Introduction
Human gliomas are the most common and malignant
primary tumors of the central nervous system (CNS). Despite the
development of surgery, chemotherapy and radiation therapy, the
median survival time of patients with this disease remains poor
(1). Glioblastoma (GBM), the most
lethal form of glioma, has a median overall survival time of only
15 months, which has remained unchanged over several years
(2). In addition to the rapid
proliferation, extensive invasion, tumor genetic heterogeneity and
treatment resistance of GBM, the poor prognosis of patients also
results from an insufficient understanding of the molecular
pathogenesis and the lack of markers for timely diagnosis and
sensitive treatments (2,3). Therefore, there is an urgent need to
explore the reliable biomarkers of GBM progression to improve the
treatment of this malignancy.
Fibroblast growth factors (FGFs) are broad-spectrum
mitogens that regulate a number of cellular functions, including
migration, proliferation, differentiation and survival (4). FGFs have been studied since the 1960s
with regards to their function in fibroblast driven growth
(5), and were formally purified and
characterized nearly a decade after first identification (6). In mammals, FGFs exert their
pleiotropic functions through binding and activating high-affinity
tyrosine kinase receptors such as the fibroblast growth factor
receptor (FGFR), which is encoded by four genes (FGFR1, FGFR2,
FGFR3 and FGFR4) and FGFRL (a truncated FGFR without an
intracellular domain) (4). The
FGF/FGFR signaling system regulates a number of biological
processes, such as embryogenesis, wound repair, tissue homeostasis
and angiogenesis (7). Dysfunction
of FGF/FGFR signaling has been observed in a variety of human
diseases, such as chronic kidney disease, congenital
craniosynostosis, dwarfism syndromes, obesity, insulin resistance
and cancer. A number of studies have demonstrated that activation
of the FGF/FGFR signaling system plays a key role in a variety of
critical steps in tumor growth and progression through its effects
on tumor and stromal cells, affecting angiogenesis, inflammation
and tumor growth (8,9).
FGFs are highly associated with the development and
progression of various human malignancies, including urothelial
cancer, multiple myeloma, prostate cancer and hepatocellular
carcinoma (4). Considering the
importance of FGF signaling, it has attracted considerable interest
in the study of multiple malignancies, where it plays a role in the
proliferation, survival, self-renewal and invasion of tumor cells
(10). However, the role of FGF
family members in GBM has been rarely explored, and most studies
examining the role of FGFs were conducted in vitro. It has
been reported that FGF2 is a oncogenic factor in glioma (11) and contributes to proliferation
(12), the vascularization of
glioma cells (13,14) and cancer stem cell self-renewal
(15). FGF9 has been shown to
potentially regulate the proliferation of human glioma cells either
in the presence or absence of endogenous growth factor expression
(16). Continued efforts to
understand the correlation of FGFs with human GBM tissues will play
a significant role in driving the discovery of novel diagnostic
approaches and new therapies.
In the present study, the RNA expression of FGF
members in human GBM tissues was first investigated by analyzing
high-throughput RNA transcriptome sequencing data, then the effect
of different FGF members on the overall survival of patients was
further clarified. The present study therefore aimed to provide
potential targets for diagnosis methods and the treatment of this
malignancy.
Materials and methods
Human GBM and adjacent non-cancerous
brain tissues (ANCBTs)
Eligible patients with newly diagnosed and
histologically confirmed GBM [World Health Organization (WHO) grade
IV astrocytoma] (17), and who had
undergone maximal safe surgery were included in the present study.
Patients with GBM who had undergone radiotherapy, chemotherapy,
immunotherapy and other new therapies were excluded from the
present study. Human cancerous tissues and ANCBTs were collected
from 12 patients with GBM admitted to the Department of
Neurosurgery at The First Affiliated Hospital of Sun Yat-sen
University (Guangzhou, China) between December, 2016 and November,
2017, with written patient consent. Molecular and
immunohistochemical biomarkers used for the diagnosis of these 12
patients with GBM included isocitrate dehydrogenase 1, glial
fibrillary acidic protein, vimentin, p53, Ki-67 and
O-6-methylguanine-DNA methyltransferase, information of which was
obtained from the medical records from The Department Pathology at
The First Affiliated Hospital of Sun Yat-sen University. Written
patient consent was also obtained for the release of potentially
personally-identifying clinical information. The present study was
approved by the Institutional Ethics Review Board of The First
Affiliated Hospital of Sun Yat-sen University [approval no.
(2016)279] and complied with all relevant ethical regulations
regarding human participants.
Analyzing high-throughput RNA
transcriptome sequencing data
Previously, our team conducted high-throughput RNA
transcriptome sequencing on tumors and adjacent tissues of the
aforementioned 12 patients with GBM. The high-throughput RNA
transcriptome sequencing was conducted and the procedure was
clearly described in our previous study (18). The raw high-throughput RNA
sequencing data was uploaded to the National Center for
Biotechnology Information database with the (accession no.
PRJNA525736). In the present study, the expression of FGF family
members in GBM and proximal tissues was analyzed using this
sequencing data. Data were mapped to the reference genome using the
software TopHat2 (v.2.1.1), and then transcript abundance was
quantified using RSEM (v.1.2.19).
Overall survival analysis
To further investigate the relationship between the
expression of FGF family members and the overall survival of
patients with GBM and glioma respectively, the ‘Survival Analysis’
tool (version 1.0) on Gene Expression Profiling Interactive
Analysis (GEPIA; http://gepia.cancer-pku.cn/index.html) was used. The
GBM and glioma datasets were respectively selected, and FGF1,
FGF17, FGF20 and FGF22 (distinguishing high and low expression
according to the median expression) were selected as the genes of
interest. GEPIA datasets were originally from TCGA database. GEPIA
used the log-rank test (sometimes termed the Mantel-Cox test) for
the hypothesis evaluation, and P<0.05 was considered to indicate
a statistically significant difference. The Cox proportional hazard
ratio and the 95% confidence interval information were also
included in the survival plot (19).
