Introduction
Superficial CD34-positive fibroblastic tumor (SCPFT)
is a rare mesenchymal tumor of intermediate malignancy, with
reported cases representing a wide range of ages from pediatrics to
the elderly, although the highest incidence is among middle-aged
people. The preoperative duration typically exceeds 1 year. SCPFT
typically manifests as a long-standing, painless mass in the
subcutaneous tissue, commonly occurring in the lower limbs,
occasionally in the upper limbs and back, and rarely in the neck,
chest, axilla, abdominal wall and breast (1). SCPFT was first reported by Carter
et al (2) in 2014 and then
described as a new entity in the 5th edition of the World Health
Organization (WHO) classification of soft tissue tumors (3). Clinically, SCPFT presents as a
slow-growing, painless, circumscribed mass that mainly occurs in
adults, and it generally involves the deep dermis and subcutaneous
tissues, showing a predilection for the lower extremities. Grossly,
SCPFT sections are firm and yellow-to-tan in color.
Microscopically, SCPFTs are composed of spindled to epithelioid
cells arranged in a fascicular pattern. The tumor cells show
apparent cellular heterogeneity and prominent nucleoli with
apparent CD34 positivity (4).
Surgical resection is the optimal treatment for most
patients, resulting in a good long-term prognosis. However,
patients with positive surgical margins may experience local
recurrence, while regional lymph node metastasis is uncommon
(1,5). However, more cases are needed for
long-term prognostic data to establish reliable indicators. The
current report presents a case of a typical SCPFT with
morphological, immunophenotypic and molecular characteristics,
revealing internal morphological heterogeneity.
Case report
A 43-year-old male patient was admitted to the
Shandong Provincial Qianfoshan Hospital (Jinan, China) in October
2022, following the discovery of a tumor in the left perineum. The
lesion, initially presenting as a mildly tender mass at the thigh
roots near the scrotum 6 months prior, exhibited slow growth
without signs of irritation or inflammation. Upon physical
examination, a contained solid mass measuring 5.5×4×3 cm was
observed. Computed tomography revealed a hypoechoic enclosed mass,
measuring 5×3.5×3 cm, at the base of the thigh (Fig. 1A). There were no abnormalities
present in the hematological laboratory tests, including peripheral
blood cell analysis, coagulation index and blood infection markers.
The tumor was surgically excised under general anesthesia,
involving complete capsule removal, and the excised tumor exhibited
a white-colored, cartilage-like consistency on the cut surface
(Fig. 1B).
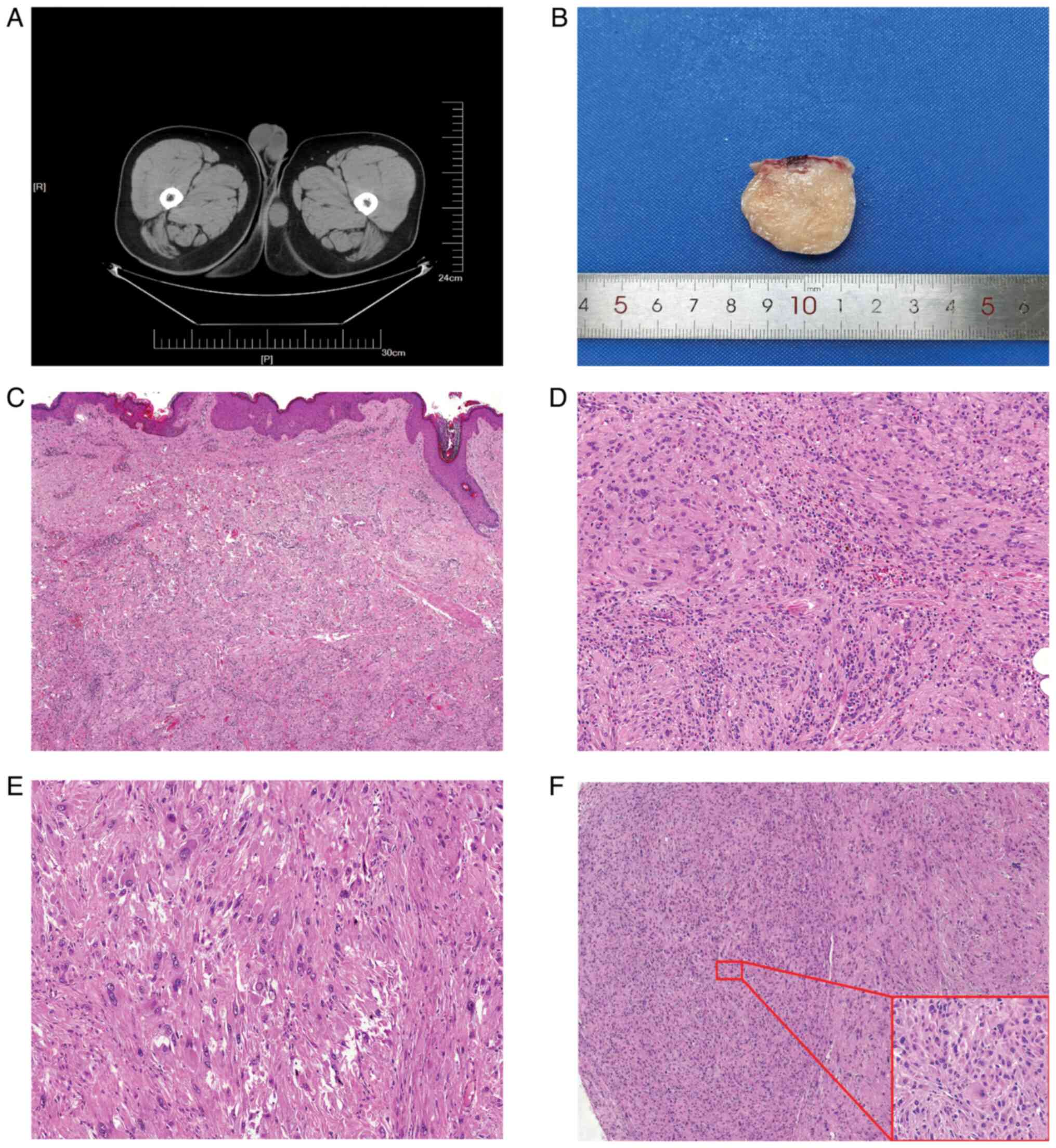 | Figure 1.(A) Gross appearance of the tumor
