Introduction
Molecularly targeted drugs and immune checkpoint
inhibitors (ICIs) are being introduced more frequently in the field
of gynecologic oncology. Although ICIs have only recently been
developed, they have been proven effective in cancer treatment. The
anti-programmed death 1 (PD-1) monoclonal antibody pembrolizumab,
combined with platinum-based chemotherapy, is especially useful for
managing persistent, recurrent, or metastatic cervical cancers
(1). Immune-related adverse effects
(irAEs) commonly occur with ICI use and generally affect the skin,
gastrointestinal tract, liver, lungs, thyroid gland, and adrenal
cortex (2). Many irAEs are
reversible and often improve with the discontinuation of ICIs.
These rarely progress to fatal conditions and often respond to
treatment. However, risk factors for the development of irAEs are
increasingly apparent, and gynecologists lack the clinical
experience of treating irAEs (3,4).
Cytokine release syndrome (CRS) is a serious irAE that develops
after ICI treatment. CRS is an inflammatory syndrome caused by an
excessive immune response that results in cytokine overproduction.
Although ICI-induced CRS is rare, the condition can be severe,
rapidly exacerbated, and life-threatening (4). CRS may develop with chimeric antigen
receptor (CAR)-modified T cells (CAR-Ts) for hematological
malignancy; however, tocilizumab (TOC), an anti-IL-6 receptor
antibody, is effective in such cases (5). In contrast, ICI-induced CRS develops
less frequently, and an effective treatment has not yet been
established.
Here, we report a case of CRS after ICI treatment
for recurrent adenocarcinoma of the uterine cervix. Treatment for
ICI-induced CRS had not yet been established; therefore, pulse
steroid therapy was initiated. TOC was administered because the CRS
was resistant to treatment, and it saved the patient's life.
Case report
A 49-year-old Japanese woman with a suspected
uterine cervical adenocarcinoma was admitted to the hospital of the
University of Occupational and Environmental Health (Kitakyushu,
Japan) in May 2021. The patient was diagnosed with International
Federation of Gynecology and Obstetrics (FIGO) stage IIB (cT2bN0M0)
uterine cervical cancer and received concurrent chemoradiotherapy
with weekly cisplatin. Eight months after the initial treatment,
pelvic recurrence was evident. One year after the initial
treatment, abdominal distention, pain, and bilateral hydronephrosis
were detected. Paclitaxel, carboplatin, and pembrolizumab were
administered every 3 weeks after ureteral stenting. After the first
dose, the patient presented with a fever of 37°C and Grade 2
neutropenia. No inflammatory response or systemic symptoms were
