Introduction
Lung cancer is the 2nd most commonly diagnosed
cancer after female breast cancer and the leading cause of cancer
death worldwide. In 2020, there were 2.2 million new lung cancer
cases and an estimated 1.8 million (18%) lung cancer-associated
deaths globally (1). Surgery is the
preferred treatment for early-stage lung cancer. With the
development of modern techniques such as computed tomography
screening and other imaging techniques, the early detection of
smaller lesions has improved, increasing the number of surgeries
(2). Lobectomy is usually
recommended for patients with stage I non-small cell lung cancer
(NSCLC) (3,4). Studies also confirmed that lobectomy
combined with mediastinal lymph node dissection has a 5-year
survival rate of ~60% (4,5). Considering that most patients with
NSCLC are older, with a median age range diagnosis of 67.7–70 years
(6,7), removing excess healthy tissue can
seriously affect the patient's quality of life (8). Thus, alternative methods are
recommended for these patients.
For those patients with severe comorbidities who
cannot undergo lobectomy, segmentectomy (ST) and sublobectomy are
currently being considered (9). In
recent years, sublobectomy [ST or wedge resection (WR)] has gained
attention since it can preserve lung function. WR is a nonanatomic
procedure that removes the cancerous lung tissue surrounded by a
margin of normal lung parenchyma. Its operation time is shorter
because, during this procedure, the pulmonary vessels and bronchus
do not need to be identified (10,11).
By contrast, ST is an anatomic excision that requires the surgeon
to carefully identify the location of the pulmonary blood vessels
and bronchi. Due to the need for careful dissection and evaluation
of intraparenchymal and hilar lymph nodes, ST is more technically
demanding than WR (12). At
present, WR is more commonly used than ST, accounting for ~80% of
sublobectomy (13), but WR is
generally considered less effective than anatomic ST for the
following two reasons: i) In WR, lymph nodes in the tumor area are
usually not removed immediately; and ii) the staple line edge of WR
is closer to the tumor than that of ST (10). Various studies have produced
inconsistent results regarding the impact of two surgical
approaches i.e., WR and ST, on overall survival (OS) and lung
cancer-specific survival (LCSS) in patients with operable NSCLC.
Multiple studies have found no notable difference in OS or
relapse-free survival between patients treated with ST and WR for
NSCLC with ground glass opacity (GGO) as the primary clinical stage
(14–17). Moreover, a meta-analysis of nine
studies published in 2016 suggested that ST is associated with a
higher OS rate than WR for patients with stage I NSCLC; however, no
difference was observed for patients with stage IA NSCLC and tumors
>2 cm in diameter (10). Another
meta-analysis of 19 studies published in 2019 concluded that OS,
LCSS and disease-free survival (DFS) after ST were markedly higher
than those after WT in patients with NSCLC (18). In addition, recent clinical studies
have shown that WR and ST are equally effective in NSCLC with a GGO
diameter of 2–3 cm, with fewer complications, faster recovery of
lung function and greater prevention of postoperative death from
causes other than NSCLC (19,20).
Still, this meta-analysis (18)
only evaluated the difference between WR and ST in survival
outcomes, and the latest studies (19,20)
pointed out that WR may have clinically significant advantages in
terms of postoperative complications, and recurrence and metastasis
rates.
In the present study, a meta-analysis was performed
to compare the outcomes of OS, LCSS, mortality, complication rate,
recurrence and metastasis rates in patients with operable NSCLC
receiving WR compared with those undergoing ST. These data further
contribute to our understanding of surgical options for patients
with NSCLC who received limited lung resection and provide more
specific recommendations for clinical decision-making.
Materials and methods
Literature search
The current meta-analysis was performed according to
the 2020 Preferred Reporting Items for Systematic Reviews and
Meta-Analyses guidelines (21). The
relevant articles were searched based on the population,
intervention, comparison, outcomes and study principle (22). PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/), Web of Science (https://webofscience.com) and Cochrane Library
(https://www.cochranelibrary.com/) were
systematically searched for potentially eligible studies published
from inception to July 2023, using the Medical Subject Headings
terms ‘carcinoma, non-small-cell lung’, ‘pneumonectomy’, ‘sublobar
resection’, ‘segmentectomy’ and ‘wedge resection’ and relevant
keywords (Table SI). The
literature retrieval and selection process were performed
independently by two investigators and results were compared once
the process was complete. Any discrepancy was solved by
discussion.
Eligibility criteria
The inclusion criteria were: i) Studies reporting on
patients with operable NSCLC; ii) the intervention group received
WR, while a control group received ST; iii) study endpoints
included OS, LCSS, mortality, recurrence, metastasis and
postoperative complications; iv) no restrictions on study design;
and v) full text available. The exclusion criteria were: i)
Reviews, case reports, dissertations, conference papers, chapters
in handbooks and editorials; ii) studies not published in an
international peer-reviewed journal; and iii) duplicate published
studies.
Data extraction
Two independent investigators performed data
extraction. Any disagreements were resolved by a third reviewer.
Data included the authors' name, publication year, study design and
location, sample size, data sources, male proportion, mean age,
stage of NSCLC, tumor size and study outcomes. The primary outcome
was the 3- or 5-year OS, LCSS, mortality and postoperative
complications; secondary outcomes included the recurrence and
metastasis rates.
Quality assessment
Due to the particularity of intervention methods,
complete double-blind, randomized controlled studies (RCTs) are
challenging. ROBINS-I was selected to assess the risk of bias and
quality of evidence in the included non-randomised studies of
interventions (23). Quality
assessment was performed in duplicate by investigators
separately.
The contents of the study evaluation included the
following: Bias due to confounding, bias in the selection of
participants into the study, bias in classification of
interventions, bias due to deviations from intended interventions,
bias due to missing data, bias in the measurement of outcomes and
bias in the selection of the reported result. The categories for
risk of bias judgments were: ‘Low risk’, ‘Moderate risk, ‘Serious
risk’ and ‘Critical risk’ of bias.
Statistical analysis
All analyses were performed using the Stata (version
15.1 SE; http://www.stata.com/stata15/). Odds ratios (ORs),
hazard ratios (HRs), risk difference (RDs) and their corresponding
95% confidence intervals (Cis) were used to compare the outcomes.
Studies providing 5-year OS numbers and 5-year OS HRs were
separated for pooled analysis. For studies that reported effective
sizes by subgroup, the subgroups were combined and the effect size
for the entire sample was calculated. Statistical heterogeneity
among the included studies was calculated using Cochran's Q-test,
and the I2 index (I2>50% and Q-test P>0.10 indicated high
heterogeneity). Given the clinical heterogeneity of the original
studies (e.g., geographic, ethnic and others), the random effects
model was used. P<0.05 was considered to indicate a
statistically significant difference. A sensitivity analysis with a
leave-one-out method was performed to assess the potential
confounding effects and the robustness of the pooled results. If
the pooled results after the exclusion of a study were inconsistent
with the original pooled ones, the study was excluded as a
potential confounder. Subgroup analysis was conducted to examine
the survival outcome complications, and the recurrence and
metastasis rates between WR and ST in different data sources
[Surveillance, Epidemiology, and End Results (SEER) database or
hospital], and tumor size (<1, 1–2, 2–3 and <3 cm).
Publication bias was identified through the funnel plot, Egger
regression and Begg tests.
