Introduction
With an estimated 900,000 new cases and 830,000
associated deaths in 2020, hepatocellular carcinoma (HCC) ranks as
the sixth most common neoplasm and the third leading cause of
cancer-related mortality worldwide (1,2).
Recent advancements in systemic chemotherapy for advanced HCC,
including immune checkpoint inhibitors (ICIs) and molecular
targeted agents, have enhanced patient outcomes (3–8). The
main elements of the tumor immune microenvironment (TIME) include
cancer cells, antigen, immune cells and cytokines. These components
interact with each other to determine the tendency of antitumor
immunity (9). ICIs exhibit
antitumor effects by reactivating the immune cells in TIME and it
is imperative to elucidate the TIME in HCC.
Lenvatinib, an oral multi-kinase inhibitor targeting
vascular endothelial growth factor receptors 1–3, fibroblast growth
factor receptors 1–4, platelet-derived growth factor receptor α,
rearranged during transfection and stem cell factor receptor, has
demonstrated anticancer efficacy (10). A global, randomized, multicenter,
open-label trial assessing the non-inferiority of lenvatinib
compared with sorafenib (REFLECT; NCT01761266) revealed that
lenvatinib significantly improved progression-free survival (PFS)
versus sorafenib in patients with previously untreated, metastatic
or advanced HCC (3). Lenvatinib is
currently approved for the treatment of HCC. Recently, Yamauchi
et al (11) described the
capability of lenvatinib to modulate the TIME in HCC.
Inflammation has an important role in cancer, and
neutrophils suppress T cell function by secreting myeloperoxidase
and arginase-1, and upregulating programmed cell death ligand 1
(12). Therefore, neutrophils
create an immunosuppressive tumor microenvironment that reduces the
efficacy of immunotherapy (13).
Lymphocytes also have a role in cytotoxic cell death, and they
produce cytokines to inhibit tumor cell growth (14). The neutrophil-to-lymphocyte ratio
(NLR) is considered a systemic marker of the balance between
adaptive immune surveillance and the inflammatory status. A high
NLR at baseline is associated with a poor prognosis in numerous
types of cancer, such as lung, thyroid, biliary tract and colon
cancer, and the dynamics of the NLR are associated with prognosis
or treatment efficacy in various cancer types, such as lung cancer,
renal cell carcinoma and gastrointestinal cancer, treated with
systemic chemotherapies such as ICIs (15–22).
It has been reported that the dynamics of the NLR reflect changes
in the TIME and capture antitumor immune responses, ultimately
being associated with clinical outcomes following immune checkpoint
blockade (23). To the best of our
knowledge, to date, no reports have evaluated the dynamics of the
NLR as a biomarker of the TIME during lenvatinib therapy in HCC.
The present study therefore investigated the dynamics of the NLR in
this context.
Patients and methods
Patients
The current prospective, single-center study
analyzed the dynamics of the NLR in patients with HCC who were
treated with lenvatinib at Aso Iizuka Hospital (Iizuka, Japan)
between May 2018 and February 2023. In total, 130 patients with
unresectable HCC who received lenvatinib treatment as first-line
treatment or post-progression treatment after other therapies,
including transarterial chemoembolization, sorafenib, and
atezolizumab plus bevacizumab, were identified. Finally, 101
patients were evaluated, after excluding 29 patients who were
observed for <12 weeks and did not have images to assess
treatment efficacy. Additionally, liver tumor biopsy samples were
obtained with consent from 9 patients treatment to assess the TIME
prior to subsequent chemotherapy treatment for progression or
discontinuation due to adverse events on lenvatinib treatment. This
study adhered to the Declaration of Helsinki guidelines and
received approval from the Iizuka Hospital Ethics Committee
(approval no. 18070). All patients provided written informed
consent. Specific written informed consent was obtained from 2
patients for the publication of their immunohistochemistry
results.
Biomarker analysis
Peripheral blood (2 ml) was obtained from the
patients at the start of treatment and at each hospital visit
during lenvatinib treatment. The NLR was a calculation based on the
absolute neutrophil count divided by the absolute lymphocyte count
determined by complete blood count differential in the peripheral
blood.
Treatment protocol
Patients received oral lenvatinib (Eisai Co., Ltd.)
based on body weight (8 mg/day for those weighing <60 kg and 12
mg/day for those weighing ≥60 kg). Reduction of the initial dose
was permitted according to the performance status (by assessment of
the level of function and capability of self-care) and the presence
of proteinuria at the start of treatment (SOT) (4–8 mg/day). Dose
adjustment, including interruption and reduction (to 8 mg/day, 4
mg/day or 4 mg every other day), was permitted during treatment
according to the performance status and adverse events. The
protocols outlined in the REFLECT trial, as prescribed by Eisai
Co., Ltd., were followed (3).
Adverse events were graded using the Common Terminology Criteria
for Adverse Events, version 4.0 (24). Grade 3 or higher adverse events or
any unacceptable grade 2 events led to a reduction in the drug dose
or interrupted treatment according to the lenvatinib administration
guidelines. Following the occurrence of an adverse event, the
lenvatinib dose was reduced or treatment was temporarily halted
until symptoms improved to grade 1 or 2, in line with Eisai Co.,
Ltd., guidelines.
