Introduction
Peripheral T cell lymphoma (PTCL) is a malignant
tumor of the lymphatic system originating from mature natural
killer or T cells, and is a type of aggressive non-Hodgkin's
lymphoma with poor prognosis (1).
It accounts for ~10% of all lymphomas in western countries and 20%
in Asian populations (1). Different
pathological types of PTCL have distinct immunophenotypes,
molecular characteristics and clinical manifestations but all show
low sensitivity to chemotherapy. PTCL-not otherwise specified (NOS)
is the most common pathological subtype of PTCL, accounting for
21–27% of PTCL cases. PTCL-NOS predominantly occurs in middle-aged
and elderly individuals and is more aggressive than B cell lymphoma
with a 5-year overall survival (OS) rate of 20–30% (2). Even with intensive treatment, 5-year
progression-free survival (PFS) rate is ~20% (3). Moreover, due to the prevalence of
underlying disease and weakened organ functions in elderly
patients, they are often unable to tolerate standard doses of
chemotherapy, which further decreases the treatment effect. Our
center, (The 940th Hospital of the Joint Logistics Support Force of
the Chinese People's Liberation Army; Lanzhou, China), successfully
treated an elderly patient with PTCL-NOS with Tet methylcytosine
dioxygenase 2 (TET2) and DNA methyltransferase 3α (DNMT3A) gene
mutations which were the two causative genes of PCTL. The patient
achieved complete remission after combined chemotherapy with
azacitidine and chidamide.
Case report
A 71-year-old male patient visited The 940th
Hospital of the Joint Logistics Support Force of the Chinese
People's Liberation Army in February 2023 due to enlargement of
lymph nodes in multiple parts of the body. There was no history of
diabetes, hypertension or heart disease. He had no symptoms such as
fatigue, fever or night sweats, but showed a weight loss >5 kg
over the past 6 months. Hematological results were as follows:
White blood cell count, 13.42×109/l (reference range,
3.5–9.5×109/l); lymphocyte count, 1.08×109/l
(reference range, 0.8–4.0×109/l); neutrophil count,
9.98×109/l (reference range, 2.0–7.0×109/l);
red blood cell count, 5.34×1012/l (reference range,
4.09–5.74×1012/l); hemoglobin, 165 g/l (reference range,
131–172 g/l); platelet count, 97×109/l (reference range,
85–303×109/l); β2-microglobulin (β2-MG), 4.47 mg/l
(reference range, 0.97–2.64 mg/l) and lactate dehydrogenase (LDH),
586 IU/l (reference range, 120–250 IU/l); hepatitis series tests
were normal (Table I). Superficial
lymph node ultrasound revealed multiple enlarged lymph nodes in
bilateral neck, axillary and inguinal areas with the largest
measuring 3.1×1.6, 2.8×1.9 and 3.3×1.6 cm at each location,
respectively, with unclear lymph node hilum structure, suggestive
of lymphoma. A neck lymph node biopsy was performed. Briefly,
tissue was fixed with 4% paraformaldehyde overnight at room
temperature, embedded in paraffin and sliced into 5-µm sections.
The sections were stained by hematoxylin and eosin staining. For
immunohistochemistry, the sections were then dewaxed by heating to
65°C followed by three washes with xylene, and rehydrated via an
ethanol series. The sections were treated with 3%
H2O2 for 10 min to block endogenous
peroxidase activity and then blocked with 5% bovine serum albumin
for 10 min at 37°C. Afterwards, sections were incubated with
primary antibodies for 1 h at 25°C and with the secondary antibody
at room temperature for 30 min at 25°C. Observation of sections at
×400 and ×200 was carried out by a BX53 biological light microscope
(Olympus Corporation). Pathology showed lymph node structure damage
with diffuse distribution of medium-sized atypical cells and
eosinophil infiltration. The tumor cells were characterized by
irregular nuclei, prominent nucleoli and scant cytoplasm (Fig. 1A). Tumor cells were negative for
EBV-encoded RNA, as well as CD10, 15, 20, 30 and 56, Multiple
Myeloma Oncogene 1 and granzyme B (Fig.