Results
Study selection and
characteristics
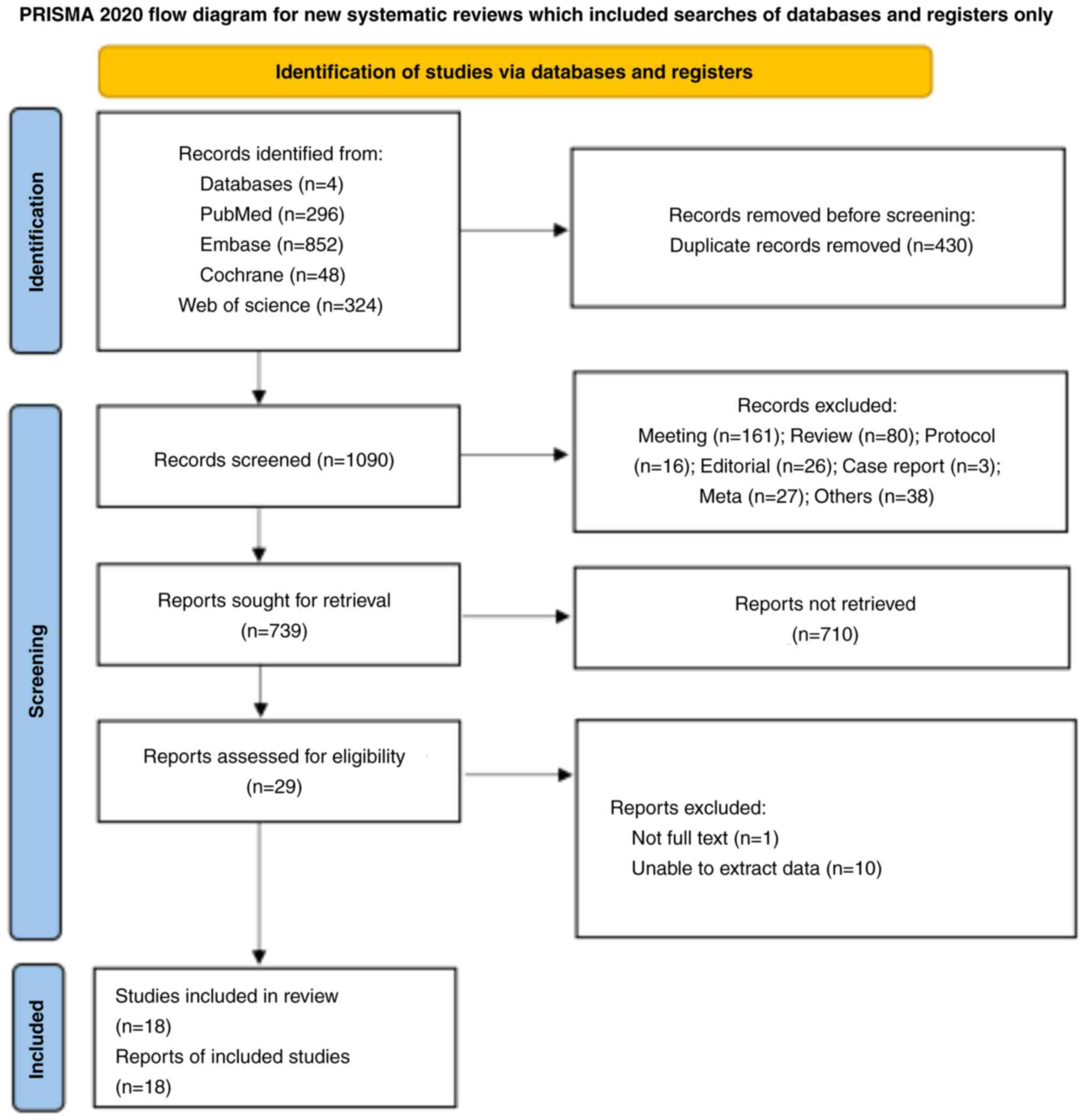
Fig. 1 presents the
study selection process. The initial search resulted in 1,520
records. After removing 430 duplicates by automation tools and
1,062 records after reviewing the title and abstract, 29 studies
were included. One study without a full text and 10 studies without
interest outcomes were further excluded. Finally, 18 studies
(19,20,24–39)
were included in the current meta-analysis.
Table I shows the
characteristics of the included studies. A total of 19,381 patients
with operable NSCLC were included in the current meta-analysis;
14,611 in the WR group and 4,770 in the ST group. Studies were
conducted in four countries, including America (n=2), China (n=10),
Germany (n=1) and Japan (n=5). All of the included studies were
retrospective. Nine studies had data from the SEER database, and
the other nine obtained oncology data from hospitals. The mean age
range of the included patients with operable NSCLC was 55.96–79.9
years. The range of female percentage was 30.3–79.09%. All of the
patients with NSCLC included in the 12 studies that provided
pathological staging of the tumor were evaluated as stage IA lung
cancer. The mean tumor size range was 1.5–4 cm.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| First author(s),
year | Country | Study design | Data sources | Sample size, n
(WR/ST) | Age, years | Female, % | Stage of NSCLC | Mean tumor size, cm
(WR/ST) | Outcomes | (Refs.) |
|---|
| Altorki et al,
2016 | US | Retrospective
study | SEER database | 160/129 | 72.66 | 57.09 | IA | 1.5/1.7 | OS, AEs, death | (24) |
| Dai et al,
2016 | China | Retrospective
study | SEER database | 3,316/769 | 68.3 | 61.27 | IA | <2 | OS, LCSS | (26) |
| Cao et al,
2018 | China | Retrospective
study | SEER database | 3,007/809 | n/a | 58.94 | IA | <3 | OS, LCSS | (25) |
| Wu et al, 2021 | China | Retrospective
study | SEER database | 995/182 | n/a | 61.43 | IA | <2 | OS, LCSS | (36) |
| Zhang et al,
2021 | China | Retrospective
study | SEER database | 736/194 | 79.9 | 60.54 | IA | <4 | OS | (19) |
| Zhao et al,
2019 | China | Retrospective
study | SEER database | 686/686 | 69 | 63.37 | IA | n/a | OS | (39) |
| Zhou et al,
2022 | China | Retrospective
study | Hospital | 100/100 | 57.45 | 44.5 | n/a | 2-3 | AEs, OS | (37) |
| Fan et al,
2020 | China | Retrospective
study | SEER database | 851/175 | n/a | 64.33 | IA | n/a | OS | (27) |
| Handa et al,
2022 | Japan | Retrospective
study | Hospital | 179/179 | 69.5 | 50.55 | IA | 1.5 | Death | (28) |
| Handa et al,
2021 | Japan | Retrospective
study | Hospital | 61/61 | 67.5 | 42.62 | IA | <2 | AEs, death, OS | (29) |
| Jiang et al,
2014 | China | Retrospective
study | Hospital | 15/19 | 64.2 | 64.71 | n/a | <1 | AEs | (30) |
| Nakamura et al,
2011 | Japan | Retrospective
study | Hospital | 84/38 | 74.18 | 38.52 | n/a | n/a | Death | (31) |
| Sienel et al,
2008 | Germany | Retrospective
study | Hospital | 31/56 | 65.67 | 29.89 | IA | n/a | Death, OS | (32) |
| Smith et al,
2013 | US | Retrospective
study | SEER database | 1,568/378 | 70 | 55.76 | IA | ≤3 | OS, LCSS | (33) |
| Mimae et al,
2021 | Japan | Retrospective
study | Hospital | 191/279 | n/a | n/a | n/a | n/a | Death, AEs, OS | (38) |
| Tsou et al,
2020 | China | Retrospective
study | Hospital | 273/57 | 55.96 | 79.09 | n/a | <1 | AEs | (34) |
| Tsutani et al,
2019 | Japan | Retrospective
study | Hospital | 60/39 | 76 | 30.3 | n/a | n/a | Death, OS | (35) |
| Wang et al,
2020 | China | Retrospective
study | SEER database | 2,298/620 | 76.7 | 56.5 | IA | ≤3 | LCSS | (20) |
Study quality
Table II shows the
risk of bias assessment results of the included studies. Only three
included studies had a moderate risk of bias, while the remaining
15 studies had a low risk. The main consideration of the three
studies with ‘moderate risk’ was a bias due to potential
confounding factors.
 | Table II.Quality evaluation by ROBINS-I. |
Table II.
Quality evaluation by ROBINS-I.
| First author(s),
year | D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall | (Refs.) |
|---|
| Altorki et al,
2016 | Low | Low | Low | Low | Low | Low | Low | Low | (24) |
| Wu et al, 2021 | Moderate | Low | Low | Low | Low | Low | Low | Moderate | (36) |
| Dai et al,
2016 | Low | Low | Low | Low | Low | Low | Low | Low | (26) |
| Cao et al,
2018 | Low | Low | Low | Low | Low | Low | Low | Low | (25) |
| Zhang et al,
2021 | Moderate | Low | Low | Low | Low | Low | Low | Moderate | (19) |
| Zhao et al,
2019 | Low | Low | Low | Low | Low | Low | Low | Low | (39) |
| Zhou et al,
2022 | Low | Low | Low | Low | Low | Low | Low | Low | (37) |
| Fan et al,
2020 | Low | Low | Low | Low | Low | Low | Low | Low | (27) |
| Handa et al,
2022 | Low | Low | Low | Low | Low | Low | Low | Low | (28) |
| Handa et al,
2021 | Low | Low | Low | Low | Low | Low | Low | Low | (29) |
| Jiang et al,
2014 | Low | Low | Low | Low | Low | Low | Low | Low | (30) |
| Nakamura et al,
2011 | Moderate | Low | Low | Low | Low | Low | Low | Moderate | (31) |
| Sienel et al,
2008 | Low | Low | Low | Low | Low | Low | Low | Low | (32) |
| Mimae et al,
2021 | Low | Low | Low | Low | Low | Low | Low | Low | (38) |
| Tsou et al,
2020 | Low | Low | Low | Low | Low | Low | Low | Low | (34) |
| Tsutani et al,
2019 | Low | Low | Low | Low | Low | Low | Low | Low | (35) |
| Wang et al,
2020 | Low | Low | Low | Low | Low | Low | Low | Low | (20) |
| Smith et al,
2013 | Low | Low | Low | Low | Low | Low | Low | Low | (33) |
OS
Data on the 3-(24,35)
and 5-year OS (19,24,27,29,32,
35,37,38),
and 5-year OS HRs (20,25,26,33,35,36,39)
were extracted from the original studies to compare the
postoperative OS of patients with operable NSCLC who received WR or
ST. For patients with operable NSCLC, there was no significant
difference in the 3- and 5-year OS numbers after WR and ST; yet,
the 5-year OS rate after ST was higher than that in the WR
group.