Evaluation of efficacy
The treatment response was assessed every 8–12 weeks
after treatment initiation using computed tomography or magnetic
resonance imaging. The antitumor response was evaluated by the
treating physician based on the modified Response Evaluation
Criteria in Solid Tumours version 1.1 (25). The disease control rate (DCR) was
defined as the sum of the rates for complete response (CR), partial
response (PR) and stable disease lasting at least 4 months. The
objective response rate (ORR; also referred to as the best
response) was defined as the sum of the PR and CR rates. Patients
were followed up every 4 weeks, and long-term treatment was
continued until disease progression or intolerable side effects
occurred.
Immunohistochemistry (IHC)
Liver tumor biopsy specimens were fixed in 10%
formalin at room temperature for 10–48 h and embedded in paraffin.
Serial sections (5 µm) were cut from the paraffin blocks and
stained with hematoxylin and eosin (hematoxylin for 3 min and eosin
for 45 sec at room temperature). CD8+ T-cell staining
was performed with a Leica Bond-III, which is an automatic and
continuous access slide-staining system that simultaneously
processes IHC protocols, using a Bond Polymer Refine Detection Kit
(Leica Biosystems). Specimens were then incubated for 30 min with
the primary antibody mouse anti-human monoclonal CD8 antibody
(clone C8/144B; 1:50; Dako; Agilent Technologies, Inc.), followed
by visualization with the Leica Bond Polymer Refine Detection kit
for 20 min at room temperature. The sections were counterstained
with hematoxylin, dehydrated and mounted. The slides were examined
under the BZ-X700 fluorescence microscope (Keyence Corporation).