1B-P). Tumor cells were positive for CD3-5 and 8, CD21
follicular reticulum and CD38 plasma cells. Ki67 index was ~40%.
These results were suggestive of PTCL-NOS. Positron emission
tomography/computed tomography (PET-CT; Siemens Biograph 64 True
Point; Siemens AG; CT scan parameters were as follows: Tube
voltage, 120 kV; automatic tube current; layer thickness, 3 mm and
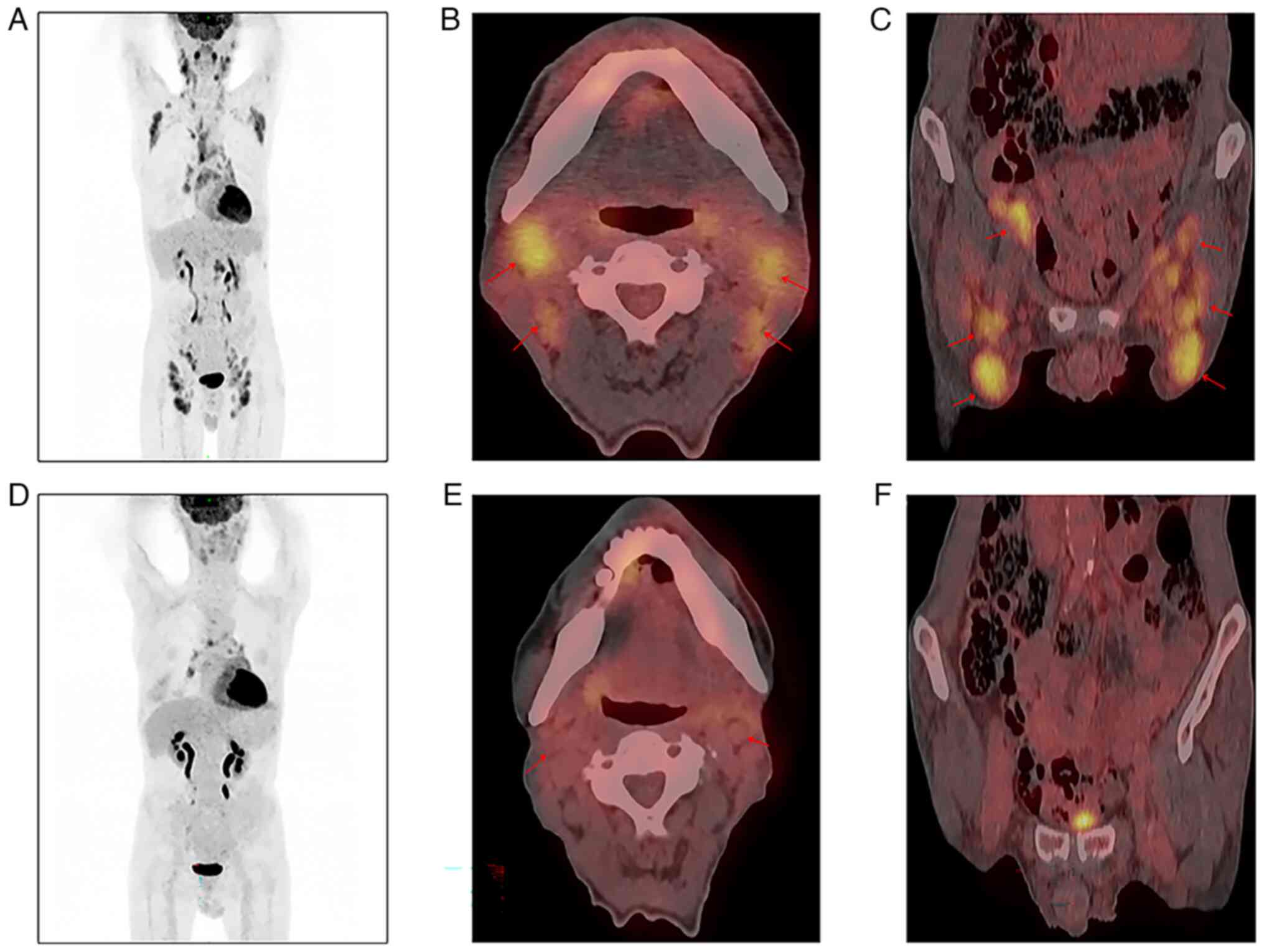
layer spacing, 0.8 mm; Fig. 2A-C)
revealed abnormally increased fluorodeoxyglucose (FDG) uptake in
multiregional lymph nodes [maximum standard uptake value (SUV) in
the neck lymph nodes, 7.79; maximum SUV in the inguinal lymph
nodes, 6.88], consistent with stage III lymphocytoma [5-point scale
(5-PS) score, 5]. FDG is a glucose analog used to evaluate glucose
metabolism by measuring uptake. The PET/CT results were assessed
according to the Deauville 5-PS criteria. The 5-PS scoring system
was used to qualitatively evaluate the treatment response as
follows: i) No uptake; ii) uptake ≤ mediastinal blood pool; iii)
uptake > mediastinal blood pool; iv) uptake moderately increased
compared with the liver uptake at any site; and v) uptake markedly
increased compared with the liver. The 5-PS scoring system was used
to qualitatively evaluate the treatment response as follows: i) no
uptake; ii) uptake ≤ mediastinal blood pool; iii) uptake >
mediastinal blood pool, but ≤ liver; iv) uptake moderately
increased compared with the liver uptake at any site; and v) uptake
markedly increased compared with the liver at any site. Scores of
4–5 were considered positive, while scores of 1–3 were considered
negative (4). The bone marrow cell
morphology examination included posterior iliac crest bone marrow