specimen presenting with a grayish-white color in section and a
tough texture. (B) Computed tomography image demonstrating a
hypoechoic, well-demarcated mass situated at the base of the thigh.
(C) Tumor cells situated beneath the dermis and exhibiting local
infiltration into the dermis (HE, ×100 magnification). (D) Tumor
cells arranged in bundles, accompanied by eosinophils and
lymphocytes in the background (HE, ×200 magnification). (E) Tumor
cells with distinct nucleoli, with visible intranuclear inclusion
bodies (HE, ×400 magnification). (F) Tumor margins featuring
cell-rich areas, as depicted in the inset (HE, ×400 magnification),
showing tumor cells with abundant cytoplasm, atypical nuclei and a
small size (HE, ×100 magnification). HE, hematoxylin and eosin. |
The tumor was cut into flat tissue blocks ~2×1.5 cm
in size and 0.3 cm in thickness. These tumor blocks were then fixed
in a solution containing 10% formaldehyde in 0.01 M
phosphate-buffered saline (PBS) for 2 h at room temperature.
Following fixation, the tissue blocks were loaded into the
Tissue-Tek VIP®6 AI Tissue Processor (Sakura Finetek
USA, Inc.) and subsequently embedded in paraffin. Formalin-fixed
paraffin-embedded sections were cut using a Leica RM 2155 Rotary
Microtome (Leica Microsystems). The subsequent dewaxing process
included sequential treatments with xylene, anhydrous ethanol, a
decreasing concentration gradient of ethanol (95, 90, 80 and 70%),
and water. Following this, the sections were immersed in Harris
hematoxylin staining solution for 5 min and then differentiated
with 0.3% acid alcohol, before being incubated with 0.6% ammonia.
Eosin staining solution was applied for 1–3 min, followed by
dehydration with ethanol and xylene. Finally, the samples were
mounted with neutral gum to prepare the slides. Postoperative
histopathological microscopy revealed that the lesion was confined
within the subcutaneous tissue, and at low magnification, the
dermis of the skin showed infiltration by tumor cells (Fig. 1C). The tumor displayed
sparse-to-moderate cellularity, featuring a densely cellular,
basophilic, nodular area at the periphery. Under high
magnification, the tumor primarily consisted of spindle-shaped to
epithelioid cells, arranged singly or in clusters within a
collagenous stroma. The tumor cells exhibited marked nuclear
pleomorphism with prominent nucleoli, abundant eosinophilic
cytoplasm and occasional multilobed nuclei or intranuclear exudate
inclusion bodies. Scattered lymphocytes and eosinophils were
observed in the interstitium (Fig. 1D
and E). Within the densely populated nodule, the tumor cells
displayed the same morphological characteristics but were
relatively smaller in size and more tightly arranged compared with
other regions (Fig. 1F).