CD8+ cell infiltration was quantified according to the
number of positively stained CD8+ tumor-infiltrating
lymphocytes (TILs) at ×400 magnification, focusing on areas with
the densest CD8+ TIL presence. A cutoff of 15.9
cells/high-power field was utilized to classify high and low
CD8+ TIL infiltration, consistent with a previous report
(26).
Statistical analysis
JMP Pro version 11 (SAS Institute Inc.) was utilized
for all statistical analyses. Data are presented as the median
(interquartile range) or mean (standard deviation). The
Kaplan-Meier method was applied for statistical testing to evaluate
overall survival (OS) time, PFS time and first objective response
time. NLR was compared at different time points using Friedman's
test with Dunn's post hoc test or a paired t-test. P<0.05 was
used to indicate a statistically significant difference.
Results
Patient characteristics
The characteristics of the 101 patients who received
lenvatinib are presented in Table
I. A total of 54 patients (53.5%) required a reduction of the
initial dose of lenvatinib. The ORR was 25.7% (26/101 patients) and
the DCR was 58.4% (59/101 patients). The median PFS time was 6.0
months [95% confidence interval (CI), 4.9–7.5] and the median OS
time was 27.9 months (95% CI, 16.5–32.8). Median time to first
objective response was 3.1 months (95% CI, 2.3–3.6 months).
 | Table I.Baseline and overall characteristics
of patients who received lenvatinib. |
Table I.
Baseline and overall characteristics
of patients who received lenvatinib.
|
Characteristics | Value |
|---|
| Number of
patients | 101 |
| Age,
yearsa | 73.0
(68.3–80.0) |
| Males/females,
n | 77/24 |
| MVI-positive,
n | 20 |
| EHS-positive,
n | 30 |
| Intrahepatic max
