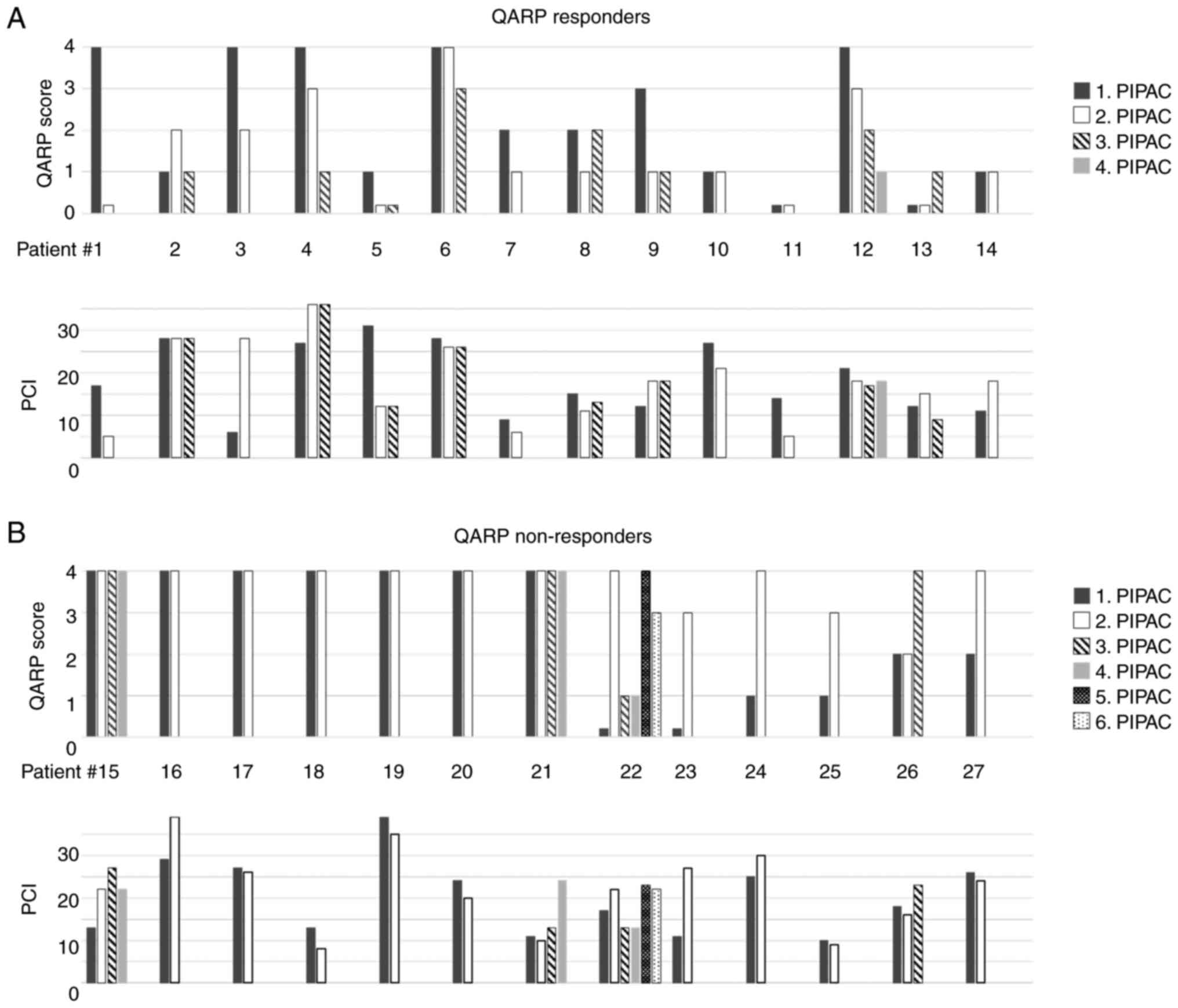
Regressive changes within the peritoneal biopsies
were determined by QARP for each PIPAC cycle. QARP scores for each
patient and procedure were plotted on a graph depicting the
development over time (Fig. 2). If
biopsies from the same procedure revealed different QARP scores,
the highest score was plotted. Nine patients showed improvement in
QARP scores over time and 5 patients had stable low QARP scores
(QARP 0 or 1) in every procedure. These patients were labelled as
‘QARP responders’ (Fig. 2A). For
comparison, matching PCI values were plotted below the QARP scores
(Fig. 2A). Thirteen patients had
either stable high QARP scores (QARP 4) or worsening scores over
time. These patients were labelled as ‘QARP non-responders’
(Fig. 2B). Once more, for
comparison, matching PCI values were plotted below the QARP scores
(Fig. 2B). Looking at the group of
responders to treatment (n=14), an improvement in QARP score was
noted from a median of QARP 2 at the first PIPAC procedure to
median QARP of 1 at the second and subsequent PIPAC procedures
(Fig. 3A, middle panel). ‘QARP
non-responders’ already started with a median QARP score of 4 at
the first PIPAC procedure and remained at that level during the
following PIPAC procedures (Fig.
