Introduction
Prostate cancer (PCa), mainly caused by genetic
mutations in basal or luminal prostate epithelial cells, accounts
for ~7% of newly diagnosed types of cancer in male patients
globally (1,2). In China, the disease burden of PCa is
heavy and its age-standardized incidence in male patients has shown
an average annual percentage increment of 2.6% from 2008 to 2012
(3,4). Since PCa is a highly
androgen-dependent disease, androgen deprivation therapy (ADT),
with the goal of reducing circulating androgen levels, is widely
applied in PCa management (5). For
patients with PCa with lymph node metastasis after extended pelvic
lymph node dissection, ADT is recommended as an early adjuvant
therapy following radical prostatectomy (6). Furthermore, ADT is the foundational
treatment for patients with high-risk stage III and IV PCa
(7,8), which may be more prevalently applied
in China since the proportion of metastatic PCa is higher in China
(25–32%) compared with the United States (19%) and the United
Kingdom (18%) (9).
ADT comprises bilateral orchiectomy as well as
gonadotropin-releasing hormone (GnRH) agonists, such as
leuprorelin, goserelin, histrelin and triptorelin, and antagonists,
such as degarelix (10,11). Bilateral orchiectomy has been
gradually replaced due to its irreversibility and side effects of
psychological trauma (8). However,
GnRH agonists, which have become increasingly administered in
clinical practice since their development, function to suppress the
secretion of testosterone by regulating luteinizing hormone and
follicle-stimulating hormone in the hypothalamic-pituitary-gonadal
axis (12,13).
Leuprorelin is a long-acting GnRH agonist that
primarily acts on the anterior pituitary, the continuous use of
which causes the desensitization of the pituitary and further
suppresses circulating gonadotrophins (14). Leuprorelin acetate microspheres were
initially developed by Takeda Pharmaceutical Company, Ltd., and
have been approved for PCa treatment in China since 2003, with the
brand name Enantone® (15). A number of studies have reported the
efficacy and safety of Enantone® leuprorelin acetate
microspheres in the treatment of PCa (16–20).
For example, a previous study demonstrated that after
Enantone® leuprorelin acetate microsphere monotherapy,
serum testosterone levels decreased over time in patients with PCa,
whose mean level at baseline, 3, 6 and 9 months was 460.2, 9.6, 8.7
and 6.8 ng/dl, respectively (16).
Furthermore, another study found that Enantone®
leuprorelin acetate microsphere ADT treatment resulted in a
favorable pathologic response in 21% of patients with high-risk
localized PCa (17). Notably,
Boennuokang® leuprorelin acetate microspheres, as the
first generic and domestic product developed by Beijing Biote
Pharmaceutical Co., Ltd., have provided another treatment option
for patients with PCa. At present, some studies have reported the
favorable efficacy of Boennuokang® leuprorelin acetate
microspheres for controlling prostate-specific antigen (PSA)
levels, improving voluntary urination and elevating the quality of
life in patients with PCa (21–23).
Nevertheless, although Boennuokang® and
Enantone® leuprorelin acetate microspheres are both
commonly used for PCa treatment in China, to the best of our
knowledge, their efficacy and safety have not been compared.
Therefore, the present study aimed to compare the efficacy and
safety profile of Boennuokang® and Enantone®
leuprorelin acetate microspheres in patients with PCa.
Materials and methods
Patients
The present retrospective, observational,
single-center study included 116 patients with PCa who received
leuprorelin acetate microspheres via injection
(Boennuokang® or Enantone®) between January
2017 and April 2022. The inclusion criteria were: i) Diagnosed with
PCa via pathological examination; ii) treated with
Boennuokang® or Enantone®; iii) aged >18
years old; and iv) with a complete medical history. The exclusion
criteria were: i) History of surgical castration or pharmacologic
endocrine therapy; ii) diagnosed with hematological malignancies or
other types of cancer; iii) suffers from dysfunction of organs,
such as heart, liver or kidneys; and iv) is missing information on
key data, such as testosterone or PSA levels. The present study
obtained approval from the Ethics Committee of Peking Union Medical
College Hospital, Peking Union Medical College, Chinese Academy of
Medical Sciences (approval no. I-23PJ473; Beijing, China), and the
committee waived the requirement for informed consent.
Treatment
At Peking Union Medical College Hospital (Beijing,
China), generic leuprorelin acetate microspheres
(Boennuokang®; Beijing Biote Pharmaceutical Co., Ltd.;
3.75 mg depot formulation) and branded leuprorelin acetate
microspheres (Enantone®; Takeda Pharmaceutical Company,
Ltd.; 3.75 mg depot formulation) were administered via injection
and were used to treat PCa. The common dosage and usage for both
Boennuokang® and Enantone® was 3.75 mg once
every 4 weeks according to the package insert. Patients who
received Boennuokang® were considered to be the test
group, while those who received Enantone® were
considered to be the reference group. Patients received appropriate
therapy (laparoscopic radical prostatectomy, endocrine therapy or
radiotherapy) based on their disease status according to the
National Comprehensive Cancer Network (NCCN) guideline (24) and other medications were
administered as required.
