Introduction
In 2020, breast cancer had overtaken lung cancer to
become the most prevalent form of cancer globally. Breast cancer
represents 11.7% of the total number of cancer cases. Among female
individuals, breast cancer constitutes 25% of all cancer cases and
16% of all cancer-related fatalities and is the leading cancer in
incidence in 159 countries (1).
Breast-conserving surgery is commonly used to treat early-stage
breast cancer. Additionally, 50 Gy in 25 fractions of radiotherapy
to the remaining breast after breast-conserving surgery can reduce
the 10-year risk of recurrence and 15-year risk of breast
cancer-related mortality (2).
Therefore, radiotherapy is the standard of care for residual breast
cancer after breast-conserving surgery. The 10-year local
recurrence rates with 42.56 Gy in 16 fractions of hypofractionated
irradiation and 50 Gy in 25 fractions of conventional irradiation
are 6.2 and 6.7%, respectively. Since hypofractionated irradiation
has shown non-inferior results to conventional irradiation
(3), 42.56 Gy in 16 fractions of
hypofractionated irradiation has recently been approved as an
option for radiotherapy.
Cahan et al first documented the incidence of
radiation-induced malignancy as a late adverse event associated
with radiotherapy in 1948 (4).
Since then, this information has become common knowledge. According
to a previous study, the standardized incidence ratios of secondary
malignancies following treatment of breast cancer are 1.08 in
non-irradiated patients and 1.23 in irradiated patients (5). They reported that the irradiated group
had an elevated risk of developing lung cancer, esophageal cancer,
thyroid cancer, and sarcoma, which were thought to be caused by
radiation. Moreover, the risk ratio for sarcoma at 10 years was
6.54, highest among all malignancies (5). Nevertheless, despite having the
highest risk ratio, the 10-year reported incidence rate of
radiation-induced sarcoma is negligible at 0.03–0.27% (6,7).
Radiation-induced sarcomas are predominantly angiosarcomas
(48.1–57.9%) (6,8). Radiation-induced liposarcomas are even
rarer and accounts for 2.4% of all radiation-induced sarcomas
(9). Herein, we report a rare case
of radiation-induced pleomorphic liposarcoma after
breast-conserving surgery and hypofractionated irradiation and a
literature review.
Case report
The patient was a 63-year-old woman with no family
history, history of alcohol consumption, or history of smoking. In
June 2012, she underwent a breast cancer checkup at Kawasaki
Medical School Hospital (Kurashiki City, Japan). She underwent a
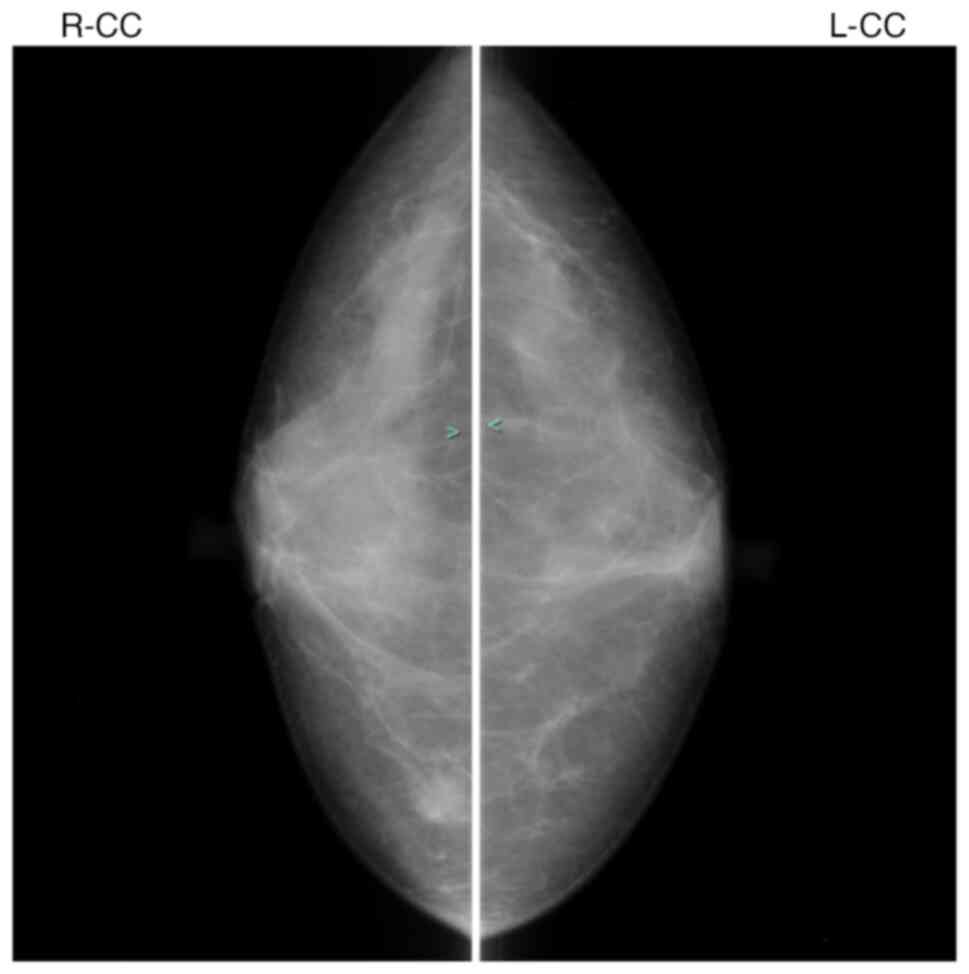
mammography and ultrasonography. Fig.