tumor size, cma | 3.1 (2.0–5.2) |
| Patients with >5
tumors, n | 50 |
| Etiology, n |
|
|
HBV | 19 |
|
HCV | 42 |
|
NBNC | 40 |
| Child-Pugh score,
n |
|
|
A | 83 |
|
B/C | 18 |
| Alb,
g/dla | 3.7 (3.3–4.1) |
| T.Bil,
g/dla | 0.8 (0.6–1.2) |
| ALBI
scorea | −2.39 (−2.75 to
−2.01) |
| BCLC stage, n |
|
|
A | 12 |
|
B | 45 |
|
C | 44 |
| Tumor
markersa |
|
|
AFP, ng/ml | 23.7
(4.2–4392.1) |
|
PIVKA-II,
mAU/ml | 189.0
(29.0–2086.0) |
| Initial dose
reduction, n (%) | 54 (53.5%) |
| ORR (CR + PR), n
(%) | 26 (25.7) |
| DCR (CR + PR + SD),
n (%) | 59 (58.4) |
| Median PFS,
monthsb | 6.0 (4.9–7.5) |
| Median OS,
monthsb | 27.9
(16.5–32.8) |
Dynamics of the NLR after treatment
with lenvatinib
The NLR values at the SOT, after 1 month of
treatment and after 3 months of treatment were 2.78±2.20, 2.61±1.86
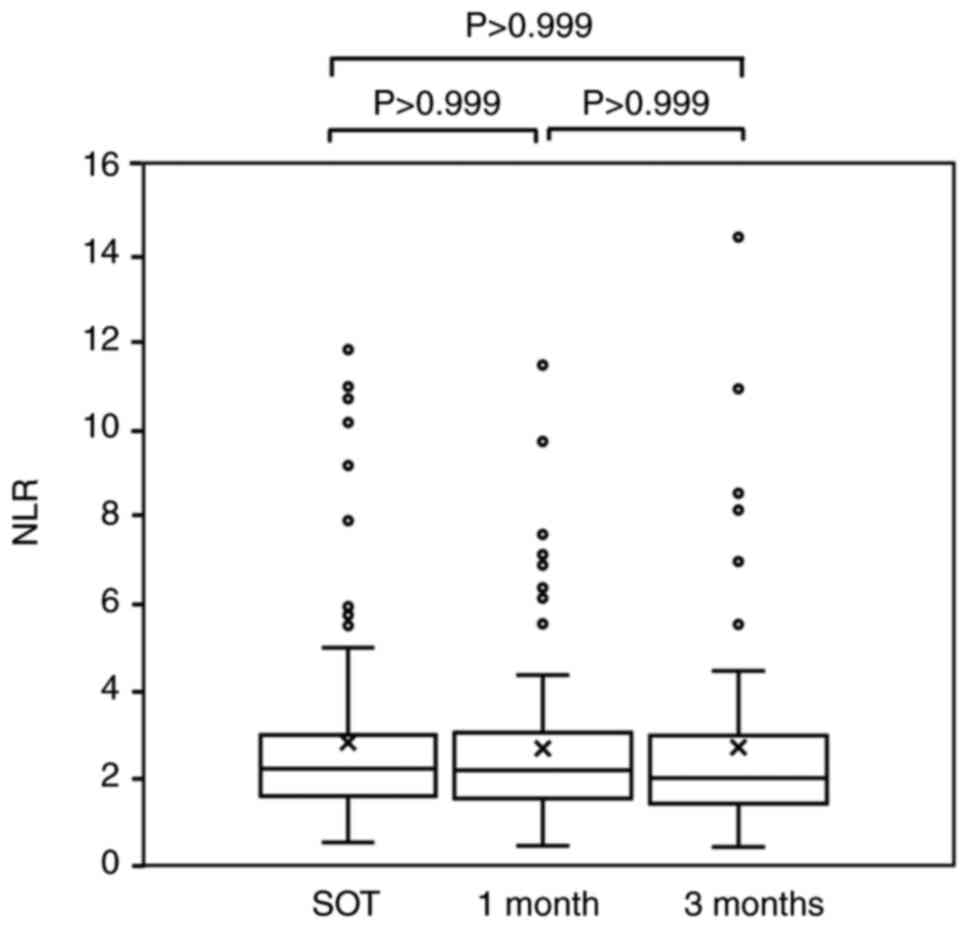
and 2.66±2.36, respectively (P=0.733; Friedman's test) (Fig. 1). Among the patients with no
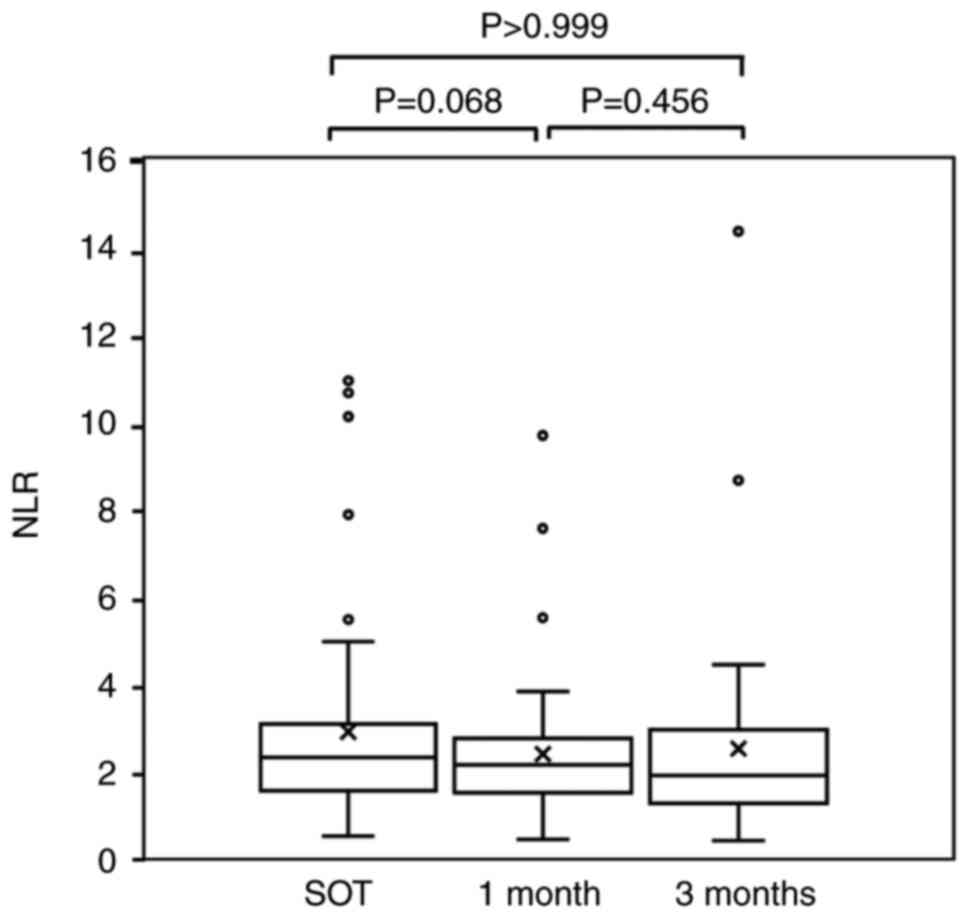
reduction of the initial dose, the NLR values at the SOT, after 1
month of treatment and after 3 months of treatment were 2.86±2.33,
2.34±0.25 and 2.48±2.26 (P=0.613; Friedman's test) (Fig. 2). There was no significant
difference between the NLR after 1 month and that at the SOT. Among
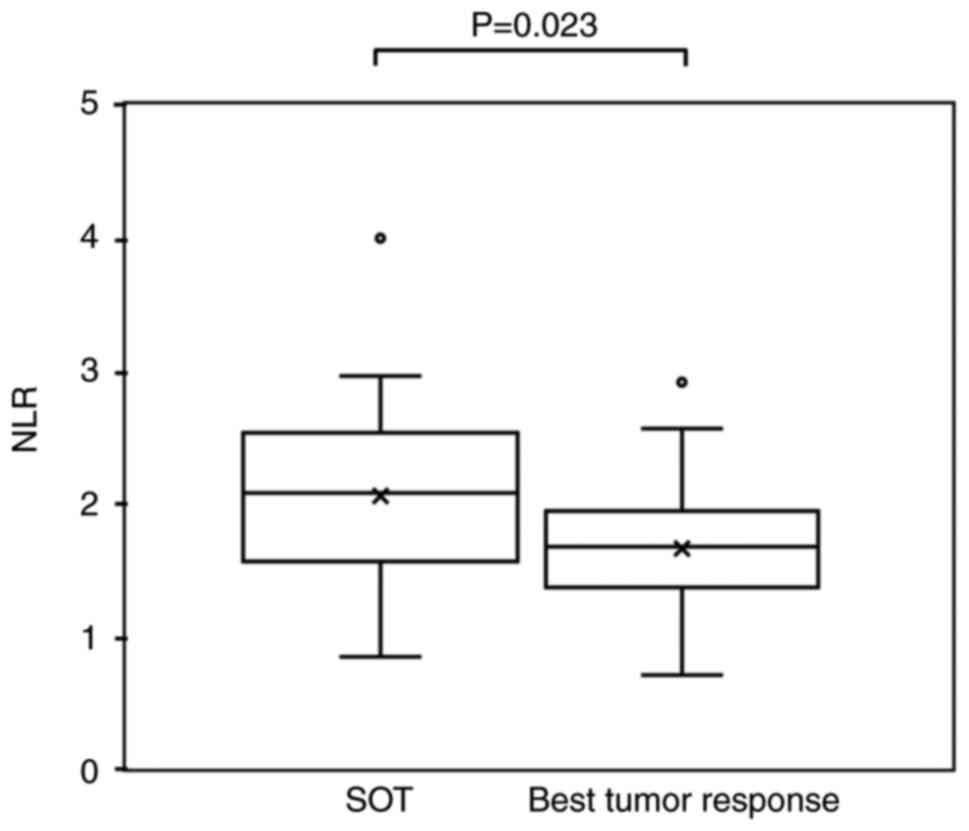
the patients with an objective response, the NLR at the time of the
best tumor response was 1.65±0.56, which was significantly lower
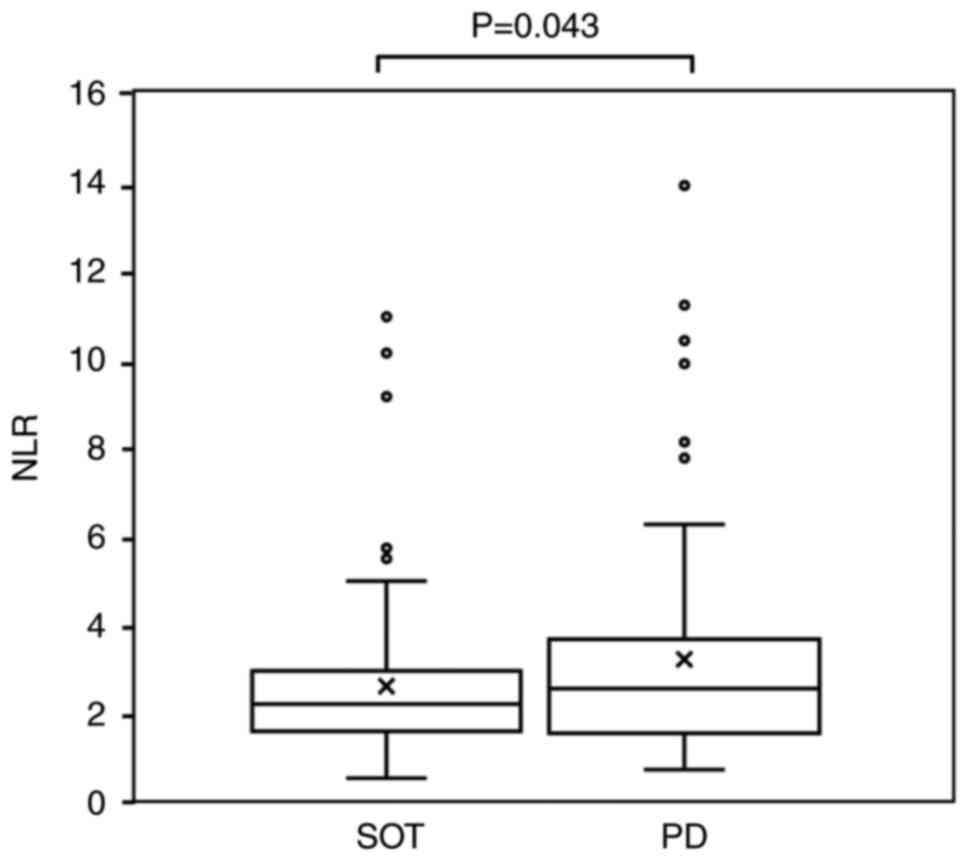
than that at the SOT (2.05±0.78) (P=0.023; Fig. 3). Among the non-responders, the NLR
was significantly higher at the time of disease progression
(3.68±3.19) compared with that at the SOT (2.78±1.79) (P=0.043;
Fig. 4).