aspirate smears were that stained with Wright-Giemsa for 2 min and
then rinsed with phosphate buffer (pH 7.0) for another 10 min at
room temperature. The image was collected by a light microscope
(Olympus BX-53; Olympus Corporation; magnification, ×1,000.) and
showed no abnormality. Next generation sequencing on a targeted
panel of 15 genes was performed by Suzhou Youqin Medical Laboratory
on formalin-fixed, paraffin-embedded tumor tissue. Sequencing
analyses revealed DNMT3A and TET2 mutations (Table II). There were no mutations in Ras
Homolog Family Member A (RHOA) or Isocitrate Dehydrogenase 2 (IDH2)
genes. The patient was diagnosed with PTCL-NOS, stage IIIB,
(5) International Prognostic Index
(IPI) score of 4. CHOEP chemotherapy regimen was administered
[cyclophosphamide, 1 g, intravenous (IV) drip, D1; liposomal
doxorubicin, 20 mg, IV drip, D1; vincristine, 2 mg, IV drip, D1;
etoposide injection, 0.1 g, IV drip, D1-3; prednisone tablets 60
mg/day, D1-5]. The chemotherapy process went smoothly, and no other
serious adverse reactions or suspected unexpected adverse reactions
occurred. The patient was discharged after treatment in February
2023. In March 2023, CHOEP chemotherapy regimen was continued as a
second-course treatment, but the patient had poor tolerance and
developed acute renal failure and cardiac insufficiency. The
condition gradually stabilized following dialysis treatment. In May
2023, a follow-up ultrasound of superficial lymph nodes showed a
slight decrease in size (largest neck lymph node, 2.1×1.3; largest
axillary lymph node, 3.0×1.4 and largest inguinal lymph node,
2.3×1.2 cm). The patient was treated with the COEP regimen combined
with chidamide [cyclophosphamide, 1 g, IV drip, D1; vincristine, 2