1 shows mammography. The presence of breast cancer was
suspected; a pathological diagnosis of breast cancer was
established based on a biopsy analysis. Contrast-enhanced magnetic
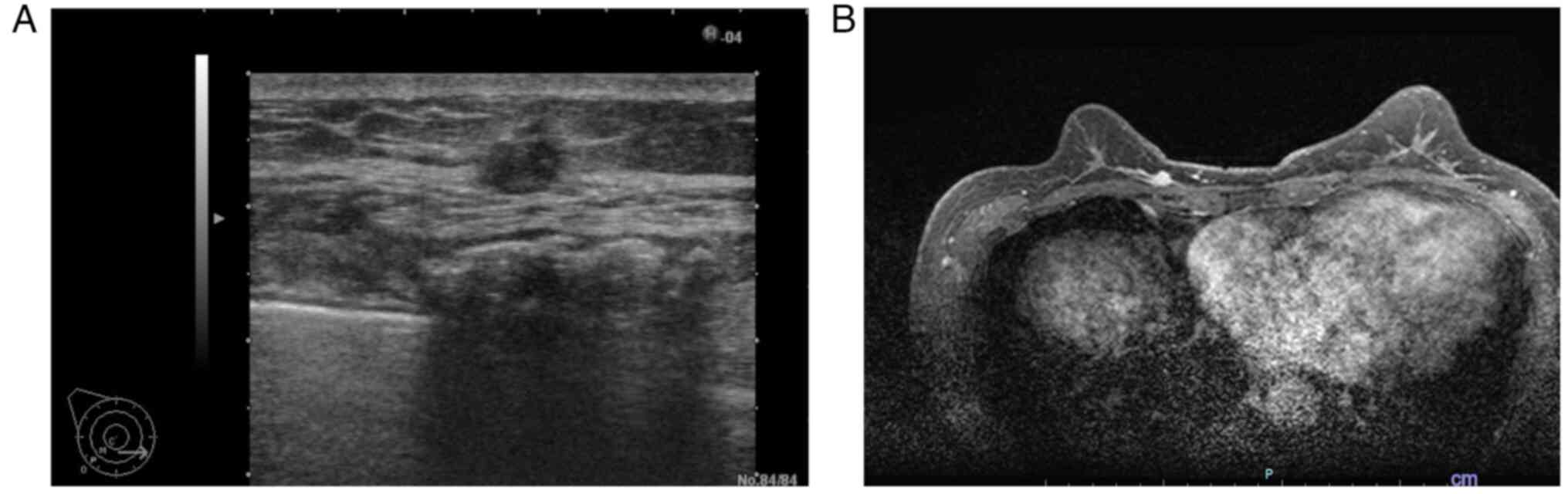
resonance imaging was performed in the same month. Fig. 2 shows ultrasonography and
contrast-enhanced magnetic resonance imaging. The tumor was staged
as cT1bN0 cStage I. In July 2012, She was admitted to our hospital
and subsequently underwent a quadrantectomy and sentinel node
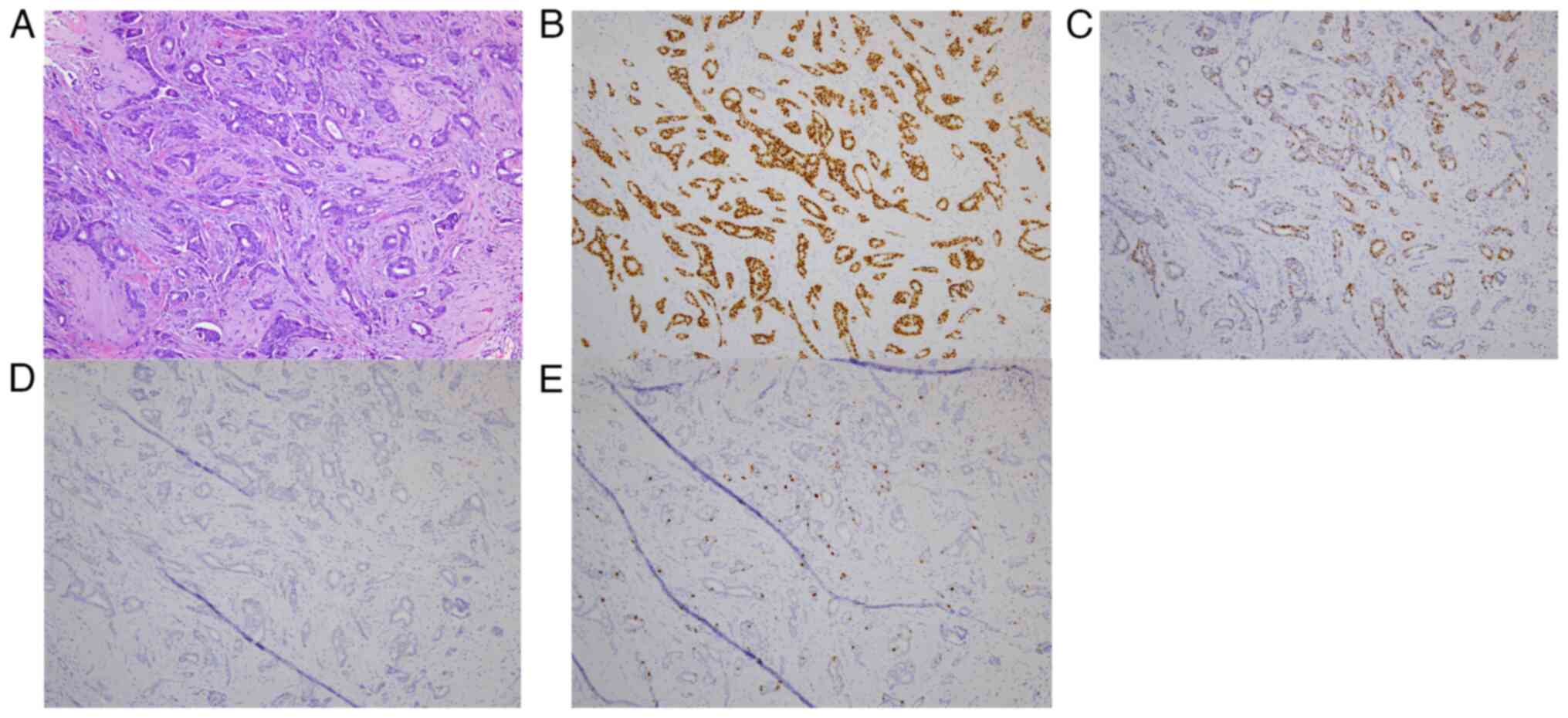
biopsy. She was pathologically diagnosed with papillotubular
carcinoma. The invasive diameter was 7 mm with negative margins.