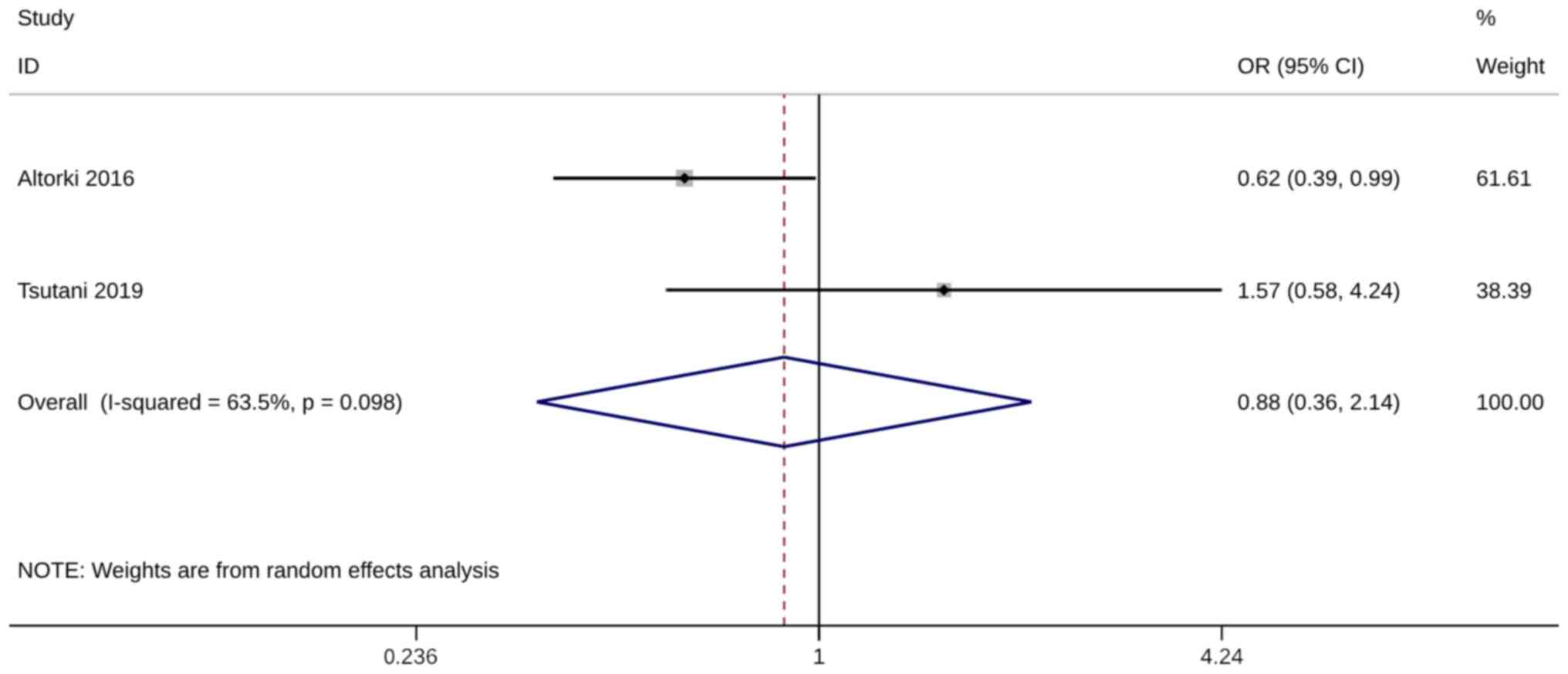
Several studies have examined the 3-year (n=2)
(24,35) and 5-year (n=8) (19,24,27,29,32,35,37,38) OS
numbers after WR or ST. The pooled analysis was only conducted when
at least two adequately powered studies were available, and no
significant difference in both survival numbers was observed
between the WR and the ST group [(3-year OS, OR=0.88; 95% CI, 0.36,
2.14; P=0.782, I2=63.5%) and (5-year OS numbers:
OR=0.90; 95% CI, 0.58, 1.40; P=0.649, I2=78.5%; Figs. 2 and 3)]. The sensitivity analysis results
indicated that the meta-analysis findings were robust, with
consistent results remaining unchanged even when excluding any
single study (Fig. S1).
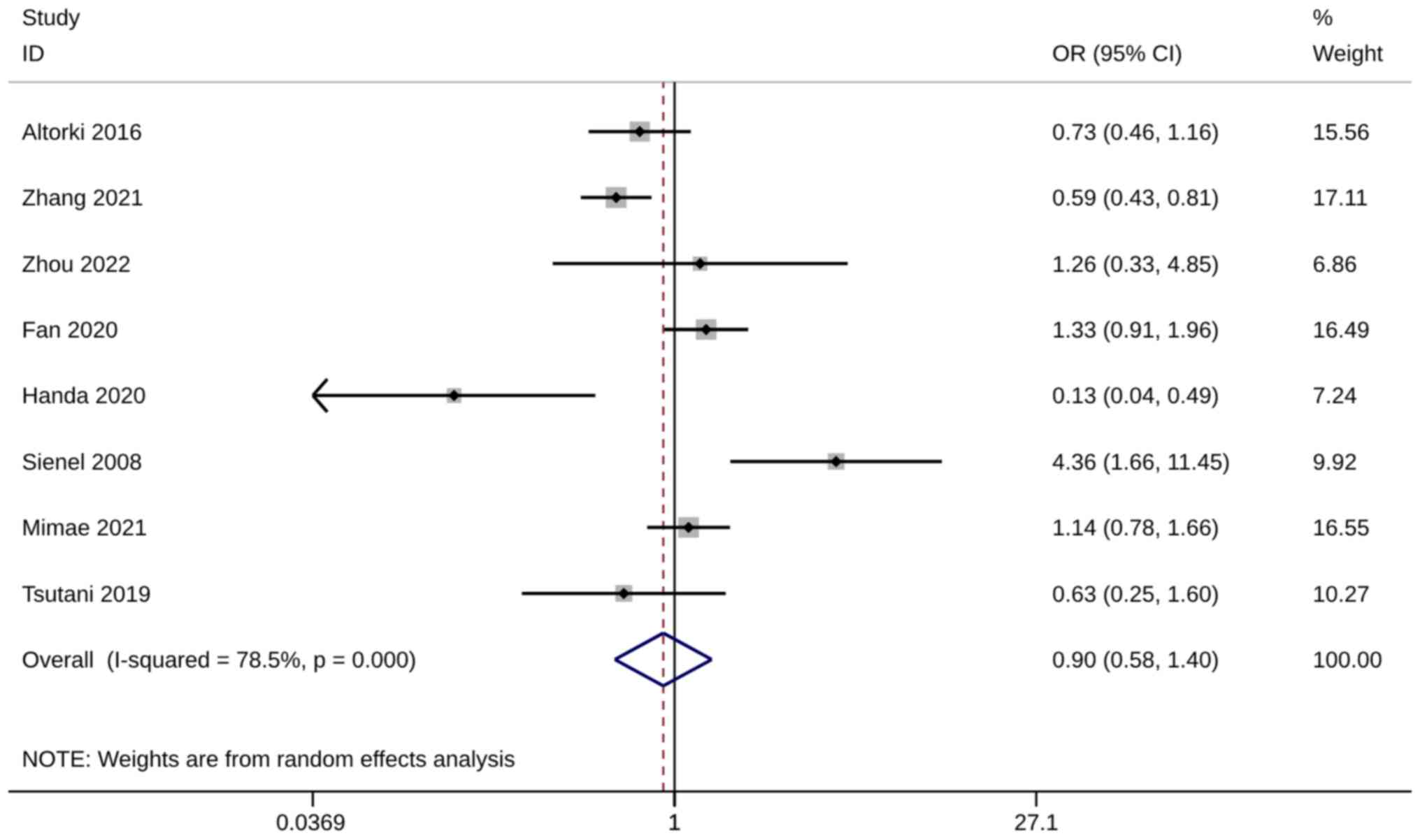
Seven studies with 11 trials (20,25,26,33,35,36,39),
including 15,413 patients with operable NSCLC, reported on the HRs
of 5-year OS. All these studies were included in the quantitative
synthesis, and meta-analysis showed that with significant
heterogeneity, 5-year OS was significantly higher after ST than
after WR (HR, 0.19; 95% CI, 0.04, 0.34; P=0.014;
I2=76.3%; Fig. 4). The
sensitivity analysis results showed that the meta-analysis results
were robust, and the advantage of ST remained unchanged when any
one study was excluded (Fig.
S2).