Paraffin sections of 5-µm were loaded into The
Discovery ULTRA (Roche Tissue Diagnostics) for automated staining.
The automated immunohistochemistry staining process utilized
solutions and antibodies from Roche Tissue Diagnostics unless
otherwise specified. Initially, slides underwent de-paraffinization
with the EZ PREP solution (cat. no. 950-100), followed by antigen
retrieval through Heat Induced Epitope Retrieval in Tris-EDTA
buffer (cat. no. 950-124) at pH 7.8 and 95°C for 40 min.
Subsequently, primary antibodies were manually applied and
incubated at 37°C for 60 min. The targets were then linked using
the OmniMap anti-rabbit (cat. no. 760-4311) and OmniMap anti-mouse
(cat. no. 760-4310) HRP-conjugated secondary antibodies.
Visualization of the different targets was achieved through DAB and
H2O2 (cat. no. 760-159). Finally, tissue
slides were counterstained with hematoxylin and mounted using a
xylene-based mounting medium. The following ready-to-use primary
antibodies were utilized: Cytokeratin [anion exchanger (AE)1/AE3;
cat. no. Kit-0009], epithelial membrane antigen (EMA; cat. no.
Kit-0011), ERG (cat. no. RMA-0748), STAT6 (cat. no. RMA-0845), CD34
(cat. no. MAB-1076), smooth muscle actin (SMA; cat. no. MAB-0890),
Desmin (cat. no. MAB-0766), S100 protein (cat. no. MAB-0697), CD10
(cat. no. MAB-0668), CD117 (cat. no. Kit-0029), discovered on
GIST-1 (DOG1; cat. no. MAB-0851), integrase interactor 1
(INI1)/SWI/SNF-related matrix-associated actin-dependent regulator
of chromatin subfamily B member 1 (SMARCB1) (cat. no. MAB-0696),
anaplastic lymphoma kinase (ALK; cat. no. MAB-0848) and Ki-67 (cat.
no. MAB-0672). All antibodies were purchased from MaximBiotech,
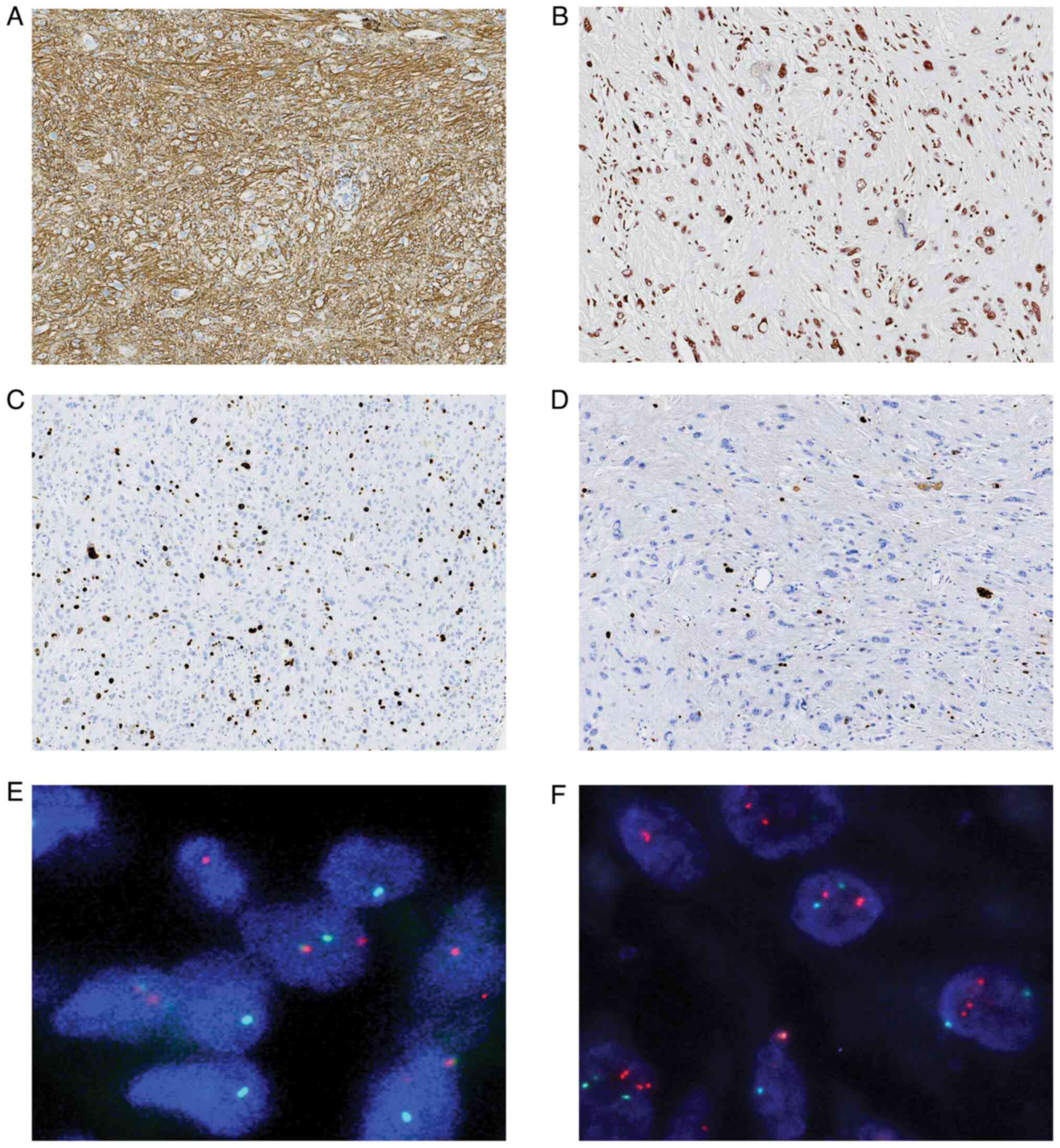
Inc. The tumor cells exhibited strong positive staining for CD34 in
a diffuse pattern (Fig. 2A).
Nuclear expression of INI1/SMARCB1 was evident in all cases
(Fig. 2B). The Ki-67 labeling index
was ~5% in most areas and ~20% in the focal cell-dense regions
