Introduction
Lung cancer is the most prevalent malignancy
worldwide, the incidence of which has increased annually among the
elderly for the past 40 years according to the surveillance,
epidemiology and end results (SEER) database (1). Treatment modalities for lung cancer
include surgery, radiotherapy, chemotherapy, targeted therapy,
antiangiogenic therapy and immunotherapy. Among these treatment
modalities, chemotherapy is the cornerstone of adjuvant or
palliative therapy. Routinely, the drugs for chemotherapy are
administered through a central venous access, such as central
venous catheters (CVCs), peripherally inserted catheters (PICCs) or
totally implanted venous access ports (TIVAPs). PICCs are often
used for short-term treatment (up to 3 months) in the perioperative
or intensive care setting, while CVCs and TIVAPs are used for
medium to long-term treatments (months to years) such as total
parenteral nutrition and chemotherapy. Potential complications of
central venous accesses usually include short-term (≤30 days of
insertion) and long-term complications (>30 days post
insertion). Short-term complications may present with symptoms of
hemorrhage, hemothorax, pneumothorax, air embolism, cardiac
arrhythmias or nerve palsy. Long-term complications mainly include
catheter migration, catheter-related thrombosis and infection
(2–4). These three types of central venous
access reduce repeated venipuncture and avoid focal venous injury
and tissue necrosis caused by repeated administration of anticancer
therapies. Furthermore, TIVAPs have lower reported rates of
catheter-related bloodstream infections (CRBSIs) than the other two
types of central access (5). TIVAPs
are also more optimal for bathing and swimming, which are
restricted with external vascular access, and may appeal to
patients concerned about the psychological implications of the
presence of visible non-implanted catheters. A meta-analysis by
Yeow et al (6) reported that
TIVAPs were superior to CVCs and PICCs in terms of complication
rate and quality of life without compromising cost-effectiveness.
However, insertion or removal of TIVAPs requires a minor surgical
operation, and long-term complications are major reasons for
removal, which include pocket infection, CRBSI, catheter-related
thrombosis and catheter migration (7–9).
According to the literature, the incidences of catheter-related
infection, thrombosis and migration were 3–10%, 1.06–11.4% and
0.05–3.5%, respectively (10–12).
Since management of complications is typically time-consuming and
costly, a risk prediction model of related events may be of great
value. However, such models are not well established at present.
The main focus of the present study is to explore the risk factors
for long-term complications after TIVAP placement and construct a
predictive model.
Machine learning, with its powerful and efficient
computational capabilities, can assist in the diagnosis of diseases
through well-trained models (13).
Thus far, machine learning has been widely used in foundation and
clinical medicine, new drug development and public health (14–16).
Machine learning-based abnormality detection overcomes the data
imbalance problems encountered in the real healthcare world
(17). To the best of the authors'
knowledge, the present study is the first to develop a machine
learning-based risk prediction model for long-term complications
associated with TIVAP implantation in patients with lung
cancer.
Materials and methods
Patients and variables
The present retrospective, low-risk study was
approved by The Ethics Committee of The First Affiliated Hospital
with Nanjing Medical University (Nanjing, China; approval no.
2022-SR-518) and informed patient consent was waived. Clinical data
between January, 2016 and December, 2018 were obtained from the
inpatient recording system. The patient inclusion criteria were as
follows: i) Aged ≥60 years (according to the World Health
Organization criteria for the age classification of older
individuals in developing countries); ii) pathologically diagnosed
with lung cancer and requiring chemotherapy; and iii) had TIVAP
implanted by a physician and a nurse in the operation room and
aided by ultrasound guided venipuncture and intracavitary
electrocardiogram (IC-ECG) guided tip localization (18,19).
Patients with large amount of missing data were excluded from the
study. There were 666 males and 236 females, with a median age of
67.23±0.52 (range 60–90) years. The primary end point in the
present study was long-term complications and all complications
were diagnosed by the current gold standard (3,20,21).
By searching the relevant literature (2,22–32),
the data collected in the present study were as follows: i)
Demographic characteristics, including age, sex, body mass index
(BMI), smoking history, thrombus history, history of catheter
placement, comorbidities, pleural effusion, cough, pathological
type based on WHO standard (33)
and tumor stage based on the 8th Edition of the TNM Classification
of the International Association for the Study of Lung Cancer
(34); ii) laboratory indicators,
including white blood cell (WBC) counts, platelet (PLT) counts,
hemoglobin (HB), D-dimer, activated partial thromboplastin time
(APTT), fibrinogen, albumin (ALB), total bilirubin and creatinine
(Cr); iii) medication for lung cancer, including platinum,
pemetrexed, bevacizumab, docetaxel, paclitaxel, radiotherapy,
leukocyte stimulant and PLT stimulant; and iv) data related to the
TIVAPs, including implantation site (right or left side of the
body), catheter length and operation time
Model development
The occurrence and detection of abnormalities is the
focus of disease prediction. Anomaly detection, also known as
outlier detection, was used to build the predictive model in the
present study (35). Anomaly
detection has a wide range of applications in various scenarios,
such as earth sciences, traffic monitoring, early diagnosis of
diseases and disease outbreak detection (36–38).