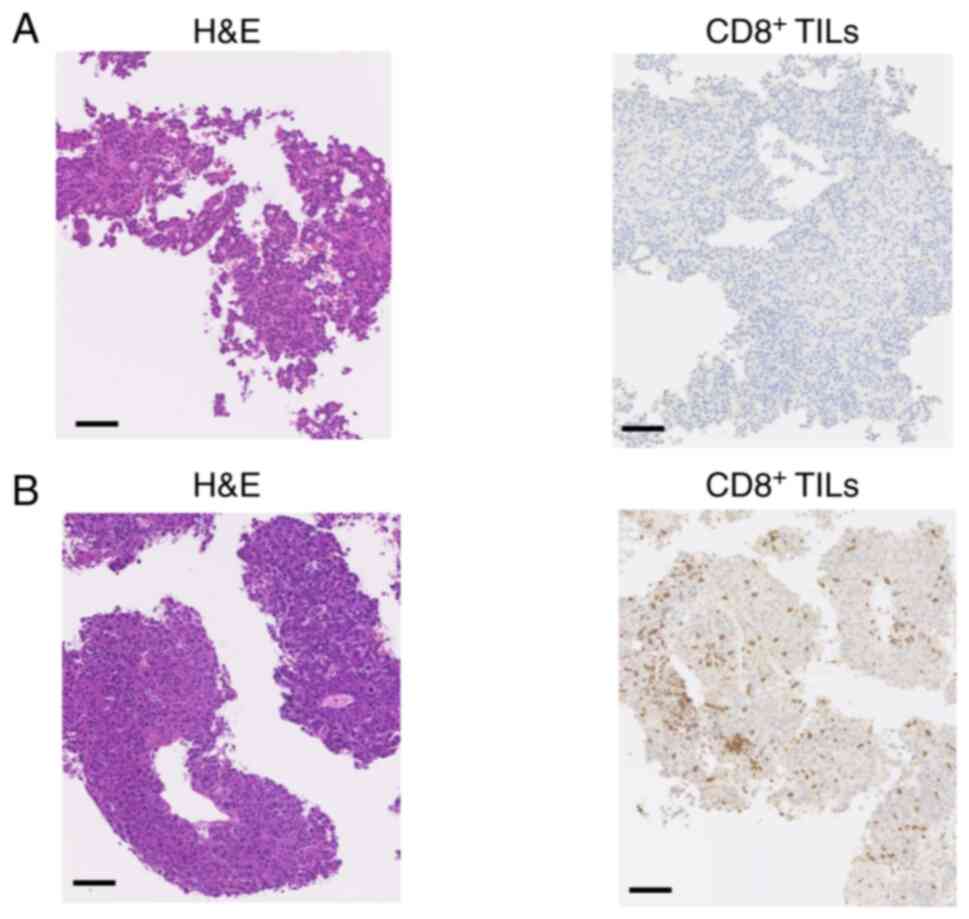
IHC for CD8+ TILs in HCC
tissues
CD8+ TIL counts were assessed by IHC
after lenvatinib treatment and prior to subsequent-line
chemotherapy in 9 patients (Table
II). In total, 5 out of 6 patients who did not respond to
lenvatinib had low CD8+ TIL counts at disease
progression. A typical case is presented in Fig. 5A (case 6). Furthermore, 2 out of the
3 patients who discontinued treatment due to adverse events had
high CD8+ TIL counts. A typical case is presented in
Fig. 5B (case 7).
 | Table II.CD8+ TIL levels after
lenvatinib treatment and prior to subsequent-line chemotherapy. |
Table II.
CD8+ TIL levels after
lenvatinib treatment and prior to subsequent-line chemotherapy.
| Case | Age, years | Sex | Etiology | Reason for
discontinuation of treatment | NLR at the start of
treatment | NLR at PD or
discontinuation of treatment | CD8+ TIL
infiltration |
|---|
| 1 | 52 | M | HBV | PD | 1.65 | 2.17 | Low |
| 2 | 59 | M | ALC | PD | 5.99 | 1.10 | Low |
| 3 | 76 | M | HCV | PD | 2.28 | 3.52 | Low |
| 4 | 87 | M | NBNC | PD | 1.87 | 5.09 | Low |
| 5 | 73 | F | HBV | PD | 0.71 | 0.85 | Low |
| 6 | 68 | M | HBV | PD | 2.39 | 4.27 | High |
| 7 | 79 | M | HCV | Proteinuria | 2.61 | 2.11 | High |
| 8 | 61 | M | HCV | Proteinuria | 7.86 | 2.71 | High |
| 9 | 69 | M | NBNC | Proteinuria | 2.62 | 2.07 | Low |
Discussion
The immune response serves a crucial role in the
progression of cancer. The most recent immunogenomic classification
of HCC was published in 2022 (27).