(Fig. 2C and D). Furthermore, the
tumor cells displayed negative staining for AE1/AE3, CD117, STAT6,
DOG1, SMA, ALK, Desmin, CD10, ERG, EMA and S-100 expression
(Figs. S1 and S2).
For fluorescence in situ hybridization
(FISH), a commercial TGFBR3-MGEA5 Dual Fusion/Translocation FISH
probe (cat. no. F.01228-01) and PRDM10 Break Apart FISH probe (cat.
no. F.01377-01) were obtained from Guangzhou Anbiping Medical
Laboratory Co., Ltd. The PRDM10 probe utilized two custom-labeled
FISH DNA probes to label the PRDM10 gene flanks on chromosome 11.
Specifically, the BAC clone D11S1083 (366 kb), positioned
centromeric to PRDM10, was labeled with FITC dUTP, while the BAC
clone RH104271 (189 kb), located telomeric to PRDM10, was labeled
with 5(6)-TRITC dUTP. In the case of the TGFBR3-MGEA5 Dual
Fusion/Translocation FISH Probe, the BAC clone BV444963-STSG24458
(872 kb) was labeled with FITC dUTP to identify the TGFBR3 gene,
and the BAC clone SHGC153008-D10S2456 (765 kb) was labeled with
5(6)-TRITC dUTP for identifying the MGEAS gene.
Paraffin sections of 4 µm were dewaxed and hydrated.
Chromosomal DNA was denatured on the slides in a 70%
formamide-2XSSC solution at 68–70°C for 2 min. Subsequently, the
slides were dehydrated and air-dried. The hybridization mixture,
containing DNA probe at a concentration of 20–50 µg/ml, was added
to the slide and covered with a cover slip. Subsequently, the
slides were incubated in a moist plastic chamber at 37°C for 6–12
h. After incubation, the slides were washed and dried before being
immersed in blocking buffer (1X PBS, 0.1% Triton-100) for 2 min.
Following this, the slides were rinsed in PBS for 5 min at room
temperature. The semi-dried slides were then treated with 100 µl of
1:100 rabbit antibiotin antibody and incubated in a humidity
chamber at 37°C for 5 min. Subsequently, the slides were washed
with PBS and immersed in 100 µl diluted antibody (FITC-conjugated
goat anti-rabbit antibody at a ratio of 1:100 in dilution buffer).