LCSS
Five studies with 11 trials reported on LCSS after
operation. HR was used to compare LCSS between groups. Compared
with the ST controls, significantly higher LCSS was observed in ST
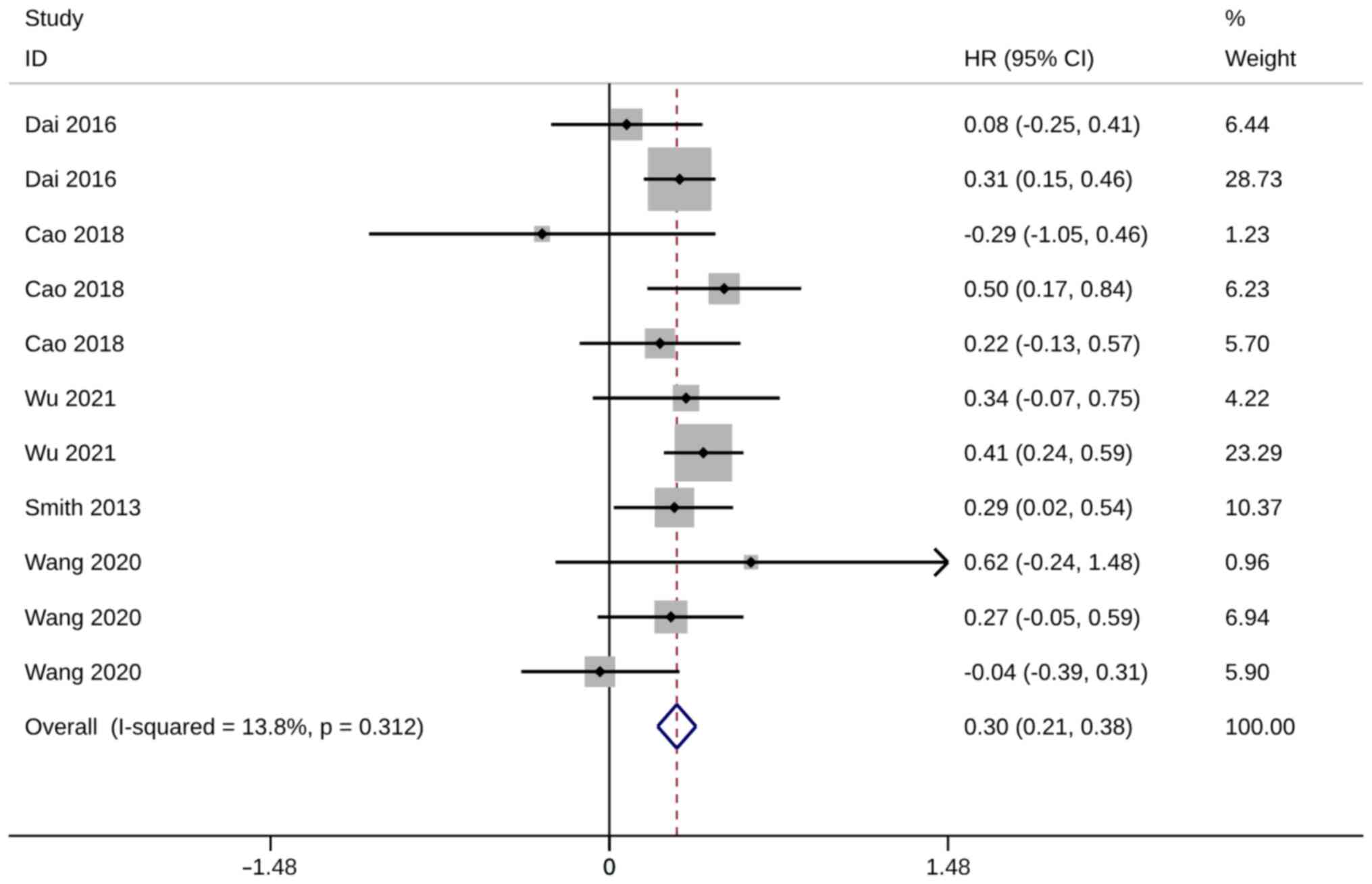
groups compared with WR groups (HR, 0.30; 95% CI, 0.21, 0.38;
P<0.01). Heterogeneity was insignificant, so the fixed-effects
model was used for the analysis (I2=13.8%; Fig. 5). Sensitivity analysis demonstrated
the robustness of meta-analysis results since the advantage of ST
remained unchanged after excluding any of these studies (Fig. S3).
Mortality
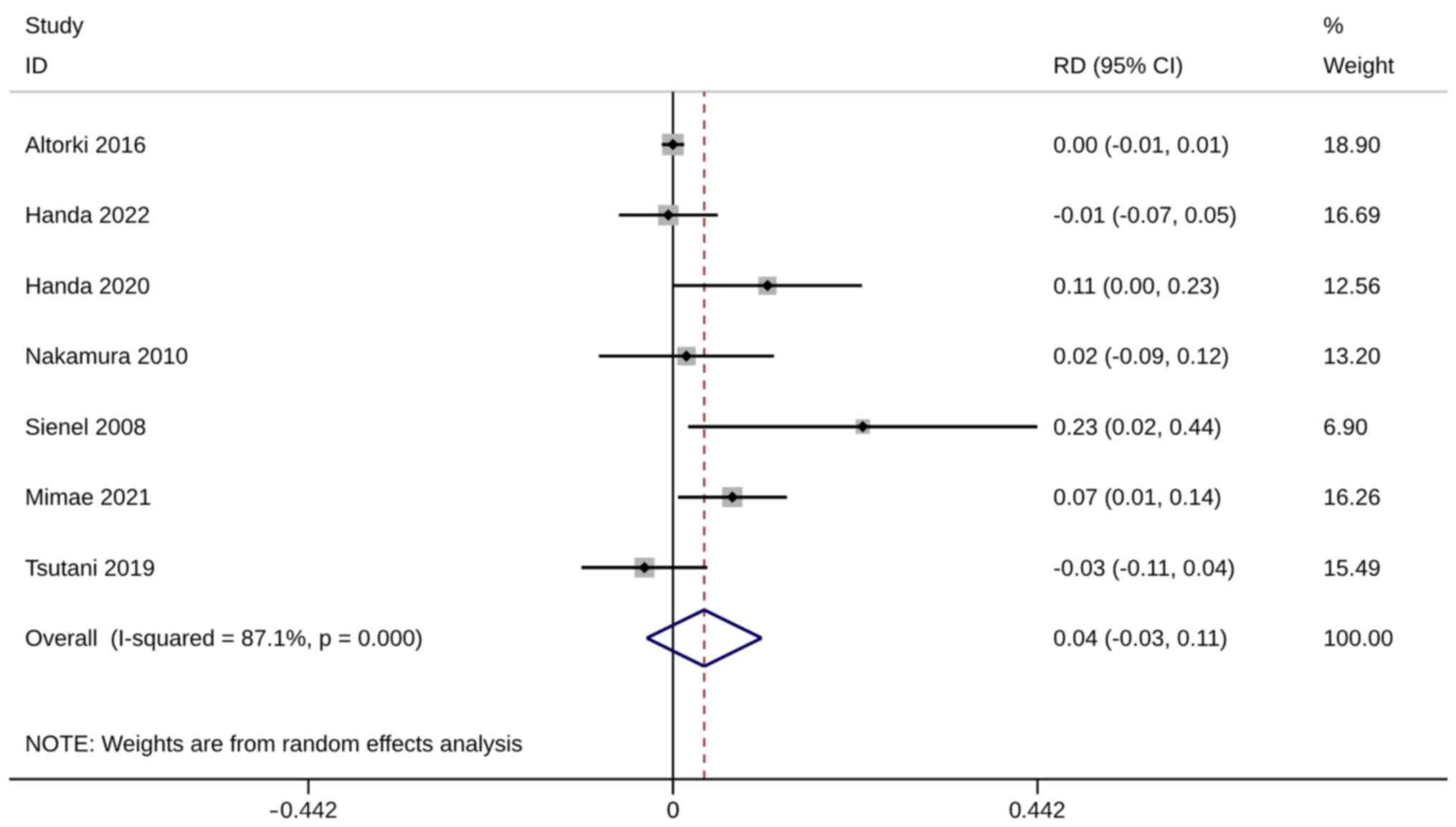
Mortality was evaluated using six studies with seven
trials. No significant difference in mortality was observed between
ST and WR groups (RD, 0.04; 95% CI, −0.03, 0.11; P=0.287).
Heterogeneity was significant so the random-effects model was used
for analysis (I2=87.1%; Fig.
6). The sensitivity analysis results were robust (Fig. S4).
Complications
Mortality was evaluated using six studies. There was
no significant RD in complication incidence between ST and WR
groups (OR, 0.72; 95% CI, 0.25, 2.07; P=0.536). Heterogeneity was
significant so the random-effects model was used for analysis
(I2=82%; Fig. 7). The
sensitivity analysis showed that when Mimae et al (38) was excluded, the pooled results were
inconsistent with the original meta-analysis results (Fig. S5). Mimae et al (38) may be the source of heterogeneity,
and meta-analysis results after exclusion showed that the
complication rate in the WR group was significantly lower than that
in the ST group (OR, 0.44; 95% CI, 0.23, 0.82; Table SI, Table SII, Table SIII).