Data collection
Clinical characteristics of patients with PCa were
screened from their medical records, which involved demographics
and disease information. Data on testosterone and PSA levels at
baseline, 1 (M1), 3 (M3), 6 (M6) and 12 months (M12) after
treatment were collected to evaluate efficacy, and data on adverse
events from baseline to M12 were collected for safety evaluation.
In addition, follow-up data including castration-resistant PCa-free
survival (CRPC-FS) and overall survival (OS) were retrieved.
CRPC-FS was defined as the time in months between the date of
diagnosis and the date of occurrence of CRPC or last follow-up. OS
was defined as the time in months between the date of diagnosis and
the date of any-cause death or last follow-up. The last follow-up
date was 7th March, 2024, and the follow-up duration ranged from 1
to 86 months.
Statistical analysis
SPSS v.26.0 (IBM Corp.) was used for data analysis
and GraphPad Prism 7.01 (Dotmatics) was used for figure
construction. The Kolmogorov-Smirnov test was used to test the
normality of continuous variables. Continuous variables that did
not follow a normal distribution are presented as the median
[interquartile range (IQR)], while those following a normal
distribution are presented as the mean ± standard deviation.
Categorical variables are presented as the number (percentage). A
Wilcoxon rank-sum test, unpaired Student's t-test, χ2
test or Fisher's exact test was used for comparisons between
groups. Specifically, the Wilcoxon rank-sum test was used to
compare non-normally distributed variables between groups.
Student's t-test was used to compare normally distributed variables
between groups. The χ2 test or Fisher's exact test was
used to compare categorical variables between groups. Kaplan-Meier
curves were generated to show CRPC-FS or OS rates, and the log-rank
test was used to compare the rates between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient selection
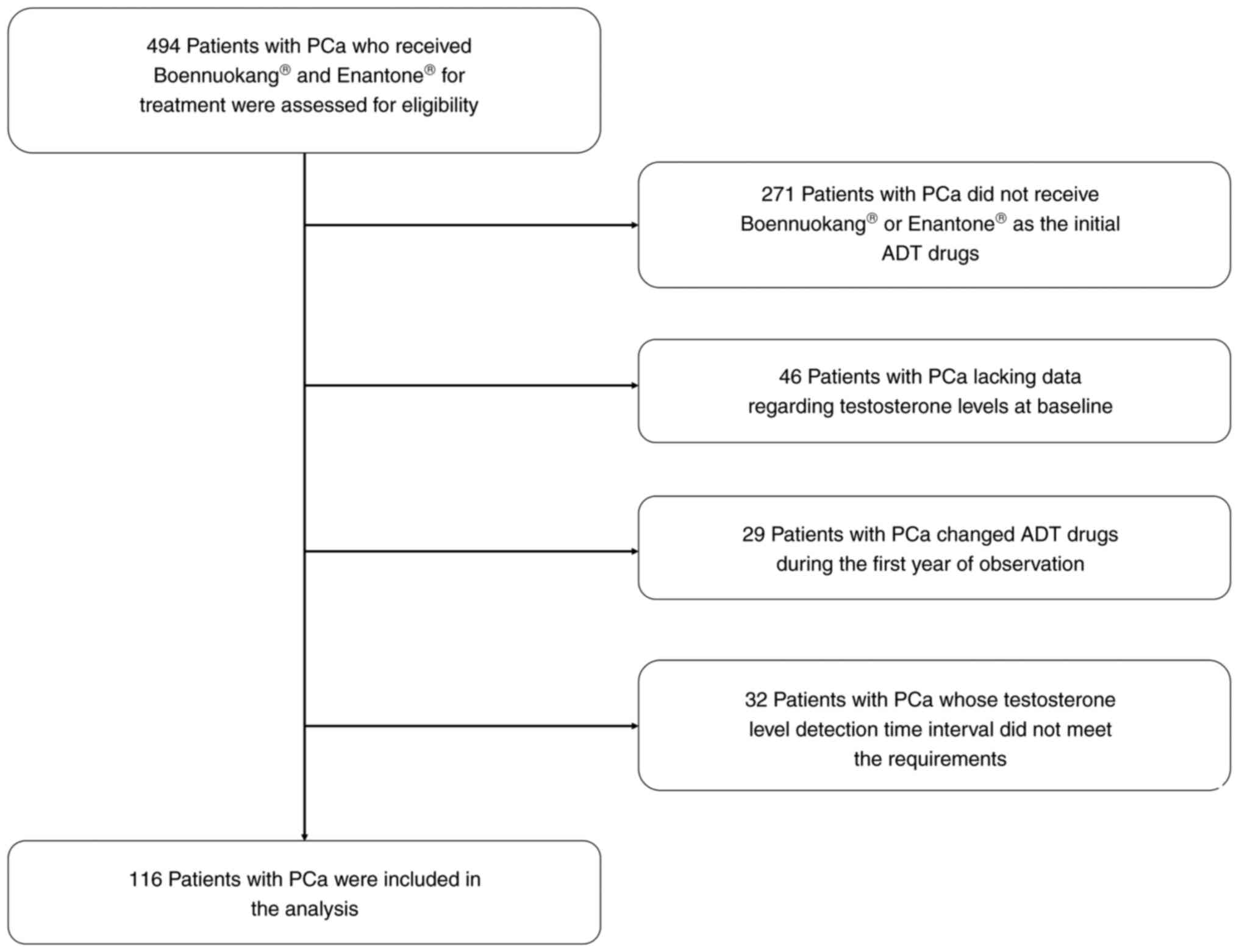
A total of 494 patients with PCa receiving
Boennuokang® or Enantone® leuprorelin acetate
microspheres for treatment were assessed. Among them, 271 patients
who did not use Boennuokang® or Enantone®
leuprorelin acetate microspheres as the initial ADT drugs, 46
patients who lacked data on testosterone levels at baseline, 29
patients who changed ADT drugs during the first year of observation
and 32 patients whose testosterone level detection time interval
did not meet the requirements were excluded. Finally, 116 patients
with PCa were included in the analysis, and the process of patient
selection with inclusion and exclusion data is shown in Fig. 1.
