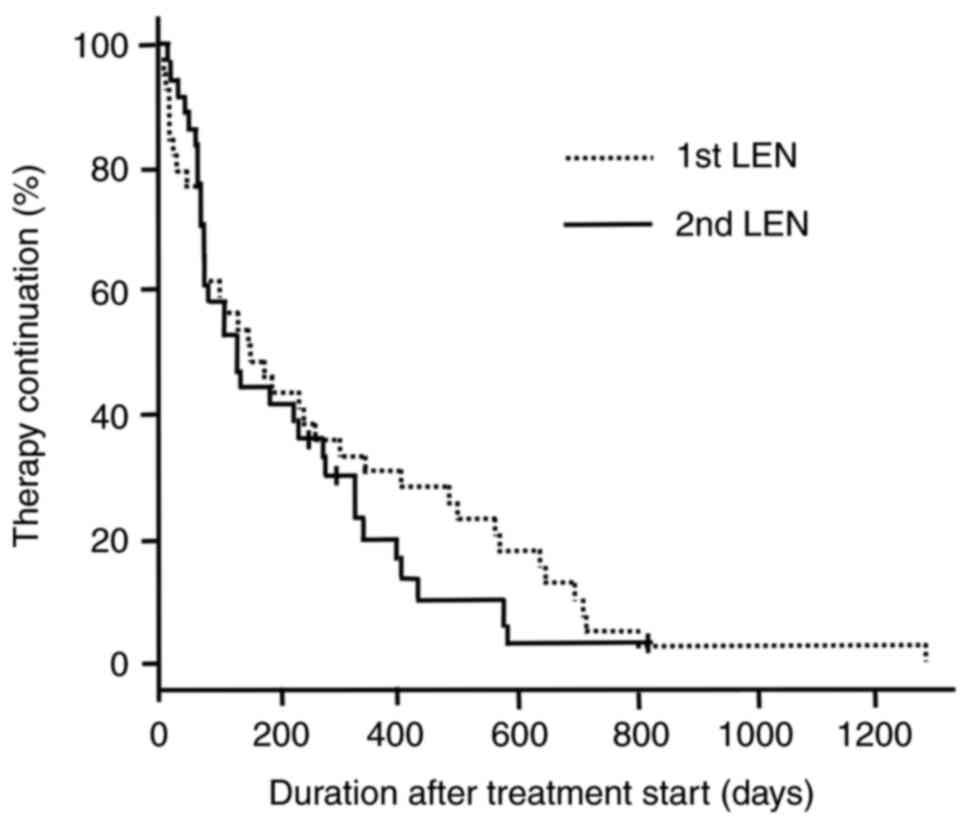
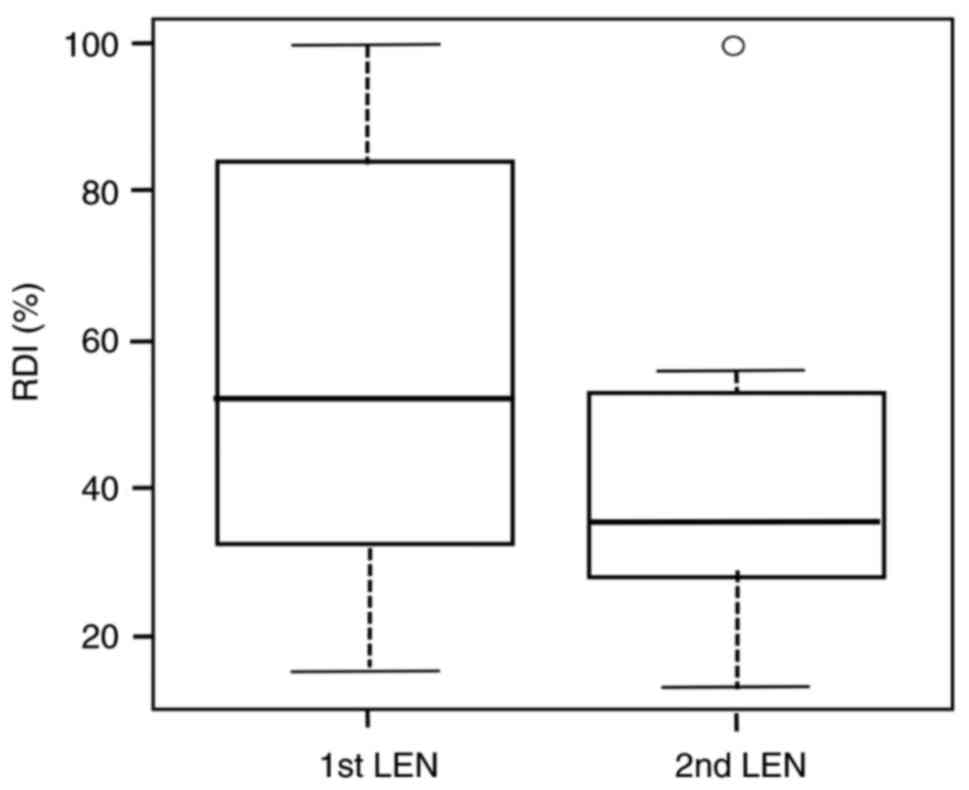
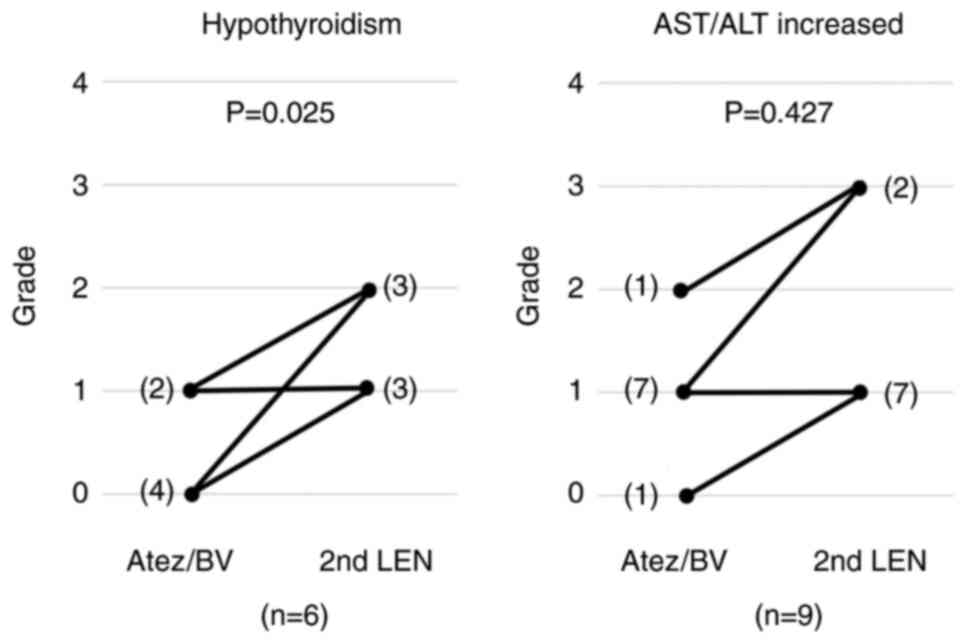
|
1
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 14:1894–1905. 2020. View Article : Google Scholar
|
|
2
|
Sonbol MB, Riaz IB, Naqvi SAA, Almquist
DR, Mina S, Almasri J, Shah S, Almader-Douglas D, Uson Junior PLS,
Mahipal A, et al: Systemic therapy and sequencing options in
advanced hepatocellular carcinoma: A systematic review and network
meta-analysis. JAMA Oncol. 6:e2049302020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeda S, Namisaki T, Tsuji Y, Fujimoto Y,
Murata K, Enomoto M, Fujinaga Y, Nishimura N, Kitagawa K, Takaya H,
et al: Initial experience with atezolizumab plus bevacizumab for
unresectable hepatocellular carcinoma: A real-world retrospective
study. Anticancer Res. 42:5465–5473. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobayashi K, Nagai H, Matsui T, Matsuda T
and Higai K: Importance of Atezolizumab plus bevacizumab
combination treatment as first-line therapy for immunological
changes in patients with unresectable hepatocellular carcinoma.