3A, right panel). For comparison, matching PCI values were
plotted below the QARP scores (Fig.
3B).
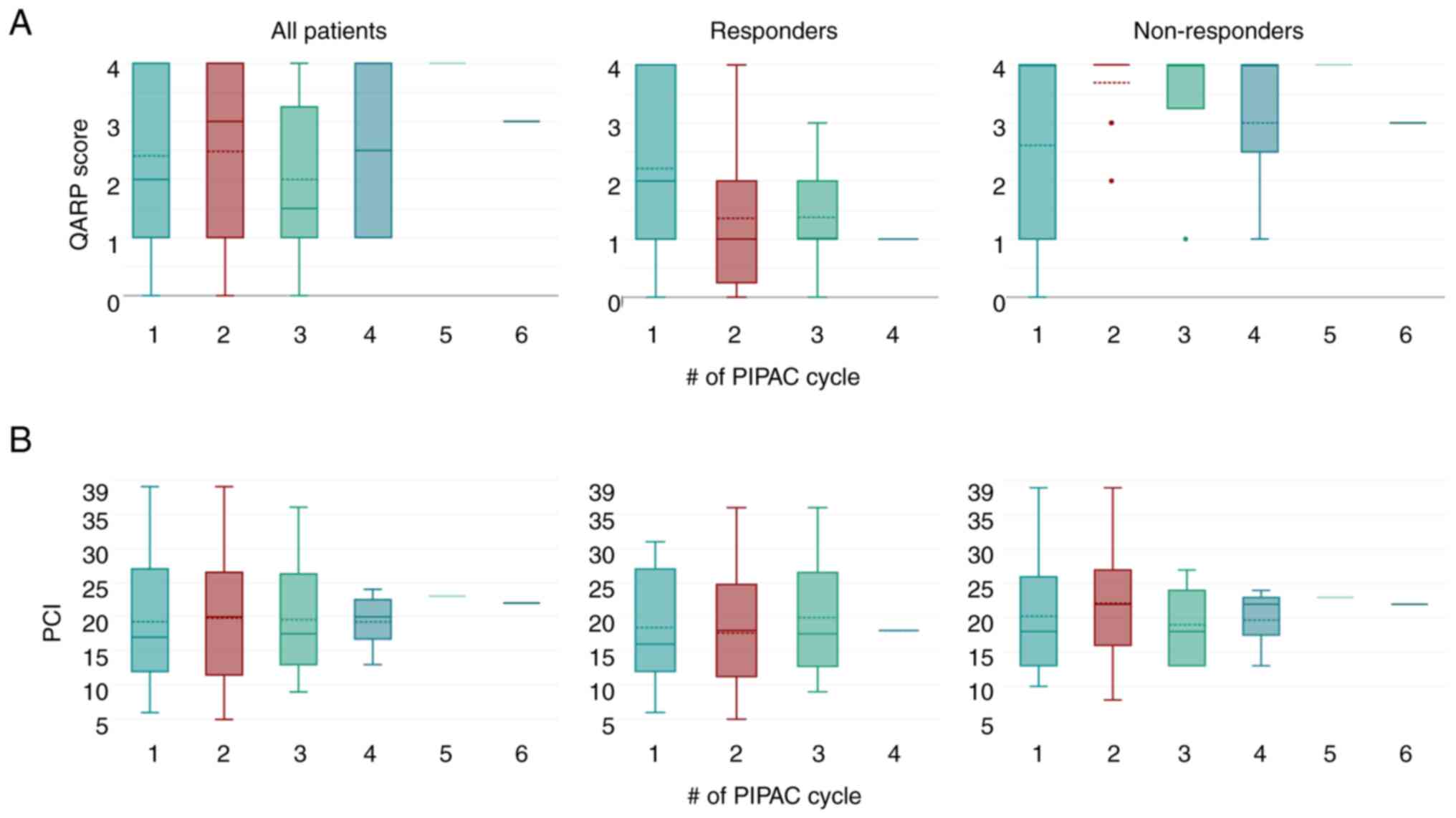 | Figure 3.QARP and PCI for entire cohort, QARP
responders and non-responders: (A) Boxplot of QARP scores (solid
transverse line depicts median, dotted transverse line depicts
mean) at each PIPAC cycle for all patients (left panel), for
patients defined as QARP responders (middle panel), for patients
defined as QARP non-responders (right panel). (B) Boxplot of PCI
(solid transverse line depicts median, dotted transverse line
depicts mean) at each PIPAC cycle for all patients (left panel),