Clinical characteristics of the test
and reference groups
The mean age of the test and reference groups was
74.2±8.8 years (ranging from 49 to 90 years) and 74.1±8.1 years
(ranging from 57 to 90 years), respectively. The mean Gleason score
was 7.6±1.2 and 7.7±0.9 in the test and reference group,
respectively. With respect to the tumor grade, 8 (12.5%), 10
(15.6%), 10 (15.6%), 7 (10.9%) and 13 (20.3%) patients in the test
group were evaluated as having International Society of Urological
Pathology (ISUP) (25) grade 1, 2,
3, 4 and 5, respectively, and the ISUP grade for the remaining 16
(25.0%) patients was unavailable. In the reference group, there
were 2 (3.8%), 6 (11.5%), 9 (17.3%), 11 (21.2%) and 8 (15.4%)
patients that were identified as having ISUP grade 1, 2, 3, 4 and
5, respectively. The ISUP grade was unknown in the remaining 16
(30.8%) patients. The percentage of patients with ISUP grade 1 and
2 was not different between the test group and the reference group
(28.1 vs. 15.3%; P=0.102). Notably, clinical characteristics,
including age, Gleason score, ISUP grade, tumor node metastasis
stage, treatment regimens, baseline testosterone levels and
baseline PSA levels, did not vary between the test group and the
reference group (all P>0.05; Table
I).
 | Table I.Clinical characteristics of patients
with prostate cancer. |
Table I.
Clinical characteristics of patients
with prostate cancer.
|
Characteristics | Test group
(n=64) | Reference group
(n=52) | P-value |
|---|
| Age (years), mean ±
SD | 74.2±8.8 | 74.1±8.1 | 0.970a |
| Gleason score, mean
± SD | 7.6±1.2 | 7.7±0.9 | 0.638a |
| ISUP grade, n
(%) |
|
| 0.369b |
| 1 | 8 (12.5) | 2 (3.8) |
|
| 2 | 10 (15.6) | 6 (11.5) |
|
| 3 | 10 (15.6) | 9 (17.3) |
|
| 4 | 7 (10.9) | 11 (21.2) |
|
| 5 | 13 (20.3) | 8 (15.4) |
|
| NA | 16 (25.0) | 16 (30.8) |
|
| ISUP grade 1&2,
n (%) | 18 (28.1) | 8 (15.3) | 0.102c |
| TNM stage, n
(%) |
|
| 0.100c |
| II | 21 (32.8) | 7 (13.5) |
|
|
III | 16 (25.0) | 18 (34.6) |
|
| IV | 11 (17.2) | 13 (25.0) |
|
| NA | 16 (25.0) | 14 (26.9) |
|
| Treatment regimens,
n (%) |
|
|
|
|
Endocrine therapy only | 34 (53.1) | 21 (40.4) | 0.172c |
|
Radiotherapy combined with
endocrine therapy | 20 (31.3) | 23 (44.2) | 0.150c |
|
Laparoscopic
radical prostatectomy combined with endocrine therapy | 10 (15.6) | 8 (15.4) | 0.972c |
Comparison of testosterone and PSA
levels between the test and reference groups
Testosterone levels at M3 (P<0.001), M6 (P=0.012)
and M12 (P<0.001) were decreased in the test group compared with
the reference group, but testosterone levels at baseline (P=0.635)
and M1 (P=0.076) were not significantly different. The detailed
testosterone levels at different timepoints in the two groups are
listed in Table II.
 | Table II.Testosterone and PSA levels of
patients with prostate cancer at different time points. |
Table II.
Testosterone and PSA levels of
patients with prostate cancer at different time points.