mg, IV drip, D1; etoposide injection, 0.1 g, IV drip, D1-3;
prednisone tablets 50 mg/day, D1-5; chidamide, 20 mg, PO orally,
twice weekly (biw)]. The treatment proceeded smoothly, and no
serious adverse reactions occurred during the treatment. In June
2023, follow-up ultrasound revealed an increase in lymph node size
(largest neck lymph node, 2.71.4; largest axillary lymph node,
3.6×1.5; largest inguinal lymph node, 4.5×1.8 cm), suggesting
progression of the primary disease. The patient received combined
treatment with chidamide + azacitidine + COP (chidamide, 20 mg, PO
biw; azacitidine, 100 mg, subcutaneous injection (SC), D1-7;
cyclophosphamide, 1 g, IV drip, D1; vincristine, 2 mg, IV drip, D1;
prednisone tablets, 50 mg PO, D1-5). After 4 weeks, follow-up
ultrasound of the lymph nodes showed notably decreased size
(largest neck lymph node, 1.4×0.6; largest axillary lymph node,
1.9×0.7; largest inguinal lymph node, 1.7×0.5 cm). PET-CT (Fig. 2D-F) scan revealed a mild increase in
FDG metabolism in multiregional lymph nodes (maximum SUV in the
neck lymph nodes, 2.65; metabolic activity in the inguinal lymph
nodes disappeared), consistent with complete remission (CR) phase
metabolic changes following lymphoma treatment (5-PS score, 2).
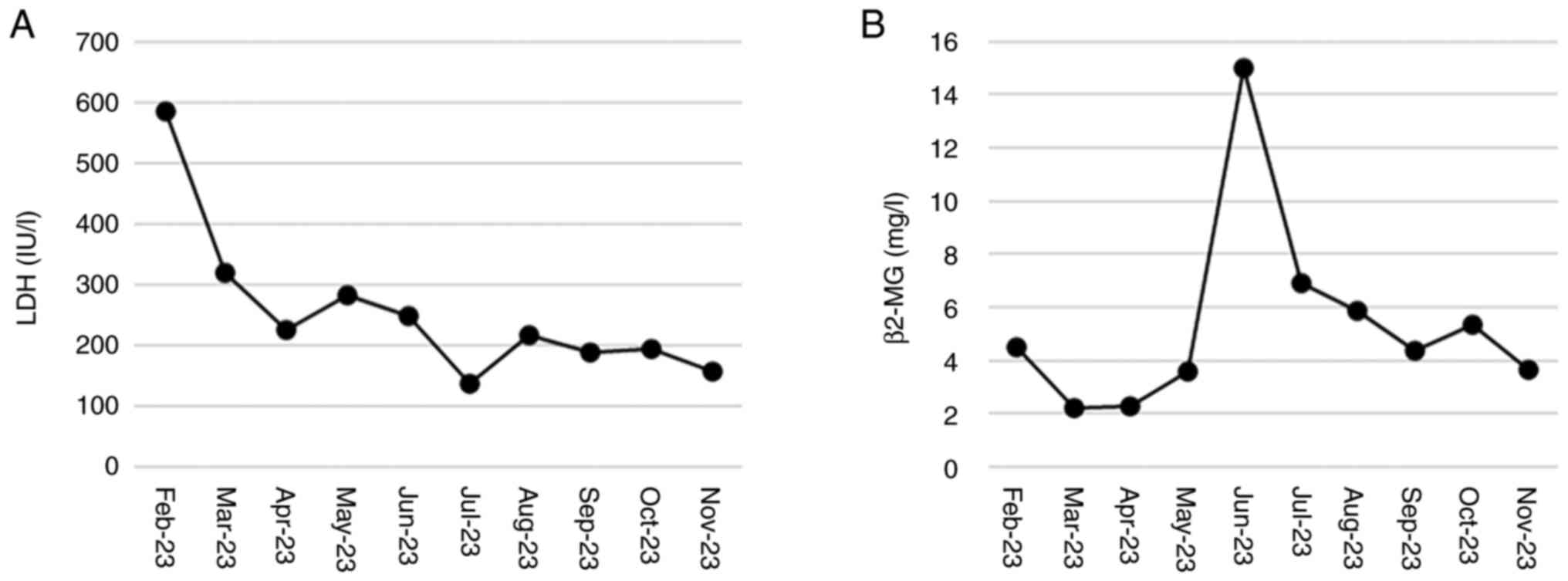
After starting treatment, β2-MG and LDH levels showed a downward
trend with the patient achieving remission (Fig. 3). Considering the patient's advanced
age and poor tolerance to conventional chemotherapy, treatment with
azacitidine in combination with chidamide was continued
(azacitidine, 100 mg, SC, D1-7, every four weeks + chidamide, 20
mg, PO biw). Follow-up was performed once a month and the patient's
condition remained stable until final follow-up in December
2023.