observed on the first day of the second treatment cycle, which was
performed as planned. On day 21 of the second treatment cycle, the
patient's serum creatinine level was elevated, and pollakiuria
ensued; therefore, the third cycle was postponed, and ureteral
restenting was performed. On day 23 of the second treatment cycle,
the patient presented to our hospital with a fever of 38°C and
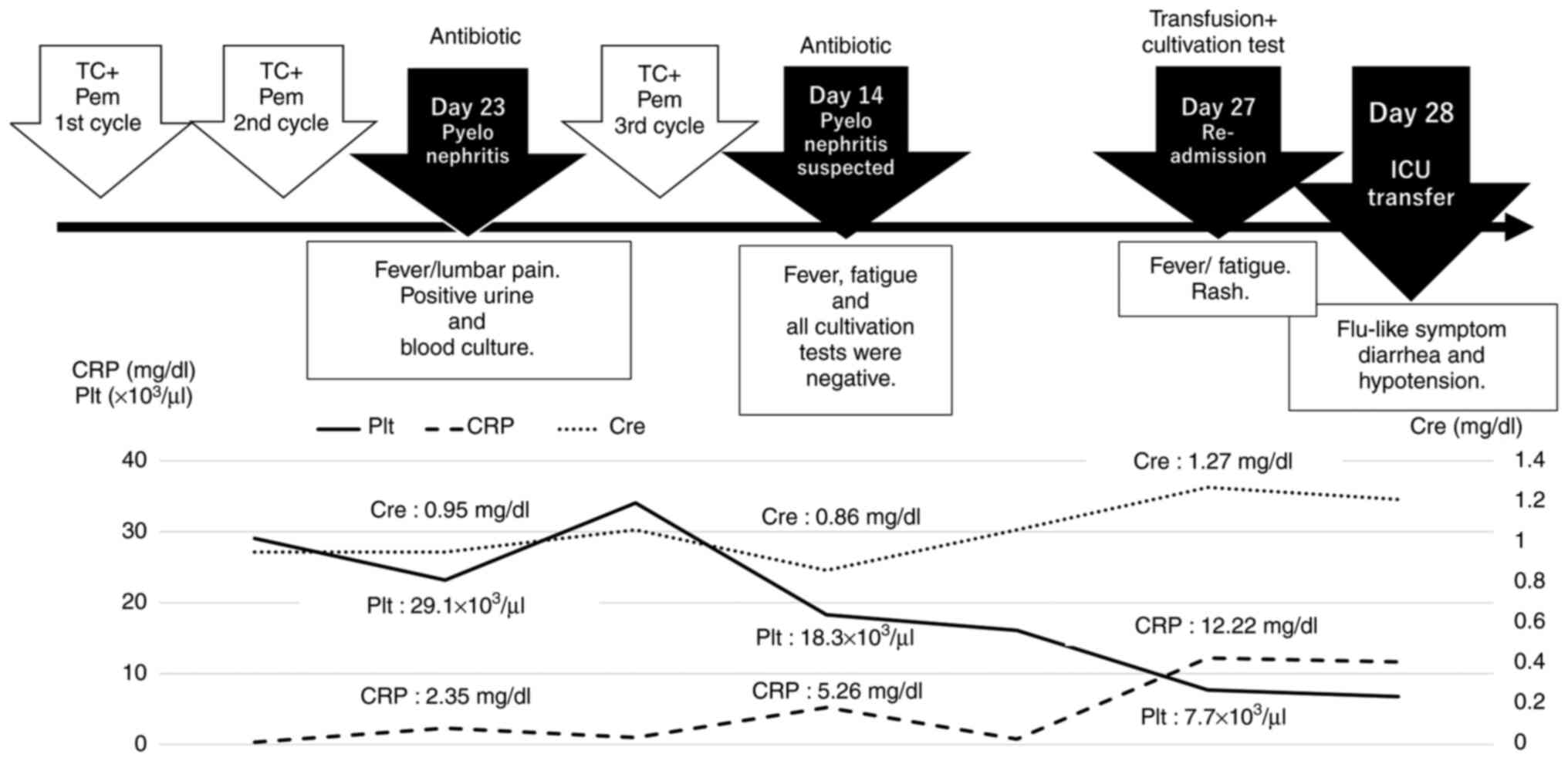
right lumbar pain (Fig. 1).
Laboratory data (L/D) revealed a white blood cell (WBC) count of
7,400/µg, neutrophilic sequestration of 68.2%, procalcitonin (PCT)
level of 0.05 ng/dl, and C-reactive protein (CRP) content of 2.35
mg/dl; an enhanced computed tomography (CT) scan showed no obvious
focus of infection. Bacteriuria was evident, indicating possible
pyelonephritis; therefore, intravenous antibiotics (cefmetazole)
were administered every 12 h. Several days after hospitalization,
the patient experienced intermittent nighttime fever, which
eventually disappeared. Blood and urine cultivation tests revealed
methicillin-sensitive Staphylococcus aureus, and bacteremia
was diagnosed in association with a uterine tract infection;
therefore, the antibiotic was changed to cefazolin. After discharge
on day 8, the fever disappeared and the blood test results
improved. The third chemotherapy cycle was administered 40 days
after the initial treatment. On day 14 of the third cycle, the
patient presented with a fever of 39°C and fatigue. L/D revealed a
WBC count of 3,700/µg, neutrophilic sequestration of 55.9%, CRP of
5.26 mg/dl, and bacteriuria. The latter result suggested a relapse
of pyelonephritis, and intravenous antibiotics (tazobactam and
piperacillin, TAZ/PIPC) were administered empirically every 8 h.
All cultivation tests (urine and blood cultures, including both
aerobic and anaerobic cultures) performed at the time of admission
to the hospital were negative. The patient's fever and general
condition improved within one week, at which time she was
discharged from the hospital. On day 27 of the third cycle, the
patient was readmitted with a fever of 40°C and fatigue. In
addition, erythema of the extremities and face, and influenza-like
symptoms, including headache, myalgia, arthralgia, and upper
respiratory tract symptoms, were observed. L/D showed a WBC count
of 3,300/µg, CRP of 12.22 mg/dl, Grade 3 liver dysfunction
associated with aspartate aminotransferase, and Grade 1
thrombocytopenia and renal dysfunction associated with an elevated
serum creatinine level. The patient was admitted for fever
evaluation and received a course of intravenous antibiotics
(TAZ/PIPC) after cultivation tests were performed.