Recurrence rate
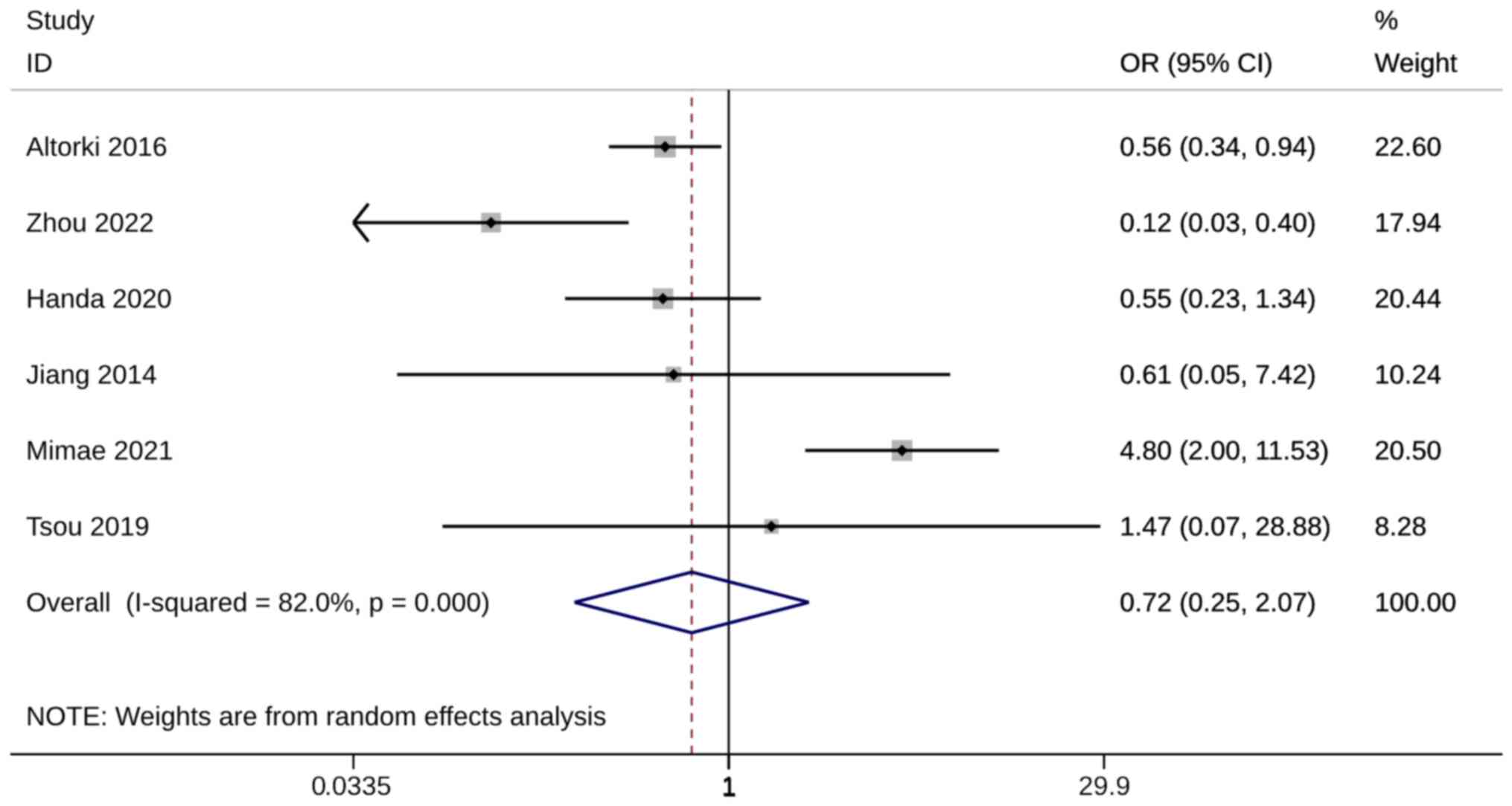
There were nine of the 18 included studies that
compared recurrence rates between groups. No difference in
recurrence rate was observed between ST and WR groups (OR, 2.15;
95% CI, 0.97, 4.74; P=0.058). Heterogeneity was significant so the
random-effects model was used for analysis (I2=57.1%;
Fig. 8). When the studies of
Altorki et al (24), Handa et al
(29) and Zhou et al (37) were excluded, the pooled results were
inconsistent with the original meta-analysis results (Fig. S6). The aforementioned three
included studies may be the source of heterogeneity, and
meta-analysis results after exclusion showed that the recurrence
rate in the WR group was significantly higher than in the ST group,
with OR >1 and 95% CI not included 1 (Table SIII).
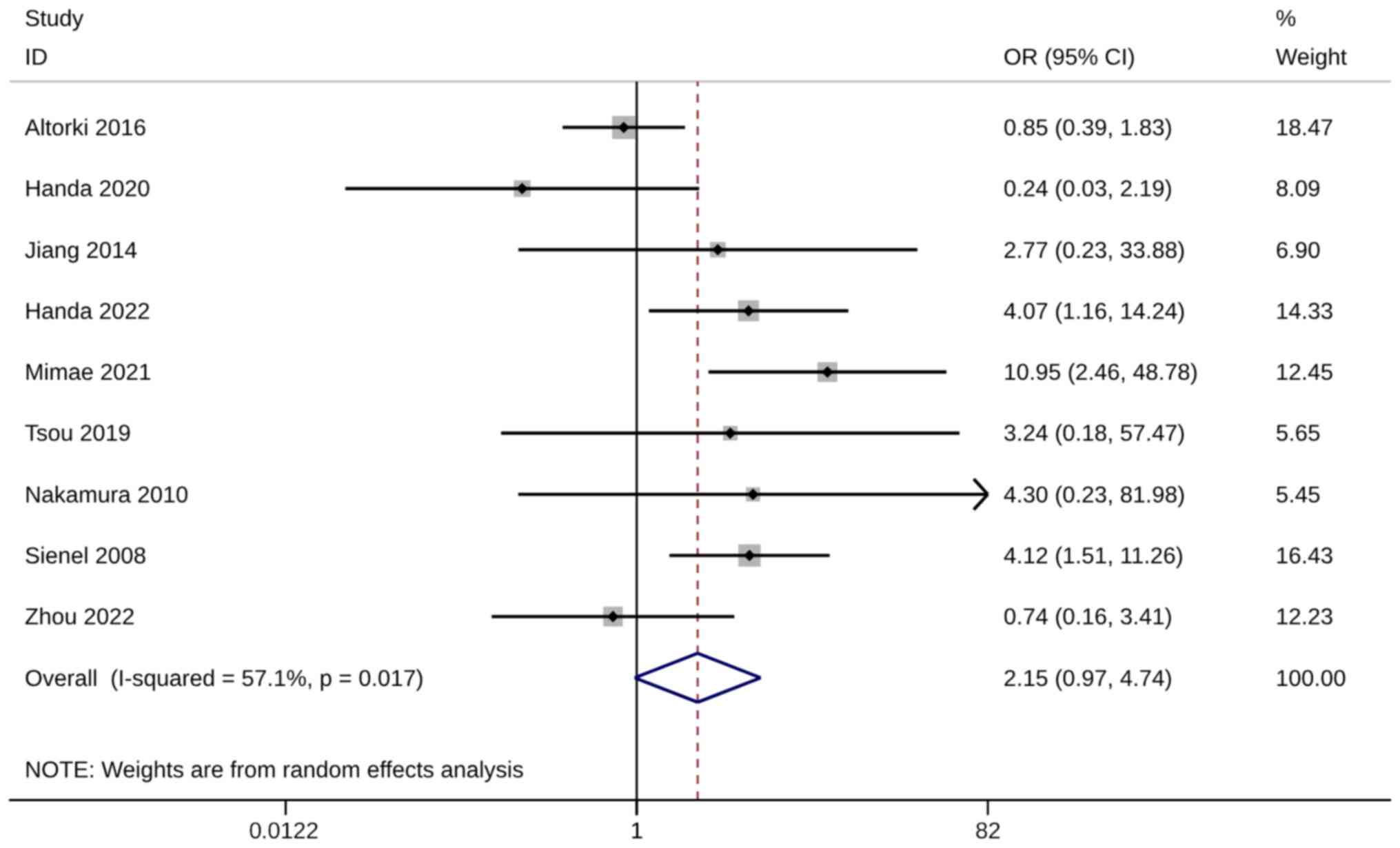
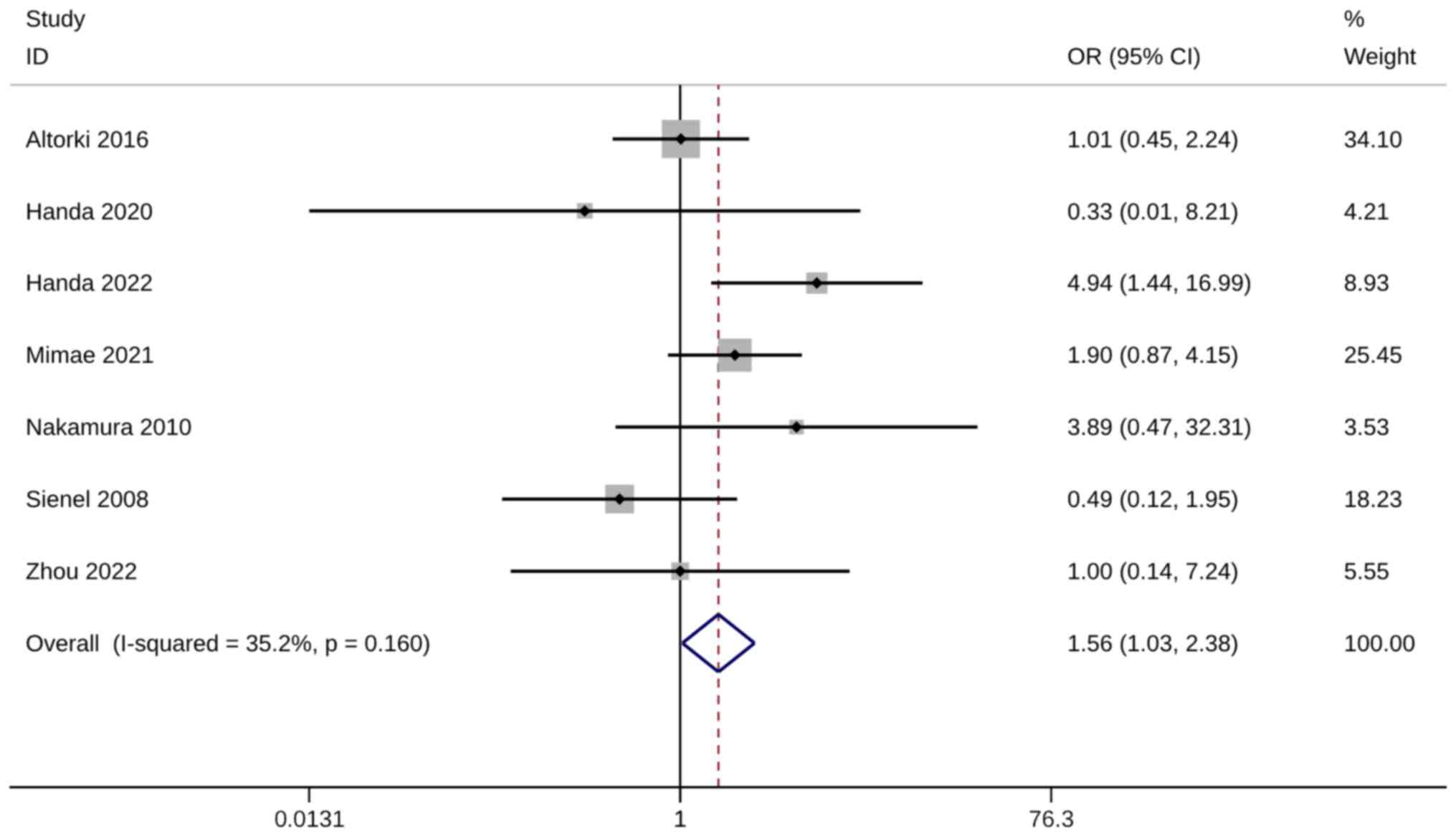
Metastasis rate
The metastasis rate was evaluated using six studies
with seven trials. A significantly higher metastasis rate was
observed in WR groups than in ST groups (OR, 1.56; 95% CI, 1.03,
2.38; P=0.037). Heterogeneity was not significant, so the
fixed-effects model was used for analysis (I2=35.2%;
Fig. 9). The sensitivity analysis
results were robust (Fig. S7).
Data sources subgroup-analysis
Given the heterogeneity of NSCLC data sources,
subgroup analysis divided studies into SEER database and hospital
groups. Considering the number of studies, all subgroup analyses
were performed using six outcome variables, excluding 3-year OS
numbers and LCSS.
In the SEER database subgroups, the 5-year OS rate
in the ST group was higher than that in the WR group (HR, 0.20; 95%
CI, 0.05, 0.35; P=0.01; I2=78.3%; n=10; Fig. S8), and the complication rate in the
WR group was lower than that in the ST group (OR, 0.56; 95% CI,
0.34, 0.94; P=0.027; n=1; Fig.
S9). No difference was observed in other outcomes (Figs. S10 and 11).
In hospital subgroups, higher recurrence (OR, 2.71;
95% CI, 1.22, 6.02; P=0.015; n=8) and metastasis rate (OR, 1.85;
95% CI, 1.12, 3.05; P=0.016; n=6) were observed in WR subgroups
compared with ST groups (Figs. S12
and 13).
Tumor size subgroup-analysis
Stratified by the average tumour size of enrolled
patients with NSCLC, the study was divided into <1, 1–2, 2–3 and
<3 cm, and not provided groups. Tumour size subgroup analyses
were only performed for 5-year OS rate and LCSS.
For patients with NSCLC with tumor size <1 cm,
LCSS in the ST group was superior to WR (HR, 0.3; 95% CI, 0.16,
0.44; P<0.01; n=4), while there was no difference in 5-year OS
rate (HR, 0.22; 95% CI, −0.03, 0.47; P=0.089; n=3). When the tumor
size was 1–2 cm, the 5-year OS rate (HR, 0.32; 95% CI, 0.20, 0.44;
P<0.01; n=3; Fig. S14) and LCSS
(OR, 0.35; 95% CI, 0.22, 0.48; P<0.01; n=4; Fig. S15) in the ST group were better than
those in the WR group. In patients with NSCLC with tumor size <3
cm, the 5-year OS rate in the WR group was higher (HR, −0.17; 95%
CI, −0.29, −0.05; P=0.005; n=1) and the LCSS was lower than that in
the ST group (HR, 0.29; 95% CI, 0.02, 0.55; P=0.032; n=1). There
was no difference in OS rate and LCSS 5 years after WR or ST in
patients with tumor size 2–3 cm (Figs.
S14 and 15).
Publication bias and sensitivity
analysis
Publication bias was assessed using a funnel plot,
Egger regression test, Begg test and trim and fill method.
According to Fig. S16, Fig. S17, Fig. S18, Fig. S19, Fig. S20, Fig. S21, Fig. S22, the Egger regression test and
the Begg test results, no publication bias was observed in all
primary or secondary outcomes (Table
SII).
The results of the sensitivity analysis found that
Mimae et al (38) may be the source
of heterogeneity in complication rate (Fig. S5), and Altorki et al (24), Handa et al (28), or Zhou et al (37) were identified as the potential
confounders of recurrence rate (Fig.
S6).
Discussion
A sublobar resection, which includes ST and WR, is