for patients defined as QARP responders (middle panel), for
patients defined as QARP non-responders (right panel). QARP,
quantitative assessment of histological regression in peritoneal
carcinomatosis; PCI, peritoneal cancer index; PIPAC, pressurized
intraperitoneal aerosol chemotherapy. |
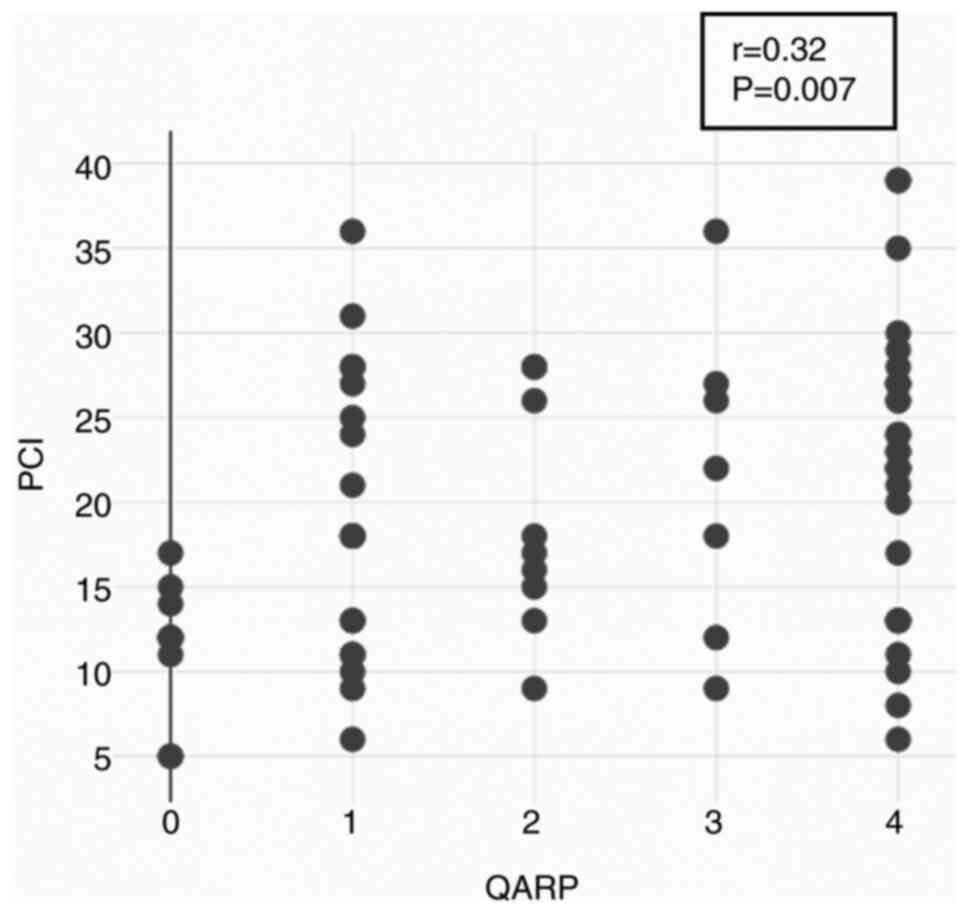
Correlation of QARP and PCI
PCI scores for each patient and procedure were
compared with QARP scores for each patient and procedure, and the
Spearman correlation was calculated. QARP and PCI showed a weak,
but significant, positive correlation (r=0.32; P=0.007), i.e.,
higher QARP scores correlated with higher PCI scores (Fig. 4).
