Introduction
Cervical cancer (CC) is the fourth most widespread
malignancy and the fourth leading cause of cancer-related death
among women worldwide (1). Despite
several advances in the screening, prevention and treatment of
cancer, more than half a million women are diagnosed with CC every
year, resulting in >300,000 deaths globally (2,3).
Cervical squamous cell cancer (CSCC) is the most common
pathological type of CC, accounting for ~70% of CC cases. Most CSCC
cases are diagnosed in an advanced stage, with a high incidence of
metastasis, resulting in unfavorable outcomes (4). Therefore, a number of studies have
been conducted to discover biological indicators related to the
progression and prognosis of CC to provide new potential
therapeutic targets in recent years (5,6).
Antiangiogenic therapy has emerged as a therapeutic target in CC,
but identification of the mechanisms affecting neovascularization
in CC are still needed.
Angiogenesis, which refers to the formation of
vessels from a preexisting vascular network, is the most common
form of tumor blood vessels and plays an essential role in tumor
growth and metastasis (7). Vascular
endothelial growth factor (VEGF)-A is a critical pro-angiogenic
factor that modulates angiogenesis by binding and interacting with
VEGF receptors (VEGFRs). Upregulation of VEGF-A in tumor tissues is
closely related to the occurrence, development and progression of
solid tumors (8). Micro-vessel
density (MVD) has become the morphological gold standard to assess
the neo-vasculature in human tumors and is significantly associated
with metastasis and prognosis in several tumor types, such as renal
cell carcinoma and ovarian cancer (9,10).
Studies have demonstrated that increased VEGF expression and MVD
are significantly correlated with poor prognosis in CC (11,12).
However, the mechanisms affecting VEGF expression and MVD have not
been well elucidated.
Reactive oxygen species (ROS), including hydrogen
peroxide (H2O2), are well-known as harmful
substances of normal cellular metabolism by inducing intracellular
oxidative stress. Antioxidant enzymes play a crucial role in
regulating intracellular redox homeostasis, protecting cells from
oxidative stress by reducing the intracellular accumulation of ROS.
Notably, the peroxiredoxins (Prxs) are a ubiquitous family of
antioxidant enzymes highly involved in various physiological
functions, including cell growth, differentiation and apoptosis
(13). It has been indicated that
Prxs are engaged in either inhibiting or promoting cancer,
depending on the cancer type and the Prx isoform (14). Prx2 is a typical Prx and plays a
dual role as both a tumor suppressor and promoter. Certain reports
have indicated that the expression of Prx2 protein is increased in
the development of CC (15,16). However, to the best of our
knowledge, no study has evaluated the potential of Prx2 in the
prognosis of CSCC.
Kang et al (17) showed that Prx2 protects VEGFR-2
against H2O2-mediated oxidative inactivation
in vascular endothelial cells. The absence of Prx2 increased
cellular H2O2 levels and VEGFR-2 was inactive
in response to VEGF stimulation. It was then further demonstrated
that Prx2 deficiency suppressed tumor angiogenesis in vivo.
These results indicated that Prx2 is an essential antioxidant
enzyme that preserves VEGF signaling by protecting VEGFR2 against
oxidative inactivation, thus promoting tumor angiogenesis. However,
to the best of our knowledge, whether Prx2 expression is associated
with angiogenesis in CC is currently unknown.
Hence, the present study aimed to investigate Prx2
expression in relation to the progression and prognosis of CSCC and
its association with angiogenesis. Prx2 expression was detected by
immunohistochemistry (IHC) staining. Then, the association of Prx2
expression with clinicopathological features, VEGF-A expression and
MVD of CSCC was analyzed. Furthermore, Kaplan-Meier and Cox
proportional hazard regression analyses were performed to explore
the prognostic factors influencing patient survival.
Materials and methods
Patients
The records of 105 patients with CSCC treated at the
Jingzhou Central Hospital, Tongji Medical College of Huazhong
University of Science and Technology (Jingzhou, China) between
January, 2015 and August, 2020 were retrospectively reviewed. The
inclusion criteria included: i) Patients with a first diagnosis of
cervical squamous cell carcinoma; and ii) patients that did not
receive chemotherapy, radiotherapy, immunotherapy or hormonal
therapy before surgery. The exclusion criteria included: i)
Patients with recurrent CC; ii) patients that received
chemotherapy, radiotherapy, immunotherapy, or hormonal therapy
before surgery; and iii) patients with CSCC combined with other
diseases, such as malignant tumors, systemic immune diseases,
infectious diseases, organ failure diseases and cancer
complications. A total of 40 adjacent peri-tumoral tissues were
selected and included as the control group.
Follow-up
The follow-up duration was defined as the time from
the diagnosis of CSCC until disease progression or death or the
cut-off date of December, 2022. The progression-free survival (PFS)
time was the interval from the surgery date to the first
documentation of disease progression or death. The median follow-up
time of the patients was 34.5 months (range, 4.3–90 months).