| Item | Test group | Reference
group | P-value |
|---|
| Testosterone levels
(ng/dl) |
|
|
|
|
Baseline |
|
|
|
|
Number of assessed
patients | 64 | 52 |
|
|
Median (IQR) | 324.00
(264.50–417.50) | 319.00
(256.50–421.00) | 0.635a |
| M1 |
|
|
|
|
Number of assessed
patients | 61 | 51 |
|
|
Median (IQR) | 32.00
(17.00–45.00) | 37.00
(26.00–47.00) | 0.076a |
| M3 |
|
|
|
|
Number of assessed
patients | 63 | 49 |
|
|
Median (IQR) | 22.00
(10.00–29.00) | 31.00
(23.50–37.00) |
<0.001a |
| M6 |
|
|
|
|
Number of assessed
patients | 63 | 51 |
|
|
Median (IQR) | 21.00
(10.00–33.00) | 31.00
(19.00–37.00) | 0.012a |
|
M12 |
|
|
|
|
Number of assessed
patients | 62 | 51 |
|
|
Median (IQR) | 15.50
(10.00–31.25) | 28.00
(22.00–37.00) |
<0.001a |
| PSA levels
(ng/ml) |
|
|
|
|
Baseline |
|
|
|
|
Number of assessed
patients | 64 | 52 |
|
|
Median (IQR) | 11.70
(8.08–35.54) | 17.43
(10.39–42.77) | 0.145a |
| M1 |
|
|
|
|
Number of assessed
patients | 57 | 48 |
|
|
Median (IQR) | 2.35
(0.86–4.34) | 2.62
(0.58–8.35) | 0.489a |
| M3 |
|
|
|
|
Number of assessed
patients | 61 | 47 |
|
|
Median (IQR) | 0.17
(0.06–0.44) | 0.24
(0.04–0.96) | 0.712a |
| M6 |
|
|
|
|
Number of assessed
patients | 60 | 47 |
|
|
Median (IQR) | 0.03
(0.01–0.14) | 0.04
(0.01–0.38) | 0.590a |
|
M12 |
|
|
|
|
Number of assessed
patients | 59 | 47 |
|
|
Median (IQR) | 0.01
(0.01–0.10) | 0.02
(0.01–0.16) | 0.444a |
PSA levels at baseline (P=0.145), M1 (P=0.489), M3
(P=0.712), M6 (P=0.590) and M12 (P=0.444) did not vary between the
test and reference groups. The detailed PSA levels at different
timepoints in the two groups are shown in Table II.
Comparison of changes in testosterone
levels between the test and reference groups
Following treatment, testosterone levels decreased
in both groups and no difference was seen in M1-baseline (P=0.441),
M3-baseline (P=0.463), M6-baseline (P=0.598) or M12-baseline
(P=0.520) changes in testosterone levels between the test and
reference groups. Specifically, the median (IQR) M1-baseline,
M3-baseline, M6-baseline and M12-baseline changes in testosterone
levels were −292.00 ng/dl (−387.50 to −239.50 ng/dl), −303.00 ng/dl
(−391.00 to −245.00 ng/dl), −306.00 ng/dl (−391.00 to −247.00
ng/dl) and −305.50 ng/dl (−388.00 to −241.75 ng/dl), respectively,
in the test group. The median (IQR) M1-baseline, M3-baseline,
M6-baseline and M12-baseline changes in testosterone levels were
−290.00 ng/dl (−389.00 to −215.00 ng/dl), −288.00 ng/dl (−385.00 to
−219.50 ng/dl), −296.00 ng/dl (−388.00 to −227.00 ng/dl) and
−293.00 ng/dl (−391.00 to −225.00 ng/dl), respectively, in the
reference group (Table III).
 | Table III.Changes in testosterone levels of
patients with prostate cancer. |
Table III.
Changes in testosterone levels of
patients with prostate cancer.
| Item | Test group | Reference
group | P-value |
|---|
| M1-baseline |
|
|
|
| Number
of assessed patients | 61 | 51 |
|
| Median
(IQR), ng/dl | −292.00 (−387.50 to
−239.50) | −290.00 (−389.00 to
−215.00) | 0.441a |
| M3-baseline |
|
|
|
| Number
of assessed patients | 63 | 49 |
|
| Median
(IQR), ng/dl | −303.00 (−391.00 to
−245.00) | −288.00 (−385.00 to
−219.50) | 0.463a |
| M6-baseline |
|
|
|
| Number
of assessed patients | 63 | 51 |
|
| Median
(IQR), ng/dl | −306.00 (−391.00 to
−247.00) | −296.00 (−388.00 to
−227.00) | 0.598a |
| M12-baseline |
|
|
|
| Number
of assessed patients | 62 | 51 |
|
| Median
(IQR), ng/dl | −305.50 (−388.00 to
−241.75) | −293.00 (−391.00 to
−225.00) | 0.520a |
Comparison of changes in PSA levels
between the test and reference groups
The test and reference groups both exhibited
decreased PSA levels after treatment, but the M1-baseline
(P=0.286), M3-baseline (P=0.144), M6-baseline (P=0.158) and
M12-baseline (P=0.270) PSA changes were not significantly different
between the test and reference groups. Specifically, the median
(IQR) M1-baseline, M3-baseline, M6-baseline and M12-baseline
changes in PSA levels in the test group were −10.00 ng/ml (−28.53
to −5.13 ng/ml), −11.60 ng/ml (−32.10 to −7.90 ng/ml), −11.78 ng/ml
(−36.70 to −8.00 ng/ml) and −11.89 ng/ml (−37.16 to −8.09 ng/ml),
respectively. Meanwhile, in the reference group, the median (IQR)
PSA changes at these time points were −14.13 ng/ml (−34.53 to −9.04
ng/ml), −16.86 ng/ml (−42.90 to −10.10 ng/ml), −16.89 ng/ml (−43.62
to −10.11 ng/ml) and −17.14 ng/ml (−43.79 to −10.07 ng/ml),
respectively (Table IV).
 | Table IV.Changes in prostate-specific antigen
levels of patients with prostate cancer. |
Table IV.