The tumor was positive for estrogen receptor (95%) and progesterone
receptor (30%) but negative for human epidermal growth factor
receptor 2 (score 0); it had Ki67 index of 7.8%. A lymph node
biopsy yielded negative results (0/2), and the patient was
diagnosed with pT1bN0, pStage IA. The pathological and
immunostaining results are shown in Fig. 3. Following surgery, in August 2012,
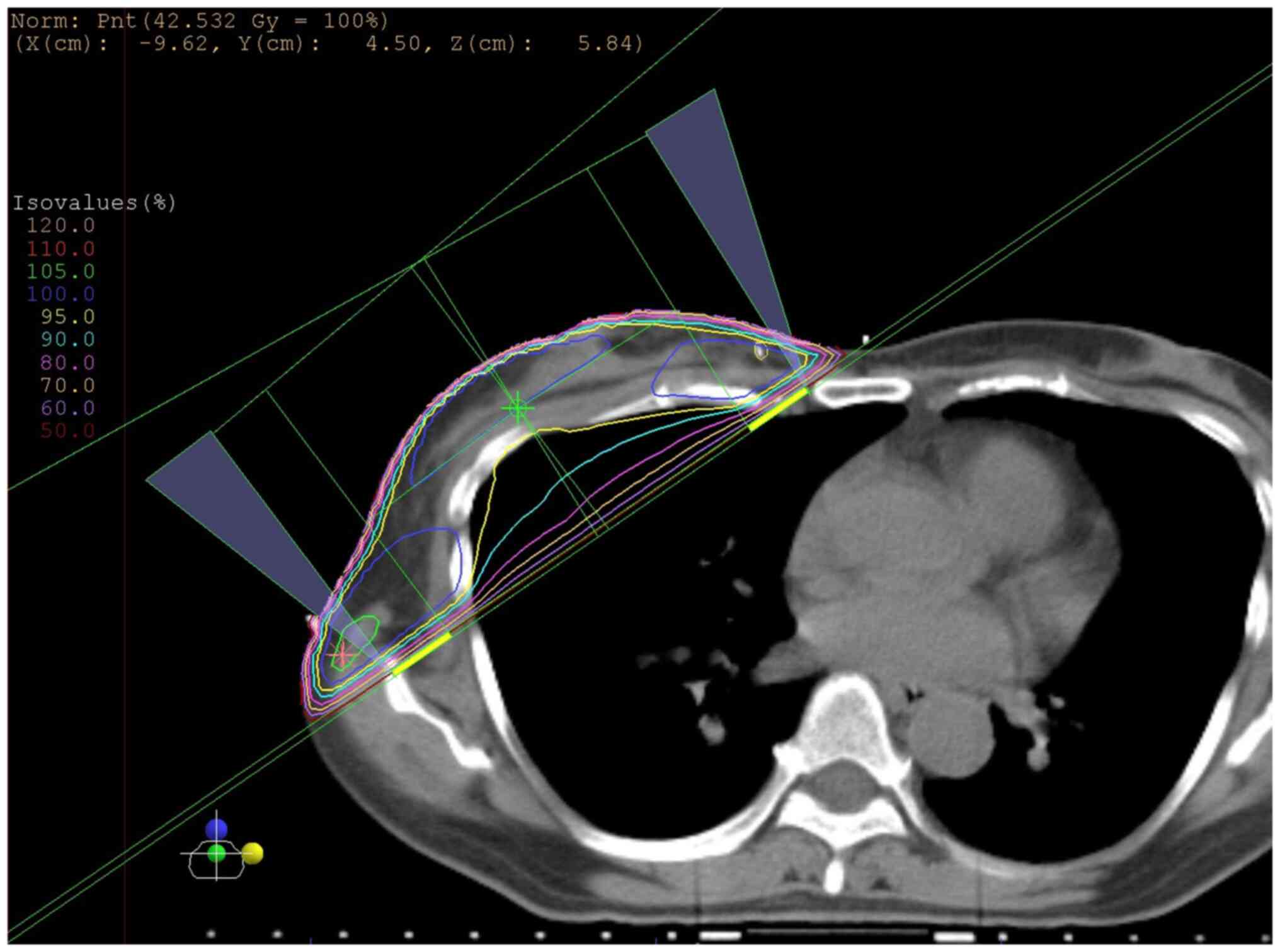
the patient received radiotherapy to the right residual breast,
with a total of 42.56 Gy in 16 fractions delivered via two
oblique-entry noncontralateralized beams using a 15° wedge
(Fig. 4). From September 2012, she
was administered hormonal therapy for five years.