Patients lost to follow-up were excluded from the present
study.
IHC
Neutral formaldehyde solution (4%) fixed (at room
temperature for 12–24 h) and paraffin-embedded CSCC tumor tissue
and adjacent non-neoplastic tissue blocks were cut into 4-µm-thick
sections, dried, deparaffinized and dehydrated in a graded ethanol
series. The antigen was retrieved by a high-pressure method using
alkaline pH (pH 8.0) for 1 min, and then washed by phosphate
buffered saline (PBS) 3 times. Then, the tissue sections were
treated with 1% hydrogen peroxide for 10 min to block endogenous
tissue peroxidase activity and non-specific protein binding. The
slides were incubated with rabbit polyclonal anti-human Prx2
antibody (Proteintech Group, Inc.; cat. no. 10545-2-AP; 1:500),
rabbit polyclonal anti-human VEGF-A antibody (ImmunoWay
Biotechnology Company; cat. no. YT5108; 1:100) and mouse monoclonal
anti-human CD34 antibody (Dako; Agilent Technologies, Inc.; clone
no. QBEnd 10; cat. no. IR632; ready-to-use) overnight at 4°C,
followed by incubation at room temperature for 30 min with the
Ultra-Sensitive S-P Kit (containing the secondary antibodies;
Fuzhou Maixin Biotech Co., Ltd.; cat. no. KIT-9710). The slides
were washed with PBS before color development using a
3,3′-diaminobenzidine substrate kit for 3–10 min and were then
counterstained with hematoxylin at room temperature for 1–2 min,
before visualization with a light microscope.
Evaluation of IHC
The immunoreactivity of Prx2 and VEGF-A was examined
by two senior pathologists blinded to the clinicopathological data.
The staining was evaluated semi-quantitatively based on the
staining intensity and percentage of positive cells. The intensity
of the stained cells was graded into four levels as follows: 0 (no
staining), 1 (weak staining: light yellow), 2 (moderate staining:
yellow-brown) and 3 (intense staining: brown). The percentage of
positive cells was graded into five levels as follows: 0 (≤5% of
cells), 1 (6–25% of cells), 2 (26–50% of cells), 3 (51–75% of
cells) and 4 (>75% of cells). The immunoreaction score of each
marker was calculated by multiplying the intensity and percentage
of positive cells. Scores ≤3 were defined as low expression and
scores >3 were described as high expression.
MVD was evaluated by detecting CD34+
cells, including the single endothelial cell or endothelial cell
cluster separated from the adjacent micro-vessels or other
connective tissue elements. The entire section was initially
scanned at a low magnification (×40-100) to identify the highest
density of CD34+ cells within the tumor samples, and
necrotic and ulcerated areas were avoided. Within these areas,
micro-vessels were manually counted in a ×200 magnified field in
five of the most vascularized regions, and the average value was
taken as the MVD count for each sample.
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM Corp.). Continuous variables are presented as the
mean ± standard deviation, and non-normally distributed variables
are presented as the median (P25-P75). The differences in Prx2
expression between cervical cancerous and normal tissues were
analyzed by Unpaired Student's t-test. The association between Prx2
expression with the clinicopathological features, VEGF-A expression
and the MVD of CSCC were compared by χ2 test. The
Kaplan-Meier method and the log-rank test was used to analyze
patient survival, and the Cox proportional hazard regression model
was used to identify the prognostic factors that influenced patient
survival. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients
The median age of the patients at surgery was 50
years (range, 33–67 years), and the tumor diameter was >4 cm in
72 cases and ≤4 cm in 33 cases. The histological grade was
well-differentiated (G1) in 30 cases, moderately differentiated
(G2) in 49 cases and lowly differentiated (G3) in 26 cases [as
determined using the 2020 World Health Organization Classification
of Female Genital Tumors (18)].
The depth of stromal invasion was superficial 1/3 in 38 cases,
middle 1/3 in 55 cases and deep 1/3 in 12 cases. There were 79
cases without LN metastasis and 26 cases with LN metastasis. The
International Federation of Gynecology and Obstetrics (FIGO) stage
was stage I in 62 cases, stage II in 17 cases and stage III in 26
cases, according to 2018 FIGO cervical cancer staging (19). A total of 55 cases exhibited lymph
vascular space invasion (LVSI) and 50 cases were without LVSI.