considered a possible alternative to lobectomy (33), especially for patients with stage I
NSCLC (40). Yet, specific target
populations and postoperative prognostic outcomes of ST and WR
remain controversial (32).
After excluding possible confounders, the findings
of the present study suggest that 5-year OS, LCSS and metastasis
rates in patients with operable NSCLC treated with WR are worse
than those in patients treated with ST. However, the incidence of
postoperative complications in WR patients was significantly lower
than in the ST treatment group. Based on current evidence, ST may
be the best option for patients with NSCLC whose tumor size range
of 1–2 cm in terms of postoperative OS and LCSS. Clinical data from
hospitals can better support the advantages of ST surgical methods
in recurrence and metastasis rates. Also, the results related to
postoperative survival in patients with NSCLC who received WR and
ST were consistent with previous meta-analyses (10,12,18).
Numerous previous studies have compared the outcomes
of patients with stage I NSCLC undergoing cuneiform and ST
(10,18,41).
The findings of the current study on OS and LCSS are consistent
with those studies. Hou et al (10)
analyzed 19 studies (3,604 patients receiving ST and 10,593
receiving WR) and concluded that survival and LCSS were higher for
ST than for WR. Mata-analysis results published by Zhang et al
(18) suggested that ST had
markedly better OS, LCSS and DFS than WR in patients with stage I
NSCLC who underwent lobectomy. Moreover, a Bayesian network
meta-analysis published in 2022 showed that ST has marked benefits
compared with WR for OS and DFS in patients with early-stage NSCLC
(41). At the same time, compared
with the aforementioned studies, the present meta-analysis included
more recent research results, and the analysis results were more
convincing. Previous studies have only included a limited number of
original documents and these documents are quite old. The present
study included 18 recent documents, which will make the findings
more convincing. In addition, the previous studies are compared in
the following paragraphs. By comparing a previously published study
(29) and review (10), it was shown that recently published
studies (29) report different
results compared with older studies (10). For example, Hou et al (10) found comparable results between ST
and WR with regards to OS when assessing studies published between
2005 and 2010, but OS was also higher for ST than WR when assessing
studies published between 2010 and 2015, which may be due to the
continuous improvement and development of ST surgical techniques. A
study published in Handa in 2022 comparing the survival outcomes of
complex ST and WR when lobectomy is not possible, lung cancer with
a ‘complex site’ solid component size ≤2.0 cm may require complex
ST instead of WR (29). This
complex ST requires the surgeon to separate and segment the
appropriate segmental bronchi, arteries and veins more peripherally
and, in some cases, to cut into the lung parenchyma. Several
intersegmental planes with safe surgical margins must be made.
Compared with WR resection of lung tumors with normal parenchymal
margins, the aforementioned procedure can effectively improve the
postoperative survival of patients. Meanwhile, a more recent study
has further findings on the size of the tumors suitable for the two
surgeries. In their meta-analysis, Hou et al (10) suggested that the larger the tumor
size for patients with stage I NSCLC, the greater the advantage of
ST over WR. Both meta-analyses were published later, and the
present study found no difference in the effect of ST and WR on
survival outcomes in patients with NSCLC with tumor sizes >2
cm.
For some types of tumors, the recurrence rate
depends primarily on the appropriateness of selecting the initial
surgical resection area (42).