To improve the accuracy of the developed model and to identify
relevant risk factors that have not yet been recognized, all data
were incorporated into the model.
The modeling process, in which machine learning
algorithms suitable for supervised learning tasks [including
Isolation Forest (iForest), Local Outlier Factor (LOF) and
one-class Support Vector Machines (one-class SVM)] were used, was
divided into steps. First, the collected data were pre-processed,
including missing value processing, feature selection and
standardization. The tools used for this step were Pandas version
1.5.2 (https://github.com/pandas-dev/pandas), Numpy version
1.26.0 (https://github.com/numpy/numpy) and Seaborn version
0.12.2 (https://github.com/mwaskom/seaborn). The dataset was
then divided into training and test sets, ensuring that model
training was performed on a representative sample of data, while
retaining an independent dataset for evaluation. Second, the model
parameters were adjusted (contamination=28/902) according to the
data distribution after initializing the model. Training was then
performed on the training set, from which patterns and
relationships between input features and implanted outcomes were
learned. During the training process, the algorithm parameters were
iteratively adjusted to minimize the prediction error using
GridSearchCV in the hyperparameter tuning. The tool used for this
step was scikit-learn (sklearn) version 1.2.2 (https://github.com/scikit-learn/scikit-learn).
Finally, a model evaluation was performed and the receiver
operating characteristic curves (ROC) were plotted. The tool for
plotting ROC was matplotlib version 3.7.1 (https://github.com/matplotlib/matplotlib). A total of
five common metrics were introduced, including accuracy, precision,
recall, F1 score and area under the curve (AUC), to evaluate the
performance of the model with the test set. In general, the higher
the accuracy, precision, and recall of the model and the closer the
F1 score is to 1, the more optimal the performance of the model. In
addition, Matthew's correlation coefficient (MCC) was introduced,
which provided a more accurate assessment of performance with the
unbalanced data sets to inform clinical decision-making and risk
management. All the models were analyzed using Python version
3.10.5 (https://www.python.org/downloads/release/python-3105/).
Statistical analysis
Continuous variables are presented as the mean ± SD,
while categorical variables are presented as numbers (n) and
frequencies (%). Comparisons were conducted using χ2
test or Fisher's exact test with scipy version 1.10.1 (https://github.com/scipy/scipy). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient clinical characteristics in
the training and test sets and the occurrence of complications
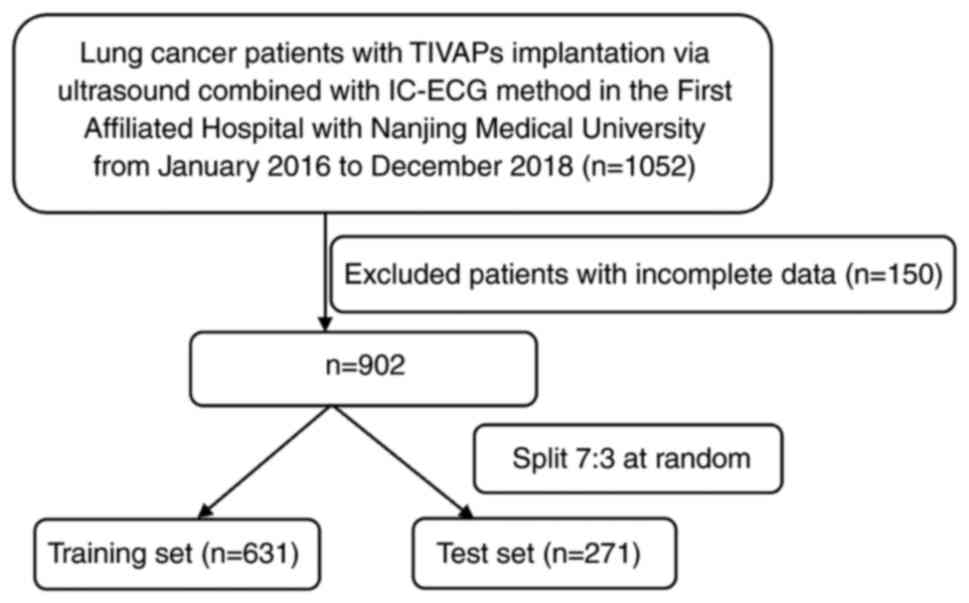
A total of 902 patients were included in the present
study (Fig. 1). As shown in
Table I, the training and test sets
consisted of 631 and 271 patients, respectively. The median age of
the training and test set was 67.35±0.42 and 67.10±0.62 years
respectively. Males accounted for 74.8% of the training set and
71.6% of the test set. A total of 28 patients (3.1%) developed
complications. There were no significant differences between the
two sets except in the number of patients administered docetaxel or
leukocyte stimulant (P<0.05; Table
I), which suggested that the feature distribution of the two
datasets was similar after data pre-processing, ensuring that the
model had good generalization ability.
 | Table I.Baseline characteristics of the
training (n=631) and test (n=271) sets. |
Table I.