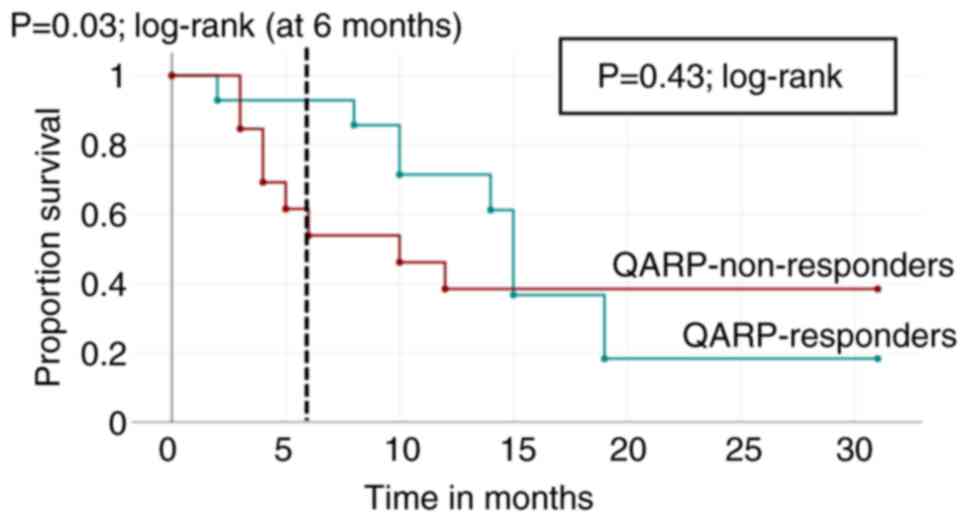
Survival curves
Survival times for QARP responders and QARP
non-responders, as defined above, were plotted on a Kaplan-Meier
graph. The survival curves diverge in the first months, with a
significant survival advantage for QARP responders at 6 months
(P=0.03; log-rank), but eventually intersect at 15 months (Fig. 5). There is no significant difference
in long-term overall survival between QARP responders and
non-responders in our cohort at the end of the observation period
(P=0.43; log-rank) (Fig. 5). For
comparison, survival curves grouped by primary cancer type were
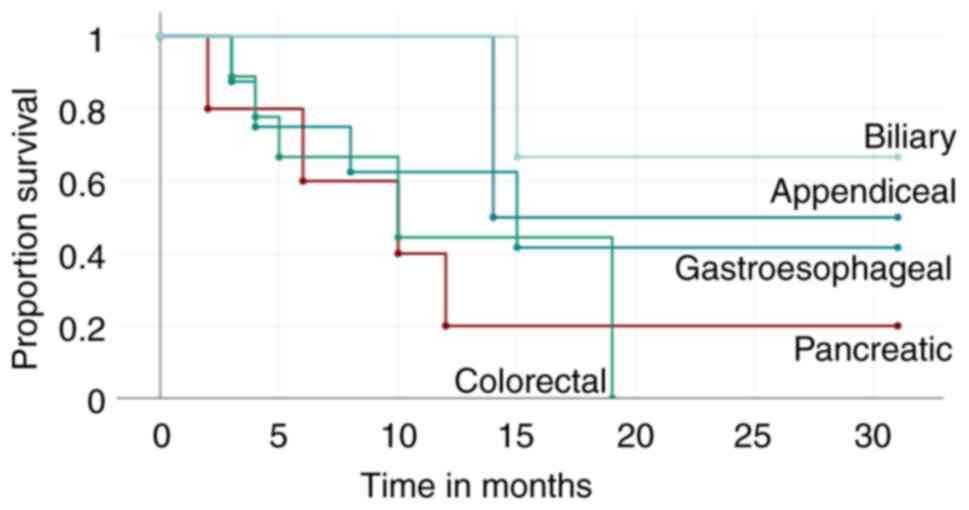
calculated (Fig. 6).
Discussion
Tumor regression scores following chemotherapy
and/or radiotherapy find applications across various primary and
metastatic cancers in assessing treatment efficacy. Notably, the
Mandard system introduced in 1994 delineates five tumor regression
grades (TRG) for esophageal carcinoma treated with preoperative
chemoradiation (32). TRG 1
signifies complete regression with no residual cancer cells
alongside fibrosis of the esophageal wall, TRG 2 indicates fibrosis
with scattered tumor cells, TRG 3 is defined as predominant
fibrosis with an increased number of residual cancer cells, TRG 4
shows a relative abundance of cancer compared to fibrosis of the
esophageal wall, and TRG 5 signifies no regressive changes
(32). In 2003, Becker et al
defined a regression score for adenocarcinoma of the
esophagogastric junction (33)
distinguishing grades as follows: grade 1a (complete tumor
regression), grade 1b (<10% of vital tumor tissue), grade 2 (10
to 50% residual tumor per tumor bed), and grade 3 (>50% of
viable tumor remaining). In 2004, Baldus et al introduced a
semiquantitative system to evaluate regressive changes after
neoadjuvant treatment of locally advanced esophageal cancers
demonstrating a favorable prognosis for patients with greater than
90% regression (34). Dworak et
al proposed a system assessing the regression of primary rectal
cancer following chemoradiation (35), while Rubbia-Brandt et al and
Blazer et al presented systems for evaluating response in
liver metastases of colorectal origin using semiquantitative
methods (36,37). In the context of assessing
peritoneal metastases from gastrointestinal carcinomas after PIPAC
therapy, Solass et al established the peritoneal regression
grading system (PRGS), confirming its reproducibility and low
interobserver variability (29,38).