Slides were then incubated in the humidity chamber at 37°C for
30–60 min. After incubation, the slides were washed again, and 60
µl of an antifade solution (composed of p-phenylenediamine at a
concentration of 10 mg/ml, 90% glycerol and propidium iodide at 1
µg/ml as a counterstain) was added to each slide. The slides were
observed under fluorescence microscope (BM4000B, Leica Microsystems
GmbH).
FISH analysis revealed a positive rearrangement of
the PRDM10 gene (Fig. 2E), whilst
TGFBR3/MGEA5 showed negative results (Fig. 2F). These findings aligned with a
diagnosis of SCPFT. Post-surgery, the patient exhibited a rapid
recovery. There is no clear academic consensus on the optimal
follow-up time after SCPFT. The patient will be monitored every 6
months during the first postoperative year and annually thereafter.
So far, the patient has not had recurrence or metastasis.
Discussion
SCPFT was initially identified and characterized in
2014 by Carter et al (2) in
an assessment of 18 cases. The tumors were observed in the
superficial subcutaneous layer, displaying notable pleomorphic
cells, a low mitotic index, absence of necrosis and widespread
strong positivity for CD34 in immunohistochemical staining. Carter
et al designated this distinctive entity as SCPFT. In the
2020 revision of the WHO Classification of Soft Tissue and Bone
Tumors, SCPFT was acknowledged as a novel tumor (3). SCPFT has garnered growing attention
from pathologists, resulting in a rise in the number of published
cases. Presently, ~150 cases of SCPFTs have been documented in the
English-language literature (5–15).
Clinically, SCPFT predominantly affects young adults
spanning an age range of 8–85 years, with a slight prevalence in
males (5,7). The primary sites of SCPFT occurrence
are commonly the lower limbs, particularly the thighs, along with
other locations such as the calves, groin, foot, Achilles tendon,
shoulder, vulva, neck, knee, buttocks and arms. The majority of
cases are situated in the deep dermis to the superficial subcutis.
Grossly, SCPFTs vary in size from 1.2-10 cm, typically exhibiting a
circumscribed border but occasionally infiltrating surrounding soft
tissues (16). Microscopically,
SCPFTs consist of spindled to epithelioid cells arranged in
fascicles or storiform formations. The cells exhibit prominent
eosinophilic cytoplasm, notable nuclear pleomorphism with prominent
macronucleoli and occasional pseudoinclusions. The interstitium
displays branching capillaries and scattered lymphocytes, mast
cells and foam cells. Mitotic figures are exceedingly rare
(<1/50 high-power fields). Immunohistochemically, SCPFT cells
consistently exhibit diffuse and strong positivity for CD34.
Recently, synaptic cell adhesion molecule 3 has been identified as
a sensitive marker expressed in 95% (56/59 cases) of SCPFT
(17). The Ki-67 index is generally
low in SCPFT, typically <5% (7).
In 2015, Hofvander et al identified recurrent
PRDM10 gene fusions for the first time in 3/28 cases of
undifferentiated pleomorphic sarcoma (UPS) with a morphological
resemblance to SCPFT (18). It was
hypothesized that these cases represented a subset of low-grade
UPS. Subsequent studies have assessed the relationship between
SCPFT and PRDM10-STT, highlighting their overlapping features and
leaning toward considering them as part of the same spectrum
(5,17,19).
Due to the presence of marked cellular pleomorphism and nuclear
atypia, an accurate diagnosis of SCPFT necessitates the exclusion
of superficially located and high-grade sarcomas with morphological
similarities (19).
The principal differential diagnoses include
atypical fibrous histiocytoma (AFH), pleomorphic hyalinizing
angiectatic tumor (PHAT), atypical fibroxanthoma (AFX), epithelioid
sarcoma (EpS), UPS, pleomorphic dermal sarcoma (PDS) and
myxoinflammatory fibroblastic sarcoma (MIFS). AFH typically
exhibits a classic fibrous histiocytoma background interspersed
with a population of atypical cells displaying nuclear
hyperchromasia and prominent nucleoli, often with abundant
cytoplasm. Dermal collagen at the tumor margins and pigmentation of
the basal layer aid distinguishes AFH from SCPFT. Although AFH
tumor cells may express CD34, it is usually focal and weak, and
they generally do not express cytokeratins (20,21).