Baseline characteristics of the
training (n=631) and test (n=271) sets.
| Variables | Training set | Test set | P-value |
|---|
| Age, years | 67.35±0.42 | 67.10±0.62 | 0.354 |
| Sex, n (%) |
|
| 1.000 |
|
Female | 159 (25.2) | 77 (28.4) |
|
|
Male | 472 (74.8) | 194 (71.6) |
|
| BMIa, n (%) |
|
| 0.532 |
| 0 | 336 (53.2) | 157 (57.9) |
|
| 1 | 38 (6.0) | 9 (3.3) |
|
| 2 | 210 (33.3) | 83 (30.6) |
|
| 3 | 47 (7.4) | 22 (8.1) |
|
| Smoking history, n
(%) |
|
| 0.976 |
| No | 251 (40.0) | 117 (43.2) |
|
|
Yes | 380 (60.0) | 154 (56.8) |
|
| Thrombosis history,
n (%) |
|
| 0.461 |
| No | 573 (90.8) | 251 (100.0) |
|
|
Yes | 58 (9.2) | 20 (0.0) |
|
| CVC history, n
(%) |
|
| 1.000 |
| No | 629 (99.7) | 271 (100.0) |
|
|
Yes | 2 (0.3) | 0 (0.0) |
|
| Comorbidities, n
(%) |
|
| 0.759 |
| No | 271 (42.9) | 125 (46.1) |
|
|
Yes | 360 (57.1) | 146 (53.9) |
|
| Pleural effusion, n
(%) |
|
| 0.421 |
| No | 358 (56.7) | 144 (53.1) |
|
|
Yes | 273 (43.3) | 127 (46.9) |
|
| Pathological
typeb, n (%) |
|
|
|
| 1 | 376 (59.6) | 153 (56.5) | 0.338 |
| 2 | 164 (26.0) | 83 (30.6) | 0.833 |
| 3 | 71 (11.3) | 30 (11.1) | 0.760 |
| 4 | 4 (0.6) | 2 (0.7) | 1.000 |
| 5 | 14 (2.2) | 8 (3.0) | 1.000 |
| Tumor
stagec, n (%) |
|
| 0.482 |
| 1 | 72 (11.4) | 47 (17.3) |
|
| 2 | 59 (9.4) | 33 (12.2) |
|
| 3 | 141 (22.3) | 58 (21.4) |
|
| 4 | 259 (41.0) | 95 (35.1) |
|
| 5 | 58 (9.2) | 23 (8.5) |
|
| WBC, n (%) |
|
| 0.758 |
| Normal,
3.5–9.5×109/l | 547 (86.7) | 228 (84.1) |
|
|
Abnormal | 84 (13.3) | 43 (15.9) |
|
| PLT, n (%) |
|
| 0.686 |
| Normal,
125–350×109/l | 562 (89.1) | 238 (87.8) |
|
|
Abnormal | 69 (10.9) | 33 (12.2) |
|
| HB, n (%) |
|
| 0.056 |
| Normal,
115–150 g/l | 490 (77.7) | 208 (76.8) |
|
|
Abnormal | 141 (22.3) | 63 (23.2) |
|
| Albumin, n (%) |
|
| 1.000 |
| Normal,
40–55 g/l | 192 (30.4) | 81 (30.0) |
|
|
Abnormal | 439 (69.6) | 190 (70.0) |
|
| Total bilirubin, n
(%) |
|
| 0.287 |
| Normal,
5.1–19 µmol/l | 587 (93.0) | 254 (93.7) |
|
|
Abnormal | 44 (7.0) | 17 (6.3) |
|
| Cr, n (%) |
|
| 0.515 |
| Normal,
41–81 µmol/l | 524 (83.0) | 211 (77.9) |
|
|
Abnormal | 107 (17.0) | 60 (22.1) |
|
| D-Dimer, n (%) |
|
| 0.438 |
| Normal,
≤0.55 mg/l | 287 (45.4) | 116 (42.8) |
|
|
Abnormal | 344 (54.5) | 155 (57.2) |
|
| Fibrinogen, n
(%) |
|
| 1.000 |
| Normal,
2–4 g/l | 412 (65.3) | 178 (65.7) |
|
|
Abnormal | 219 (34.7) | 93 (34.3) |
|
| APTT, n (%) |
|
| 0.929 |
| Normal,