The PRGS hinges on the presence of residual tumor cells and the
extent of histological regressive features, paralleling the basis
of the QARP score. Viable tumor cells are characterized by
hyperchromatic nuclei, eosinophilic cytoplasm, mitotic figures,
and/or apoptosis. Conversely, regressive changes involve fibrosis,
mucin devoid of cells, necrosis, and inflammatory changes with
giant cells. The PRGS distinguishes complete response (absence of
tumor cells), major response (predominant regressive changes),
minor response (mainly viable tumor cells) and no response (no
regressive changes) (29).
Our new scoring system combines elements of the PRGS
with a semiquantitative assessment of tumor cells versus regressive
changes. It calculates the percentage of tumor cells and regressive
changes in each tumor focus, assigning scores based on the
proportion of viable tumor cells per focus paralleling established
cancer regression scores, such as the regression scores according
to Becker et al (33) and
Baldus et al (34). Tissue
surrounding a focus with no signs of regression pointing toward
previous tumor cells is excluded from quantitative assessment.
Biopsies showing neither tumor cells nor clear regressive features
denoting a treatment response of previous tumor cells are graded as
‘x’ (indeterminable), avoiding overestimation of treatment
response. Fortunately, such instances were rare. Given multiple
biopsies were taken during each PIPAC procedure, there was
sufficient material to assign at least one QARP score to each
patient and procedure. As biopsies were routinely taken from
different abdominal quadrants, we sometimes found-in the same
patient-biopsies with regressive features without tumor cells,
while others had nearly completely viable tumor cells. This raised
the question of differing treatment responses or whether areas
without tumor cells and only regressive changes were initially not
involved by peritoneal cancer, but simply reflected the response of
‘normal’ non-diseased peritoneum to prior treatment. The PIPAC
procedure itself can result in chemical peritonitis and subsequent
histological changes of the non-diseased peritoneum, which can be
difficult to distinguish from complete regression. To prevent
overestimation of treatment response, we routinely selected the
highest score as a reference score when comparing scores over time.
Thus, a potential misinterpretation of reactive change in
non-diseased peritoneum as complete regression was avoided. When
using PRGS in the past, we frequently found ourselves struggling
with the assignment of the highest score, PRGS 4, indicating ‘no
response’, as fibrous tissue can be part of the ‘normal’ stroma of
a viable tumor or sign of fibrotic regressive changes. Therefore,
biopsies with a small amount of connective tissue within the tumor
focus could be interpreted as PRGS 4 assuming it was normal tumor
stroma, or interpreted as PRGS 3, assuming we were looking at
fibrosis suggesting a minimal regressive response. As
differentiating between these two can be challenging, we were
risking erroneous lower scores by categorizing biopsies as ‘partial
response’ rather than ‘no response’. In addition, we were wondering
if it was actually clinically relevant to discriminate between ‘no
response’ and a minimal response which would be displayed as two
different scores, namely PRGS 4 and PRGS 3. To overcome this
limitation, we decided to create a five-tiered system, with the
highest QARP score, grade 4, translating to over 75% of viable
tumor cells per focus, thus grouping patients with a minimal and no
response together. As semiquantitative scores are routinely used at
our institute, introduction of the QARP score was seamless and it
was adopted readily by our pathologists.