Statistical analysis
The data were analyzed using paired Student's t-test
or χ2 test. Statistical analysis was performed using
GraphPad Prism 8.0 (Dotmatics) and SPSS (version 25.0; IBM Corp.)
statistical software. P<0.05 was considered to indicate a
statistically significant difference.
Results
FGF1, FGF17, FGF20 and FGF22 were
differentially expressed in human GBMs and ANCBTs at the RNA
level
Human cancerous GBM tissues and ANCBTs were
collected from 12 patients. The median age of these patients was
45.5 years (range, 29–59 years), and there were 7 (58.3%) male and
5 (41.7%) female patients. All patients were diagnosed with GBM
(WHO grade IV astrocytoma) and underwent maximal surgical
resection. The clinicopathological characteristics of the patients
with GBM are shown in Table I.
 | Table I.Clinicopathological characteristics
of the patients with glioblastoma included in the present
study. |
Table I.
Clinicopathological characteristics
of the patients with glioblastoma included in the present
study.
| Patient no. | Age, years | Sex | Stage | Therapy | IDH-1 | GFAP | Vimentin | p53 | Ki-67, % | MGMT |
|---|
| 1 | 59 | Male | IV | Resection | - | + | + | + | +, 20 | + |
| 2 | 56 | Female | IV | Resection | - | + | + | + | +, 40 | - |
| 3 | 29 | Male | IV | Resection | - | + | + | + | +, 60 | + |
| 4 | 36 | Female | IV | Resection | - | + | + | + | +, 60 | + |
| 5 | 54 | Male | IV | Resection | - | + | + | + | +, 50 | + |
| 6 | 45 | Male | IV | Resection | - | + | + | + | +, 30 | + |
| 7 | 44 | Male | IV | Resection | - | + | + | + | +, 60 | + |
| 8 | 59 | Female | IV | Resection | - | + | + | + | +, 20 | - |
| 9 | 52 | Male | IV | Resection | - | + | + | - | +, 80 | + |
| 10 | 46 | Male | IV | Resection | - | + | + | + | +, 70 | + |
| 11 | 29 | Female | IV | Resection | - | + | + | + | +, 50 | + |
| 12 | 37 | Female | IV | Resection | - | + | + | + | +, 30 | - |
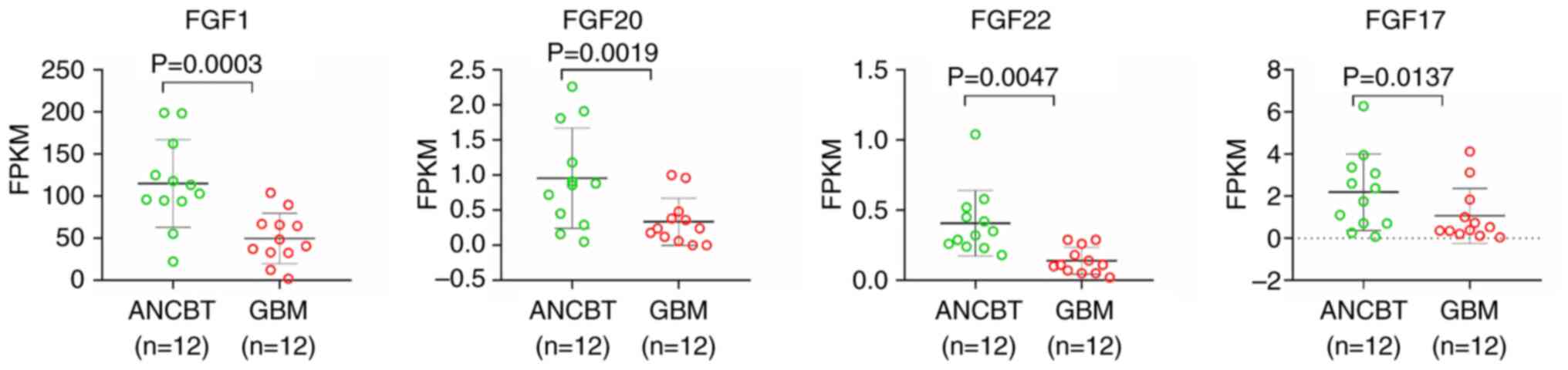
A panoramic visualization analysis was performed on
the RNA expression of all members of the FGF family in human GBM
tissues and ANCBTs (Table II). The
result showed that the expression levels of four members, FGF1,
FGF17, FGF20 and FGF22, were significantly lower in GBM tissues
compared with the adjacent tissues, with a difference of >2
times (P<0.05; Fig. 1 and
Table II). However, the expression
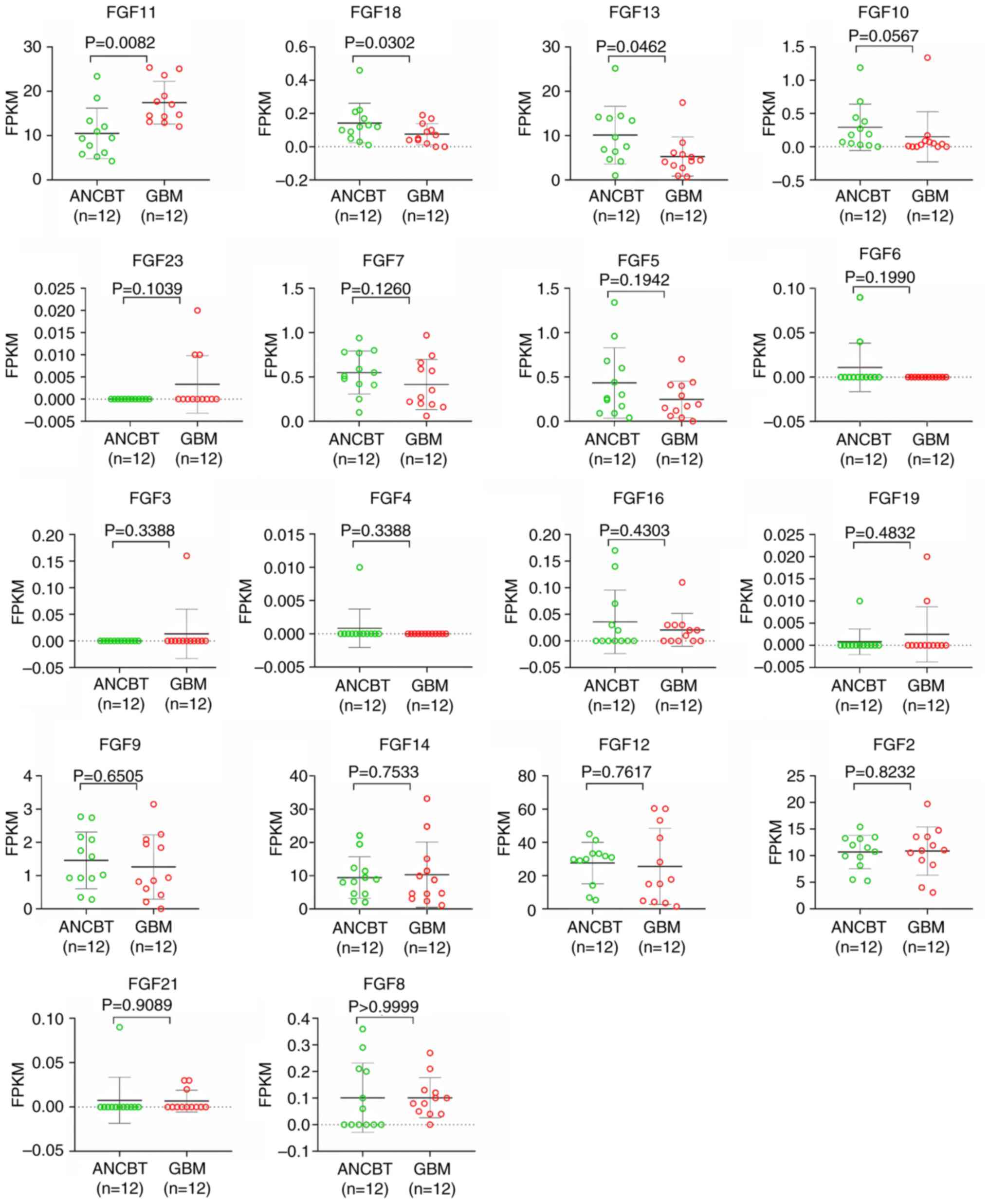
of other FGF members in human GBM tissues and ANCBTs did not
achieve both a difference of >2 times and statistical