Anticancer Res. 43:4601–4609. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 24:1163–1173. 2018. View Article : Google Scholar
|
|
7
|
Ogushi K, Chuma M, Uojima H, Hidaka H,
Numata K, Kobayashi S, Hirose S, Hattori N, Fujikawa T, Nakazawa T,
et al: Safety and Efficacy of lenvatinib treatment in Child-Pugh A
and B patients with unresectable hepatocellular carcinoma in
clinical practice: A multicenter analysis. Clin Exp Gastroenterol.
13:385–396. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwamoto H, Suzuki H, Shimose S, Niizeki T,
Nakano M, Shirono T, Okamura S, Noda Y, Kamachi N, Nakamura T, et
al: weekends-off lenvatinib for unresectable hepatocellular
carcinoma improves therapeutic response and tolerability toward
adverse events. Cancers (Basel). 12:10102020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoo C, Kim JH, Ryu MH, Park SR, Lee D, Kim
KM, Shim JH, Lim YS, Lee HC, Lee J, et al: Clinical outcomes with
multikinase inhibitors after progression on first-line atezolizumab
plus bevacizumab in patients with advanced hepatocellular
carcinoma: A multinational multicenter retrospective study. Liver
Cancer. 10:107–114. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aoki T, Kudo M, Ueshima K, Morita M,
Chishina H, Takita M, Hagiwara S, Ida H, Minami Y, Tsurusaki M and
Nishida N: Exploratory analysis of lenvatinib therapy in patients
with unresectable hepatocellular carcinoma who have failed prior
PD-1/PD-L1 checkpoint blockade. Cancers (Basel). 12:30482020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiraoka A, Kumada T, Tada T, Hirooka M,
Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E,
Fukunishi S, et al: Lenvatinib as second-line treatment after
atezolizumab plus bevacizumab for unresectable hepatocellular
carcinoma: Clinical results show importance of hepatic reserve
function. Oncology. 101:624–633. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Cancer Institute, . US Department
Of Health And Human Services: Common terminology criteria for
adverse events (CTCAE). Version 5.0, 2017. https://ctepCancer. Gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf
|
|
13
|
Tamai T, Hayato S, Hojo S, Suzuki T,
Okusaka T, Ikeda K and Kumada H: Dose finding of lenvatinib in
subjects with advanced hepatocellular carcinoma based on population
pharmacokinetic and exposure-response analyses. J Clin Pharmacol.
57:1138–1147. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SE, Kim KA, Lee H and Park J: Risk of
developing hypothyroidism with the use of tyrosine kinase
inhibitors and immune checkpoint inhibitors. Cancer Epidemiol.
81:1022652022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Makita N and Iiri T: Tyrosine kinase
inhibitor-induced thyroid disorders: A review and hypothesis.
Thyroid. 23:151–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmadieh H and Salti I: Tyrosine kinase
inhibitors induced thyroid dysfunction: A review of its incidence,
pathophysiology, clinical relevance, and treatment. Biomed Res Int.
2013:7254102013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muto H, Kuzuya T, Kawabe N, Ohno E,
Funasaka K, Nagasaka M, Nakagawa Y, Miyahara R, Shibata T,
Hashimoto S, et al: Clinical outcomes with lenvatinib in patients
previously treated with atezolizumab/bevacizumab for advanced
hepatocellular carcinoma. Anticancer Res. 43:4673–4682. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Otsubo K, Nakatomi K, Furukawa R, Ashida
K, Yoneshima Y, Nakanishi Y and Okamoto I: Two cases of late-onset
secondary adrenal insufficiency after discontinuation of nivolumab.
Ann Oncol. 28:3106–3107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muir CA, Clifton-Bligh RJ, Long GV,
Scolyer RA, Lo SN, Carlino MS, Tsang VHM and Menzies AM: Thyroid
immune-related adverse events following immune checkpoint inhibitor
treatment. J Clin Endocrinol Metab. 18:e3704–e3713. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ichimura T, Ichikura D, Hinata M, Hida N
and Baba T: Thyroid dysfunction with atezolizumab plus bevacizumab
after Lenvatinib in hepatocellular carcinoma: A case series. SAGE
Open Med Case Rep. 11:2050313X2311644882023.PubMed/NCBI
|