25–31.3 sec | 473 (75.0) | 194 (71.6) |
|
|
Abnormal | 158 (25.0) | 77 (28.4) |
|
| Implant
sited, n (%) |
|
| 0.590 |
| 1 | 274 (43.4%) | 112 (41.3%) |
|
| 2 | 307 (48.7%) | 137 (50.6%) |
|
| 3 | 16 (2.5%) | 4 (1.5%) |
|
| 4 | 34 (5.4%) | 18 (6.6%) |
|
| Depth, cm | 23.58±0.27 | 23.36±0.43 | 0.231 |
| Time, min | 11.34±0.32 | 11.28±0.46 | 0.448 |
| Treatments n
(%) |
|
|
|
|
Platinum |
|
| 1.000 |
| No | 54 (8.6) | 19 (7.0) |
|
|
Yes | 577 (91.4) | 252 (93.0) |
|
|
Pemetrexed |
|
| 0.870 |
| No | 261 (41.4) | 123 (45.4) |
|
|
Yes | 370 (58.6) | 148 (54.6) |
|
|
Bevacizumab |
|
| 0.858 |
| No | 598 (94.8) | 262 (96.7) |
|
|
Yes | 33 (5.2) | 9 (3.3) |
|
|
Docetaxel |
|
| 0.049e |
| no | 559 (88.6) | 239 (88.2) |
|
|
yes | 72 (11.4) | 32 (11.8) |
|
|
Paclitaxel |
|
| 0.661 |
| No | 467 (74.0) | 193 (71.2) |
|
|
Yes | 164 (26.0) | 78 (28.8) |
|
|
Radiotherapy |
|
| 1.000 |
| No | 526 (83.4) | 224 (82.7) |
|
|
Yes | 105 (16.6) | 47 (17.3) |
|
|
Leukocyte-stimulant |
|
| 0.012e |
| No | 173 (27.4) | 61 (22.5) |
|
|
Yes | 458 (72.6) | 210 (77.5) |
|
|
PLT-stimulant |
|
| 0.874 |
| No | 525 (83.2) | 222 (81.9) |
|
|
Yes | 106 (16.8) | 49 (18.1) |
|
| Complications n
(%) |
|
|
|
| No | 610 (96.7) | 264 (97.4) |
|
|
Yes | 21 (3.3) | 7 (2.6) |
|
Feature selection; correlation
analysis with heatmaps
Seaborn was used to construct correlation-based
heatmaps (Fig. 2), to perform full
factor analysis and to determine any correlations with the
occurrence of complications. According to the heatmap, the factors
that may be associated with complications include history of
thrombosis, comorbidities, pleural fluid, adenocarcinoma, tumor
stage, APTT, BMI, site and time of implantation, WBC, HB, D-dimer,
ALB, Cr, antineoplastic agents and leukocyte stimulants. Among
these factors, those with a correlation coefficient of ≥0.05 were
BMI, HB, implantation time, docetaxel and leukocyte stimulants.
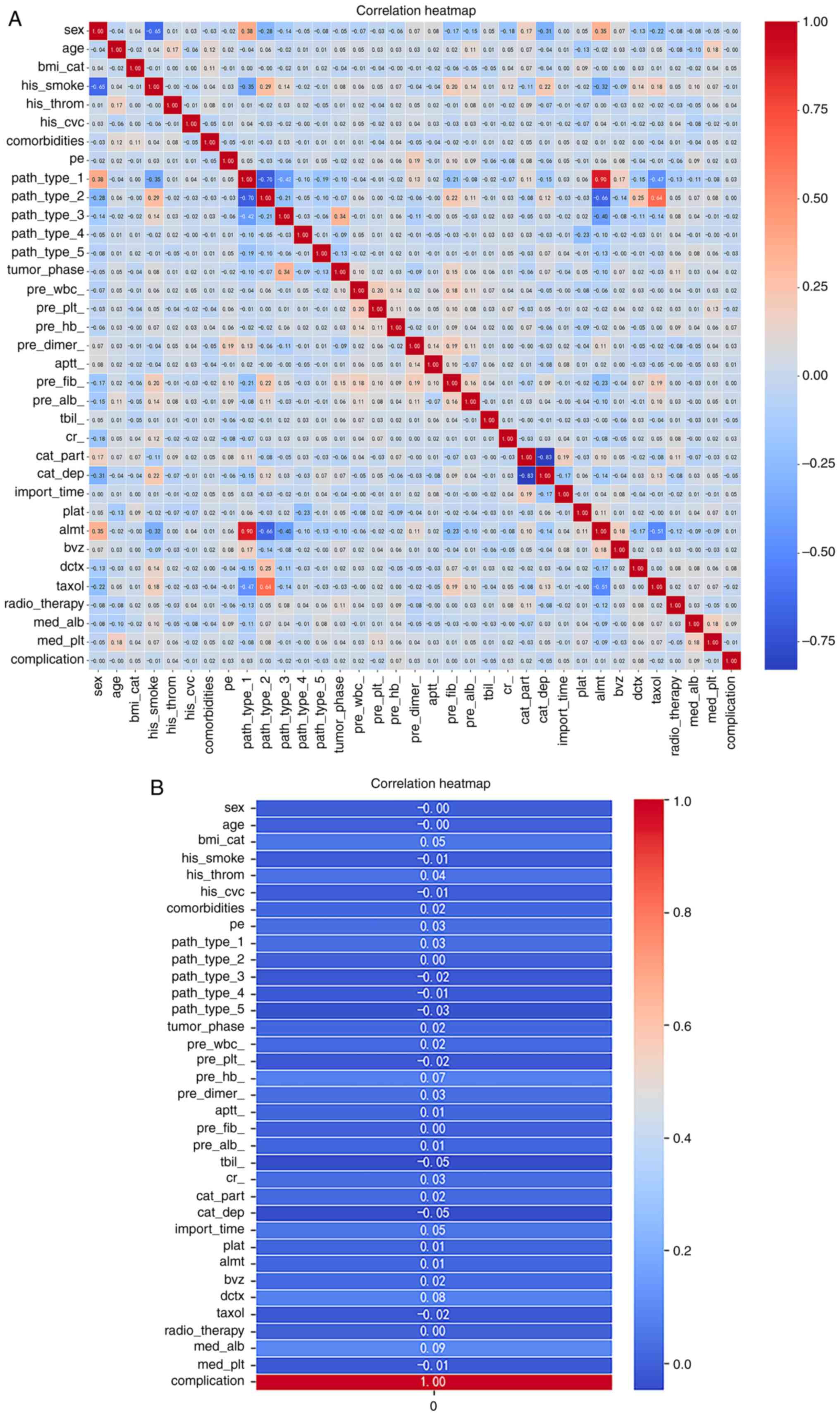 | Figure 2.Correlation-based heatmap. (A)
Seaborn was used to construct correlation-based heatmaps to perform
full factor analysis and to determine any correlations with the
occurrence of complications. (B) The detailed heatmap shows that
the history of thrombosis, comorbidities, pleural fluid,
adenocarcinoma, tumor phase, APTT, BMI, site and time of
implantation, WBC, HB, D-dimer, ALB, Cr, antineoplastic agents and
leukocyte stimulants may be associated with the development of