This study reported that ICI treatment was likely to initiate a
response in 65% of HCC cases in the non-inflammatory group and in
35% of cases in the inflammatory group. The inflammatory group was
characterized by robust interferon signaling and cytolytic
activity, upregulated effector molecules of cytotoxic T cells, and
increased checkpoint molecule levels and CD8+ T-cell
counts. HCC is influenced by the TIME, which has been reported to
benefit from immune checkpoint blockade treatment (28).
Clinical trials and preclinical studies
investigating the immunomodulatory effects of antiangiogenic agents
on the tumor microenvironment have highlighted enhanced maturation
of dendritic cells, improved trafficking and function of T cells,
and reversal of immunosuppression that is induced by hypoxia or
immunosuppressive cells (29–31).
Further in vivo and in vitro studies have illustrated
that molecular targeted agents enhance antitumor immunity by
promoting the polarization of tumor-associated macrophages to an M1
phenotype (32–34), enhancing the infiltration and
functions of CD4+ and CD8+ T cells (35,36),
reducing the numbers of regulatory T cells (37–39),
and reversing the suppressive functions of myeloid-derived
suppressor cells in the tumor microenvironment (40,41).
Lenvatinib has also been demonstrated to modulate the TIME
(11,42–44).
It is important to evaluate the TIME in the treatment of HCC.
However, previous studies have required liver tumor biopsy tissues
to be obtained, highlighting the need for a non-invasive biomarker
for predicting treatment response.
The NLR is a simple and inexpensive measure of the
balance between adaptive immune surveillance and the inflammatory
status (16). Tada et al
(45) reported that a high NLR was
associated with negative outcomes (PFS, ORR, and DCR) in patients
who received lenvatinib for HCC. However, the dynamics of the NLR
in patients with unresectable HCC treated with lenvatinib have not
been thoroughly investigated.
In the present study, the NLR decreased at the time
of the best tumor response among the patients with an objective
response, indicating an inflammatory condition, whereas NLR
elevation at the time of disease progression suggested a
non-inflammatory condition. There was notably less CD8+
TIL infiltration in liver tumor tissue at the time of disease
progression in patients who did not respond to lenvatinib. The
results suggest that NLR may be useful for assessing the TIME and
treatment efficacy.
Recently, the combination of an ICI and a vascular
endothelial growth factor inhibitor (atezolizumab plus bevacizumab)
and the combination of the ICIs tremelimumab and durvalumab were
approved as systemic therapy options for patients with advanced HCC
(4,8). If the tumor is inflamed prior to ICI
treatment, the response to ICI treatment might be improved.
Therefore, switching to ICI treatment early or before disease
progression after lenvatinib administration could improve
prognosis.
The limitations of the present study include
included the small number of patients due to the single-center
design and the lack of observation of tumor tissue over time. The
NLR can be influenced by numerous factors, including age, body mass
index, steroidal drugs, viral hepatitis, alcoholic fatty liver and
diabetes (46,47). The present study encompassed
advanced HCC cases with varying stages and levels of liver
function. Matching patients according to these factors is not
feasible when analyzing a small case series. These factors warrant
consideration, and randomized controlled trials should be conducted
in future. Nevertheless, despite the limitations of the present
study, NLR dynamics may be recognized in future as a useful marker
of the TIME.
In conclusion, the NLR at the time of the best tumor
response was lower than that at the SOT among the patients with a
PR or CR. Among the non-responders, the NLR was higher at the time
of disease progression than at the SOT. These findings suggest the
potential of lenvatinib as an immunomodulator. Further studies
exploring the impact of different treatment methods on the TIME of
HCC and further studies with larger sample sizes are required to
investigate the TIME in patients with advanced HCC.
Acknowledgements
The authors are grateful to Ms. Yukie Ishibashi
(Department of Hepatology, Iizuka Hospital, Iizuka, Japan) for
assistance with manuscript preparation.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AK, MY, AM and KM designed the study. AK, YK and KT
assisted with data analyses. YO performed pathological
examinations, including immunostaining. AK wrote the initial draft
of the manuscript. MY contributed to data analysis and
interpretation. MY, AM and KM assisted in the preparation and
critical review of the manuscript. AK and MY confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript and agree to be accountable for all
aspects of the work.
Ethics approval and consent to
participate
The study was performed in accordance with the
principles and ethical guidelines of the 1975 Declaration of
Helsinki. The study received approval from the Aso Iizuka Hospital
Ethics Committee (Iizuka, Japan; approval no. 18070). All patients
provided written informed consent.
Patient consent for publication
Written informed consent was obtained from two
patients for the publication of their immunohistochemistry
results.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
ICI
|
immune checkpoint inhibitor
|
|
TIME
|
tumor immune microenvironment
|
|
PFS
|
progression-free survival
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
DCR
|
disease control rate
|
|
ORR
|
objective response rate
|
|
IHC
|
immunohistochemistry
|
|
TIL
|
tumor-infiltrating lymphocyte
|
|
CI
|
confidence interval
|
|
SOT
|
start of treatment
|
References
|
1
|
Caldwell S and Park SH: The epidemiology
of hepatocellular cancer: From the perspectives of public health
problem to tumor biology. J Gastroenterol. 44:96–101. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus Bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Becht R, Kiełbowski K and Wasilewicz MP:
New opportunities in the systemic treatment of hepatocellular
carcinoma-today and tomorrow. Int J Mol Sci. 25:14562024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abou-Alfa GK, Lau G, Kudo M, Chan SL,
Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De
Toni EN, et al: Tremelimumab plus Durvalumab in unresectable
hepatocellular carcinoma. NEJM Evid. 1:EVIDoa21000702022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Locy H, de Mey S, de Mey W, De Ridder M,
Thielemans K and Maenhout SK: Immunomodulation of the tumor
microenvironment: Turn foe into friend. Front Immunol. 9:29092018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cabanillas ME and Habra MA: Lenvatinib:
Role in thyroid cancer and other solid tumors. Cancer Treat Rev.