significance (Fig. 2 and Table II). To the best of our knowledge,
these finding are the first to show the whole expression level of
FGF family members in human GBM tissues and ANCBTs.
 | Table II.Expression of the FGF family members
in the high-throughput RNA sequencing data obtained from 12 pairs
of human GBM tissues and ANCBTs. |
Table II.
Expression of the FGF family members
in the high-throughput RNA sequencing data obtained from 12 pairs
of human GBM tissues and ANCBTs.
| ID | Symbol | GBM, average
FPKM | ANCBT, average
FPKM | GBM/ANCBT, fold
change | P-value |
|---|
|
ENSG00000113578 | FGF1 | 49.8383 | 115.1533 | 2.3105 | 0.0003 |
|
ENSG00000078579 | FGF20 | 0.3350 | 0.9567 | 2.8557 | 0.0019 |
|
ENSG00000070388 | FGF22 | 0.1392 | 0.4067 | 2.9222 | 0.0047 |
|
ENSG00000158815 | FGF17 | 1.0692 | 2.1883 | 2.0468 | 0.0137 |
|
ENSG00000161958 | FGF11 | 17.4600 | 10.5225 | 0.6027 | 0.0082 |
|
ENSG00000156427 | FGF18 | 0.0758 | 0.1425 | 1.8791 | 0.0302 |
|
ENSG00000129682 | FGF13 | 5.2875 | 10.1283 | 1.9155 | 0.0462 |
|
ENSG00000070193 | FGF10 | 0.1500 | 0.2917 | 1.9444 | 0.0567 |
|
ENSG00000118972 | FGF23 | 0.0033 | 0.0000 | 0.0000 | 0.1039 |
|
ENSG00000140285 | FGF7 | 0.4150 | 0.5500 | 1.3253 | 0.1260 |
|
ENSG00000138675 | FGF5 | 0.2475 | 0.4342 | 1.7542 | 0.1942 |
|
ENSG00000111241 | FGF6 | 0.0000 | 0.0108 | 0.0108/0 | 0.1990 |
|
ENSG00000075388 | FGF4 | 0.0000 | 0.0008 | 0.0008/0 | 0.3388 |
|
ENSG00000186895 | FGF3 | 0.0133 | 0.0000 | 0.0000 | 0.3388 |
|
ENSG00000196468 | FGF16 | 0.0208 | 0.0358 | 1.7200 | 0.4303 |
|
ENSG00000162344 | FGF19 | 0.0025 | 0.0008 | 0.3333 | 0.4382 |
|
ENSG00000102678 | FGF9 | 1.2600 | 1.4583 | 1.1574 | 0.6505 |
|
ENSG00000102466 | FGF14 | 10.3017 | 9.4483 | 0.9172 | 0.7533 |
|
ENSG00000114279 | FGF12 | 25.5267 | 27.5492 | 1.0792 | 0.7617 |
|
ENSG00000138685 | FGF2 | 10.8842 | 10.6917 | 0.9823 | 0.8232 |
|
ENSG00000105550 | FGF21 | 0.0067 | 0.0075 | 1.1250 | 0.9089 |
|
ENSG00000107831 | FGF8 | 0.1017 | 0.1017 | 1.0000 | 1.0000 |
FGF1, FGF17 and FGF22 expression had a
notable influence on the survival of patients with glioma
The impact of the expression of the aforementioned
four FGF genes (FGF1, FGF17, FGF20 and FGF22) on the survival of
patients with GBM and glioma was respectively analyzed using data
from The Cancer Genome Atlas (TCGA) database. In TCGA dataset, the
high FGF groups represented patients with ≥50% FGF expression
compared with all patients, and the low FGF groups represented
patients with <50% FGF expression compared with all patients.