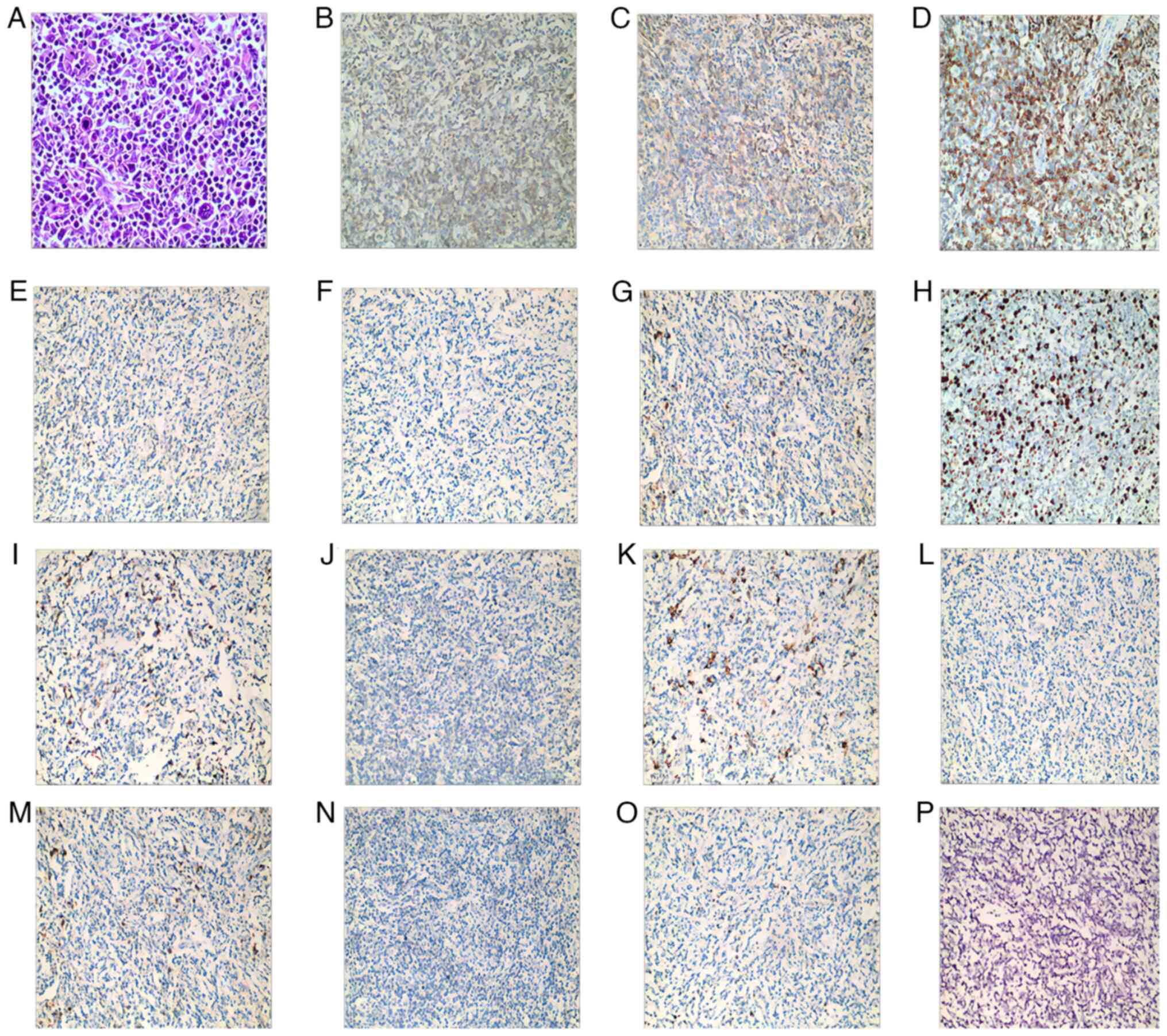 | Figure 1.Histological morphology and
immunohistochemical staining of the tumor. (A) Diffuse distribution
of medium-sized atypical cells (magnification, ×400). The tumor
cells were positive for (B) CD3 (magnification, ×200), (C) CD4
(magnification, ×200), (D) CD5 (magnification, ×200), (E) CD8
(magnification, ×200), (F) CD21 (magnification, ×200) and (G) CD38
(magnification, ×200). (H) Ki67 index was ~40% (magnification,
×200). Tumor cells were negative for (I) CD10 (magnification,
×200), (J) CD15 (magnification, ×200), (K) CD20 (magnification,
×200), (L) CD30 (magnification, ×200), (M) CD56 (magnification,
×200), (N) granzyme B (magnification, ×200), (O) multiple myeloma
oncogene 1 (magnification, ×200) and (P) EBV-encoded RNA
(magnification, ×200). |
 | Table I.Laboratory data at the time of the
initial visit. |
Table I.
Laboratory data at the time of the
initial visit.
| Parameter | Result |
|---|
| White blood cell
count |
13.32×109/l |
| Hemoglobin | 165.00 g/l |
| Platelet count |
97.00×109/l |
| Lymphocyte count | 1.08
×109/l |
| Lactate
dehydrogenase | 586.00 IU/l |
| β2-microglobulin | 4.47 mg/l |
| Creatinine | 68.00 µmol/l |
| Uric acid | 305.00 µmol/l |
| Hepatitis B
virus | Negative |
| Hepatitis C
virus | Negative |
| Globulin | 38.80 g/l |
 | Table II.Test results of lymphoma-associated