A limitation of our study is the rather small and
mixed patient cohort. This is not surprising, as patients with
peritoneal carcinomatosis form a heterogenous group due to the
different tumor origins. In addition, PIPAC is far from being the
standard of care for patients with peritoneal metastases despite
its growing acceptance and evidence for its usefulness. PIPAC is
only performed at specialized centers and, as a result, only a
fraction of patients who would be candidate for it, are actually
offered PIPAC treatment. We cannot show a significant difference in
survival between what we defined as QARP responders and
non-responders which might be due to the small number of patients
in our study. Interestingly, the survival curves diverge in the
first months and at 6 months, from the time of the first PIPAC,
there is still a significant difference in survival suggesting that
there might be a survival advantage early after initiation of PIPAC
treatment when comparing QARP responders and non-responders. Given
our small cohort, we did not stratify our patients further
according to the primary tumor origin which will be the goal of a
future study. However, even in our small cohort, we find a
significant correlation between QARP and PCI, a well-established
score that has been used for decades in patients with peritoneal
metastases and has been shown to be of prognostic relevance by
itself (28). In contrast to the
PRGS, we employed a five-tiered system which can allow for enhanced
subgroup differentiation, especially when analyzing larger number
of patients. Future studies with larger patient cohorts will be
able to further delineate the prognostic value of the QARP score
and confirm its usefulness.
In conclusion, QARP, our newly developed regression
score for peritoneal carcinomatosis following PIPAC, represents an
advancement built upon the foundation of the established PRGS.
Throughout our patient cohort, QARP proved to be a useful tool in
quantifying treatment response post-PIPAC. Further studies
incorporating larger patient cohorts will be able to further
delineate the prognostic value of the QARP score and confirm its
usefulness.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MA and JCS conceptualized the study. MA and EW
performed the histological analysis. MA, JPR, DECB, AP and JCS
collected, analyzed and interpreted the data. MA, JPR and JCS wrote
the original draft of the manuscript. DECB, AP and EW reviewed and
edited the manuscript. JCS supervised the project. MA, JPR, DECB
and JCS confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki, and was approved by Institutional Ethics
Committee of Ärztekammer Westfalen-Lippe (approval no.
2022-850-f-S, date of approval: October 1, 2023). Written informed
consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HIPEC
|
hyperthermic intraperitoneal
chemotherapy
|
|
PIPAC
|
pressurized intraperitoneal aerosol
chemotherapy
|
|
PCI
|
peritoneal cancer index
|
|
PRGS
|
peritoneal regression grading
score
|
|
QARP
|
quantitative assessment of
histological regression in peritoneal carcinomatosis
|
|
H&E
|
hematoxylin and eosin
|
|
PAS
|
periodic acid-Schiff
|
|
BSA
|
body surface area
|
|
TRG
|
tumor regression grade
|
References
|
1
|
Arnold M, Park JY, Camargo MC, Lunet N,
Forman D and Soerjomataram I: Is gastric cancer becoming a rare
disease? A global assessment of predicted incidence trends to 2035.
Gut. 69:823–829. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koppe MJ, Boerman OC, Oyen WJ and
Bleichrodt RP: Peritoneal carcinomatosis of colorectal origin:
Incidence and current treatment strategies. Ann Surg. 243:212–222.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugarbaker PH, Landy D and Pascal R:
Intraperitoneal chemotherapy for peritoneal carcinomatosis from
colonic or appendiceal cystadenocarcinoma: Rationale and results of
treatment. Prog Clin Biol Res. 354B:141–170. 1990.PubMed/NCBI
|
|
4
|
Khomyakov V, Ryabov A, Ivanov A, Bolotina
L, Utkina A, Volchenko N and Kaprin A: Bidirectional chemotherapy
in gastric cancer with peritoneal metastasis combining intravenous
XELOX with intraperitoneal chemotherapy with low-dose cisplatin and
Doxorubicin administered as a pressurized aerosol: An open-label,
Phase-2 study (PIPAC-GA2). Pleura Peritoneum. 1:159–166. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tempfer CB, Rezniczek GA, Ende P, Solass W
and Reymond MA: Pressurized Intraperitoneal Aerosol Chemotherapy
with Cisplatin and Doxorubicin in Women with Peritoneal
Carcinomatosis: A Cohort Study. Anticancer Res. 35:6723–6729.