complications. APTT, activated partial thromboplastin time; BMI,
body mass index; WBC, white blood cell; HB, hemoglobin; ALB,
albumin; Cr, creatinine. |
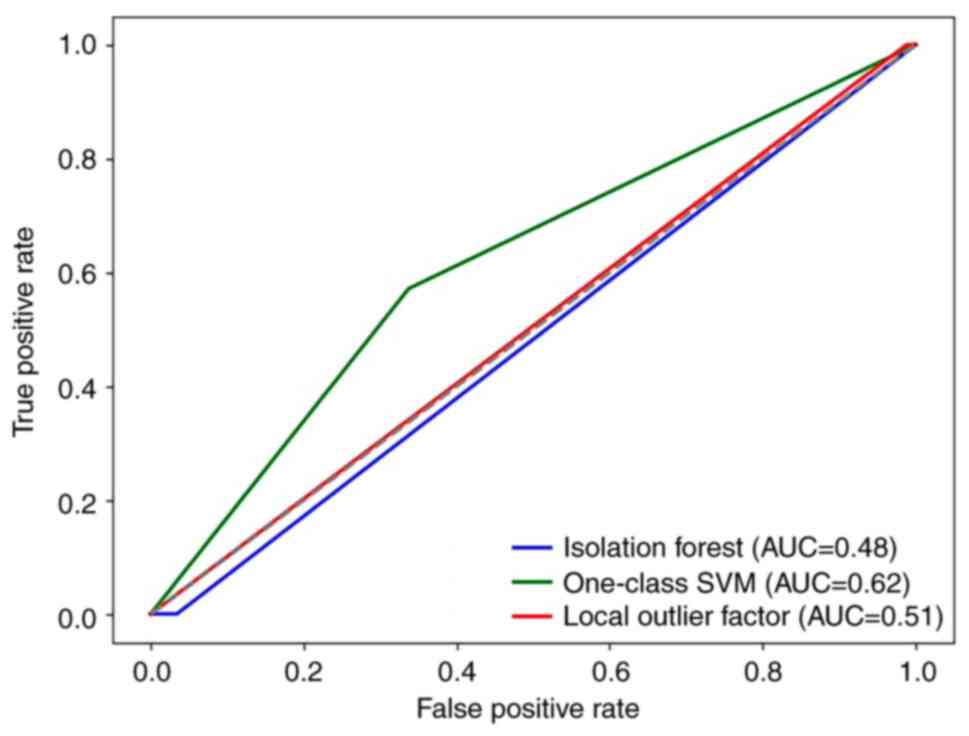
One-class SVM model performs the best
among the three models
The performance of the one-class SVM model (MCC,
0.078; AUC, 0.62; accuracy, 66.0%) was significantly superior than
the iForest (MCC, 0.015; AUC, 0.48; accuracy, 94.0%) and LOF (MCC,
−0.017; AUC, 0.51; accuracy, 96.0%; Fig. 3 and Table II) models. The classification
reports for patients in category 0 (without complications) and
category 1 (with complications) provided more detailed performance
metrics (Table II). Overall, the
one-class SVM model had a positive MCC, a relatively balanced
recall and performed well in the task.
 | Table II.Comparison of the performance metrics
of the three models. |
Table II.
Comparison of the performance metrics
of the three models.
| Algorithm | Precision | F1 score | Recall | MCC | AUC | Accuracy, % |
|---|
| iForest |
|
|
| 0.015 | 0.48 | 94.0 |
| 0 | 0.97 | 0.97 | 0.97 |
|
|
|
| 1 | 0.00 | 0.00 | 0.00 |
|
|
|
| One-class SVM |
|
|
| 0.078 | 0.62 | 66.0 |
| 0 | 0.98 | 0.79 | 0.66 |
|
|
|
| 1 | 0.04 | 0.08 | 0.57 |
|
|
|
| LOF |
|
|
| −0.017 | 0.51 | 96.0 |
| 0 | 0.97 | 0.98 | 0.99 |
|
|
|
| 1 | 0.00 | 0.00 | 0.00 |
|
|
|
Discussion
Different algorithms have different performances for
specific datasets. In the present study, three common anomaly
detection algorithms were applied to the same training set and
their performances were compared. These three algorithms were
chosen due to the following: i) iForest excels at identifying
outliers by constructing random trees and isolating anomalies in
shorter paths. iForest does not assume any potential data
distributions and can effectively handle large datasets (39); ii) LOF is sensitive to local
context, which is important in the healthcare setting as subtle
changes may indicate abnormalities. Moreover, LOF is less affected
by noise and different densities, which is consistent with the
inherent variability of medical data (40); and iii) one-class SVM can implement
boundary learning, it builds a hyperplane around most data points
to isolate a few classes (anomalies). Additionally, one-class SVM
is flexible to capture the complex relationships between risk
factors by tweaking the kernel function and performs well when
dealing with unbalanced datasets (41,42).