One day after hospitalization, the patient suddenly
developed hypotension after a bout of diarrhea, and LD showed a PCT
level of 13.00 ng/dl. Disseminated intravascular coagulation (DIC)
secondary to septic shock was suspected, based on the elevated CRP
level and the presence of thrombocytopenia, and the patient was
admitted to the intensive care unit for circulation dynamics
assessment (Table I). Steroids
(prednisolone 0.5 mg/kg/day) and catecholamines were administered
for shock recovery. All cultivation tests yielded negative results,
and a CT scan showed only a portion of bowel edema. Therefore,
bacterial infection was discounted. L/D and echocardiography
revealed MOF, including liver dysfunction, heart failure [left
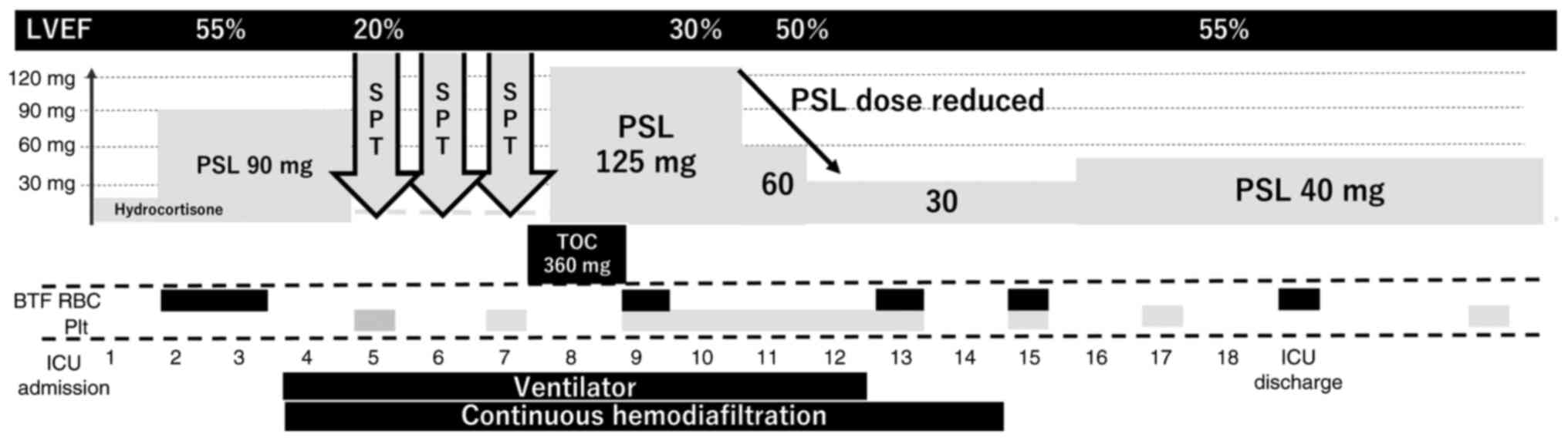
ventricular ejection fraction (LVEF) decreased to 20%], and kidney
dysfunction following rhabdomyolysis (Fig. 2). Systemic symptoms associated with
irAEs were initially suspected, and the steroid dose was increased
to 2 mg/kg/day (prednisolone). Chest radiography revealed a
decrease in the permeability of the lower lung and cardiac
enlargement, with no elevation in muscle or brain troponin or
creatine kinase levels. High-flow nasal cannula oxygen therapy was
initiated with a fraction of inspired oxygen (FiO2)
concentration of 100% and a flow rate of 50 l/min; however,
hypercarbonemia was exacerbated, and intratracheal intubation was
required. Renal dysfunction resulting from rhabdomyolysis caused
renal failure and progression of acidosis; therefore, continuous
hemodiafiltration (CHDF) was initiated. Considering that high-dose
steroid therapy had little effect and -multiorgan failure (MOF)
persisted, close examination for irAEs was continued. Causes of
thrombocytopenia other than DIC were considered, and pancytopenia
was determined to be the principal clinical condition associated
with comorbid anemia. Eventually, the patient was diagnosed with
hemophagocytic syndrome associated with CRS, based on elevated
serum ferritin and soluble interleukin-2 receptor levels. Steroid
pulse therapy was administered for 3 of the 5 days in the ICU;
however, the effect was negligible. L/D showed elevated amylase and
lipase levels and a CT scan revealed acute pancreatitis. CRS
continued to affect other organs; therefore, the anti-IL-6-receptor
mAb, tocilizumab, was administered on the 8th day in the ICU. The
steroid pulse therapy was discontinued, and the steroid dose was
reduced to 3.5 mg/kg/day of prednisolone. Two days after TOC
administration, L/D showed improvements in the levels of liver
enzymes, creatinine kinase, and lipase. Blood pressure measurements
improved and catecholamines were discontinued on the same day. The
chest radiography results and oxygenation levels improved. The
patient was extubated on day 4, and CHDF was discontinued after 6
days. The steroid dose was tapered from 40 to 20 mg of prednisolone
by 10 mg each week to prevent irAE relapse. Thereafter, L/D showed
improvement of MOF, and the patient was transferred from the ICU on
the 19th day after admission. The steroid was changed to
methylprednisolone for oral administration, and the dose was
reduced by 2.5–5 mg/day every 2 weeks. The patient was discharged
on the 31st day after admission.