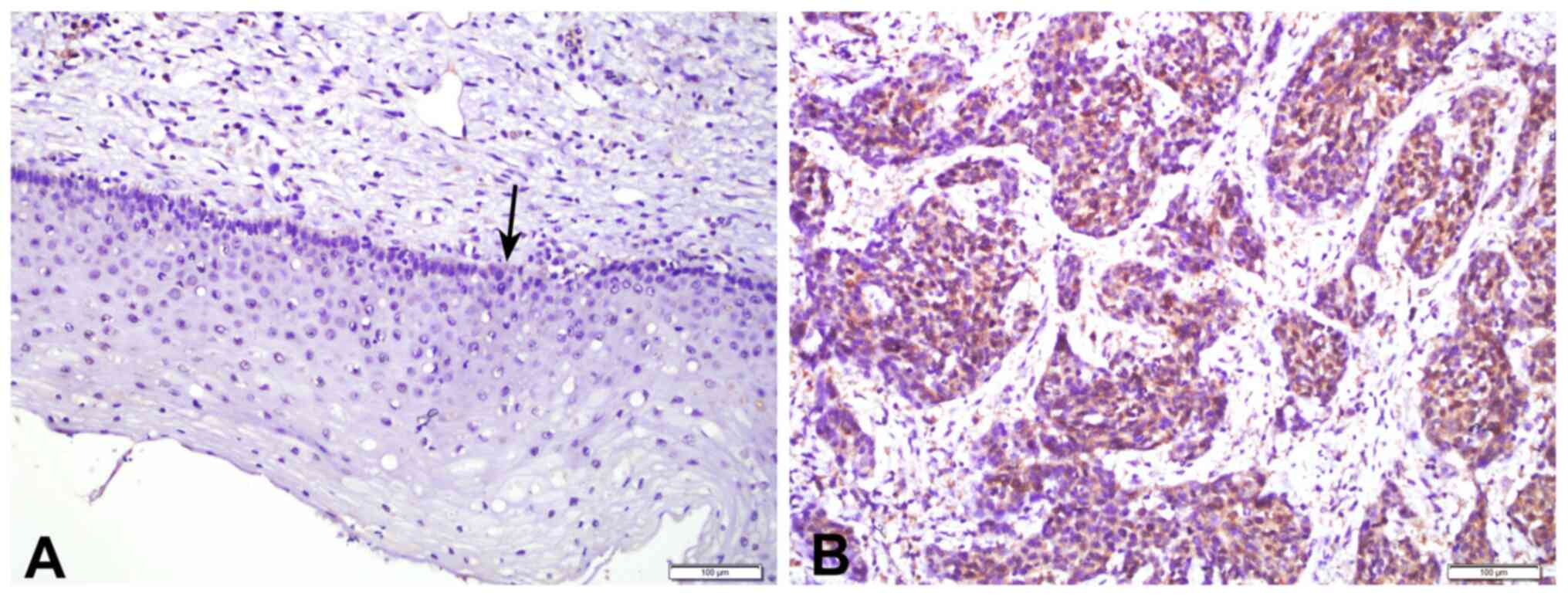
Expression of Prx2 in CSCC and normal
cervical squamous tissues
The expression levels of Prx2 in 105 CSCC tissues
and 40 adjacent peri-tumoral tissues were analyzed by IHC. Positive
staining of the Prx2 protein was mainly observed in the cytoplasm
of both CSCC tumor cells and the basal layer cells of normal
tissues (Fig. 1). The expression of
Prx2 in CSCC tissues was significantly higher than that in adjacent
peri-tumoral tissues (P<0.001; Table I).
 | Table I.Expression of Prx2 in cervical cancer
tissues and adjacent normal tissues. |
Table I.
Expression of Prx2 in cervical cancer
tissues and adjacent normal tissues.
| Tissue type | No. | Prx2 expression,
median (P25-P75) | P-value |
|---|
| Adjacent normal
tissue | 40 | 2.00 (2.00–3.00) |
<0.001a |
| Cervical cancer
tissue | 105 | 8.00 (4.00–9.00) |
|
Association of Prx2 expression with
clinicopathological features and angiogenesis in CSCC
Based on Prx2 immunoreactivity, 69.5% (73/105) of
CSCC tissue samples exhibited high Prx2 expression and 30.5%
(32/105) exhibited low Prx2 expression. The association of Prx2
expression with the patient clinicopathological features of CSCC is
shown in Table II. It was found
that high Prx2 expression was associated with a higher depth of
stromal invasion (P=0.023) and occurrence of LVSI (P=0.044). By
contrast, Prx2 expression was not associated with age, tumor size,
histological grade, LN metastasis or FIGO stage (all
P>0.05).
 | Table II.Association of Prx2 expression with
clinicopathological features, VEGF-A expression and MVD in cervical
squamous cell cancer. |
Table II.