Changes in prostate-specific antigen
levels of patients with prostate cancer.
| Item | Test group | Reference
group | P-value |
|---|
| M1-baseline |
|
|
|
| Number
of assessed patients | 57 | 48 |
|
| Median
(IQR), ng/ml | −10.00 (−28.53 to
−5.13) | −14.13 (−34.53 to
−9.04) | 0.286a |
| M3-baseline |
|
|
|
| Number
of assessed patients | 61 | 47 |
|
| Median
(IQR), ng/ml | −11.60 (−32.10 to
−7.90) | −16.86 (−42.90 to
−10.10) | 0.144a |
| M6-baseline |
|
|
|
| Number
of assessed patients | 60 | 47 |
|
| Median
(IQR), ng/ml | −11.78 (−36.70 to
−8.00) | −16.89 (−43.62 to
−10.11) | 0.158a |
| M12-baseline |
|
|
|
| Number
of assessed patients | 59 | 47 |
|
| Median
(IQR), ng/ml | −11.89 (−37.16 to
−8.09) | −17.14 (−43.79 to
−10.07) | 0.270a |
Comparison of long-term outcomes
between the test and reference groups
CRPC-FS did not differ between the test group and
the reference group (P=0.550). In detail, the 1-, 3- and 5-year
CRPC-FS rates in the test group were 90.2, 78.6 and 78.6%, and
these were 90.7, 76.2 and 64.3% in the reference group (Fig. S1A). OS did not vary between the
test and reference groups (P=0.437). Specifically, the 1-, 3- and
5-year OS rates in the test group were 98.4, 95.0 and 95.0%, and
these were 97.1, 89.9 and 81.7% in the reference group (Fig. S1B).
Comparison of adverse events between
the test and reference groups
Generally, the adverse events were controllable in
both the test and reference groups. The most common adverse events
in the test group were fatigue (20.3%), weight gain (17.2%) and
sweating (15.6%). Similarly, the most common adverse events in the
reference group were sweating (21.2%), weight gain (19.2%) and
fatigue (17.3%). Notably, no significant difference was observed in
the frequency of each adverse event, including fatigue (P=0.681),
weight gain (P=0.776), sweating (P=0.442), elevated blood lipids
(P=0.478), lower urinary tract symptoms (P=0.690), elevated blood
glucose levels (P=0.699), elevated transaminase levels
(P>0.999), tachycardia (P>0.999) and bloody stool (P=0.448),
between the test and reference groups. Furthermore, 1 patient in
the reference group experienced bloody stool, which was induced by
radiation enteritis (Table V).
 | Table V.Adverse events. |
Table V.
Adverse events.
| Events | Test group, n (%)
(n=64) | Reference group, n
(%) (n=52) | P-value |
|---|
| Fatigue | 13 (20.3) | 9 (17.3) | 0.681a |
| Weight gain | 11 (17.2) | 10 (19.2) | 0.776a |
| Sweating | 10 (15.6) | 11 (21.2) | 0.442a |
| Elevated blood
lipid | 7 (10.9) | 8 (15.4) | 0.478a |
| LUTS | 4 (6.3) | 2 (3.8) | 0.690b |
| Elevated blood
glucose | 3 (4.7) | 4 (7.7) | 0.699b |
| Elevated
transaminase | 2 (3.1) | 1 (1.9) |
>0.999b |
| Tachycardia | 1 (1.6) | 1 (1.9) |
>0.999b |
| Bloody stool | 0 (0.0) | 1 (1.9) | 0.448b |
Subgroup analysis
Further subgroup analysis was conducted in patients
receiving different treatment regimens, which showed that, in
patients with PCa treated with endocrine therapy only and patients
who received laparoscopic radical prostatectomy combined with
endocrine therapy, PSA levels at baseline, M1, M3, M6 and M12 were
not significantly different between the test and reference groups
(all P>0.050). In patients receiving radiotherapy combined with
endocrine therapy, PSA levels at baseline (P<0.001) and M1
(P=0.004) were decreased in the test group compared with the
reference group, but there was no difference in PSA levels between
the two groups at M3, M6 and M12 (Table VI).
 | Table VI.Subgroup analysis of
prostate-specific antigen levels. |
Table VI.
Subgroup analysis of
prostate-specific antigen levels.