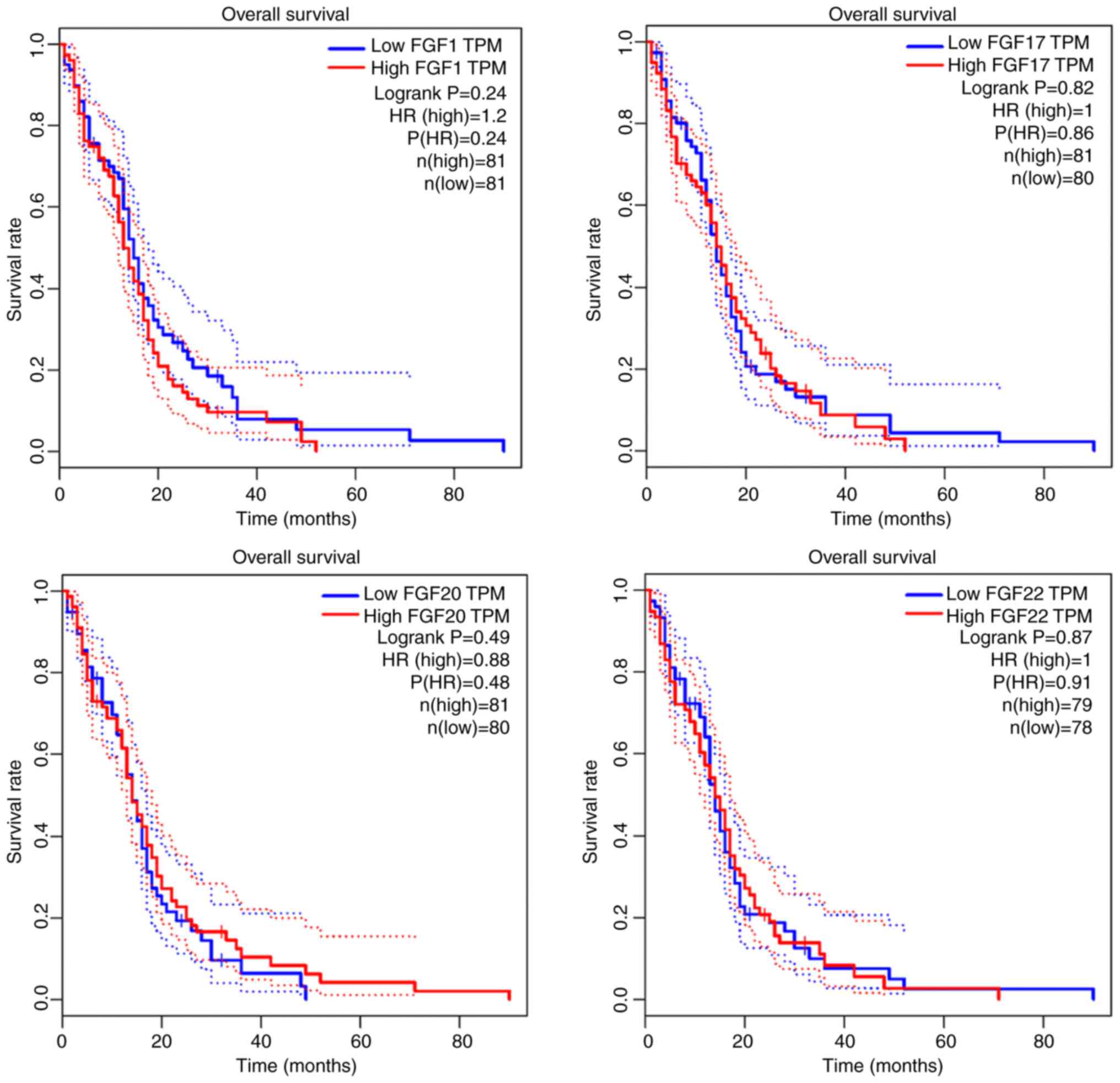
The expression levels of FGF1 (P=0.24), FGF17 (P=0.82), FGF20
(P=0.49) and FGF20 (P=0.87) were not significantly correlated with
the overall survival of the patients with GBM (Fig. 3). The relationship between these
aforementioned FGFs and the overall survival of patients with
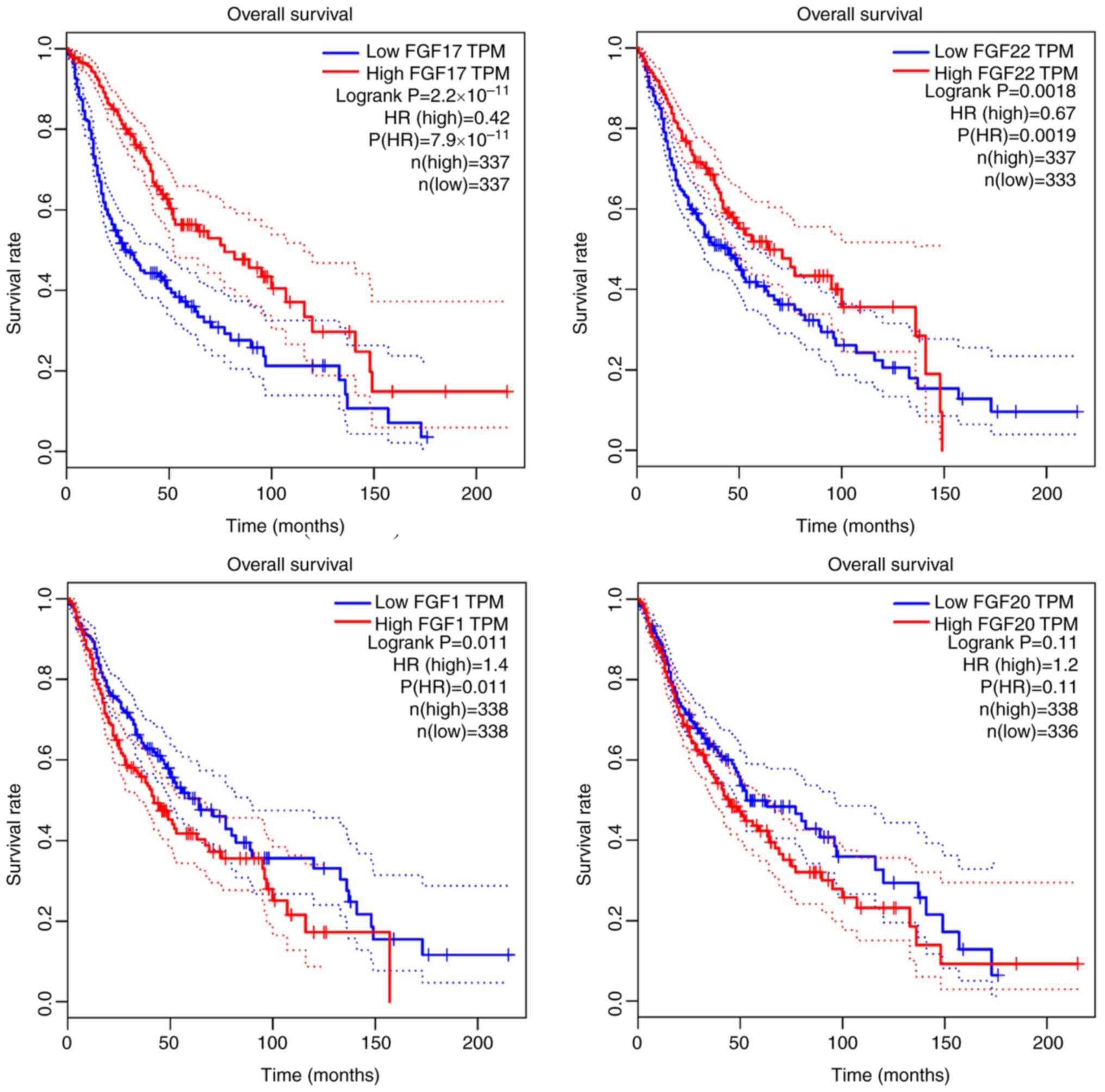
glioma was then investigated. It was found that patients with
glioma with low FGF1 expression achieved a longer survival time
than patients with high FGF1 expression. Conversely, high
expression of FGF17 and FGF22 indicated a longer survival time. In
particular, the expression of FGF17 was most closely related to the
survival of patients with glioma (P<0.001) (Fig. 4).
Discussion
The FGF family contains 22 members, which are
typically divided into seven subfamilies based on evolutionary
relationships, sequence similarity and biochemical functions,
including the FGF1 (FGF1 and FGF2), FGF4 (FGF4, FGF5 and FGF6),
FGF7 (FGF3, FGF7, FGF10 and FGF22), FGF8 (FGF8, FGF17 and FGF18),
FGF9 (FGF9, FGF16 and FGF20), FGF19 (FGF19, FGF21 and FGF23) and
FGF11 (FGF11, FGF12, FGF13 and FGF14) subfamilies (4,20). The
results of the present study showed that the FGF1, FGF20, FGF22 and
FGF17 expression levels were lower in GBM tissues compared with
adjacent tissues, with a >2 times difference.
Among the FGF family members, FGF2 is one of the
best characterized FGFs present in the CNS, and plays an important
role in astrocyte proliferation, migration and maturation (21–23).
FGF2 also promotes glioma cell growth, vascularization and cancer
stem cell self-renewal (10).
Experimental studies using FGF2-specific antisense oligonucleotides
or antibodies to block glioma cell proliferation (24) and angiogenesis (25) have highlighted the relevance of the
therapeutic targeting of FGF2 to increase survival time in glioma
animal models. However, the expression of FGF2 in patients with GBM
has not been focused upon. In the present study, a significant
difference in FGF2 expression was not observed between tumor and
adjacent tissues in patients with GBM. The expression of FGF2 was
not correlated with the overall survival of patients with GBM.