Association of Prx2 expression with
clinicopathological features, VEGF-A expression and MVD in cervical
squamous cell cancer.
|
|
| Prx2 expression |
|
|---|
|
|
|
|
|
|---|
| Feature | No. of patients
(n=105) | Low (n=32) | High (n=73) | P-value |
|---|
| Age, n |
|
|
|
|
| <50
years | 50 | 12 | 38 | 0.171 |
| ≥50
years | 55 | 20 | 35 |
|
| Mean tumor size, cm ±
SD | - | 4.138±1.148 | 4.056±1.262 | 0.756 |
| Histological grade,
n |
|
|
|
|
| G1 | 30 | 14 | 16 | 0.566 |
| G2 | 49 | 7 | 42 |
|
| G3 | 26 | 11 | 15 |
|
| Depth of stromal
invasion, n |
|
|
|
|
|
Superficial 1/3 | 38 | 16 | 22 | 0.022a |
| Middle
1/3 | 55 | 15 | 40 |
|
| Deep
1/3 | 12 | 1 | 11 |
|
| LN metastasis,
n |
|
|
|
|
| No | 79 | 24 | 55 | 0.970 |
|
Yes | 26 | 8 | 18 |
|
| FIGO stage, n |
|
|
|
|
| I | 62 | 15 | 47 | 0.076 |
| II | 17 | 9 | 8 |
|
|
III | 26 | 8 | 18 |
|
| LVSI, n |
|
|
|
|
| No | 50 | 20 | 30 | 0.043a |
|
Yes | 55 | 12 | 43 |
|
| VEGF-A expression,
n |
|
|
|
|
|
Low | 38 | 15 | 23 | 0.133 |
|
High | 67 | 17 | 50 |
|
| MVD, n |
|
|
|
|
|
Low | 32 | 15 | 17 | 0.015a |
|
High | 73 | 17 | 56 |
|
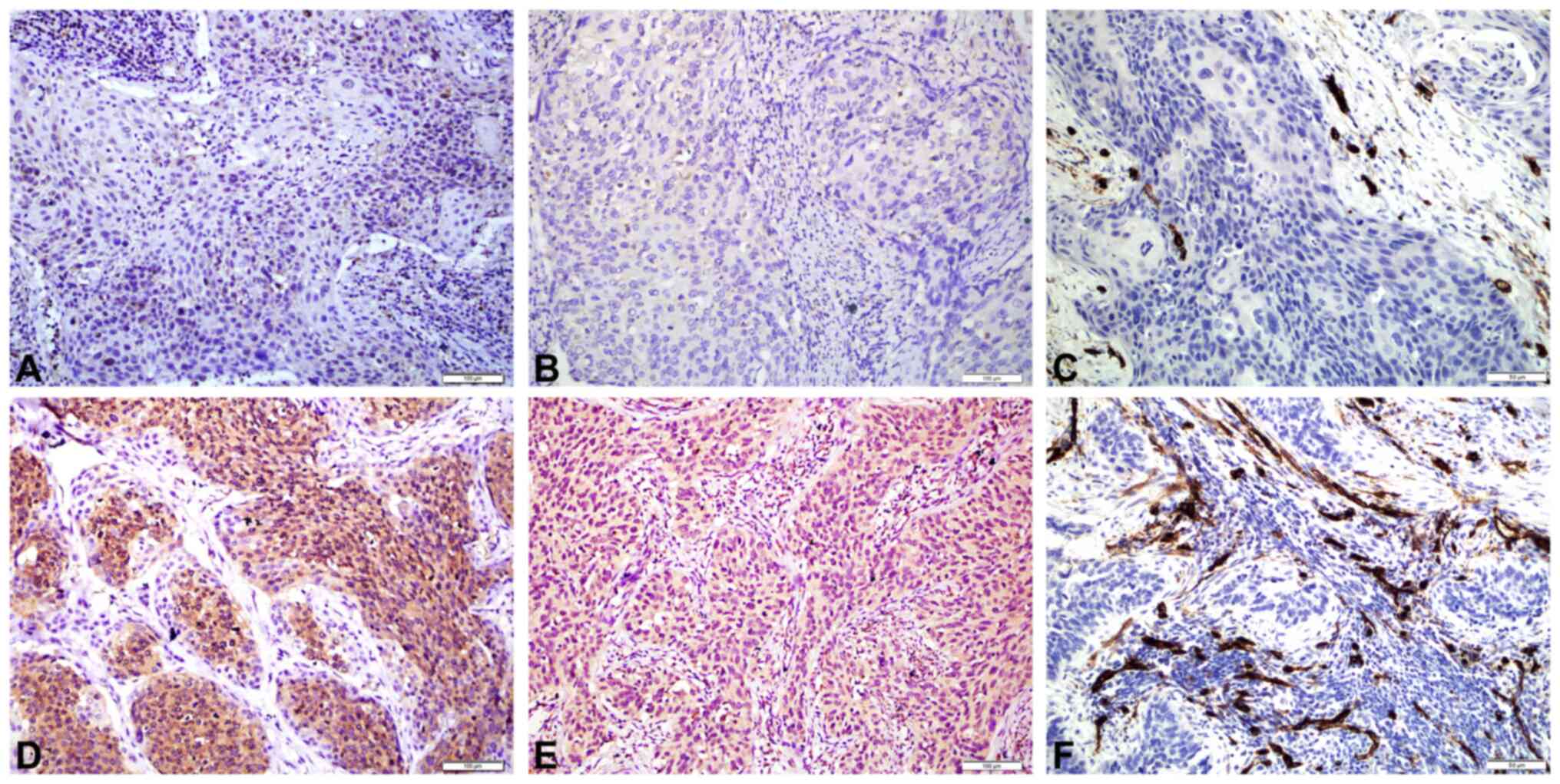
Expression of Prx2 (Fig.