Unfortunately, in November 2020, she sought medical
attention at her local hospital because she discovered hardening of
the tissue beneath the skin of the right breast eight years after
radiotherapy. A biopsy was performed and breast cancer recurrence
was suspected. In December 2020, she was referred to our hospital;
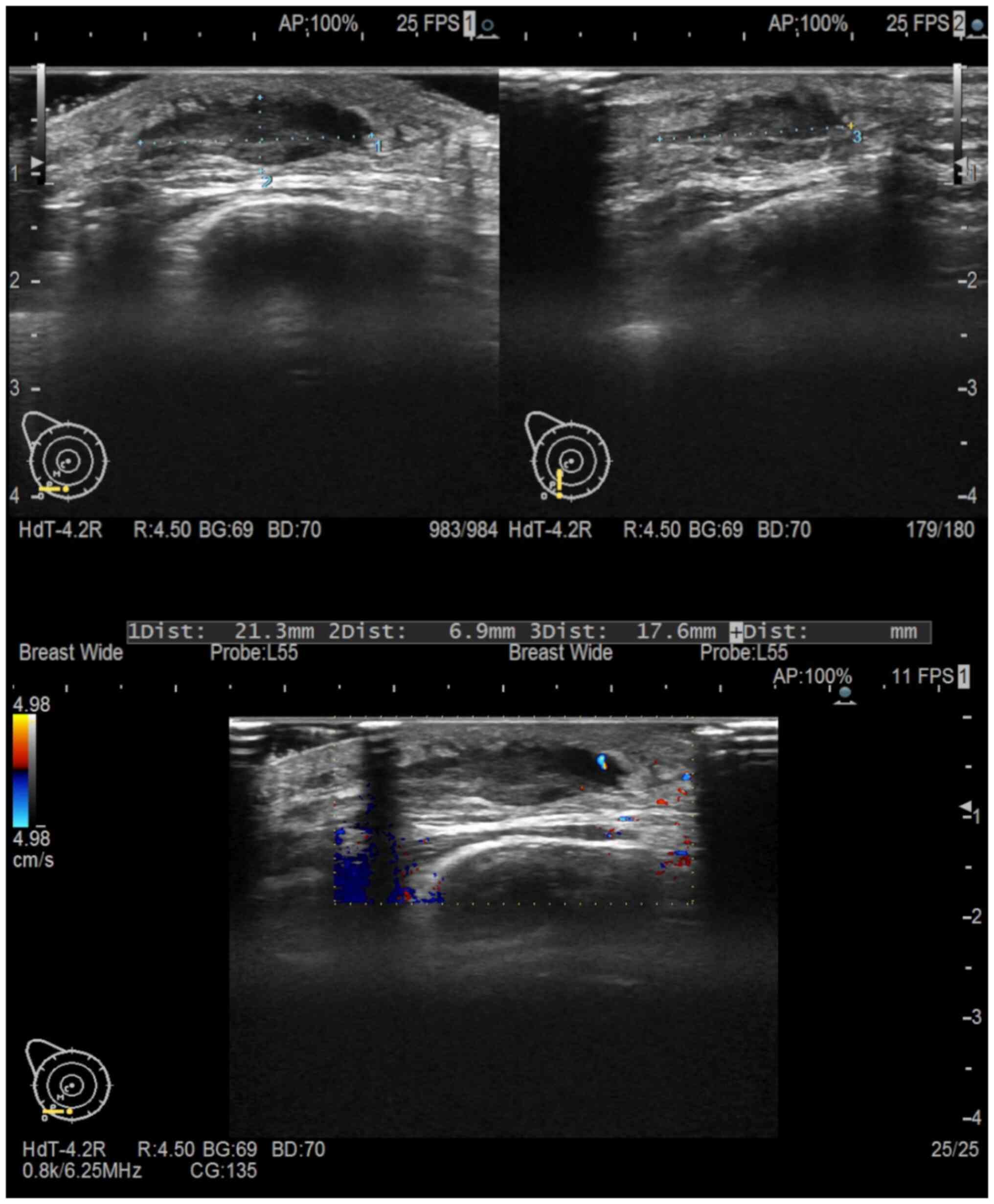
ultrasonography revealed a hypoechoic mass measuring 21×7×17 mm in
the lower part of the right breast containing internal blood flow
(Fig. 5). We re-evaluated the
biopsy result; the pathology was less likely to be an epithelial
malignancy. Therefore an excisional biopsy was performed to
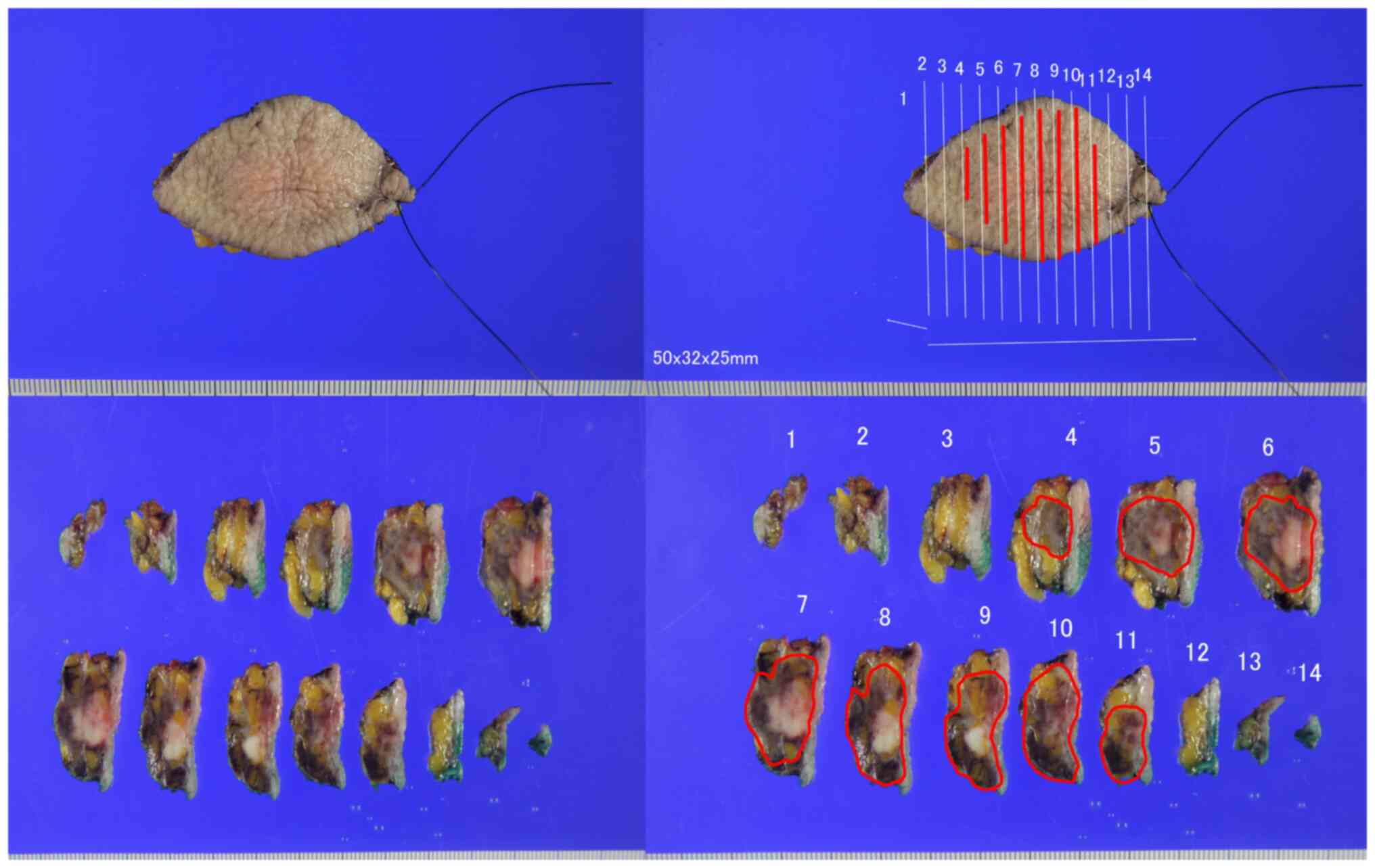
validate the diagnosis made in December 2020. The gross,
pathological, and immunostaining results are presented in Fig. 6, Fig.