2015.PubMed/NCBI
|
|
6
|
Demtroder C, Solass W, Zieren J, Strumberg
D, Giger-Pabst U and Reymond MA: Pressurized intraperitoneal
aerosol chemotherapy with oxaliplatin in colorectal peritoneal
metastasis. Colorectal Dis. 18:364–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alyami M, Gagniere J, Sgarbura O,
Cabelguenne D, Villeneuve L, Pezet D, Quenet F, Glehen O, Bakrin N
and Passot G: Multicentric initial experience with the use of the
pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the
management of unresectable peritoneal carcinomatosis. Eur J Surg
Oncol. 43:2178–2183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khosrawipour T, Khosrawipour V and
Giger-Pabst U: Pressurized intra peritoneal aerosol chemotherapy in
patients suffering from peritoneal carcinomatosis of pancreatic
adenocarcinoma. PLoS One. 12:e01867092017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Falkenstein TA, Gotze TO, Ouaissi M,
Tempfer CB, Giger-Pabst U and Demtroder C: First clinical data of
pressurized intraperitoneal aerosol chemotherapy (PIPAC) as salvage
therapy for peritoneal metastatic biliary tract cancer. Anticancer
Res. 38:373–378. 2018.PubMed/NCBI
|
|
10
|
Horvath P, Beckert S, Struller F,
Konigsrainer A and Reymond MA: Pressurized intraperitoneal aerosol
chemotherapy (PIPAC) for peritoneal metastases of pancreas and
biliary tract cancer. Clin Exp Metastasis. 35:635–640. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hubner M, Alyami M, Villeneuve L,
Cortes-Guiral D, Nowacki M, So J and Sgarbura O; ISSPP PIPAC study
groupL, : Consensus guidelines for pressurized intraperitoneal
aerosol chemotherapy: Technical aspects and treatment protocols.
Eur J Surg Oncol. 48:789–794. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hubner M, Teixeira Farinha H, Grass F,
Wolfer A, Mathevet P, Hahnloser D and Demartines N: Feasibility and
safety of pressurized intraperitoneal aerosol chemotherapy for
peritoneal carcinomatosis: A retrospective cohort study.
Gastroenterol Res Pract. 2017:68527492017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reymond MA, Hu B, Garcia A, Reck T,
Köckerling F, Hess J and Morel P: Feasibility of therapeutic
pneumoperitoneum in a large animal model using a microvaporisator.
Surg Endosc. 14:51–55. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Solaß W, Hetzel A, Nadiradze G, Sagynaliev
E and Reymond MA: Description of a novel approach for
intraperitoneal drug delivery and the related device. Surg Endosc.
26:1849–1855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Solass W, Kerb R, Mürdter T, Giger-Pabst
U, Strumberg D, Tempfer C, Zieren J, Schwab M and Reymond MA:
Intraperitoneal chemotherapy of peritoneal carcinomatosis using
pressurized aerosol as an alternative to liquid solution: First
evidence for efficacy. Ann Surg Oncol. 21:553–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solass W, Herbette A, Schwarz T, Hetzel A,
Sun JS, Dutreix M and Reymond MA: Therapeutic approach of human
peritoneal carcinomatosis with Dbait in combination with
capnoperitoneum: Proof of concept. Surg Endosc. 26:847–852. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Giorgio A, Macri A, Ferracci F, Robella
M, Visaloco M, De Manzoni G, Sammartino P, Sommariva A, Biacchi D,
Roviello F, et al: 10 Years of pressurized intraperitoneal aerosol
chemotherapy (PIPAC): A systematic review and meta-analysis.
Cancers (Basel). 15:11252023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daniel SK, Sun BJ and Lee B: PIPAC for
Gastrointestinal Malignancies. J Clin Med. 12:67992023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grass F, Vuagniaux A, Teixeira-Farinha H,
Lehmann K, Demartines N and Hubner M: Systematic review of
pressurized intraperitoneal aerosol chemotherapy for the treatment
of advanced peritoneal carcinomatosis. Br J Surg. 104:669–678.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tempfer CB, Giger-Pabst U, Seebacher V,
Petersen M, Dogan A and Rezniczek GA: A phase I, single-arm,
open-label, dose escalation study of intraperitoneal cisplatin and
doxorubicin in patients with recurrent ovarian cancer and
peritoneal carcinomatosis. Gynecol Oncol. 150:23–30. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Odendahl K, Solass W, Demtroder C,
Giger-Pabst U, Zieren J, Tempfer C and Reymond MA: Quality of life
of patients with end-stage peritoneal metastasis treated with
pressurized intraperitoneal aerosol chemotherapy (PIPAC). Eur J
Surg Oncol. 41:1379–1385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nadiradze G, Giger-Pabst U, Zieren J,
Strumberg D, Solass W and Reymond MA: Pressurized intraperitoneal
aerosol chemotherapy (PIPAC) with low-dose cisplatin and
doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg.