2A and D) and VEGF-A (Fig. 2B and
E), and MVD (Fig 2C and F) were
analyzed in the 105 CSCC tissue samples. Positive staining of
VEGF-A was mainly found in the cytoplasm of tumor cells. Increased
Prx2 expression was associated with high MVD (P=0.016), while
VEGF-A expression was not associated with Prx2 expression
(P>0.05) (Table II).
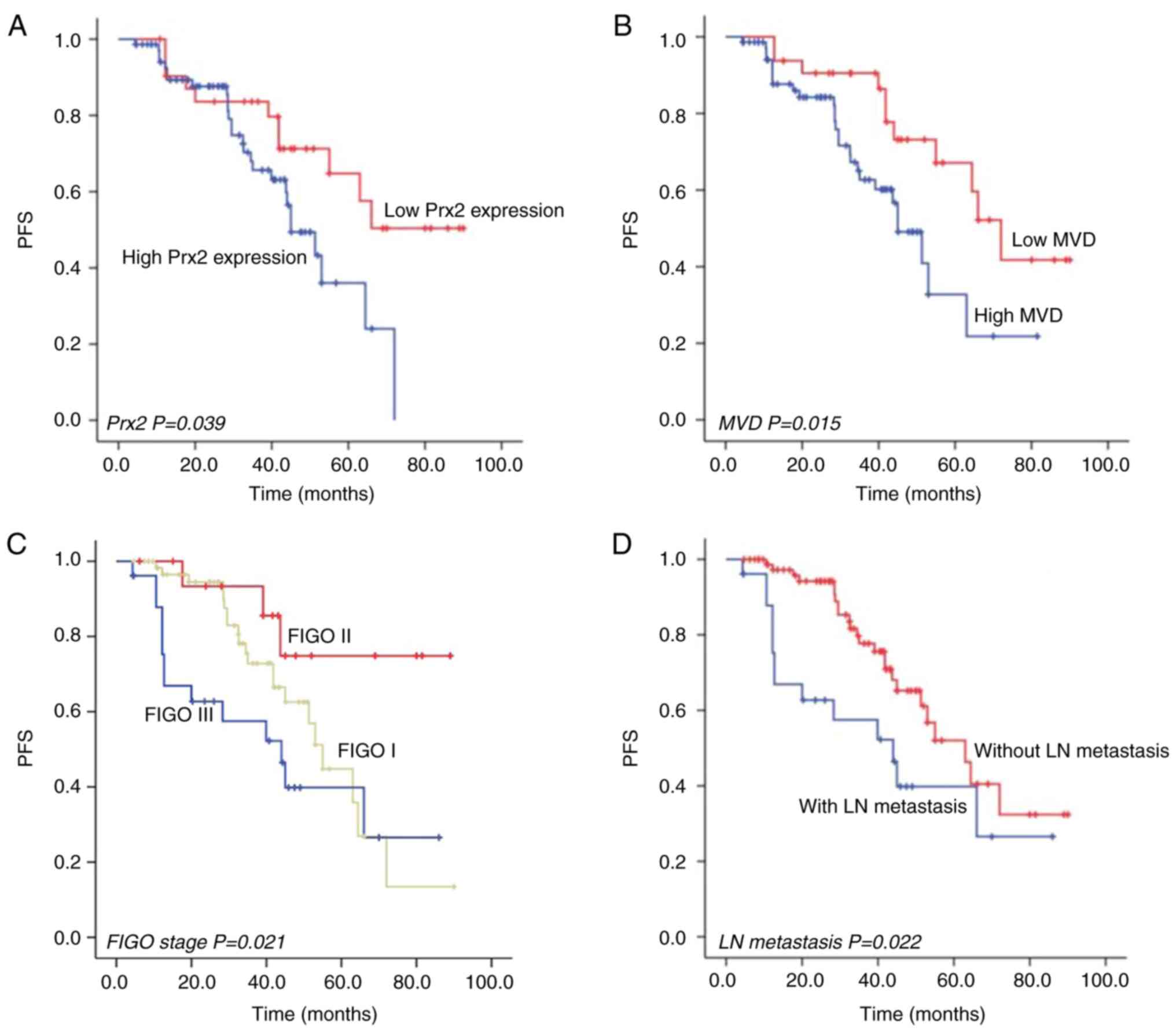
Survival analysis
The median follow-up time of the selected patients
was 34.5 months (range, 4.3–90 months). Kaplan-Meier plus log-rank
and Cox proportional hazard regression model analyses were used to
evaluate the risk factors of PFS of patients with CSCC.