gene mutations in the patient. |
Table II.
Test results of lymphoma-associated
gene mutations in the patient.
| Gene | Mutation site | Nucleotide
change | Variant allele
frequency, % |
|---|
| DNMT3A | Exon 20 | c.2408G>A | 3.40 |
|
| Exon 23 | c.2645G>T | 31.70 |
| TET2 | Exon
3 | c.2725C>T | 22.10 |
|
|
| c.1337delT | 22.70 |
|
| Exon
5 | c.3594G>A | 2.50 |
|
| Exon
9 | c.4045A>T | 4.50 |
Discussion
PTCL-NOS is the most common type of T cell tumor and
is characterized by high heterogeneity and aggressiveness. PTCL-NOS
can involve any site but typically occurs within lymph nodes. A
total of ~85% of patients present with stage III–IV disease,
accompanied by involvement of the bone marrow, spleen and other
extranodal sites (2,6). B symptoms including unexplained fever,
drenching night sweats and ≥10% weight loss over the previous 6
months are common, and some patients may exhibit increased
eosinophil and hemophagocyte counts. The cytological features are
highly variable, often exhibiting a mixture of medium and small
cells with irregular nuclear shapes (2,6). One
of the primary characteristics of the immunophenotype is the
absence of one or more T cell markers, most commonly the loss of
CD5 and CD7 expression (7).
Furthermore, in ~40% of cases, there is a presence of CD4 and CD8
double negativity or co-expression (7).