42:47–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamauchi M, Ono A, Amioka K, Fujii Y,
Nakahara H, Teraoka Y, Uchikawa S, Fujino H, Nakahara T, Murakami
E, et al: Lenvatinib activates anti-tumor immunity by suppressing
immunoinhibitory infiltrates in the tumor microenvironment of
advanced hepatocellular carcinoma. Commun Med (Lond). 3:1522023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oberg HH, Wesch D, Kalyan S and Kabelitz
D: Regulatory interactions between neutrophils, tumor cells and T
cells. Front Immunol. 10:16902019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valero C, Lee M, Hoen D, Weiss K, Kelly
DW, Adusumilli PS, Paik PK, Plitas G, Ladanyi M, Postow MA, et al:
Pretreatment neutrophil-to-lymphocyte ratio and mutational burden
as biomarkers of tumor response to immune checkpoint inhibitors.
Nat Commun. 12:7292021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding PR, An X, Zhang RX, Fang YJ, Li LR,
Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH, et al: Elevated preoperative
neutrophil to lymphocyte ratio predicts risk of recurrence
following curative resection for stage IIA colon cancer. Int J
Colorectal Dis. 25:1427–1433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu N, Jian Y, Wang Y and Tian W:
Evaluation of neutrophil-to-lymphocyte ratio and calcitonin
concentration for predicting lymph node metastasis and distant
metastasis in patients with medullary thyroid cancer. Mol Clin
Oncol. 6:629–634. 2018.PubMed/NCBI
|
|
16
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moschetta M, Uccello M, Kasenda B, Mak G,
McClelland A, Boussios S, Forster M and Arkenau HT: Dynamics of
neutrophils-to-lymphocyte ratio predict outcomes of PD-1/PD-L1
blockade. Biomed Res Int. 2017:15068242017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho KM, Park H, Oh DY, Kim TY, Lee KH, Han
SW, Im SA, Kim TY and Bang YJ: Neutrophil-to-lymphocyte ratio,
platelet-to-lymphocyte ratio, and their dynamic changes during
chemotherapy is useful to predict a more accurate prognosis of
advanced biliary tract cancer. Oncotarget. 8:2329–2341. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soda H, Ogawara D, Fukuda Y, Tomono H,
Okuno D, Koga S, Taniguchi H, Yoshida M, Harada T, Umemura A, et
al: Dynamics of blood neutrophil-related indices during nivolumab
treatment may be associated with response to salvage chemotherapy
for non-small cell lung cancer: A hypothesis-generating study.
Thorac Cancer. 10:341–346. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie X, Liu J, Yang H, Chen H, Zhou S, Lin
H, Liao Z, Ding Y, Ling L and Wang X: Prognostic value of baseline
neutrophil-to-lymphocyte ratio in outcome of immune checkpoint
inhibitors. Cancer Invest. 37:265–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin J, Yang L, Liu D and Li W: Association
of the neutrophil to lymphocyte ratio and clinical outcomes in
patients with lung cancer receiving immunotherapy: A meta-analysis.
BMJ Open. 10:e0350312020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang N, Jiang J, Tang S and Sun G:
Predictive value of neutrophil-lymphocyte ratio and
platelet-lymphocyte ratio in non-small cell lung cancer patients
treated with immune checkpoint inhibitors: A meta-analysis. Int
Immunopharmacol. 85:1066772020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang M, Canzoniero JV, Rosner S, Zhang G,
White JR, Belcaid Z, Cherry C, Balan A, Pereira G, Curry A, et al:
Peripheral blood immune cell dynamics reflect antitumor immune
responses and predict clinical response to immunotherapy. J
Immunother Cancer. 10:e0046882022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Japanese translation of common terminology
criteria for adverse events (CTCAE) version 4.0. JCOG. 2009.
|
|
25
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuwano A, Yada M, Miyazaki Y, Tanaka K,
Kurosaka K, Ohishi Y, Masumoto A and Motomura K: Tumor-infiltrating
CD8+ T cells as a biomarker for chemotherapy efficacy in
unresectable hepatocellular carcinoma. Oncol Lett. 25:2592023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Montironi C, Castet F, Haber PK, Pinyol R,
Torres-Martin M, Torrens L, Mesropian A, Wang H, Puigvehi M, Maeda
M, et al: Inflamed and non-inflamed classes of HCC: A revised
immunogenomic classification. Gut. 72:129–140. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao X, Huang H, Wang Y, Pan C, Yin S, Zhou
L and Zheng S: Tumor immune microenvironment characterization in
hepatocellular carcinoma identifies four prognostic and
immunotherapeutically relevant subclasses. Front Oncol.