| Subgroup | Test group | Reference
group | P-value |
|---|
| Endocrine therapy
only |
|
|
|
| Number
of assessed patients | 34 | 21 |
|
|
Baseline, median (IQR),
ng/ml | 24.93
(8.52–80.05) | 14.85
(5.60–171.00) | 0.872a |
| M1,
median (IQR), ng/ml | 2.86
(0.69–6.43) | 1.45
(0.35–13.85) | 0.420a |
| M3,
median (IQR), ng/ml | 0.22
(0.09–1.14) | 0.11
(0.03–0.96) | 0.409a |
| M6,
median (IQR), ng/ml | 0.06
(0.02–0.53) | 0.05
(0.01–0.80) | 0.812a |
| M12,
median (IQR), ng/ml | 0.04
(0.01–0.23) | 0.10
(0.01–1.18) | 0.784a |
| Radiotherapy
combined with endocrine therapy |
|
|
|
| Number
of assessed patients | 20 | 23 |
|
|
Baseline, median (IQR),
ng/ml | 9.27
(6.39–15.83) | 19.23
(14.10–32.60) |
<0.001a |
| M1,
median (IQR), ng/ml | 2.08
(1.05–3.17) | 3.60
(2.50–8.40) | 0.004a |
| M3,
median (IQR), ng/ml | 0.18
(0.03–0.59) | 0.43
(0.08–1.43) | 0.054a |
| M6,
median (IQR), ng/ml | 0.01
(0.01–0.09) | 0.04
(0.01–0.38) | 0.074a |
| M12,
median (IQR), ng/ml | 0.01
(0.01–0.06) | 0.02
(0.01–0.12) | 0.174a |
| Laparoscopic
radical prostatectomy combined with endocrine therapy |
|
|
|
| Number
of assessed patients | 10 | 8 |
|
|
Baseline, median (IQR),
ng/ml | 10.10
(8.10–30.08) | 7.83
(3.32–50.90) | 0.570a |
| M1,
median (IQR), ng/ml | 2.49
(1.05–3.29) | 1.09
(0.15–3.40) | 0.570a |
| M3,
median (IQR), ng/ml | 0.06
(0.01–0.14) | 0.01
(0.01–0.04) | 0.100a |
| M6,
median (IQR), ng/ml | 0.01
(0.00–0.02) | 0.01
(0.01–0.01) | 0.785a |
| M12,
median (IQR), ng/ml | 0.01
(0.00–0.01) | 0.01
(0.01–0.80) | 0.525a |
Discussion
At present, there are few clinical reports
describing Boennuokang® leuprorelin acetate microspheres
in the treatment of PCa, and their use has been reported in only
three previous studies (21–23).
As reported in one randomized, controlled study, patients with PCa
treated with Boennuokang® leuprorelin acetate
microspheres plus bicalutamide and radiotherapy had decreased PSA
levels compared with those treated with radiotherapy alone, with a
mean PSA level of 15.69±3.57 and 22.38±5.24 ng/ml, respectively, at
M2 after treatment (21).
Similarly, another study noted that the combination of
Boennuokang® leuprorelin acetate microspheres and
intensity-modulated radiotherapy resulted in an improved PSA
suppression effect and elevated 1/2-year survival rates compared
with intensity-modulated radiotherapy alone in patients with PCa
(22). Furthermore, a previous
study examined administration of Boennuokang®
leuprorelin acetate microspheres in patients with PCa complicated
with urinary retention. After 16 weeks of treatment, the maximum
urinary flow rate was improved and the prostate volume was reduced
in these patients (23). The
aforementioned studies indicate the positive clinical efficacy of
Boennuokang® leuprorelin acetate microspheres in PCa
treatment (21–23). In the current study, both
testosterone and PSA levels were reduced after
Boennuokang® leuprorelin acetate microsphere treatment
in patients with PCa, and the median (IQR) M12-baseline
testosterone and PSA changes reached −305.50 ng/dl (−388.00 to
−241.75 ng/dl) and −11.89 ng/ml (−37.16 to −8.09 ng/ml),
respectively. This positive performance could be explained by
leuprorelin acetate microspheres possessing a resistance to
proteolytic enzymes and a high affinity to the GnRH receptor, and
therefore leuprorelin acetate microspheres effectively inhibits the
pituitary-gonadal system (15).
Concerning the application of Enantone®
leuprorelin acetate microspheres in patients with PCa, one case
report described a patient with PCa with lung metastases receiving
Enantone® leuprorelin acetate microsphere injection and
oral bicalutamide as the first-line therapy, who achieved a stable
disease state during the 3-year follow-up (26). Furthermore, another study reported
that the administration of Enantone® leuprorelin acetate
microspheres plus flutamide once a month resulted in a decreased
testosterone concentration and PSA levels in 16 patients with PCa
(27). Similarly, the present study
revealed that treatment with Enantone® leuprorelin
acetate microspheres resulted in decreased testosterone and PSA
levels in patients with PCa. Specifically, the median (IQR)
M12-baseline testosterone and PSA changes were −293.00 ng/dl
(−391.00 to −225.00 ng/dl) and −17.14 ng/ml (−43.79 to −10.07
ng/ml), respectively. This could also be explained by the drug
acting as a GnRH agonist (with a strong inhibiting role against
gonadotropin secretion) as aforementioned (14).
To the best of our knowledge, the present study was
the first to compare the treatment efficacy of
Boennuokang® and Enantone® leuprorelin
acetate microspheres in patients with PCa. It was observed that
M1-baseline, M3-baseline, M6-baseline or M12-baseline testosterone
and PSA changes were not varied between the two groups. This may
have been due to Boennuokang® and Enantone®
leuprorelin acetate microspheres sharing similar components,
including the active substance leuprorelin acetate and polymers of
lactic acids, resulting in their similar treatment efficacy
(15). Furthermore, it was noted
that testosterone levels at M3, M6 and M12 were decreased in
patients with PCa who received Boennuokang® leuprorelin
acetate microspheres compared with those who received
Enantone® leuprorelin acetate microspheres. These
findings may be affected by the more advanced microsphere
preparation process, as Boennuokang® was launched later,
or by the relatively small sample size. Therefore, further studies
with a larger sample size are required for validation.