FGF1 (also termed acidic FGF) is an
autocrine/paracrine hormone that has historically been considered
as a mitogen and has also attracted attention as an antidiabetic
agent (26,27). In addition, FGF1 also promotes the
repair process of damaged vessels (28). Thus, FGF1 could promote
proliferation and resistance to chemotherapy in human pancreatic
cancer cell lines (29). The
characteristic ability of FGF1 promoting tumor cell proliferation
and chemotherapy resistance may explain the finding that glioma
patients with low FGF1 expression had longer overall survival times
than patients with high FGF1 expression. However, the specific
impact of FGF1 on glioma cells still needs further study. High
expression of FGF1 has been found in non-small cell lung, ovarian,
breast and prostate malignancies (30–32).
The results of the present study showed that FGF1 expression was 2
times lower in GBM tissues compared with adjacent tissues, which
was inconsistent with a previous result indicating that FGF1 was
upregulated in human glioma tissues and cell lines (33). Thus, further studies are needed to
compare the expression levels of FGF1 in human GBM tissues and
ANCBTs. The present study found that GBM patients with high FGF1
expression (n=81) did not achieve a significantly different
survival time compared with GBM patients with low FGF1 expression
(n=81). However, glioma patients with low FGF1 expression (n=338)
had longer overall survival times compared with glioma patients
with high FGF1 expression (n=338). The paradox with regard to the
different actions of FGF1 expression on GBM and glioma maybe due to
the relatively small number of patients with GBM that were
included.
FGF17, a member of the FGF8/17/18 subfamily,
contains an N-terminal cleaved signal peptide and can activate IIIc
splice variants of FGFR 1–3 and FGFR4 (20). FGF17 is commonly expressed in the
brain, endometrium, adrenal glands, thyroid and spleen (34). A recent study showed that FGF17 can
induce the proliferation of oligodendrocyte progenitor cells in the
brain, thereby improving memory in mice (35). It has also been shown that FGF17 is
highly expressed in prostate cancer and aberrantly expressed in
acute leukemia (34,36). However, to the best of our
knowledge, there have been no reports considering the effect of
FGF17 expression on overall survival in GBM. In the present study,
the results of the RNA sequencing analysis showed that FGF17
expression was >2 times higher in adjacent tissues compared with
tumor tissues in patients with GBM. In addition, the results of
TCGA data analysis demonstrated that glioma patients with high
FGF17 expression (n=337) achieved a longer survival time compared
with glioma patients with low FGF17 expression (n=337). However,
GBM patients with high FGF17 expression (n=81) did not achieve a
significantly different survival time compared with GBM patients
with low FGF17 expression (n=80). This is maybe due to small number
of patients with GBM that were included, and further studies
including greater numbers may change the association between the
expression of FGF17 and the overall survival of patients with
GBM.
FGF20 is a paracrine growth factor, the orthologs of
which are highly conserved among vertebrates (37). FGF20 is predominantly expressed in
the brain (38) and has being
suggested to be a vital factor involved in brain development and
neuronal homeostasis (39). FGF20
could enhance the survival of dopaminergic neurons in Parkinson's
disease, and this means that FGF20 may have a neuroprotective
function in the brain (40). A
previous study found that FGF20 expression was upregulated in
glioma cells following treatment with glucocorticoids (GCs), while
a reduction in FGF20 expression in glioma cells significantly
blocked the effect of GCs on macrophage polarization (41). However, to the best of our
knowledge, there has been no research examining FGF20 expression in
patients with GBM and it remains to be determined whether FGF20
expression is associated with the prognosis of these patients. In
the present study, the results showed that FGF20 expression in
human GBM tissues was nearly 3-fold lower than that in ANCBTs.
However, the results from TCGA database analysis did not identify a
relationship between the FGF20 expression level and the overall
survival of patients with GBM and glioma, respectively.
FGF22, a target-derived presynaptic organizer
critical in the formation of novel excitatory synapses during
development and the organization of synapses in adult brains
(42,43), has become a crucial endogenous
contributor of detour circuit formation (44). Previous studies have shown that
FGF22 plays a critical role as a presynaptic organizer in the
formation of new synapses in adult remodeling spinal cords
(45) and that delivery of FGF22
may promote organization of the presynapse, promoting the
plasticity of healthy supraspinal axons and thereby contributing to
the recovery of function in incomplete spinal cord injury (44). However, there have been no studies
examining the relationship between FGF22 and GBM and whether FGF22
expression affects the survival of patients with GBM. In the
present study it was shown that the FGF22 expression level in
adjacent tissues was nearly three times higher than that in GBM
tissues. In addition, following analysis of TCGA data, it was found
that GBM patients with high FGF22 expression (n=79) did not achieve
a significantly different survival time compared with GBM patients
with low FGF22 expression (n=78). However, it was found that glioma
patients with high FGF22 expression (n=337) had a longer survival
time than glioma patients with low FGF22 expression (n=333).
In summary, in the present study, the RNA expression
levels of FGF family members in human GBM tissues were first
investigated by analyzing high-throughput RNA transcriptome
sequencing data. It was found that the expression levels of four
members, FGF1, FGF20, FGF22 and FGF17, were >2 times lower in
GBM tissues compared with adjacent tissues. The different
expression levels of FGF1, FGF17, FGF20 and FGF22 did not have a
significant influence on the overall survival of the patients with
GBM. However, it was identified that patients with glioma with low
FGF1 expression achieved longer overall survival time than patients
with high FGF1 expression. By contrast, high expression of FGF17
and FGF22 indicated a longer overall survival time. The expression
level of FGF17 was most closely related to the longer overall
survival time of patients with glioma. The present study is
therefore expected to provide a certain research basis for clinical
gene therapy of these malignancies.
However, there are some limitations to the present
study. The present study mainly focused on the RNA levels of FGFs
in patients with GBM through analyzing RNA transcriptome sequencing
data, and the FGF protein levels were therefore not detected in the
present study. The protein levels of FGFs in human GBM will be
examined in a further study. In addition, further research is still
needed to explore whether and how FGF1, FGF17, FGF20 and FGF22
affect the proliferation or invasion abilities of human GBM.