With the development of gene sequencing technology,
genetic analysis of PTCL has improved, and the molecular
pathogenesis is being increasingly uncovered. Epigenetic mechanisms
serve a crucial role in the development of many tumors, including
PTCL. Abnormal DNA methylation and histone acetylation can activate
oncogenes, promoting the progression of PTCL (8,9).
Several mutations in epigenetic modifier genes have been reported
in PTCL, such as TET2, IDH2-R172, RHOA, IDH2 and DNMT3A (10,11).
Among these, DNMT3A, IDH2, and TET2 mutations are the most common
in angioimmunoblastic T cell lymphoma (AITL) and PTCL-NOS. These
mutations are associated with disease progression (10,11).
TET2 mutation is a common driving factor in myeloid and lymphoid
system tumors, which can cause malignant lymphoma by disrupting the
conversion of 5-methylcytosine to 5-hydroxymethylcytosine (12). Mice with TET2 mutation are prone to
T cell diseases, such as PTCL, predominantly originating from
follicular helper T (TFH) cells, which may be associated with the
effect of TET2 on T cell polarization (13–15).
TET2 loss promotes CD4+ T-cell differentiation
manifested by strong skewing towards TFH/Th17 phenotypes (15). DNMT3A mutation is common in myeloid
malignancy and can lead to abnormal DNA methylation patterns,
thereby altering expression of genes involved in cell
differentiation or regulating hematopoietic function. The specific
mechanisms of DNMT3A mutation in PTCL are not clear. Experiments
have shown that DNMT3A+/− mice have an increased risk of
developing CD8-positive lymphomas when p53 expression is
downregulated (16). In PTCL,
DNMT3A mutation occurs more frequently in AITL compared with other
subtypes, with ~30% overlapping with TET2 mutation, while in
PTCL-NOS, the incidence of DNMT3A with TET2 mutation is 4–10%
(17,18). The patient in the present case had
both DNMT3A and TET2 mutations.
Currently, the first-line treatment for PTCL is
based on anthracyclines (usually CHOP or CHOEP). However, the
outcomes are not satisfactory, as nearly half of patients fail to
achieve CR and the efficacy is short-lived and prone to relapse.
High-dose chemotherapy followed by autologous stem cell
transplantation as consolidation therapy can improve prognosis,
with 2-year event-free survival (EFS) rate of 40–50% (19–21).
However, this benefit is observed primarily in patients who are
tolerant and highly responsive to the treatment.
Relapsed/refractory PTCL-NOS has median EFS and OS typically <6
months (22,23). Considering the adverse factors of
age and comorbidities, this highlights the importance of developing
new therapies, especially for elderly patients who may be
intolerant to chemotherapy (24).
To address the recurrent epigenetic changes in PTCL, histone
deacetylase inhibitor (HDACi) and hypomethylating agents (HMA) are
employed in the treatment of PTCL. HDACi has shown significant
benefits in 20–25% of PTCL-NOS cases, with chidamide monotherapy
for relapsed/refractory PTCL showing an efficacy rate of 28% and a
median OS of 21.4 months (25).
Moreover, it exhibits synergistic effects with chemotherapy agents,
enhancing chemosensitivity, and combination therapy has advantages
in response rate and long-term survival (25). The HMA, azacitidine monotherapy (SC)
for TET2-mutated relapsed/refractory AITL has an overall response
rate (ORR) of 75% and a CR rate of 50% (26). Azacitidine (PO) in combination with
CHOP as a first-line treatment for PTCL has a CR rate of up to 75%,
and 2-year PFS and OS rates of 65.8 and 68.4%, respectively
(27). The dual epigenetic
regulating treatment (HDACi + HMA) shows advantages in ORR and CR.
In a study of azacitidine (PO) in combination with romidepsin in
the treatment of PTCL, among the 25 enrolled patients, 13 were
relapsed/refractory PTCL and 17 were AITL or TFH phenotype PTCL.