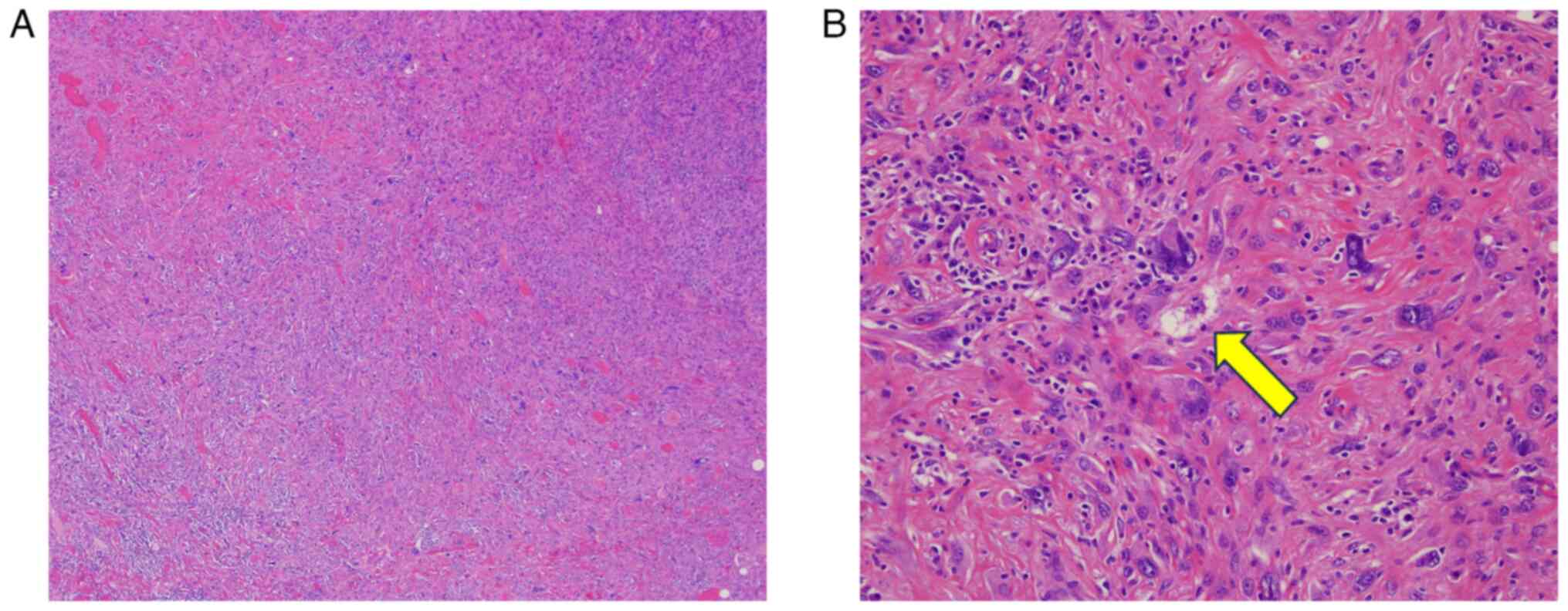
7, Fig. 8. Pathologically, the
tumor contained infiltrating spindle-shaped cells without a capsule
containing pleomorphic cells. Lipoblasts were also observed and
tended to differentiate into adipose tissue, leading to a diagnosis
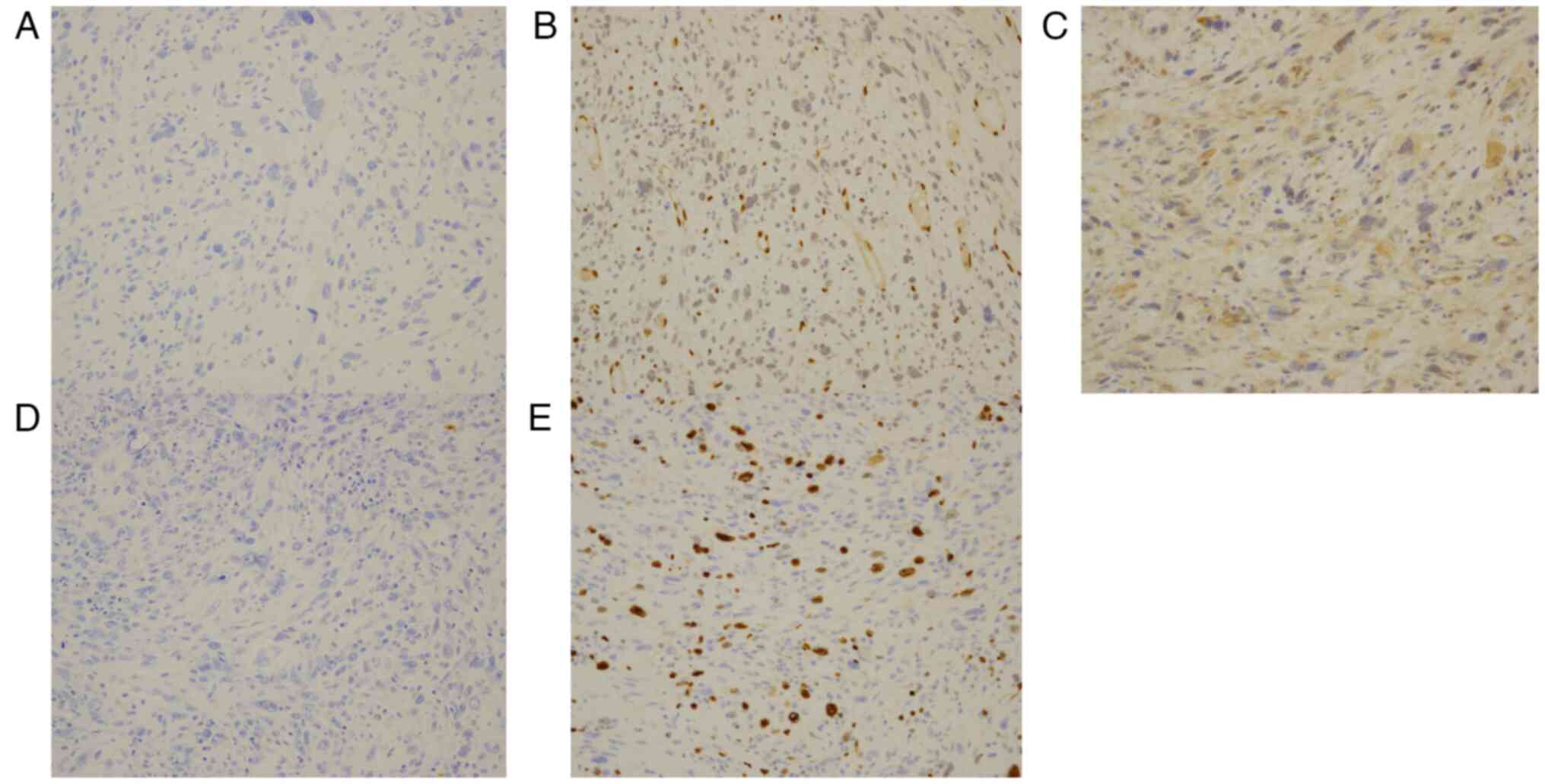
of pleomorphic liposarcoma. Immunostaining of the tumor cells
showed negativity for cytokeratin AE1/AE3, ERG, MDM2, and S-100
protein; the Ki-67 index was approximately 20%. In January 2021,
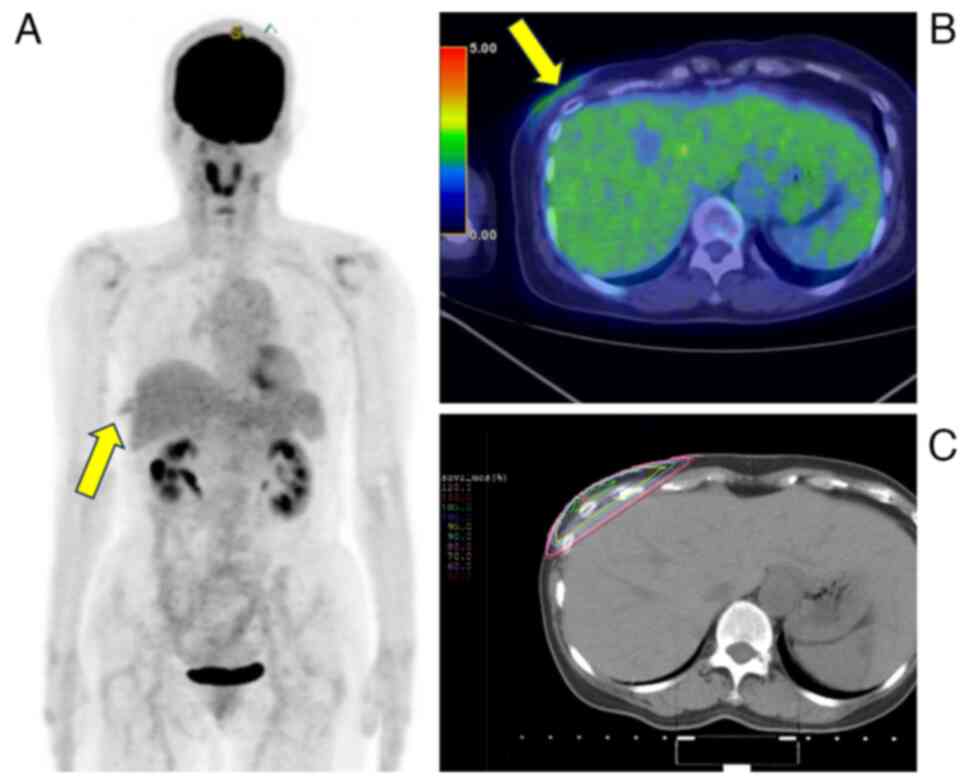
18F-fluorodeoxyglucose positron emission
tomography/computed tomography was performed. The results and
radiotherapy fields are presented in Fig. 9. Pale accumulation of
18F-fluorodeoxyglucose was detected in the right chest
wall, which was interpreted as a postoperative change owing to the
resection biopsy. The tumor was observed in an irradiated field and
no distant metastases were observed. Owing to the close margins of
the tumor, an enlargement resection involving a postoperative bed
was performed in January 2021. Following extensive resection,
pathology revealed no residual disease. Her last follow-up
appointment was in March 2023. The patient maintained a
recurrence-free survival period of three years and two months,
during which no adjuvant therapy was administered.
Discussion
The initial diagnostic criteria for
radiation-induced sarcoma were proposed by Cahan et al
(4) and have undergone subsequent
modifications over time. Currently, a more comprehensive range of
criteria is employed to identify radiation-induced malignancies,
encompassing more than just sarcoma (10). The criteria are as follows: i) tumor
develops within the irradiated area; ii) a significant amount of
time has passed since radiotherapy, preferably more than four
years; iii) treated and induced tumors are histologically distinct;
and iv) tissue from which the induced tumor grows is normal in
terms of its metabolism and genetics before being exposed to
radiation. The current case satisfied these diagnostic criteria for
radiation-induced malignancy because it developed inside the
irradiated area, there was a latency period of eight years,
histological evidence supporting this diagnosis, and the tissue
from which the tumor originated showed no aberrant signs prior to
radiation exposure.