 | Table I.Laboratory test results at intensive
care unit admission. |
Table I.
Laboratory test results at intensive
care unit admission.
| Parameter | Value |
|---|
| Biochemistry |
|
| Total
bilirubin | 0.4 mg/dl |
|
Albumin | 1.9 g/dl |
| Aspartate
aminotransferase | 438 U/l |
| Alanine
aminotransferase | 77 U/l |
| Lactate
dehydrogenase | 2,731 U/l |
|
Amylase | 151 U/l |
|
Creatinine kinase | 2,846 U/l |
| Blood
urea nitrogen | 28 mg/dl |
|
Creatinine | 1.52 mg/dl |
|
Sodium | 135 mmol/l |
|
Chlorine | 102 mmol/l |
|
Potassium | 3.1 mmol/l |
|
C-reactive protein | 13.19 mg/dl |
|
Procalcitonin | 13.00 ng/ml |
| Blood cell count |
|
| White
blood cell | 4,300/µl |
|
Hemoglobin | 7.0 g/dl |
|
Platelet |
4.7×103/µl |
| Coagulation |
|
| Prothrombin
time-international normalized ratio | 1.33 |
| Activated
partial thromboplastin time | 52.4 sec (control:
27.5 sec) |
Discussion
The anti-programmed death 1 (PD-1) monoclonal
antibody pembrolizumab combined with platinum-based chemotherapy is
effective for persistent, recurrent, or metastatic cervical cancer
(1), and pembrolizumab use for the
treatment of cervical cancer is widespread in Japan. The use of
ICIs can result in irAEs and concurrent systemic immune reactions.
Risk factors for the development of irAEs are becoming increasingly
apparent; however, the establishment of CRS in these cases is rare
(3,4). In the present study, the patient was
diagnosed with CRS, rhabdomyolysis, pancreatitis, enteritis, and
erythema multiforme, which were identified through examination and
imaging tests. In addition, hemophagocytic syndrome and myocarditis
were suspected; however, these findings could not be verified
without bone marrow puncture or myocardial biopsy (6,7). CRS,
which has been recognized as an irAE, is mainly caused by rapid
immune activation induced by CAR-Ts (5). ICI-induced CRS, particularly that
involving PD-1 or PDL-1 inhibitors, is rare, and to our knowledge,
PD-1 inhibitor-induced CRS in relation to uterine cervical cancer
has not yet been reported. CRS is mainly driven by the T
cell-derived interferon-gamma (IFN-γ), which stimulates macrophages
to produce proinflammatory substances such as interleukin 6 (IL-6)
and tumor necrosis factor-alpha (TNF-α) (8). The initial clinical aspects of CRS
include fever of 38°C or higher and influenza-like symptoms
(headache, chills, and myalgia), after which CRS can progress to
life-threatening hypovolemic shock, hypoxia, and end-organ
dysfunction (5). A fever ≥38°C is
the main symptom of CRS, and hypotension and respiratory failure
requiring ventilator support indicate an increased severity of the
condition (5,9). In its initial phase, CRS has
characteristics similar to those of severe infections, including
hypovolemia and disseminated intravascular coagulation (10). In this case, CRS was not identified
at the time of admission; the patient was diagnosed with septic
shock resulting from pyelonephritis, and antimicrobial therapy was
initiated. IFN-γ level should be used as a biomarker to
differentiate CRS from infections because IFN-γ is not generally
elevated in sepsis cases (4). An
accurate testing approach for differentiating between these
conditions is therefore required.