Kaplan-Meier analysis showed that patients with high Prx2
expression (log-rank test, P=0.039; Fig. 3A), high MVD (log-rank test, P=0.015;
Fig. 3B), a higher FIGO stage
(log-rank test, P=0.021; Fig. 3C)
and LN metastasis (log-rank test, P=0.022; Fig. 3D) had a shorter PFS time than
patients with low Prx2 expression, low MVD, a lower FIGO stage and
without LN metastasis, respectively. Cox proportional hazard
regression analysis revealed that expression of Prx2 [hazard ratio
(HR), 2.551; 95% confidence interval (CI), 1.056–6.162; P=0.037],
MVD (HR, 2.436; CI, 1.034–5.735; P=0.042) and FIGO stage (HR,
1.543; CI, 1.027–2.319; P=0.037) were identified as independent
factors for PFS (Table III).
 | Table III.Univariate and multivariate analysis
of predictive factors for progression-free survival in patients
with cervical squamous cell cancer. |
Table III.
Univariate and multivariate analysis
of predictive factors for progression-free survival in patients
with cervical squamous cell cancer.
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factor | No. of patients
(n=105) | Univariate analysis
P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
<50 | 50 | 0.108 | 0.785
(0.353–1.744) | 0.552 |
|
≥50 | 55 |
|
|
|
| Tumor size, cm |
|
|
|
|
| ≤4 | 33 | 0.091 |
|
|
|
>4 | 72 |
|
|
|
| Histological
grade |
|
|
|
|
| G1 | 30 | 0.414 | 1.202
(0.700–2.062) | 0.504 |
| G2 | 49 |
|
|
|
| G3 | 26 |
|
|
|
| Depth of stromal
invasion |
|
|
|
|
|
Superficial 1/3 | 38 | 0.368 | 1.029
(0.577–1.833) | 0.924 |
| Middle
1/3 | 55 |
|
|
|
| Deep
1/3 | 12 |
|
|
|
| LVSI |
|
|
|
|
| No | 50 | 0.799 | 0.856
(0.408–1.797) | 0.681 |
|
Yes | 55 |
|
|
|
| LN metastasis |
|
|
|
|
| No | 79 | 0.022a |
|
|
|
Yes | 26 |
|
|
|
| FIGO stage |
|
|
|
|
| I | 62 | 0.021a | 1.543
(1.027–2.319) | 0.037b |
| II | 17 |
|
|
|
|
III | 26 |
|
|
|
| Prx2
expression |
|
|
|
|
|
Low | 32 | 0.039a | 2.551
(1.056–6.162) | 0.037b |
|
High | 73 |
|
|
|
| VEGF-A
expression |
|
|
|
|
|
Low | 38 | 0.789 | 0.765
(0.384–1.526) | 0.448 |
|
High | 67 |
|
|
|
| MVD |
|
|
|
|
|
Low | 32 | 0.015a | 2.436
(1.034–5.735) | 0.042b |
|
High | 73 |
|
|
|
Discussion
The Prxs are a class of antioxidant enzymes known to
either facilitate or inhibit tumorigenesis depending on the cancer
type and Prx isoform (13,14). Thus far, six Prx isoforms have been
revealed in mammals and are categorized according to the number and
position of the conserved Cys residue and the type of disulfide
bond formed during the catalytic cycle. These include typical 2-Cys
Prx (Prx 1–4), atypical 2-Cys Prx (Prx 5) and 1-Cys Prx (Prx 6)
(13). Numerous studies have
demonstrated that these Prx isoforms are closely related to cancer
development, and the expression of the Prx family member is altered
in different types of cancer (20,21).
Prx2 is a typical Prx and plays a dual role in
tumorigenesis, depending on the tumor type. Decreasing expression
of Prx2 resulted in metastasis of melanoma cells to the lungs or
other organs, suggesting a tumor suppressor role of Prx2 in
melanoma (22). Conversely, in
other studies, Prx2 was shown to be increased in various types of
human cancer, including gastric and colorectal cancer (23,24).
This suggested that Prx2 may be a tumor promoter and could be a
potential target for treatment in these tumor types. Kim et
al (15) evaluated the
expression patterns of the Prx family and found that the Prx2
protein was upregulated in the development of CC. In another study,
Zhu et al (16) revealed
that the Prx2 protein was also frequently upregulated in CSCC. In
parallel with these studies, the Prx2 protein was also found to be
upregulated in CSCC in the present study. The results of the
present study further demonstrated that upregulation of Prx2 was
associated with a greater depth of stromal invasion and LVSI of
CSCC, indicating that Prx2 might play a role in the progression and
invasion of CSCC. However, the mechanisms through which Prx2
participates in the progression and invasion of CSCC and its
correlation with the prognosis of CSCC require further
investigation.