The reliable safety profile of leuprorelin acetate
microspheres has been previously reported (28–31);
fatigue, weight gain, hot flushes and sweating are the common
adverse events recorded in patients with PCa who receive
leuprorelin acetate microsphere treatment (28–31).
The present study also showed good tolerance of
Boennuokang® and Enantone® leuprorelin
acetate microspheres in patients with PCa, and the most common
adverse events, including fatigue, weight gain and sweating, were
consistent with previous studies (28,29),
while hot flushes were not observed in this study, which may be
explained as follows: It was a retrospective observational study
and the adverse events may be underestimated. Furthermore, the
incidence of adverse events was not statistically different between
the Boennuokang® and Enantone® leuprorelin
acetate microsphere groups, indicating that they had a similar
safety profile in patients with PCa.
The present study has some limitations. First, it
was a retrospective, observational, single-center study, and the
comparable treatment efficacy of Boennuokang® and
Enantone® leuprorelin acetate microspheres should be
validated in further multicenter, randomized, controlled studies.
Second, the adverse events were only collected within a short-term
follow-up period, and the long-term side effects of both
Boennuokang® and Enantone® leuprorelin
acetate microspheres were hard to monitor. Given that ADT can be
continuously used for maintenance in patients with PCa (12), the long-term side effects should be
investigated in further studies. Third, given that the dosage of
leuprorelin acetate microspheres was fixed, data on drug
concentrations and metabolism were not collected in the present
study, and these require further investigation. Fourth, the
percentage of patients with ISUP grade 1 and 2 in the test group
was numerically higher than that in the reference group (without
statistical significance), which could potentially lead to
relatively better clinical outcomes in the test group compared to
the reference group.
In conclusion, Boennuokang® leuprorelin
acetate microspheres may have a comparable efficacy for
testosterone and PSA suppression with similar tolerance compared
with Enantone® leuprorelin acetate microspheres in
patients with PCa. However, the findings need further validation
through a randomized controlled study with a larger sample size and
longer follow-up duration.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by National High Level Hospital
Clinical Research Funding (grant nos. 2022-PUMCH-B-009 and
2022-PUMCH-A-063).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZZ, YZ, WY, TF and ZL contributed to the study
conception and design. Material preparation, and data collection
and analysis were performed by ZZ, YZ and WY. ZZ, YZ, WY, TF and ZL
contributed to the first draft of the manuscript and commented on
previous versions of the manuscript. TF and ZL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study obtained approval from the Ethics
Committee of Peking Union Medical College Hospital, Peking Union
Medical College, Chinese Academy of Medical Sciences (approval no.
I-23PJ473; Beijing, China), and the committee waived the
requirement for informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rebello RJ, Oing C, Knudsen KE, Loeb S,
Johnson DC, Reiter RE, Gillessen S, Van der Kwast T and Bristow RG:
Prostate cancer. Nat Rev Dis Primers. 7:92021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sekhoacha M, Riet K, Motloung P, Gumenku
L, Adegoke A and Mashele S: Prostate cancer review: Genetics,
diagnosis, treatment options, and alternative approaches.
Molecules. 27:57302022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun D, Li H, Cao M, He S, Lei L, Peng J
and Chen W: Cancer burden in China: Trends, risk factors and
prevention. Cancer Biol Med. 17:879–895. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiu H, Cao S and Xu R: Cancer incidence,
mortality, and burden in China: A time-trend analysis and
comparison with the United States and United Kingdom based on the
global epidemiological data released in 2020. Cancer Commun (Lond).
41:1037–1048. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Williams IS, McVey A, Perera S, O'Brien
JS, Kostos L, Chen K, Siva S, Azad AA, Murphy DG, Kasivisvanathan
V, et al: Modern paradigms for prostate cancer detection and
management. Med J Aust. 217:424–433. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mottet N, van den Bergh RCN, Briers E, Van
den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N,
Gandaglia G, Gillessen S, et al: EAU-EANM-ESTRO-ESUR-SIOG
guidelines on prostate cancer-2020 update. Part 1: Screening,
diagnosis, and local treatment with curative intent. Eur Urol.
79:243–262. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Desai K, McManus JM and Sharifi N:
Hormonal therapy for prostate cancer. Endocr Rev. 42:354–373. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crawford ED, Heidenreich A, Lawrentschuk
N, Tombal B, Pompeo ACL, Mendoza-Valdes A, Miller K, Debruyne FMJ
and Klotz L: Androgen-targeted therapy in men with prostate cancer:
Evolving practice and future considerations. Prostate Cancer
Prostatic Dis. 22:24–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Y, Mo M, Wei Y, Wu J, Pan J, Freedland
SJ, Zheng Y and Ye D: Epidemiology and genomics of prostate cancer
in Asian men. Nat Rev Urol. 18:282–301. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwamoto H, Izumi K, Makino T and Mizokami
A: Androgen deprivation therapy in high-risk localized and locally
advanced prostate cancer. Cancers (Basel). 14:18032022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu EM and Aragon-Ching JB: Advances with
androgen deprivation therapy for prostate cancer. Expert Opin
Pharmacother. 23:1015–1033. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Merseburger AS, Hammerer P, Rozet F,
Roumeguère T, Caffo O, da Silva FC and Alcaraz A: Androgen
deprivation therapy in castrate-resistant prostate cancer: How
important is GnRH agonist backbone therapy? World J Urol.