In summary, iForest, LOF and one-class SVM were selected in the
present study due to their effectiveness in detecting anomalies,
robustness to noise, processing high-dimensional data and capturing
complex relationships.
The early identification of high-risk groups for
long-term complications is important to improve the quality of life
of patients with cancer and to reduce the waste of medical
resources. To the best of our knowledge, the present study was the
first to present a model that has been built on top of an anomaly
detection algorithm. The results of the present study indicated
that the one-class SVM model had the highest performance with an
MCC, AUC and accuracy of 0.078, 0.62 and 66.0%, respectively.
Reducing the occurrence of complications has always been the focus
of healthcare professionals. To date, a number of factors such as
catheter material, age, BMI, severe coughing, time interval from
first use to placement, site of placement, hypoalbuminemia and
leukopenia, have been identified in patients with cancer suffering
from TIVAP-related complications (26,31,43–46).
Due to data sparseness, the present study was not
able to evaluate the contribution of each variable to
classification accuracy. However, the correlation-based heat map
suggested that BMI, HB, implantation time, docetaxel and leukocyte
stimulants may be closely related to the occurrence of long-term
complications following port implantation. These findings were
consistent with the real-world observations such that the
occurrence and progression of an outcome event are often influenced
by a combination of several factors. A previous study assessing the
risk of venous thromboembolism in patients with cancer included
BMI, HB and WBC count in the risk score (47), suggesting that the relationship
between BMI, HB, leukocyte stimulants and catheter related
thrombosis should be further studied using prediction models.
Adverse reactions to antitumor drugs should also be noted. For
instance, docetaxel may cause bone marrow suppression, manifesting
as neutropenia, thrombocytopenia or anemia (48). This will undoubtedly increase the
incidence of TIVAP-related infections. Hypoalbuminemia was found to
be an independent risk factor for infections (26). However, the relationship between
albumin correction and improved prognosis was not definitively
identified; it can be further explored in larger datasets in the
future. In the present study, the duration of implantation was
shown to be associated with the development of long-term
complications. However, a multicenter prospective French cohort
study (ONCOCIP) showed that an average surgery duration of 25 min
was not a risk factor (49).
Therefore, this factor needs to be verified by further
research.
In conclusion, a machine learning-based prediction
model for the long-term complications associated with TIVAPs in
patients with lung cancer was developed in the present study. The
model will help to identify individuals at high risk of
complications, which can improve their quality of life and prevent
unnecessary waste of medical resources. However, the present study
did have several limitations. First, since it was a single-center
retrospective study, the generalizability of the conclusions was
limited. Second, all of the participants were older (aged ≥60
years) and had a small number of complications. Third, no
predictive model was developed for specific complications, which
was mainly due to the low complication rate in the dataset.
Therefore, predictive models for specific complications among
individuals of different ages with other diseases should be
developed in the future by building larger sample sets or
conducting multicenter collaborative studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the China Postdoctoral
Science Foundation (grant no. 2023M731584), the Young Scholars
Fostering Fund of the First Affiliated Hospital of Nanjing Medical
University (grant no. PY2021015), the Study on the Effect and
Health Economics Evaluation of Home Cardiac Rehabilitation for
Heart Failure Based on Digital Medicine (grant no. M2022032) and
the Individual Institutional Breakthrough Theory: Theoretical
Construction and Testing Based on Local Practice (grant no.
72072083).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
JJ, XTF, WHZ, YYZ and BQF contributed to the study
design. XTF, ZYX and MW contributed to the data acquisition and
table organization;. XTF contributed to data analysis. XTF
contributed to writing the original draft. JJ contributed to review
and editing. YYZ contributed to funding acquisition. JJ, XTF, YYZ
and BQF confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The research protocol was approved by The Ethics
Committee of the First Affiliated Hospital with Nanjing Medical
University (Nanjing, China; approval no. 2022-SR-518) and patient
informed consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abdel-Rahman O: Changing epidemiology of
elderly small cell lung cancer patients over the last 40 years; a
SEER database analysis. Clin Respir J. 12:1093–1099. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gallieni M, Pittiruti M and Biffi R:
Vascular access in oncology patients. CA Cancer J Clin. 58:323–346.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silberzweig JE, Sacks D, Khorsandi AS and
Bakal CW; Society of Interventional Radiology Technology Assessment
Committee, : Reporting standards for central venous access. J Vasc
Interv Radiol. 14((9 Pt 2)): S443–S452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nayeemuddin M, Pherwani AD and Asquith JR:
Imaging and management of complications of central venous
catheters. Clin Radiol. 68:529–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maki DG, Kluger DM and Crnich CJ: The risk
of bloodstream infection in adults with different intravascular
devices: A systematic review of 200 published prospective studies.