VEGF-A, typically referred to as VEGF, is the
primary mediator of tumor angiogenesis in the VEGF family in the
vast majority of solid tumors. The receptors, VEGFR-1 and VEGFR-2,
bind VEGF-A in vascular endothelial cells. Among these two
receptors, VEGFR-2 is predominant in stimulating angiogenic
signaling pathways by reacting with ROS (25,26).
Kang et al (17) explored
the endogenous antioxidant enzymes that modulate VEGFR-2 function
and found that Prx2 was a specific antioxidant enzyme protecting
VEGFR-2 against H2O2-mediated oxidative
inactivation in vascular endothelial cells. The absence of Prx2
increased cellular H2O2 levels and VEGFR-2
became inactive in response to VEGF stimulation. It was further
demonstrated that Prx2 deficiency suppressed tumor angiogenesis
in vivo. Zhang et al (27) also demonstrated that Prx2 is
involved in vasculogenic mimicry formation by targeting VEGFR2
activation in colorectal cancer. These results indicated that Prx2
is an essential antioxidant enzyme that preserves VEGF signaling by
protecting VEGFR2 against oxidative inactivation, thus promoting
tumor angiogenesis. However, whether Prx2 expression is correlated
with angiogenesis in CSCC remains unknown.
MVD is the morphological gold standard to assess the
neo-vasculature in human tumors and is significantly associated
with metastasis and prognosis in several tumor types (9,10).
CD34 is a sensitive and commonly used vascular endothelial marker,
which is more resistant to formalin fixation and stains deeper in
neoplastic endothelium than normal endothelium (28). In the present study, the Prx2
expression level was compared with VEGF-A expression and MVD, and
the results showed that increased Prx2 expression was associated
with high MVD, but not with VEGF-A expression. These results
indicated that increased Prx2 expression may regulate tumor
angiogenesis in CSCC, but not regulate VEGF-A expression.
Risk factors that affect the prognosis of patients
with CSCC were further analyzed in the present study. Kaplan-Meier
analysis revealed that patients with high Prx2 expression, high
MVD, a higher FIGO stage and LN metastasis had a shorter PFS time
compared with patients with low Prx2 expression, low MVD, a lower
FIGO stage and without LN metastasis, respectively. Furthermore,
the Cox proportional hazard regression analysis demonstrated that
Prx2 expression, MVD and LN metastasis were independent factors for
PFS among the aforementioned variables. However, the mechanisms by
which Prx2 influences tumor angiogenesis in CSCC require further
investigation. In addition, due to the limited number of patients
included in the present study, a more extensive study is needed,
particularly one that includes a prolonged follow-up time to
analyze overall survival.
In conclusion, the present study revealed that Prx2
was upregulated in CSCC. Increased expression of Prx2 was
associated with the depth of stromal invasion, LVSI and MVD in
CSCC, which may be related to poor prognosis. Therefore, Prx2 may
be a potential biomarker for predicting CSCC prognosis and a novel
target for antiangiogenic therapy in CSCC; however, further studies
are required to elucidate the mechanisms of Prx2.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KZ and MH contributed to the concept and design of
the study and were major contributors to writing the manuscript. RY
contributed to data acquisition, analysis and interpretation. TZ,
JL and MH provided technical support and performed the histological
examination. All authors read and approved the final version of the
manuscript. KZ and MH confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by The Medical Ethics
Committee of Jingzhou Central Hospital, Tongji Medical College of
Huazhong University of Science and Technology (Jingzhou, China;
approval no. 2020123001). Written informed consent was obtained
from each patient regarding the use of tissues for ex vivo
experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN and Gaffney DK: Cervical cancer: A global health
crisis. Cancer. 123:2404–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim AW, Ramirez AJ, Hamilton W, Sasieni P,
Patnick J and Forbes LJ: Delays in diagnosis of young females with
symptomatic cervical cancer in England: An interview-based study.