33:1079–1085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raja T, Sud R, Addla S, Sarkar KK, Sridhar
PS, Talreja V, Jain M and Patil K: Gonadotropin-releasing hormone
agonists in prostate cancer: A comparative review of efficacy and
safety. Indian J Cancer. 59:S142–S159. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Persad R: Leuprorelin: A leading role in
advanced prostate cancer therapy. Hosp Med. 64:360–363. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilson AC, Meethal SV, Bowen RL and Atwood
CS: Leuprolide acetate: A drug of diverse clinical applications.
Expert Opin Investig Drugs. 16:1851–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shim M, Bang WJ, Oh CY, Lee YS and Cho JS:
Effectiveness of three different luteinizing hormone-releasing
hormone agonists in the chemical castration of patients with
prostate cancer: Goserelin versus triptorelin versus leuprolide.
Investig Clin Urol. 60:244–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McKay RR, Xie W, Ye H, Fennessy FM, Zhang
Z, Lis R, Calagua C, Rathkopf D, Laudone VP, Bubley GJ, et al:
Results of a randomized phase II trial of intense androgen
deprivation therapy prior to radical prostatectomy in men with
high-risk localized prostate cancer. J Urol. 206:80–87. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizokami A, Ueno S, Fukagai T, Ito K,
Ehara H, Kinbara H, Origasa H, Usami M, Namiki M and Akaza H:
Global update on defining and treating high-risk localized prostate
cancer with leuprorelin: An Asian perspective. BJU Int. 99 (Suppl
1):S6–S9; discussion 17–18. 2007. View Article : Google Scholar
|
|
19
|
Suzuki K, Namiki M, Fujimoto T,
Takabayashi N, Kudou K and Akaza H: Efficacy and safety of
leuprorelin acetate 6-month depot in prostate cancer patients: A
phase III, randomized, open-label, parallel-group, comparative
study in Japan. Jpn J Clin Oncol. 45:1168–1174. 2015.PubMed/NCBI
|
|
20
|
Chung BH, Horie S and Chiong E: Clinical
studies investigating the use of leuprorelin for prostate cancer in
Asia. Prostate Int. 8:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li WL, Shi QB, Han WH and Li CM: Influence
on efficacy and immune function in patients with prostate cancer by
using intensity-modulated radiotherapy combined with endocrine
therapy. Pract J Cancer. 34:1275–1279. 2019.(In Chinese).
|
|
22
|
Su JQ, Xin JH, Zhao XR and Guan MG: The
effect of intensity-modulated radiotherapy combined with endocrine
therapy on prostate cancer. Guide Chin Med. 5:110–112. 2022.(In
Chinese).
|
|
23
|
Ling KN, Yang JR, Li BJ, Li J, Ji ZQ and
Liu JZ: A study on the clinical efficacy of medical castration in
treatment of prostate cancer complicated with urinary retention.
Chin J Clin Med. 15:340–344. 2022.(In Chinese).
|
|
24
|
Mohler JL, Armstrong AJ, Bahnson RR,
D'Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano
CS, Horwitz EM, et al: Prostate cancer, version 1.2016. J Natl
Compr Canc Netw. 14:19–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA; Grading Committee, : The 2014
international society of urological pathology (ISUP) consensus
conference on gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu LX, Lei L, Zhu YC, Du KQ, Li XF, Chen
HF, Wang WX and Xu CW: A prostate cancer patient with isolated lung
metastases: A case report. Transl Cancer Res. 9:2064–2068. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Tao D and Wang S: Clinical
evaluation of enantone in the treatment of prostate cancer.
Zhonghua Zhong Liu Za Zhi. 19:218–220. 1997.(In Chinese).
PubMed/NCBI
|
|
28
|
Padula GD, Zelefsky MJ, Venkatraman ES,
Fuks Z, Lee HJ, Natale L and Leibel SA: Normalization of serum
testosterone levels in patients treated with neoadjuvant hormonal
therapy and three-dimensional conformal radiotherapy for prostate
cancer. Int J Radiat Oncol Biol Phys. 52:439–443. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marberger M, Kaisary AV, Shore ND, Karlin
GS, Savulsky C, Mis R, Leuratti C and Germa JR: Effectiveness,
pharmacokinetics, and safety of a new sustained-release leuprolide
acetate 3.75-mg depot formulation for testosterone suppression in
patients with prostate cancer: A phase III, open-label,
international multicenter study. Clin Ther. 32:744–757. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
George DJ, Saad F, Cookson MS, Saltzstein
DR, Tutrone R, Bossi A, Brown B, Selby B, Lu S, Buckley D, et al:
Impact of concomitant prostate cancer medications on efficacy and
safety of relugolix versus leuprolide in men with advanced prostate
cancer. Clin Genitourin Cancer. 21:383–392. e22023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
LBA02-09 EMBARK, . A Phase 3 randomized
study of enzalutamide or placebo plus leuprolide acetate and
enzalutamide monotherapy in high-risk biochemically recurrent
prostate cancer. J Urol. 210:224–226. 2023. View Article : Google Scholar
|