There are three subtypes of liposarcoma: i) atypical
lipomatous tumor/well-differentiated liposarcoma, dedifferentiated
liposarcoma, ii) myxoid liposarcoma, and iii) pleomorphic
liposarcoma (11). Among them,
pleomorphic liposarcoma is the least prevalent type, accounting for
<5% of liposarcomas (12).
Nevertheless, it is considered to be the most aggressive variant.
Historically, it has been characterized by a histological makeup
consisting of pleomorphic cells combined with varying amounts of
lipoblasts. However, in recent years, the morphologic spectrum of
dedifferentiated liposarcomas has expanded to include rare variants
containing lipoblasts, called ‘homologous adipoblastic
differentiation (13)’. Therefore,
excluding the possibility of dedifferentiated liposarcomas, when
defining pleomorphic liposarcomas, is crucial (14). Upon gross examination, pleomorphic
liposarcomas often manifest as large masses that may be well
circumscribed, infiltrative, or multinodular. The sliced surface of
the tumor has a yellow-white coloration with potentially noticeable
patches of necrosis. Microscopically, pleomorphic liposarcomas
consist of a mixture of undifferentiated pleomorphic epithelial or
spindle-shaped cells with varying amounts of adipocyte components.
Unlike the diagnostic technique used for most adipocytic neoplasms,
which does not require lipoblast identification, the presence of
lipoblasts is necessary to diagnose pleomorphic liposarcomas
(14). Moreover,
immunohistochemistry plays a limited role in diagnosing pleomorphic
liposarcomas because it exhibits a nonspecific immunological
profile and demonstrates variable levels of expression of SMA,
desmin, and CD34 (14). Similarly,
the current case exhibited pleomorphic cells with mixed lipoblasts,
which aligns with the historical criteria for diagnosing
pleomorphic liposarcoma. Additionally, the immunostaining results
indicated the tumor was unlike epithelial tumors, angiosarcoma and
atypical lipomatous tumor/well-differentiated liposarcoma and
dedifferentiated liposarcoma, based on the negative myoepithelial
marker cytokeratin AE1/AE3, ERG, and MDM2, respectively.
Furthermore, negative staining for S-100 protein indicates a
decreased likelihood of nervous system malignancies and malignant
melanomas. Although the S-100 protein can also be detected in
adipocytes to help identify lipoblasts, which play a crucial role
in diagnosing pleomorphic liposarcoma (14), it was negative in this case.
Yap et al reported that the cumulative
incidence rate of secondary sarcomas up to 15 years was 3.2 per
1,000 patients who underwent radiotherapy and 2.3 per 1,000
patients who did not receive radiotherapy. Moreover, the incidence
of secondary sarcomas was significantly higher in patients who
underwent radiotherapy (P=0.001) (15). The group that underwent radiotherapy
had 87 occurrences of secondary sarcomas, of which 44 were located
within the irradiated area and considered radiation-induced
sarcomas. Of the 44 radiation-induced sarcomas, angiosarcomas
accounted for the majority (56.8%); liposarcomas were not
documented (15). A large-scale
study by Mirjolet et al included 125 patients with
radiation-induced sarcoma. Angiosarcoma was the most common
radiation-induced sarcoma, accounting for 68 (54.4%) cases. The
number of liposarcoma cases was few, accounting for three cases
(2.4%) (9). Pleomorphic
liposarcoma, the least common subtype of liposarcoma, is regarded
as quite rare among radiation-induced sarcomas.
We searched the cases of pleomorphic liposarcoma
following radiotherapy in PubMed using the terms ‘pleomorphic
liposarcoma’ and ‘radiotherapy’, or ‘pleomorphic liposarcoma’ and
‘radiation’. There were four reports (16–19):
one case of pleomorphic liposarcoma after radiotherapy for breast
cancer (16); one case after
treating medulloblastoma with Gorlin's syndrome (17); one case of a lesion on the left calf
after radiotherapy for epithelioid sarcoma (18); and one case of a lesion on the right
buttock after radiotherapy for rectal cancer with Muir-Torre
syndrome (19). These results are
summarized in Table I. Including
our case, two of the five cases have occurred after breast cancer
treatment. At first sight, pleomorphic liposarcoma seems more
likely to occur after radiotherapy for breast cancer. However, this
can be a result of the high incidence of breast cancer and its
favorable prognosis. Consequently, a substantial number of cases
can be monitored for at least several years following radiotherapy,
which may have contributed to the rise in detecting rare cases.
 | Table I.Reported cases of radiation-induced
pleomorphic liposarcoma. |
Table I.
Reported cases of radiation-induced
pleomorphic liposarcoma.
| Author | Age (years) | Location | Primary disease | Radiation dose
(Gy) | Genetic disease | Time from irradiation
to the onset (years) | (Refs.) |
|---|
| Arbabi, et
al | 68 | Left axillary | Left breast
cancer | 45 | Negative | 10 | (16) |
| O'Mally, et
al | 26 | Right scalp | Medulloblastoma | 40 | Gorlin's
syndrome | 26 | (17) |
| Orosz, et
al | 24 | Left calf | Epithelioid
sarcoma | 57 | Negative | 8 | (18) |
| Yozu, et
al | 74 | Right buttock | Rectal cancer | Not stated | Muir-Torre
syndrome | 12 | (19) |
| Present study | 71 | Right chest wall | Right breast
cancer | 42.56 | Negative | 8 | - |
Systematic reviews have indicated poor prognosis of
radiation-induced sarcomas, with a 5-year overall survival rate of
27–48% (7). Prognosis is correlated
with tumor size, with an average survival period of 80 months for
tumors <2 cm and 20 months for tumors >5 cm (7). In this case, palpation resulted in
early identification of the tumor, and subsequent diagnosis and
therapy were promptly administered at an early stage, leading to a
favorable outcome.