There is an idea that patients who undergo limited resection, WR or
ST, have a markedly increased risk of intrathoracic recurrence
(43,44). Another view is that there is no
notable difference in survival between patients with stage I NSCLC
undergoing sublobectomy (cuneiform resection and ST) and standard
lobectomy, especially for early NSCLC (45,46).
NSCLC is a malignancy that affects mainly the elderly, and the
removal of excess healthy tissue can seriously affect the patient's
quality of life (6,8). The present meta-analysis aimed not to
select the best surgical resection between WR and ST, but to
clarify whether WR has the same survival, recurrence and metastasis
rates as ST as WR preserves more normal tissue (14). The current results suggest that WR,
which preserves more normal tissue, may not be prioritized when
both resection methods are suitable for patients.
For patients with NSCLC, the choice of postoperative
recurrence is more relevant than the choice of surgical resection
margin. Sato et al (47)
demonstrated that the accurate identification of adequate surgical
margins plays a crucial role in preventing recurrence at the
margins. Although no conclusion has been reached, it is
hypothesised that 20-mm incision margin for a full lung and 15-mm
incision margin for a depleted lung are the appropriate choices
(48). Tsutani et al (49) compared cancer control between ST and
WR in patients with clinical stage IA NSCLC and found that 36/195
(18.5%) patients undergoing WR and 14/262 (5.3%) patients
undergoing ST experienced recurrence after surgery. ST has better
tumor control than WR. Suzuki et al (50) reported that the median incision
margin of pathological surgery was 15 mm, and the 5-year
recurrence-free survival rate was 99.7%. Sublobectomy with adequate
surgical margins provides adequate local control and
recurrence-free survival for clinically resectable lung cancer.
Zhou et al (37) showed that WR may
be better suited for peripheral lesions, whereas ST is better
suited for deeper lesions that cannot be accessed by WR.
Adequate lymph node dissection is the key to
preventing metastasis and recurrence. For patients with early NSCLC
≥3 cm in diameter, evaluated lymph nodes in patients undergoing
sublobectomy are associated with better OS and LCSS (51). Baig et al (52) reported that ST has a higher
long-term survival rate than WR for second primary lung cancer
after a prior lobectomy. WR for second primary lung cancer markedly
improves OS when adequate lymph node dissection is performed.
The results of the study showed that the outcomes of
WR in terms of survival, recurrence and metastasis were not
favourable; however, a study by Handa et al (29) included demonstrated generally lower
toxicity and a significantly lower incidence of postoperative
complications in WR as compared to the ST group. Regarding
short-term surgical outcomes such as surgical time, surgical blood
loss and postoperative hospital stay, WR is less damaging than ST
(29). Similar to previous
findings, WR had 3.15 fewer complications than ST. Notably, there
were no serious postoperative complications, and postoperative
mortality was comparable to ST (29), which also provides surgical options
for patients with early NSCLC who are older or have severe
comorbidities.
The present study has some limitations. First, a
large amount of retrospective data can bring uncertainty and
concerns to a conclusion; thus, more prospective studies and RCTs
are needed to make up for this shortcoming. Second, a great
heterogeneity in the included studies may lead to some bias. Third,
specific surgical methods for different ST and WR, such as complex
ST and simple ST included in the studies, were not discussed. Also,
due to limited original data, the current study did not conduct a
subgroup analysis of patients of different ages, which could have
further refined the target population of different surgical
methods.
A meta-analysis, which was published in 2019,
incorporated 15 studies, four of which were prospective (18). Although the number of studies
included in the present study is lower than that of the study of
Zhang et al (18), the time range
of the literature search has been extended from before April 30,
2018 to July 2023. The 16 studies included in the Bayesian
meta-analysis published in 2022 were all retrospective studies,
similar to those in the current study (41). Therefore, in terms of the included
studies, it is hypothesised that the present meta-analysis is just
as compelling as previous meta-analyses. As a result, it
contributes to the exploration of postoperative recurrence and
metastasis of NSCLC, which is somewhat innovative.
The data of the current study suggest that the
postoperative survival rate of patients with operable NSCLC after
WT is not equivalent to that of ST; however, the postoperative
complication rate of WT is lower than that of ST. The equivalence
of recurrence rate and distant metastasis rate remains
controversial. Practitioners should not blindly use WT instead of
ST in clinical practice. More prospective studies and RCTs are
needed to further analyse the differences in recurrence and
metastasis rates between ST and WR surgical modalities.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
JX, SX and SW conceived the study idea and designed
the study. SX provided administrative support and study materials
or patients. JX, SW, XW, WX, YHu and YHua collected and assembled
the data. JH, SW and XW analyzed and interpreted the data. All
authors contributed to the writing of the manuscript. JX, SW and SX
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forquer JA, Fakiris AJ, McGarry RC, Cheung
MK, Watson C, Harkenrider M, Henderson MA and Lo SS: Treatment
options for stage I non-small-cell lung carcinoma patients not
suitable for lobectomy. Expert Rev Anticancer Ther. 9:1443–1153.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scott WJ, Howington J, Feigenberg S,
Movsas B and Pisters K; American College of Chest Physicians, :
Treatment of non-small cell lung cancer stage I and stage II: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132 (Suppl):S234–S242. 2007. View Article : Google Scholar
|
|
4
|
Nakamura H, Kazuyuki S, Kawasaki N,
Taguchi M and Kato H: History of limited resection for non-small
cell lung cancer. Ann Thorac Cardiovasc Surg. 11:356–362.
2005.PubMed/NCBI
|
|
5
|
Martini N, Bains MS, Burt ME, Zakowski MF,
McCormack P, Rusch VW and Ginsberg RJ: Incidence of local
recurrence and second primary tumors in resected stage I lung
cancer. J Thorac Cardiovasc Surg. 109:120–129. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jatoi A, Schild SE, Foster N, Henning GT,
Dornfeld KJ, Flynn PJ, Fitch TR, Dakhil SR, Rowland KM, Stella PJ,
et al: A phase II study of cetuximab and radiation in elderly
and/or poor performance status patients with locally advanced
non-small-cell lung cancer (N0422). Ann Oncol. 21:2040–2044. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heiden BT, Eaton DB Jr, Engelhardt KE,
Chang SH, Yan Y, Patel MR, Kreisel D, Nava RG, Meyers BF, Kozower
BD and Puri V: Analysis of delayed surgical treatment and oncologic
outcomes in clinical stage I non-small cell lung cancer. JAMA Netw
Open. 4:e21116132021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Celli JP, Spring BQ, Rizvi I, Evans CL,
Samkoe KS, Verma S, Pogue BW and Hasan T: Imaging and photodynamic
therapy: Mechanisms, monitoring, and optimization. Chem Rev.
110:2795–2838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao C, Gupta S, Chandrakumar D, Tian DH,
Black D and Yan TD: Meta-analysis of intentional sublobar
resections versus lobectomy for early stage non-small cell lung
cancer. Ann Cardiothorac Surg. 3:134–141. 2014.PubMed/NCBI
|
|
10
|
Hou B, Deng XF, Zhou D, Liu QX and Dai JG:
Segmentectomy versus wedge resection for the treatment of high-risk
operable patients with stage I non-small cell lung cancer: A
meta-analysis. Ther Adv Respir Dis. 10:435–443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding H, Song N, Zhang P, Jiang G and Wang
H: Wedge resection plus adequate lymph nodes resection is
comparable to lobectomy for small-sized non-small cell lung cancer.
Front Oncol. 12:10229042022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Detterbeck FC, Mase VJ Jr, Li AX, Kumbasar
U, Bade BC, Park HS, Decker RH, Madoff DC, Woodard GA, Brandt WS
and Blasberg JD: A guide for managing patients with stage I NSCLC:
Deciding between lobectomy, segmentectomy, wedge, SBRT and
ablation-part 2: Systematic review of evidence regarding resection
extent in generally healthy patients. J Thorac Dis. 14:2357–2386.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding H, Wang H, Xu L, Song N and Jiang G:
Survival and resected lymph node number during sublobar resection
for n0 non-small cell lung cancer 2 cm or less. Ann Thorac Surg.
107:1647–1655. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsutani Y, Miyata Y, Nakayama H, Okumura
S, Adachi S, Yoshimura M and Okada M: Appropriate sublobar
resection choice for ground glass opacity-dominant clinical stage
IA lung adenocarcinoma: Wedge resection or segmentectomy. Chest.