Mayo Clin Proc. 81:1159–1171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeow M, Soh S, Yap R, Tay D, Low YF, Goh
SSN, Yeo CS and Lo ZJ: A systematic review and network
meta-analysis of randomized controlled trials on choice of central
venous access device for delivery of chemotherapy. J Vasc Surg
Venous Lymphat Disord. 10:1184–1191.e8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walser EM: Venous access ports:
Indications, implantation technique, follow-up, and complications.
Cardiovasc Intervent Radiol. 35:751–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tabatabaie O, Kasumova GG, Eskander MF,
Critchlow JF, Tawa NE and Tseng JF: Totally implantable venous
access devices: A review of complications and management
strategies. Am J Clin Oncol. 40:94–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Voog E, Campion L, du Rusquec P, Bourgeois
H, Domont J, Denis F, Emmanuel E, Dupuis O, Ganem G, Lafont C, et
al: Totally implantable venous access ports: A prospective
long-term study of early and late complications in adult patients
with cancer. Support Care Cancer. 26:81–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lebeaux D, Fernández-Hidalgo N, Chauhan A,
Lee S, Ghigo JM, Almirante B and Beloin C: Management of infections
related to totally implantable venous-access ports: Challenges and
perspectives. Lancet Infect Dis. 14:146–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wildgruber M, Borgmeyer S, Haller B,
Jansen H, Gaa J, Kiechle M, Meier R, Ettl J and Berger H:
Short-term and long-term outcome of radiological-guided insertion
of central venous access port devices implanted at the forearm: A
retrospective monocenter analysis in 1704 patients. Eur Radiol.
25:606–616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rouzrokh M, Shamsian BS, Tabari A,
Mahmoodi M, Kouranlo J, Manafzadeh G, Arzanian MT, Fallah F, Anoush
M and Gorji FA: Totally implantable subpectoral vs. subcutaneous
port systems in children with malignant diseases. Arch Iran Med.
12:389–394. 2009.PubMed/NCBI
|
|
13
|
Peiffer-Smadja N, Rawson TM, Ahmad R,
Buchard A, Georgiou P, Lescure FX, Birgand G and Holmes AH: Machine
learning for clinical decision support in infectious diseases: A
narrative review of current applications. Clin Microbiol Infect.
26:584–595. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reel PS, Reel S, Pearson E, Trucco E and
Jefferson E: Using machine learning approaches for multi-omics data
analysis: A review. Biotechnol Adv. 49:1077392021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vamathevan J, Clark D, Czodrowski P,
Dunham I, Ferran E, Lee G, Li B, Madabhushi A, Shah P, Spitzer M
and Zhao S: Applications of machine learning in drug discovery and
development. Nat Rev Drug Discov. 18:463–477. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mooney SJ and Pejaver V: Big data in
public health: Terminology, machine learning, and privacy. Annu Rev
Public Health. 39:95–112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alloqmani A, Abushark YB and Khan AI:
Anomaly detection of breast cancer using deep learning. Arab J Sci
Eng. 12:1–26. 2023.PubMed/NCBI
|
|
18
|
Pittiruti M, Pelagatti F and Pinelli F:
Intracavitary electrocardiography for tip location during central
venous catheterization: A narrative review of 70 years of clinical
studies. J Vasc Access. 22:778–785. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Z, Zheng X, Zhen Y, Liu X, Lin F, Ye Z
and Liu P: Efficacy, safety, and cost-effectiveness of
intracavitary electrocardiography-guided catheter tip placement for
totally implantable venous access port. Ann Vasc Surg. 83:168–175.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bell T and O'Grady NP: Prevention of
central line-associated bloodstream infections. Infect Dis Clin
North Am. 31:551–559. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baskin JL, Pui CH, Reiss U, Wilimas JA,
Metzger ML, Ribeiro RC and Howard SC: Management of occlusion and
thrombosis associated with long-term indwelling central venous
catheters. Lancet. 374:159–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang YF, Lo AC, Tsai CH, Lee PY, Sun S,
Chang TH, Chen CC, Chang YS and Chen JR: Higher complication risk
of totally implantable venous access port systems in patients with
advanced cancer-a single institution retrospective analysis.
Palliat Med. 27:185–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goltz JP, Schmid JS, Ritter CO, Knödler P,
Petritsch B, Kirchner J, Hahn D and Kickuth R: Identification of
risk factors for catheter-related thrombosis in patients with
totally implantable venous access ports in the forearm. J Vasc
Access. 13:79–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma LI, Liu Y, Wang J, Chang Y, Yu L and
Geng C: Totally implantable venous access port systems and
associated complications: A single-institution retrospective
analysis of 2,996 breast cancer patients. Mol Clin Oncol.