20:367–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gockel I, Jansen-Winkeln B, Haase L, Rhode
P, Mehdorn M, Niebisch S, Moulla Y, Lyros O, Lordick F, Schierle K,
et al: Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in
gastric cancer patients with peritoneal metastasis (PM): Results of
a single-center experience and register study. J Gastric Cancer.
18:379–391. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sgarbura O, Hubner M, Alyami M, Eveno C,
Gagniere J, Pache B, Pocard M, Bakrin N and Quénet F: Oxaliplatin
use in pressurized intraperitoneal aerosol chemotherapy (PIPAC) is
safe and effective: A multicenter study. Eur J Surg Oncol.
45:2386–2391. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alyami M, Bonnot PE, Mercier F, Laplace N,
Villeneuve L, Passot G, Bakrin N, Kepenekian V and Glehen O:
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for
unresectable peritoneal metastasis from gastric cancer. Eur J Surg
Oncol. 47:123–127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugarbaker PH and Jablonski KA: Prognostic
features of 51 colorectal and 130 appendiceal cancer patients with
peritoneal carcinomatosis treated by cytoreductive surgery and
intraperitoneal chemotherapy. Ann Surg. 221:124–132. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giger-Pabst U, Demtroder C, Falkenstein
TA, Ouaissi M, Götze TO, Rezniczek GA and Tempfer CB: Pressurized
intraperitoneal aerosol chemotherapy (PIPAC) for the treatment of
malignant mesothelioma. BMC Cancer. 18:4422018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harmon RL and Sugarbaker PH: Prognostic
indicators in peritoneal carcinomatosis from gastrointestinal
cancer. Int Semin Surg Oncol. 2:32005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Solass W, Sempoux C, Detlefsen S, Carr NJ
and Bibeau F: Peritoneal sampling and histological assessment of
therapeutic response in peritoneal metastasis: Proposal of the
peritoneal regression grading score (PRGS). Pleura Peritoneum.
1:99–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hubner M, Somashekhar SP, Teixeira Farinha
H, Abba J, Rao RG, Alyami M and Willaert W: Treatment response
after pressurized intra peritoneal aerosol chemotherapy (PIPAC) for
peritoneal metastases of colorectal originf. Ann Surg Open.
3:e2032022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kurtz F, Struller F, Horvath P, Solass W,
Bosmuller H, Königsrainer A and Reymond MA: Feasibility, safety,
and efficacy of pressurized intraperitoneal aerosol chemotherapy
(PIPAC) for peritoneal metastasis: A registry study. Gastroenterol
Res Pract. 2018:27439852018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mandard AM, Dalibard F, Mandard JC, Marnay
J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G,
et al: Pathologic assessment of tumor regression after preoperative
chemoradiotherapy of esophageal carcinoma. Clinicopathologic
correlations. Cancer. 73:2680–2686. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Becker K, Mueller JD, Schulmacher C, Ott
K, Fink U, Busch R, Böttcher K, Siewert JR and Höfler H:
Histomorphology and grading of regression in gastric carcinoma
treated with neoadjuvant chemotherapy. Cancer. 98:1521–1530. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baldus SE, Monig SP, Schroder W, Metzger
R, Lang S, Zirbes TK, Thiele J, Müller RP, Dienes HP, Hölscher AH
and Schneider PM: Regression of oesophageal carcinomas after
neoadjuvant radiochemotherapy: Criteria of the histopathological
evaluation. Pathologe. 25:421–427, (In German). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dworak O, Keilholz L and Hoffmann A:
Pathological features of rectal cancer after preoperative
radiochemotherapy. Int J Colorectal Dis. 12:19–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rubbia-Brandt L, Giostra E, Brezault C,
Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE,
Soubrane O, et al: Importance of histological tumor response
assessment in predicting the outcome in patients with colorectal
liver metastases treated with neo-adjuvant chemotherapy followed by
liver surgery. Ann Oncol. 18:299–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blazer DG III, Kishi Y, Maru DM, Kopetz S,
Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, et al:
Pathologic response to preoperative chemotherapy: A new outcome end
point after resection of hepatic colorectal metastases. J Clin
Oncol. 26:5344–5351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Solass W, Sempoux C, Carr NJ, Bibeau F,
Neureiter D, Jäger T, Di Caterino T, Brunel C, Klieser E, Fristrup
CW, et al: Reproducibility of the peritoneal regression grading
score for assessment of response to therapy in peritoneal
metastasis. Histopathology. 74:1014–1024. 2019. View Article : Google Scholar : PubMed/NCBI
|