Moreover, the overall survival curves were obtained through the
GEPIA analysis tool, which generates survival curves based on data
from TCGA database. In addition, since whole datasets could not be
obtained from all research within TCGA and as FGF expression was
only tested in 12 patients in The First Affiliated Hospital of Sun
Yat-sen University, survival outcomes in this study could only be
obtained via the GEPIA analysis tool, which may have led to a bias
in the overall survival data analysis.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Natural Science
Foundation Youth Project of China (grant no. 82103254).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Author's contributions
KZ designed the study, analyzed the sequencing data
and wrote the manuscript. JX and BZ made contributions to the
analysis and interpretation of data. KZ and JX confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Human GBM tissues and adjacent non-cancerous brain
tissues were collected from the Neurosurgery Department of the
First Affiliated Hospital of Sun Yat-sen University (Guangzhou,
China) with written patient consent. The present study was approved
by The Institutional Ethics Review Board of the First Affiliated
Hospital of Sun Yat-sen University [approval no. (2016)279] and
complied with all relevant ethical regulations regarding human
participants.
Patient consent for publication
Written patient consent was obtained for the
publication of potentially personally-identifying clinical
information.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng J, Meng J, Zhu L and Peng Y:
Exosomal noncoding RNAs in Glioma: Biological functions and
potential clinical applications. Mol Cancer. 19:662020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alifieris C and Trafalis DT: Glioblastoma
multiforme: Pathogenesis and treatment. Pharmacol Ther. 152:63–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie Y, Su N, Yang J, Tan Q, Huang S, Jin
M, Ni Z, Zhang B, Zhang D, Luo F, et al: FGF/FGFR signaling in
health and disease. Signal Transduct Target Ther. 5:1812020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Foley JF, Kennedy BJ and Ross JD: A factor
from HeLa cells promoting colonial growth of human fibroblast-like
cells in culture. Cancer Res. 23:368–371. 1963.PubMed/NCBI
|
|
6
|
Gospodarowicz D: Localisation of a
fibroblast growth factor and its effect alone and with
hydrocortisone on 3T3 cell growth. Nature. 249:123–127. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Presta M, Chiodelli P, Giacomini A,
Rusnati M and Ronca R: Fibroblast growth factors (FGFs) in cancer:
FGF traps as a new therapeutic approach. Pharmacol Ther.
179:171–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giacomini A, Chiodelli P, Matarazzo S,
Rusnati M, Presta M and Ronca R: Blocking the FGF/FGFR system as a
‘two-compartment’ antiangiogenic/antitumor approach in cancer
therapy. Pharmacol Res. 107:172–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Presta M, Dell'Era P, Mitola S, Moroni E,
Ronca R and Rusnati M: Fibroblast growth factor/fibroblast growth
factor receptor system in angiogenesis. Cytokine Growth Factor Rev.
16:159–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jimenez-Pascual A, Mitchell K,
Siebzehnrubl FA and Lathia JD: FGF2: A novel druggable target for
glioblastoma? Expert Opin Ther Targets. 24:311–318. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haley EM and Kim Y: The role of basic
fibroblast growth factor in glioblastoma multiforme and
glioblastoma stem cells and in their in vitro culture. Cancer Lett.
346:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bian XW, Du LL, Shi JQ, Cheng YS and Liu
FX: Correlation of bFGF, FGFR-1 and VEGF expression with
vascularity and malignancy of human astrocytomas. Anal Quant Cytol
Histol. 22:267–274. 2000.PubMed/NCBI
|
|
13
|
Joy A, Moffett J, Neary K, Mordechai E,
Stachowiak EK, Coons S, Rankin-Shapiro J, Florkiewicz RZ and
Stachowiak MK: Nuclear accumulation of FGF-2 is associated with
proliferation of human astrocytes and glioma cells. Oncogene.
14:171–183. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toyoda K, Tanaka K, Nakagawa S, Thuy DH,
Ujifuku K, Kamada K, Hayashi K, Matsuo T, Nagata I and Niwa M:
Initial contact of glioblastoma cells with existing normal brain
endothelial cells strengthen the barrier function via fibroblast
growth factor 2 secretion: A new in vitro blood-brain barrier
model. Cell Mol Neurobiol. 33:489–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pollard SM, Yoshikawa K, Clarke ID, Danovi
D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M, et
al: Glioma stem cell lines expanded in adherent culture have
tumor-specific phenotypes and are suitable for chemical and genetic
screens. Cell Stem Cell. 4:568–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyagi N, Kato S, Terasaki M, Shigemori M
and Morimatsu M: Fibroblast growth factor-2 and −9 regulate
proliferation and production of matrix metalloproteinases in human
gliomas. Int J Oncol. 12:1085–1090. 1998.PubMed/NCBI
|
|
17
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao X, Xia X, Li F, Zhang M, Zhou H, Wu X,
Zhong J, Zhao Z, Zhao K, Liu D, et al: Circular RNA-encoded
oncogenic E-cadherin variant promotes glioblastoma tumorigenicity
through activation of EGFR-STAT3 signalling. Nat Cell Biol.
23:278–291. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45((W1)):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ornitz DM and Itoh N: The Fibroblast
Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol.