4:456–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vidal M, Genillon JP, Forestier E,
Trouiller S, Pereira B, Mrozek N, Aumeran C and Lesens O: Outcome
of totally implantable venous-access port-related infections. Med
Mal Infect. 46:32–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Skummer P, Kobayashi K, DeRaddo JS,
Blackburn T, Schoeneck M, Patel J and Jawed M: Risk factors for
early port infections in adult oncologic patients. J Vasc Interv
Radiol. 31:1427–1436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YC, Lin PL, Chou WH, Lin CP and Huang
CH: Long-term outcomes of totally implantable venous access
devices. Support Care Cancer. 25:2049–2054. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuruta S, Goto Y, Miyake H, Nagai H,
Yoshioka Y, Yuasa N and Takamizawa J: Late complications associated
with totally implantable venous access port implantation via the
internal jugular vein. Support Care Cancer. 28:2761–2768. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin WY, Lin CP, Hsu CH, Lee YH, Lin YT,
Hsu MC and Shao YY: Right or left? Side selection for a totally
implantable vascular access device: A randomised observational
study. Br J Cancer. 26:932–937. 2017. View Article : Google Scholar
|
|
30
|
Tian L, Li W, Su Y, Gao H, Yang Q, Lin P,
Wang L, Zeng J and Li Y: Risk factors for central venous access
device-related thrombosis in hospitalized children: A systematic
review and meta-analysis. Thromb Haemost. 121:625–640. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Intagliata E, Basile F and Vecchio R:
Totally implantable catheter migration and its percutaneous
retrieval: Case report and review of the literature. G Chir.
37:n211–n215. 2017.PubMed/NCBI
|
|
32
|
Chen Y, Tsang YS, Chou X, Hu J and Xia Q:
A lung cancer patient with deep vein thrombosis: A case report and
literature review. BMC Cancer. 19:2852019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 world health organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Foorthuis R: On the nature and types of
anomalies: A review of deviations in data. Int J Data Sci Anal.
12:297–331. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arnaout R, Curran L, Zhao Y, Levine JC,
Chinn E and Moon-Grady AJ: An ensemble of neural networks provides
expert-level prenatal detection of complex congenital heart
disease. Nat Med. 27:882–891. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim B, Kwon K, Oh C and Park H:
Unsupervised anomaly detection in MR images using multicontrast
information. Med Phys. 48:7346–7359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karadayi Y, Aydin MN and Ogrenci AS:
Unsupervised anomaly detection in multivariate spatio-temporal data
using deep learning: Early detection of COVID-19 outbreak in Italy.
IEEE Access. 8:164155–164177. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo Y, Jiang X, Tao L, Meng L, Dai C, Long
X, Wan F, Zhang Y, van Dijk J, Aarts RM, et al: Epileptic seizure
detection by cascading isolation forest-based anomaly screening and
easyensemble. IEEE Trans Neural Syst Rehabil Eng. 30:915–924. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin CH, Hsu KC, Johnson KR, Luby M and
Fann YC: Applying density-based outlier identifications using
multiple datasets for validation of stroke clinical outcomes. Int J
Med Inform. 132:1039882019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mei S and Zhu H: A novel one-class SVM
based negative data sampling method for reconstructing
proteome-wide HTLV-human protein interaction networks. Sci Rep.
5:80342015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schölkopf B, Platt JC, Shawe-Taylor J,
Smola AJ and Williamson RC: Estimating the support of a
high-dimensional distribution. Neural Comput. 13:1443–1471. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wildgruber M, Lueg C, Borgmeyer S, Karimov
I, Braun U, Kiechle M, Meier R, Koehler M, Ettl J and Berger H:
Polyurethane versus silicone catheters for central venous port
devices implanted at the forearm. Eur J Cancer. 59:113–124. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu M, Deng L, Zhu Y, Li Y, Wang F, Li H
and Zhou Y: Risk factors of catheter-related infection in unplanned
extubation of totally implantable venous-accessportsin tumor
patients. Emerg Med Int. 2022:42353162022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Narducci F, Jean-Laurent M, Boulanger L,
El Bedoui S, Mallet Y, Houpeau JL, Hamdani A, Penel N and Fournier
C: Totally implantable venous access port systems and risk factors
for complications: A one-year prospective study in a cancer centre.
Eur J Surg Oncol. 37:913–918. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Y, Li LL, Xu L, Feng DD, Cao Y, Mao
XY, Zheng J, Jin F and Chen B: Comparison between arm port and
chest port for optimal vascular access port in patients with breast
cancer: A systematic review and meta-analysis. Biomed Res Int.
2020:90829242020.PubMed/NCBI
|
|
47
|
Khorana AA, Kuderer NM, Culakova E, Lyman
GH and Francis CW: Development and validation of a predictive model
for chemotherapy-associated thrombosis. Blood. 111:4902–4907. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wabont G, Bergeron S, Gautier S and Barus
R: Sex differences in serious adverse drug reactions in patients
receiving immunotherapy, targeted therapy, or chemotherapy: A
disproportionality analysis of the VigiBase(R). Eur J Clin
Pharmacol. 78:1355–1356. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Decousus H, Bourmaud A, Fournel P,
Bertoletti L, Labruyere C, Presles E, Merah A, Laporte S, Stefani
L, Piano FD, et al: Cancer-associated thrombosis in patients with
implanted ports: A prospective multicenter French cohort study
(ONCOCIP). Blood. 132:707–716. 2018. View Article : Google Scholar : PubMed/NCBI
|