Br J Gen Pract. 64:e602–e610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tewari KS, Sill MW, Long HJ III, Penson
RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao
MM, et al: Improved survival with bevacizumab in advanced cervical
cancer. N Engl J Med. 370:734–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minion LE and Tewari KS: Cervical
cancer-state of the science: From angiogenesis blockade to
checkpoint inhibition. Gynecol Oncol. 148:609–621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viallard C and Larrivée B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Majidpoor J and Mortezaee K: Angiogenesis
as a hallmark of solid tumors-clinical perspectives. Cell Oncol
(Dordr). 44:715–737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iakovlev VV, Gabril M, Dubinski W,
Scorilas A, Youssef YM, Faragalla H, Kovacs K, Rotondo F, Metias S,
Arsanious A, et al: Microvascular density as an independent
predictor of clinical outcome in renal cell carcinoma: An automated
image analysis study. Lab Invest. 92:46–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bais C, Mueller B, Brady MF, Mannel RS,
Burger RA, Wei W, Marien KM, Kockx MM, Husain A and Birrer MJ; NRG
Oncology/Gynecologic Oncology Group, : Tumor microvessel density as
a potential predictive marker for bevacizumab benefit: GOG-0218
biomarker analyses. J Natl Cancer Inst. 109:djx0662017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan Y, Min SJ, Xu DQ, Shen Y, Yan HY,
Wang Y, Wang W and Tan YJ: Expressions of VEGF and miR-21 in tumor
tissues of cervical cancer patients with HPV infection and their
relationships with prognosis. Eur Rev Med Pharmacol Sci.
22:6274–6279. 2018.PubMed/NCBI
|
|
12
|
Hu X, Liu H, Ye M and Zhu X: Prognostic
value of microvessel density in cervical cancer. Cancer Cell Int.
18:1522018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nicolussi A, D'Inzeo S, Capalbo C,
Giannini G and Coppa A: The role of peroxiredoxins in cancer. Mol
Clin Oncol. 6:139–153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim Y and Jang HH: The role of
peroxiredoxin family in cancer signaling. J Cancer Prev. 24:65–71.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim K, Yu M, Han S, Oh I, Choi YJ, Kim S,
Yoon K, Jung M and Choe W: Expression of human peroxiredoxin
isoforms in response to cervical carcinogenesis. Oncol Rep.
21:1391–1396. 2009.PubMed/NCBI
|
|
16
|
Zhu H, Tao X, Zhou L, Sheng B and Zhu X
and Zhu X: Expression of thioredoxin 1 and peroxiredoxins in
squamous cervical carcinoma and its predictive role in NACT. BMC
Cancer. 19:8652019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang DH, Lee DJ, Lee KW, Park YS, Lee JY,
Lee SH, Koh YJ, Koh GY, Choi C, Yu DY, et al: Peroxiredoxin II is
an essential antioxidant enzyme that prevents the oxidative
inactivation of VEGF receptor-2 in vascular endothelial cells. Mol
Cell. 44:545–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
International Agency for Research on
Cancer (IARC), . WHO classification of tumours editorial board: WHO
classification of female genital tumour. 5th edition. IARC; Lyon:
2020
|
|
19
|
Salvo G, Odetto D, Pareja R, Frumovitz M
and Ramirez PT: Revised 2018 international federation of gynecology
and obstetrics (FIGO) cervical cancer staging: A review of gaps and
questions that remain. Int J Gynecol Cancer. 30:873–878. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Hu X, Ye M and Zhu X: The prognostic
values of the peroxiredoxins family in ovarian cancer. Biosci Rep.
38:BSR201806672018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Wang P, Hu W and Chen D: New
insights into the roles of peroxiredoxins in cancer. Biomed
Pharmacother. 164:1148962023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee DJ, Kang DH, Choi M, Choi YJ, Lee JY,
Park JH, Park YJ, Lee KW and Kang SW: Peroxiredoxin-2 represses
melanoma metastasis by increasing E-Cadherin/β-Catenin complexes in
adherens junctions. Cancer Res. 73:4744–4757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Zhao Y, Luo W, Chen S, Lin F,
Zhang X, Fan S, Shen X, Wang Y and Liang G: Celastrol induces
ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in
gastric cancer cells. Theranostics. 10:10290–10308. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi J, Zhou L, Huang HS, Peng L, Xie N,
Nice E, Fu L, Jiang C and Huang C: Repurposing oxiconazole against
colorectal cancer via PRDX2-mediated autophagy arrest. Int J Biol
Sci. 18:3747–3761. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung AS, Lee J and Ferrara N: Ferrara.
targeting the tumour vasculature: Insights from physiological
angiogenesis. Nat Rev Cancer. 10:505–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abid MR, Spokes KC, Shih SC and Aird WC:
NADPH oxidase activity selectively modulates vascular endothelial
growth factor signaling pathways. J Biol Chem. 282:35373–35385.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang S, Fu Z, Wei J, Guo J, Liu M and Du
K: Peroxiredoxin 2 is involved in vasculogenic mimicry formation by
targeting VEGFR2 activation in colorectal cancer. Med Oncol.
32:4142015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vieira SC, Silva BB, Pinto GA, Vassallo J,
Moraes NG, Santana JO, Santos LG, Carvasan GA and Zeferino LC: CD34
as a marker for evaluating angiogenesis in cervical cancer. Pathol
Res Pract. 201:313–318. 2005. View Article : Google Scholar : PubMed/NCBI
|