4:215–266. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Flanders KC, Lüdecke G, Renzing J, Hamm C,
Cissel DS and Unsicker K: Effects of TGF-betas and bFGF on
astroglial cell growth and gene expression in vitro. Mol Cell
Neurosci. 4:406–417. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou YJ, Yu AC, Garcia JM, Aotaki-Keen A,
Lee YL, Eng LF, Hjelmeland LJ and Menon VK: Astrogliosis in
culture. IV. Effects of basic fibroblast growth factor. J Neurosci
Res. 40:359–370. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reuss B, Dono R and Unsicker K: Functions
of fibroblast growth factor (FGF)-2 and FGF-5 in astroglial
differentiation and blood-brain barrier permeability: Evidence from
mouse mutants. J Neurosci. 23:6404–6412. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loilome W, Joshi AD, ap Rhys CM,
Piccirillo S, Vescovi AL, Gallia GL and Riggins GJ: Glioblastoma
cell growth is suppressed by disruption of Fibroblast Growth Factor
pathway signaling. J Neurooncol. 94:359–366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stan AC, Nemati MN, Pietsch T, Walter GF
and Dietz H: In vivo inhibition of angiogenesis and growth of the
human U-87 malignant glial tumor by treatment with an antibody
against basic fibroblast growth factor. J Neurosurg. 82:1044–1052.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gasser E, Sancar G, Downes M and Evans RM:
Metabolic Messengers: Fibroblast growth factor 1. Nat Metab.
4:663–671. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sancar G, Liu S, Gasser E, Alvarez JG,
Moutos C, Kim K, van Zutphen T, Wang Y, Huddy TF, Ross B and Dai Y:
FGF1 and insulin control lipolysis by convergent pathways. Cell
Metab. 34:171–183.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Uriel S, Brey EM and Greisler HP:
Sustained low levels of fibroblast growth factor-1 promote
persistent microvascular network formation. Am J Surg. 192:604–609.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El-Hariry I, Pignatelli M and Lemoine NR:
FGF-1 and FGF-2 regulate the expression of E-cadherin and catenins
in pancreatic adenocarcinoma. Int J Cancer. 94:652–661. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao D, Lu Y, Yang C, Zhou X and Xu Z:
Activation of FGF receptor signaling promotes invasion of
non-small-cell lung cancer. Tumour Biol. 36:3637–3642. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
King ML, Lindberg ME, Stodden GR, Okuda H,
Ebers SD, Johnson A, Montag A, Lengyel E, MacLean Ii JA and Hayashi
K: WNT7A/β-catenin signaling induces FGF1 and influences
sensitivity to niclosamide in ovarian cancer. Oncogene.
34:3452–3462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zubor P, Hatok J, Moricova P, Kajo K,
Kapustova I, Mendelova A, Racay P and Danko J: Gene expression
abnormalities in histologically normal breast epithelium from
patients with luminal type of breast cancer. Mol Biol Rep.
42:977–988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ke J, Yao YL, Zheng J, Wang P, Liu YH, Ma
J, Li Z, Liu XB, Li ZQ, Wang ZH and Xue YX: Knockdown of long
non-coding RNA HOTAIR inhibits malignant biological behaviors of
human glioma cells via modulation of miR-326. Oncotarget.
6:21934–2149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katoh M and Katoh M: Comparative genomics
on FGF8, FGF17, and FGF18 orthologs. Int J Mol Med. 16:493–496.
2005.PubMed/NCBI
|
|
35
|
Iram T, Kern F, Kaur A, Myneni S,
Morningstar AR, Shin H, Garcia MA, Yerra L, Palovics R, Yang AC, et
al: Young CSF restores oligodendrogenesis and memory in aged mice
via Fgf17. Nature. 605:509–515. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heer R, Douglas D, Mathers ME, Robson CN
and Leung HY: Fibroblast growth factor 17 is over-expressed in
human prostate cancer. J Pathol. 204:578–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen J, Wang X, Hu J, Du J, Dordoe C, Zhou
Q, Huang W, Guo R, Han F, Guo K, et al: FGF20 Protected Against BBB
disruption after traumatic brain injury by upregulating junction
protein expression and inhibiting the inflammatory response. Front
Pharmacol. 11:5906692021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boshoff EL, Fletcher EJR and Duty S:
Fibroblast growth factor 20 is protective towards dopaminergic
neurons in vivo in a paracrine manner. Neuropharmacology.
137:156–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dono R: Fibroblast growth factors as
regulators of central nervous system development and function. Am J
Physiol Regul Integr Comp Physiol. 284:R867–R881. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Niu J, Xie J, Guo K, Zhang X, Xia F, Zhao
X, Song L, Zhuge D, Li X, Zhao Y and Huang Z: Efficient treatment
of Parkinson's disease using ultrasonography-guided rhFGF20
proteoliposomes. Drug Deliv. 25:1560–1569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cai X, Tao W and Li L: Glioma cell-derived
FGF20 suppresses macrophage function by activating β-catenin. Cell
Signal. 89:1101812022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dabrowski A, Terauchi A, Strong C and
Umemori H: Distinct sets of FGF receptors sculpt excitatory and
inhibitory synaptogenesis. Development. 142:1818–1830. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pasaoglu T and Schikorski T: Presynaptic
size of associational/commissural CA3 synapses is controlled by
fibroblast growth factor 22 in adult mice. Hippocampus. 26:151–160.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aljović A, Jacobi A, Marcantoni M, Kagerer
F, Loy K, Kendirli A, Bräutigam J, Fabbio L, Van Steenbergen V,
Pleśniar K, et al: Synaptogenic gene therapy with FGF22 improves
circuit plasticity and functional recovery following spinal cord
injury. EMBO Mol Med. 15:e161112023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jacobi A, Loy K, Schmalz AM, Hellsten M,
Umemori H, Kerschensteiner M and Bareyre FM: FGF22 signaling
regulates synapse formation during post-injury remodeling of the
spinal cord. EMBO J. 34:1231–1243. 2015. View Article : Google Scholar : PubMed/NCBI
|