PHAT shares many common features with SCPFT, including a
superficial location, marked pleomorphism of tumor cells,
inconspicuous mitotic activity, pseudo-inclusions in the nucleus
and diffuse CD34+ staining. However, PHAT has
distinctive features, such as thick-walled ectopic vessels with
marked perivascular hyalinization (22). AFX typically occurs on sun-exposed
skin in elderly patients, featuring highly pleomorphic cells with
hyperchromatic nuclei, atypical mitoses and abundant cytoplasm,
commonly arranged in a spindly architecture. Multinucleated giant
cells and solar elastosis are often present in AFX specimens. AFX
stains positive for several markers, including CD10 and p53, but is
negative or focally positive for CD34 (23). EpS shares certain immunophenotypic
similarities with SCPFT, exhibiting diffuse or patchy expression of
cytokeratin and CD34 in ~50% of cases. However, EpS is
characterized by epithelioid to spindled cells with central
pseudogranulomatous architecture in the classic type, and
predominant epithelioid and rhabdoid cells in the proximal type,
distinguishing it from SCPFT. Loss of nuclear expression of SMARCB1
is a hallmark feature of EpS, present in the vast majority of cases
(24). UPS typically occurs in
middle-aged and older patients, originating in deeper tissues and
morphologically showing marked pleomorphism, heterogeneity and
aberrant mitosis. UPS may express CD34 focally, but not diffusely,
in contrast to SCPFTs (25). PDS
may infiltrate the subcutis, potentially causing confusion with
SCPFT. PDS exhibits true pleomorphism and heterogeneity, with
common appearances of mitotic figures and necrosis, similar to AFX.
PDS expresses CD10 but not CD34 diffusely (26). Finally, MIFS typically exhibits a
multinodular growth pattern with a variable combination of three
morphologic zones: Myxoid, hyalinized and inflammatory. MIFS tumor
cells can range from plump spindle cells to histiocytoid or
epithelioid cells, featuring enlarged basophilic or eosinophilic
macronucleoli reminiscent of virallyinfected nuclei. Variable
expression of CD34 is not uncommon in MIFS and can be prominent in
certain cases. Certain subset cases may involve molecular genetic
alterations, such as MGEA5 or BRAF rearrangements (27).
SCPFT is considered to originate from
CD34+ fibroblasts, which are a subset of fibroblasts and
are assumed to be mesenchymal stem cells. A series of fibroblastic
tumors that exhibit CD34+ activity, including
leiomyosarcoma and primary fibrosarcoma, are believed to originate
from CD34+ fibroblasts (28). CD34 is a transmembrane
phosphoglycoprotein with a molecular weight of ~115 kDa, which was
first discovered on hematopoietic stem and progenitor cells
(29). CD34 has also been
identified as a marker of several types of non-hematopoietic cells,
including fibroblast progenitors and endothelial precursors
(30). The exact functions of CD34
proteins remain complex and multifaceted. Researchers have proposed
potential roles for CD34 in promoting cell proliferation and
preventing cell differentiation (31). Thus, in SCPFT, CD34 maintains a high
self-renewal capacity of fibroblasts, which means that they can
continuously undergo cell division and generate more cells. On the
other hand, CD34 is associated with endothelial progenitor cells
and angiogenesis (32).
In the present study, a panel of antibodies was used
for immunohistochemical staining to assist in the diagnosis and
differential diagnosis. Concurrently, molecular analysis indicated
the presence of a PRDM10 gene rearrangement, thereby confirming the
diagnosis of SCPFT. While numerous SCPFT cases have been
documented, it is important to note two key points in this specific
case. Firstly, SCPFT usually develops in the thigh; however, in
this instance, the tumor was located near the scrotum in the groin,
which is a less common site for SCPFT. Secondly, various regions
within the tumor exhibited distinct levels of proliferative
activity. Notably, the cell-dense nodules displayed higher
proliferative activity compared with other regions, as highlighted
by an elevated Ki-67 proliferation index. This demonstrates that
intra-tumor heterogeneity and the potential of local recurrence
exists in SCPFT, indicating that clinicians should adjust their
follow-up strategy to increase the frequency of follow-up visits
when required.