In this case, the patient presented with high fever
and fatigue on day 14 after PD-1 inhibitor administration, and
influenza-like symptoms (headache, sore throat, and myalgia) on day
28. Subsequently, acute heart failure occurred owing to low cardiac
output, respiratory failure that required ventilator support, renal
failure, and hypotension. Clinical symptoms matched those of Grade
4 CRS (9). The symptoms improved
with steroid pulse therapy (prednisolone equivalent to 1,000 mg)
and TOC (anti-IL-6 antibody) administration, and relapse was not
observed once the steroids were tapered off. Relapses occur in
greater than 40% of initial CRS cases (4); therefore, careful observation should
be maintained after the initial signs of CRS improvement. In
contrast, ICI re-administration after Grade 1 or 2 CRS with steroid
therapy does not trigger relapse (4). There is no clear consensus regarding
ICI rechallenge after CRS. Owing to the lack of evidence for safe
rechallenge after Grade 4 CRS, the patient did not receive ICI
readministration. Although steroid administration for irAEs is
effective, the tumor-suppressive effect of steroid administration
is controversial. The development of irAEs after ICI administration
may prolong progression-free and overall survival (11); however, the need for and dosage of
steroids during CRS therapy can affect tumor-suppressive outcomes
(12). Excessive anti-inflammatory
therapy for irAEs may lead to tumor growth and recurrence.
Therefore, to prevent the relapse of irAEs or CRS, it might be
safer to use anti-inflammatory therapy only during the relapse
period as opposed to continuously.
Serum ferritin and soluble interleukin-2 receptor
(sIL-2R) levels were measured as indicators of hemophagocytic
lymphohistiocytosis (HLH), and thrombocytopenia suggestive of HLH
or macrophage activation syndrome (MAS) was noted. HLH associated
with rheumatic disease is termed MAS, whereas that associated with
other triggers, including malignancy and infection, is called
secondary HLH (sHLH) (13). In
addition, CRS and HLH can overlap, and HLH can be considered a
symptom of CRS after ICI administration (12,14).
The diagnostic criteria for HLH include elevated serum ferritin and
sIL-2R levels (7). In addition,
ferritinemia of ≥10,000 µg/l has a sensitivity of 90% and a
specificity of 96% for MAS, and hyper-ferritinemia may indicate CRS
(4). If CRS is suspected in
clinical situations, the measurement of ferritin and IL-2R levels
may help in the diagnosis.
Interleukin-6 (IL-6) has been suggested as an
important mediator of CRS; however, whether IL-6 levels predict CRS
severity remains unclear (4,9,15).
In addition, IL-6 cannot be measured in real time in a clinical
setting or during treatment; therefore, its relevance in clinical
diagnosis and therapeutic use is low (9). IL-6 analyses were performed three
times: 2 days before TOC administration, on each administration
day, and 3 days after administration. The respective values were
45.3, 48.1, and 6,380 pg/ml, and each level increased immediately
after TOC administration. It is unclear whether severity in an
individual patient can be predicted based on serum cytokine levels;
therefore, analysis of cytokine levels may not be sufficient to
evaluate therapeutic effects in clinical settings (4).
IL-6 overexpression induced by functional genetic
variants in the IL-6 gene, radiation therapy, and vaccines
[e.g., the messenger ribonucleic acid (mRNA) coronavirus 2019
vaccine] trigger CRS development in patients receiving ICIs;
however, the mechanism of the related CRS pathogenesis has not been
elucidated (4,16). CRS is a consequence of an immune
response that may cause adverse events in regions other than the
tumors and target organs. Therefore, ICI re-administration after
irAEs was considered after evaluating the risk of recurrence in
each organ, and ICI re-administration after Grade 4 irAEs
(hypotension requiring vasopressors or hypoxemia requiring
ventilation with high-flow oxygen) should be permanently
discontinued (17).
We encountered a case of CRS induced by ICI
administration for recurrent uterine cervical cancer in which TOC
was used to resuscitate the patient. The systemic manifestations of
CRS varied, and it was difficult to distinguish CRS from infection
or sepsis. TOC has been reported to be effective in the treatment
of severe CRS, which was corroborated by the present case.