145:66–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fiorelli A, Caronia FP, Daddi N, Loizzi D,
Ampollini L, Ardò N, Ventura L, Carbognani P, Potenza R and
Ardissone F: Sublobar resection versus lobectomy for stage I
non-small cell lung cancer: An appropriate choice in elderly
patients? Surg Today. 46:1370–1382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamatake D, Yoshida Y, Miyahara S,
Yamashita S, Shiraishi T and Iwasaki A: Surgical outcomes of lung
cancer measuring less than 1 cm in diameter. Interact Cardiovasc
Thorac Surg. 15:854–858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kent MS, Mandrekar SJ, Landreneau R,
Nichols F, Foster NR, DiPetrillo TA, Meyers B, Heron DE, Jones DR,
Tan AD, et al: A nomogram to predict recurrence and survival of
high-risk patients undergoing sublobar resection for lung cancer:
An analysis of a multicenter prospective study (ACOSOG Z4032). Ann
Thorac Surg. 102:239–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Liu C, Tan Z and Zhang T:
Segmentectomy versus wedge resection for stage I non-small cell
lung cancer: A meta-analysis. J Surg Res. 243:371–379. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Lin G and Li J: Comparative
effectiveness of lobectomy, segmentectomy, and wedge resection for
pathological stage I non-small cell lung cancer in elderly
patients: A population-based study. Front Surg. 8:6527702021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Sun Y, Li H, Bao M, Liu X, Jiang
G, Ye C and Hu Y: Surgical modality for stage IA non-small cell
lung cancer among the elderly: Analysis of the surveillance,
epidemiology, and end results database. J Thorac Dis. 12:6731–6742.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aslam S and Emmanuel P: Formulating a
researchable question: A critical step for facilitating good
clinical research. Indian J Sex Transm Dis AIDS. 31:47–50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sterne JA, Hernán MA, Reeves BC, Savović
J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT,
Boutron I, et al: ROBINS-I: A tool for assessing risk of bias in
non-randomised studies of interventions. BMJ. 355:i49192016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Altorki NK, Kamel MK, Narula N, Ghaly G,
Nasar A, Rahouma M, Lee PC, Port JL and Stiles BM: Anatomical
segmentectomy and wedge resections are associated with comparable
outcomes for patients with small cT1N0 non-small cell lung cancer.
J Thorac Oncol. 11:1984–1992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao J, Yuan P, Wang Y, Xu J, Yuan X, Wang
Z, Lv W and Hu J: Survival rates after lobectomy, segmentectomy,
and wedge resection for non-small cell lung cancer. Ann Thorac
Surg. 105:1483–1491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai C, Shen J, Ren Y, Zhong S, Zheng H, He
J, Xie D, Fei K, Liang W, Jiang G, et al: Choice of surgical
procedure for patients with non-small-cell lung cancer ≤1 cm or
>1 to 2 cm among lobectomy, segmentectomy, and wedge resection:
A population-based study. J Clin Oncol. 34:3175–3182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan X, Liang Y, Bai Y, Yang C and Xu S:
Conditional survival rate estimates of lobectomy, segmentectomy and
wedge resection for stage IA1 non-small cell lung cancer: A
population-based study. Oncol Lett. 20:1607–1618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Handa Y, Tsutani Y, Mimae T, Miyata Y,
Shimada Y, Ito H, Nakayama H, Ikeda N and Okada M: A multicenter
study of complex segmentectomy versus wedge resection in clinical
stage 0-IA non-small cell lung cancer. Clin Lung Cancer.
23:393–401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Handa Y, Tsutani Y, Mimae T, Miyata Y and
Okada M: Surgical procedure selection for stage I lung cancer:
Complex segmentectomy versus wedge resection. Clin Lung Cancer.
22:e224–e233. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang W, Pang X, Xi J, Chen X, Wang Q,
Qian C and Fan H: Clinical outcome of subcentimeter non-small cell
lung cancer after surgical resection: Single institution experience
of 105 patients. J Surg Oncol. 110:233–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura H, Taniguchi Y, Miwa K, Adachi Y,
Fujioka S, Haruki T, Takagi Y and Yurugi Y: Comparison of the
surgical outcomes of thoracoscopic lobectomy, segmentectomy, and
wedge resection for clinical stage I non-small cell lung cancer.
Thorac Cardiovasc Surg. 59:137–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sienel W, Dango S, Kirschbaum A, Cucuruz
B, Hörth W, Stremmel C and Passlick B: Sublobar resections in stage
IA non-small cell lung cancer: Segmentectomies result in
significantly better cancer-related survival than wedge resections.
Eur J Cardiothorac Surg. 33:728–734. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smith CB, Swanson SJ, Mhango G and
Wisnivesky JP: Survival after segmentectomy and wedge resection in
stage I non-small-cell lung cancer. J Thorac Oncol. 8:73–78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsou KC, Hsu HH, Tsai TM, Chen KC and Chen
JS: Clinical outcome of subcentimeter non-small cell lung cancer
after VATS resection: Single institute experience with 424
patients. J Formos Med Assoc. 119:399–405. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsutani Y, Kagimoto A, Handa Y, Mimae T,
Miyata Y and Okada M: Wedge resection versus segmentectomy in
patients with stage I non-small-cell lung cancer unfit for
lobectomy. Jpn J Clin Oncol. 49:1134–1142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu L, Zhao W, Chen T and Yang Y: Surgical
choice for patients with stage I non-small-cell lung cancer ≤2 cm:
An analysis from surveillance, epidemiology, and end results
database. J Cardiothorac Surg. 16:1912021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou Y, Yu T, Zhang Y, Qian L and Xia Q:
Comparison of surgical outcomes and prognosis between wedge
resection and simple Segmentectomy for GGO diameter between 2 cm
and 3 cm in non-small cell lung cancer: A multicenter and
propensity score matching analysis. BMC Cancer. 22:712022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mimae T, Saji H, Nakamura H, Okumura N,
Tsuchida M, Sonobe M, Miyazaki T, Aokage K, Nakao M, Haruki T, et
al: Survival of octogenarians with early-stage non-small cell lung
cancer is comparable between wedge resection and
lobectomy/segmentectomy: JACS1303. Ann Surg Oncol. 28:7219–7227.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao M, Lu T, Huang Y, Yin J, Jiang T, Li
M, Yang X, Zhan C, Feng M and Wang Q: Survival and long-term
cause-specific mortality associated with stage IA lung
adenocarcinoma after wedge resection vs. segmentectomy: A
population-based propensity score matching and competing risk
analysis. Front Oncol. 9:5932019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi Y, Wu S, Ma S, Lyu Y, Xu H, Deng L and
Chen X: Comparison between wedge resection and
lobectomy/segmentectomy for early-stage non-small cell lung cancer:
A bayesian meta-analysis and systematic review. Ann Surg Oncol.
29:1868–1879. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hoebeke PB, Rottey S, Van Heddeghem N,
Villeirs G, Pauwels P, Schrauwen W, Ceulemans P and Monstrey S:
One-stage penectomy and phalloplasty for epithelioid sarcoma of the
penis in an adolescent: Part 2. Eur Urol. 51:1744–1747. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stish BJ, Hallemeier CL, Olivier KR,
Harmsen WS, Allen MS and Garces YI: Long-term outcomes and patterns
of failure after surgical resection of small-cell lung cancer. Clin
Lung Cancer. 16:e67–e73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Patnaik SK, Kannisto E, Knudsen S and
Yendamuri S: Evaluation of microRNA expression profiles that may
predict recurrence of localized stage I non-small cell lung cancer
after surgical resection. Cancer Res. 70:36–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu Y, Han C, Wang Z, Gong L, Liu J, Chong
Y, Liu X, Liang N and Li S: An externally-validated dynamic
nomogram based on clinicopathological characteristics for
evaluating the risk of lymph node metastasis in small-size
non-small cell lung cancer. Front Oncol. 10:13222020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Altorki NK, Yip R, Hanaoka T, Bauer T, Aye
R, Kohman L, Sheppard B, Thurer R, Andaz S, Smith M, et al:
Sublobar resection is equivalent to lobectomy for clinical stage 1A
lung cancer in solid nodules. J Thorac Cardiovasc Surg.
147:754–762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sato M, Kobayashi M, Kojima F, Tanaka F,
Yanagiya M, Kosaka S, Fukai R and Nakajima J: Effect of
virtual-assisted lung mapping in acquisition of surgical margins in
sublobar lung resection. J Thorac Cardiovasc Surg.
156:1691–1701.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kadota K, Nitadori JI, Sima CS, Ujiie H,
Rizk NP, Jones DR, Adusumilli PS and Travis WD: Tumor spread
through air spaces is an important pattern of invasion and impacts
the frequency and location of recurrences after limited resection
for small stage I lung adenocarcinomas. J Thorac Oncol. 10:806–814.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tsutani Y, Handa Y, Shimada Y, Ito H,
Ikeda N, Nakayama H, Yoshimura K and Okada M: Comparison of cancer
control between segmentectomy and wedge resection in patients with
clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc
Surg. 162:1244–1252.e1. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Suzuki K, Watanabe SI, Wakabayashi M, Saji
H, Aokage K, Moriya Y, Yoshino I, Tsuboi M, Nakamura S, Nakamura K,
et al: A single-arm study of sublobar resection for ground-glass
opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg.
163:289–301.e2. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
He Z, Li Z, Xu S, Wu W, Zhu Q, Wang J, Wen
W and Chen L: Prognostic significance of lymph node count removed
at sublobar resection in pathologic stage IA non-small-cell lung
cancer: A population-based analysis. Clin Lung Cancer. 22:e563–e73.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Baig MZ, Razi SS, Stroever S, Weber JF,
Connery CP and Bhora FY: Anatomic resection has superior long-term
survival compared with wedge resection for second primary lung
cancer after prior lobectomy. Eur J Cardiothorac Surg.
59:1014–1020. 2021. View Article : Google Scholar : PubMed/NCBI
|