10:6105132021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramjiawan RR, Griffioen AW and Duda DG:
Anti-angiogenesis for cancer revisited: Is there a role for
combinations with immunotherapy? Angiogenesis. 20:185–204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hegde PS, Wallin JJ and Mancao C:
Predictive markers of anti-VEGF and emerging role of angiogenesis
inhibitors as immunotherapeutics. Semin Cancer Biol. 52:117–124.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kwilas AR, Donahue RN, Tsang KY and Hodge
JW: Immune consequences of tyrosine kinase inhibitors that
synergize with cancer immunotherapy. Cancer Cell Microenviron.
2:e6772015.PubMed/NCBI
|
|
32
|
Sprinzl MF, Reisinger F, Puschnik A,
Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A,
Galle PR, Schuchmann M, et al: Sorafenib perpetuates cellular
anticancer effector functions by modulating the crosstalk between
macrophages and natural killer cells. Hepatology. 57:2358–2368.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei X, Tang C, Lu X, Liu R, Zhou M, He D,
Zheng D, Sun C and Wu Z: MiR-101 targets DUSP1 to regulate the
TGF-β secretion in sorafenib inhibits macrophage-induced growth of
hepatocarcinoma. Oncotarget. 6:18389–18405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Farsaci B, Donahue RN, Coplin MA, Grenga
I, Lepone LM, Molinolo AA and Hodge JW: Immune consequences of
decreasing tumor vasculature with antiangiogenic tyrosine kinase
inhibitors in combination with therapeutic vaccines. Cancer Immunol
Res. 2:1090–1102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Romero AI, Chaput N, Poirier-Colame V,
Rusakiewicz S, Jacquelot N, Chaba K, Mortier E, Jacques Y,
Caillat-Zucman S, Flament C, et al: Regulation of CD4(+)NKG2D(+)
Th1 cells in patients with metastatic melanoma treated with
sorafenib: Role of IL-15Rα and NKG2D triggering. Cancer Res.
74:68–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sunay MM, Foote JB, Leatherman JM, Edwards
JP, Armstrong TD, Nirschl CJ, Hicks J and Emens LA: Sorafenib
combined with HER-2 targeted vaccination can promote effective T
cell immunity in vivo. Int Immunopharmacol. 46:112–123. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chuang HY, Chang YF, Liu RS and Hwang JJ:
Serial low doses of sorafenib enhance therapeutic efficacy of
adoptive T cell therapy in a murine model by improving tumor
microenvironment. PLoS One. 9:e1099922014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen ML, Yan BS, Lu WC, Chen MH, Yu SL,
Yang PC and Cheng AL: Sorafenib relieves cell-intrinsic and
cell-extrinsic inhibitions of effector T cells in tumor
microenvironment to augment antitumor immunity. Int J Cancer.
134:319–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cabrera R, Ararat M, Xu Y, Brusko T,
Wasserfall C, Atkinson MA, Chang LJ, Liu C and Nelson DR: Immune
modulation of effector CD4+ and regulatory T cell function by
sorafenib in patients with hepatocellular carcinoma. Cancer Immunol
Immunother. 62:737–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang CJ, Yang YH, Chiu CJ, Lu LC, Liao
CC, Liang CW, Hsu CH and Cheng AL: Targeting tumor-infiltrating
Ly6G+ myeloid cells improves sorafenib efficacy in mouse orthotopic
hepatocellular carcinoma. Int J Cancer. 142:1878–1889. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kwilas AR, Ardiani A, Donahue RN, Aftab DT
and Hodge JW: Dual effects of a targeted small-molecule inhibitor
(cabozantinib) on immune-mediated killing of tumor cells and immune
tumor microenvironment permissiveness when combined with a cancer
vaccine. J Transl Med. 12:2942014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kimura T, Kato Y, Ozawa Y, Kodama K, Ito
J, Ichikawa K, Yamada K, Hori Y, Tabata K, Takase K, et al:
Immunomodulatory activity of lenvatinib contributes to antitumor
activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci.
109:3993–4002. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu J, Fang P, Wang C, Gu M, Pan B, Guo W,
Yang X and Wang B: The immunomodulatory activity of lenvatinib
prompts the survival of patients with advanced hepatocellular
carcinoma. Cancer Med. 10:7977–7987. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu M, Zhang X, Gao X, Sun S, Wei X, Hu X,
Huang C, Xu H, Wang B, Zhang W, et al: Lenvatinib enhances T cell
immunity and the efficacy of adoptive chimeric antigen
receptor-modified T cells by decreasing myeloid-derived suppressor
cells in cancer. Pharmacol Res. 174:1058292021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tada T, Kumada T, Hiraoka A, Michitaka K,
Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama
K, et al: Neutrophil-to-lymphocyte ratio is associated with
survival in patients with unresectable hepatocellular carcinoma
treated with lenvatinib. Liver Int. 40:968–976. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alkhouri N, Morris-Stiff G, Campbell C,
Lopez R, Tamimi TA, Yerian L, Zein NN and Feldstein AE: Neutrophil
to lymphocyte ratio: A new marker for predicting steatohepatitis
and fibrosis in patients with nonalcoholic fatty liver disease.
Liver Int. 32:297–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wróblewska A, Lorenc B, Cheba M, Bielawski
KP and Sikorska K: Neutrocyte-to-lymphocyte ratio predicts the
presence of a replicative hepatitis C virus strand after therapy
with direct-acting antivirals. Clin Exp Med. 19:401–406. 2019.
View Article : Google Scholar : PubMed/NCBI
|