Mirjolet et al. documented that the mean dose
administered to the radiation-induced sarcoma was 47.8 Gy, which is
high (9). Owing to recent
advancements in intensity-modulated radiation therapy and
volumetric-modulated arc therapy, focusing high doses on a specific
treatment area is currently feasible, which has led to increased
dispersion of low doses. However, as for the occurrence of
radiation-induced sarcomas, reducing the high-dose volume can be
effective even if the low-dose volume increases. Furthermore,
radiation-induced sarcomas are also observed after hypofractionated
irradiation for the breast. Veiga et al. found that the risk
ratio for overall sarcoma in the hypofractionated group was 1.3
(95% confidence interval: 0.3–6.3) compared with the standard
fractionated group (P=0.73), indicating no significant difference.
Nevertheless, the limited number of hypofractionated
radiation-induced sarcoma cases resulted in an expansion of the 95%
confidence interval (20). In this
regard, the accumulation of cases and research on the frequency of
radiation-induced sarcomas caused by hypofractionated irradiation
are issues to be addressed in the future.
In conclusion, postoperative radiotherapy after
breast-conserving surgery for breast cancer is an established
medical practice that improves prognosis. On the other hand,
radiation-induced sarcomas occasionally occur after treatment of
breast cancer, partly due to the high incidence of breast cancer
and its favorable prognosis. This report describes a rare
pleomorphic liposarcoma that occurred after hypofractionated
radiation. Thus, follow-up examination of patients with
radiation-treated breast cancer is necessary for early detection of
radiation-induced sarcomas.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KW collected data and drafted the manuscript. RTo
and YK collected data. TM collected pathological figures. KW, RTo,
YK, TM, RTa, NT and KK participated in the study design and read
and approved the final manuscript. KW and KK confirm the
authenticity of all the raw data. KK supervised this study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Kawasaki Medical School (Kurashiki, Japan; approval no.
6314-00). The study was conducted in accordance with the principles
of the Declaration of Helsinki.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Darby S, McGale P, Correa C, Taylor
C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, et al:
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: Meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whelan TJ, Pignol JP, Levine MN, Julian
JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H,
et al: Long-term results of hypofractionated radiation therapy for
breast cancer. N Engl J Med. 362:513–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cahan WG, Woodard HQ, Higinbotham NL,
Stewart FW and Coley BL: Sarcoma arising in irradiated bone: Report
of eleven cases. 1948. Cancer. 82:8–34. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grantzau T and Overgaard J: Risk of second
non-breast cancer among patients treated with and without
postoperative radiotherapy for primary breast cancer: A systematic
review and meta-analysis of population-based studies including
522,739 patients. Radiother Oncol. 121:402–413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kirova YM, Vilcoq JR, Asselain B,
Sastre-Garau X and Fourquet A: Radiation-induced sarcomas after
radiotherapy for breast carcinoma: A large-scale single-institution
review. Cancer. 104:856–863. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sheth GR, Cranmer LD, Smith BD,
Grasso-Lebeau L and Lang JE: Radiation-induced sarcoma of the
breast: A systematic review. Oncologist. 17:405–418. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Lalla V, Tolba M, Khosrow-Khavar F,
Baig A, Freeman C and Panet-Raymond V: Radiation-induced sarcomas
of the breast: A review of a 20-year single-center experience.
Cureus. 15:e380962023.PubMed/NCBI
|
|
9
|
Mirjolet C, Diallo I, Bertaut A, Veres C,
Sargos P, Helfre S, Sunyach MP, Truc G, Le Pechoux C, Paumier A, et
al: Treatment related factors associated with the risk of breast
radio-induced-sarcoma. Radiother Oncol. 171:14–21. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khanna L, Prasad SR, Yedururi S,
Parameswaran AM, Marcal LP, Sandrasegaran K, Tirumani SH, Menias CO
and Katabathina VS: Second malignancies after radiation therapy:
Update on pathogenesis and cross-sectional imaging findings.
Radiographics. 41:876–894. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fletcher CDM; World Health Organization
and International Agency for Research on Cancer, : WHO
classification of tumours of soft tissue and bone. 4th edition.
World Health, Lyon Organization classification of tumoursIARC
Press; pp. 4682013
|
|
12
|
Oliveira AM and Nascimento AG: Pleomorphic
liposarcoma. Semin Diagn Pathol. 18:274–285. 2001.PubMed/NCBI
|
|
13
|
Mariño-Enríquez A, Fletcher CD, Dal Cin P
and Hornick JL: Dedifferentiated liposarcoma with ‘homologous’
lipoblastic (pleomorphic liposarcoma-like) differentiation:
Clinicopathologic and molecular analysis of a series suggesting
revised diagnostic criteria. Am J Surg Pathol. 34:1122–1131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anderson WJ and Jo VY: Pleomorphic
liposarcoma: Updates and current differential diagnosis. Semin
Diagn Pathol. 36:122–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yap J, Chuba PJ, Thomas R, Aref A, Lucas
D, Severson RK and Hamre M: Sarcoma as a second malignancy after
treatment for breast cancer. Int J Radiat Oncol Biol Phys.
52:1231–1237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arbabi L and Warhol MJ: Pleomorphic
liposarcoma following radiotherapy for breast carcinoma. Cancer.
49:878–880. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Malley S, Weitman D, Olding M and Sekhar
L: Multiple neoplasms following craniospinal irradiation for
medulloblastoma in a patient with nevoid basal cell carcinoma
syndrome. Case report. J Neurosurg. 86:286–288. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Orosz Z, Rohonyi B, Luksander A and Szántó
J: Pleomorphic liposarcoma of a young woman following radiotherapy
for epithelioid sarcoma. Pathol Oncol Res. 6:287–291. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yozu M, Symmans P, Dray M, Griffin J, Han
C, Ng D, Parry S and Wong K: Muir-Torre syndrome-associated
pleomorphic liposarcoma arising in a previous radiation field.
Virchows Arch. 462:355–360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Veiga LHS, Vo JB, Curtis RE, Mille MM, Lee
C, Ramin C, Bodelon C, Aiello Bowles EJ, Buist DSM, Weinmann S, et
al: Treatment-related thoracic soft tissue sarcomas in US breast
cancer survivors: A retrospective cohort study. Lancet Oncol.
23:1451–1464. 2022. View Article : Google Scholar : PubMed/NCBI
|