Considering that ICI-induced CRS is a rare but severe complication,
fever and other systemic conditions following ICI administration
should be monitored. We hope this case may help gynecologist to
understand ICI-induced CRS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MS and YK collected the clinical data, wrote the
manuscript and confirmed the authenticity of all the raw data. AT
and MM acquired and interpreted the clinical data. SHa, YS, SHi,
MH, RT, KH, HH, TU, TK, YM and KY contributed to acquisition and
interpretation of data and revised the original manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This case report was approved by the Ethics
Committee of Medical Research of the University of Occupational and
Environmental Health (Fukuoka, Japan; approval no. H30-160).
Patient consent for publication
Written informed consent for publication of the
clinical data was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Colombo N, Dubot C, Lorusso D, Caceres MV,
Hasegawa K, Shapira-Frommer R, Tewari KS, Salman P, Hoyos Usta E,
Yañez E, et al: Pembrolizumab for persistent, recurrent, or
metastatic cervical cancer. N Engl J Med. 385:1856–1867. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tay SH, Toh MMX, Thian YL, Vellayappan BA,
Fairhurst AM, Chan YH, Aminkeng F, Bharwani LD, Huang Y, Mak A and
Wong ASC: Cytokine release syndrome in cancer patients receiving
immune checkpoint inhibitors: A case series of 25 patients and
review of the literature. Front Immunol. 13:8070502022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chennamadhavuni A, Abushahin L, Jin N,
Presley CJ and Manne A: Risk factors and biomarkers for
immune-related adverse events: A practical guide to identifying
high-risk patients and rechallenging immune checkpoint inhibitors.
Front Immunol. 13:7796912022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palaskas N, Lopez-Mattei J, Durand JB,
Iliescu C and Deswal A: Immune checkpoint inhibitor myocarditis:
Pathophysiological characteristics, diagnosis, and treatment. J Am
Heart Assoc. 9:e0137572020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rajapakse P and Andanamala H:
Hemophagocytic lymphohistiocytosis secondary to immune checkpoint
inhibitor therapy. World J Oncol. 13:49–52. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frey N and Porter D: Cytokine release
syndrome with chimeric antigen receptor T cell therapy. Biol Blood
Marrow Transplant. 25:e123–e127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu LL, Skribek M, Harmenberg U and
Gerling M: Systemic inflammatory syndromes as life-threatening side
effects of immune checkpoint inhibitors: Case report and systematic
review of the literature. J Immunother Cancer. 11:e0058412023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimabukuro-Vornhagen A, Gödel P, Subklewe
M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B and von
Bergwelt-Baildon MS: Cytokine release syndrome. J Immunother
Cancer. 6:562018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furubayashi N, Minato A, Negishi T,
Sakamoto N, Song Y, Hori Y, Tomoda T, Harada M, Tamura S, Miura A,
et al: Association between immune-related adverse events and
efficacy and changes in the relative eosinophil count among
patients with advanced urothelial carcinoma treated by
pembrolizumab. Cancer Manag Res. 14:1641–1651. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arbour KC, Mezquita L, Long N, Rizvi H,
Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL,
et al: Impact of baseline steroids on efficacy of programmed cell
Death-1 and programmed death-ligand 1 blockade in patients with
non-small-cell lung cancer. J Clin Oncol. 36:2872–2878. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carter SJ, Tattersall RS and Ramanan AV:
Macrophage activation syndrome in adults: Recent advances in
pathophysiology, diagnosis and treatment. Rheumatol (Oxf Engl).
58:5–17. 2019. View Article : Google Scholar
|
|
14
|
Panelli MC, White R, Foster M, Martin B,
Wang E, Smith K and Marincola FM: Forecasting the cytokine storm
following systemic interleukin (IL)-2 administration. J Transl Med.
2:172004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doessegger L and Banholzer ML: Clinical
development methodology for infusion-related reactions with
monoclonal antibodies. Clin Transl Immunology. 4:e392015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ceschi A, Noseda R, Palin K and Verhamme
K: Immune checkpoint inhibitor-related cytokine release syndrome:
Analysis of WHO global pharmacovigilance database. Front Pharmacol.
11:5572020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schneider BJ, Naidoo J, Santomasso BD,
Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino
JM, Chau I, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: ASCO
guideline update. J Clin Oncol. 39:4073–4126. 2021. View Article : Google Scholar : PubMed/NCBI
|