In conclusion, the present study describes a case of
SCPFT located in the groin, which was confirmed by using
immunohistochemistry and FISH. Moreover, tumor heterogeneity was
identified in this case. Despite an increasing number of reported
SCPFT cases, further accumulation of cases is essential to unravel
the intrinsic mechanisms and clinical characteristics of SCPFT.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JS was the first-visit doctor who designed the study
and the main writer of the manuscript. SH was the surgeon who
acquired and analyzed the patient's clinical data. XY is a
diagnostic pathologist who performed the pathological assessment of
the patient and participated in the writing of the manuscript. All
authors have read and approved the final version of the manuscript.
JS and SH confirm the authenticity of all the raw data
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the First Affiliated Hospital of Shandong First
Medical University (Jinan, China; approval no. 2023-S434).
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salah HT, D'Ardis JA, Baek D, Duran J,
Schwartz MR, Ayala AG and Ro JY: Case of recurrent superficial
CD34-positive fibroblastic tumor. J Cutan Pathol. 50:477–480. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carter JM, Weiss SW, Linos K, DiCaudo DJ
and Folpe AL: Superficial CD34-positive fibroblastic tumor: Report
of 18 cases of a distinctive low-grade mesenchymal neoplasm of
intermediate (borderline) malignancy. Mod Pathol. 27:294–302. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
WHO Classification of Tumours, . Soft
Tissue and Bone Tumours. IARC Press; France: 2020
|
|
4
|
Salah HT, D'ardis JA, Baek D, Schwartz MR,
Ayala AG and Ro JY: Superficial CD34-positive fibroblastic tumor
(SCPFT): A review of pathological and clinical features. Ann Diagn
Pathol. 58:1519372022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson WJ, Mertens F, Mariño-Enríquez A,
Hornick JL and Fletcher CD: Superficial CD34-positive fibroblastic
tumor: A clinicopathologic, immunohistochemical, and molecular
study of 59 cases. Am J Surg Pathol. 46:1329–1339. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Molnar SL, Mott M, White E and De
Las Casas LE: Superficial CD34-positive fibroblastic tumor:
Cytologic features, tissue correlation, ancillary studies, and
differential diagnosis of a recently described soft tissue
neoplasm. Diagn Cytopathol. 44:926–930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lao IW, Yu L and Wang J: Superficial
CD34-positive fibroblastic tumour: A clinicopathological and
immunohistochemical study of an additional series. Histopathology.
70:394–401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perret R, Michal M, Carr RA, Velasco V,
Švajdler M, Karanian M, Meurgey A, Paindavoine S, Soubeyran I,
Coindre JM, et al: Superficial CD34-positive fibroblastic tumor and
PRDM10-rearranged soft tissue tumor are overlapping entities: A
comprehensive study of 20 cases. Histopathology. 79:810–825. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sood N and Khandelia BK: Superficial
CD34-positive fibroblastic tumor: A new entity; case report and
review of literature. Indian J Pathol Microbiol. 60:377–380. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rekhi B, Banerjee D, Gala K and Gulia A:
Superficial CD34-positive fibroblastic tumor in the forearm of a
middle-aged patient: A newly described, rare soft-tissue tumor.
Indian J Pathol Microbiol. 61:421–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamada T, Katsuki N, Hosokawa Y, Ayano Y
and Ikeda M: Additional case of superficial CD34-positive
fibroblastic tumor in a Japanese patient. J Dermatol. 46:e134–e136.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Batur S, Ozcan K, Ozcan G, Tosun I and
Comunoglu N: Superficial CD34 positive fibroblastic tumor: Report
of three cases and review of the literature. Int J Dermatol.
58:416–422. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding L, Xu WJ, Tao XY, Zhang L and Cai ZG:
Clinicopathological features of superficial CD34-positive
fibroblastic tumor. World J Clin Cases. 9:2739–2750. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li SY, Zhang HL and Bai YZ: Superficial
CD34-positive fibroblastic tumor on the chest wall of an 8-year-old
girl: A case report and literature review. Pediatr Hematol Oncol.
38:602–608. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mao X, Sun YY, Deng ML, Ma T and Yu L:
Superficial CD34-positive fibroblastic tumor: Report of two cases
and review of literature. Int J Clin Exp Pathol. 13:38–43.
2020.PubMed/NCBI
|
|
16
|
Andrei V, Haefliger S and Baumhoer D:
Superficial mesenchymal tumours expressing epithelial markers on
immunohistochemistry: Diagnostic clues and pitfalls. Semin Diagn
Pathol. 40:238–245. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Puls F, Carter JM, Pillay N, McCulloch TA,
Sumathi VP, Rissler P, Fagman H, Hansson M, Amary F, Tirabosco R,
et al: Overlapping morphological, immunohistochemical and genetic
features of superficial CD34-positive fibroblastic tumor and
PRDM10-rearranged soft tissue tumor. Mod Pathol. 35:767–776. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hofvander J, Tayebwa J, Nilsson J,
Magnusson L, Brosjö O, Larsson O, von Steyern FV, Mandahl N,
Fletcher CD and Mertens F: Recurre2nt PRDM10 gene fusions in
undifferentiated pleomorphic sarcoma. Clin Cancer Res. 21:864–869.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao M, Yin X, He H, Fan Y, Ru G and Meng
X: Recurrent PRDM10 fusions in superficial CD34-positive
fibroblastic tumors: A clinicopathologic and molecular study of 10
additional cases of an emerging novel entity. Am J Clin Pathol.
159:367–378. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rayyan MM, Aboushelib M, Sayed NM, Ibrahim
A and Jimbo R: Comparison of interim restorations fabricated by
CAD/CAM with those fabricated manually. J Prosthet Dent.
114:414–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaddu S, McMenamin ME and Fletcher CD:
Atypical fibrous histiocytoma of the skin: Clinicopathologic
analysis of 59 cases with evidence of infrequent metastasis. Am J
Surg Pathol. 26:35–46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Michal M, Kazakov DV, Hadravský L, Agaimy
A, Švajdler M, Kuroda N and Michal M: Pleomorphic hyalinizing
angiectatic tumor revisited: All tumors manifest typical
morphologic features of myxoinflammatory fibroblastic sarcoma,
further suggesting 2 morphologic variants of a single entity. Ann
Diagn Pathol. 20:40–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gru AA and Cruz DJ: Atypical
fibroxanthoma: A selective review. Semin Diagn Pathol. 30:4–12.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kohashi K, Yamada Y, Hotokebuchi Y,
Yamamoto H, Taguchi T, Iwamoto Y and Oda Y: ERG and SALL4
expressions in SMARCB1/INI1-deficient tumors: A useful tool for
distinguishing epithelioid sarcoma from malignant rhabdoid tumor.
Hum Pathol. 46:225–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kodera K, Hoshino M, Takahashi S, Hidaka
S, Kogo M, Hashizume R, Imakita T, Ishiyama M, Ogawa M and Eto K:
Surgical management of primary undifferentiated pleomorphic sarcoma
of the rectum: A case report and review of the literature. World J
Surg Oncol. 20:1992022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ríos-Viñuela E, Serra-Guillén C, Llombart
B, Requena C, Nagore E, Traves V, Guillén C, Vázquez D and
Sanmartín O: Pleomorphic dermal sarcoma: A retrospective study of
16 cases in a dermato-oncology centre and a review of the
literature. Eur J Dermatol. 30:545–553. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ieremia E and Thway K: Myxoinflammatory
fibroblastic sarcoma: Morphologic and genetic updates. Arch Pathol
Lab Med. 138:1406–1411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Díaz-Flores L, Gutiérrez R, García MP,
Sáez FJ, Díaz-Flores L Jr, Valladares F and Madrid JF: CD34+
stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve
and a principal source of mesenchymal cells. Location, morphology,
function and role in pathology. Histol Histopathol. 29:831–870.
2014.PubMed/NCBI
|
|
29
|
Tindle RW, Nichols RA, Chan L, Campana D,
Catovsky D and Birnie GD: A novel monoclonal antibody BI-3C5
recognises myeloblasts and non-B non-T lymphoblasts in acute
leukaemias and CGL blast crises, and reacts with immature cells in
normal bone marrow. Leuk Res. 9:1–9. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brown J, Greaves MF and Molgaard HV: The
gene encoding the stem cell antigen, CD34, is conserved in mouse
and expressed in haemopoietic progenitor cell lines, brain, and
embryonic fibroblasts. Int Immunol. 3:175–184. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Radu P, Zurzu M, Paic V, Bratucu M,
Garofil D, Tigora A, Georgescu V, Prunoiu V, Pasnicu C, Popa F, et
al: CD34-Structure, functions and relationship with cancer stem
cells. Medicina (Kaunas). 59:9382023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krause DS, Fackler MJ, Civin CI and May
WS: CD34: Structure, biology, and clinical utility. Blood. 87:1–13.
1996. View Article : Google Scholar : PubMed/NCBI
|