The ORR and CR rates for this regimen were 61 and 48%,
respectively. In the AITL and TFH phenotype PTCL subgroups, ORR and
CR rates were 80 and 67%, respectively, indicating superior
efficacy (28). The regimen of
chidamide combined with azacytidine + CHOP has been proved to be
feasible and safe in the treatment of PTCL in a phase 2 trial
(29). In the present case, the
elderly patient was initially treated with first-line CHOEP
chemotherapy regimen and did not achieve an ideal effect, with
superficial lymph nodes showing enlargement and cardiac and renal
function failure occurred due to the toxicity of the chemotherapy.
Considering the patient's DNA methylation-associated TET2 and
DNMT3A mutations, chidamide in combination with azacitidine was
used for the treatment with dose-reduced chemotherapy, without
anthracyclines. The outcome was equally encouraging with CR
achieved as the dose-reduced chemotherapy regimen was also
effective. The patient was subsequently treated with a
chemotherapy-free regimen of chidamide + azacitidine. During the
follow-up period, the condition remained in a stable state.
Therefore, it was hypothesized that chidamide + azacitidine is more
effective for patients with PTCL-NOS with associated mutations of
epigenetic regulator genes, and these mutations may serve as
biomarkers for the treatment of chidamide in combination with
azacitidine. However, this should be validated using a large sample
size prospective study.
To the best of our knowledge, there are few reports
(29) of chidamide in combination
with azacitidine in the treatment of PTCL-NOS. The present case
demonstrated good short-term treatment effects, and the long-term
efficacy of this regimen was also promising. The present case may
provide new treatment ideas for elderly patients with PTCL who are
intolerant or insensitive to chemotherapy, and also facilitate
development of new treatment regimens for PTCL.
Acknowledgements
Not applicable.
Funding
The study was supported by a grant from The Gansu Province
Innovation Base and Talent Plan (Gansu Province Leukemia Clinical
Research Center; grant no. 21JR7RA015).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FX, RZ, HT, JM, HL and TW contributed to study
conception and design. FX, RZ, HT and JM collected data. HL and TW
confirm the authenticity of all the raw data. HL wrote the
manuscript. TW edited the manuscript. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bisig B, Savage KJ and De Leval L:
Pathobiology of nodal peripheral T-cell lymphomas: Current
understanding and future directions. Haematologica. 108:3227–3243.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weisenburger DD, Savage KJ, Harris NL,
Gascoyne RD, Jaffe ES, MacLennan KA, Rüdiger T, Pileri S, Nakamura
S, Nathwani B, et al: Peripheral T-cell lymphoma, not otherwise
specified: A report of 340 cases from the international peripheral
T-cell lymphoma project. Blood. 117:3402–3408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zain JM: Aggressive T-cell lymphomas: 2019
Updates on diagnosis, risk stratification, and management. Am J
Hematol. 94:929–946. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuertes S, Setoain X, Lopez-Guillermo A,
Carrasco JL, Rodríguez S, Rovira J and Pons F: Interim FDG PET/CT
as a prognostic factor in diffuse large B-cell lymphoma. Eur J Nucl
Med Mol Imaging. 40:496–504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian
Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group;
European Mantle Cell Lymphoma Consortium, ; et al: Recommendations
for initial evaluation, staging, and response assessment of Hodgkin
and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol.
32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vose J, Armitage J and Weisenburger D;
International T-Cell Lymphoma Project, : International peripheral
T-cell and natural killer/T-cell lymphoma study: Pathology findings
and clinical outcomes. J Clin Oncol. 26:4124–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Went P, Agostinelli C, Gallamini A,
Piccaluga PP, Ascani S, Sabattini E, Bacci F, Falini B, Motta T,
Paulli M, et al: Marker expression in peripheral T-cell lymphoma: A
proposed clinical-pathologic prognostic score. J Clin Oncol.
24:2472–2479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bates SE: Epigenetic therapies for cancer.
N Engl J Med. 383:650–663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tigu AB and Bancos A: The role of
epigenetic modifier mutations in peripheral T-cell lymphomas. Curr
Issues Mol Biol. 45:8974–8988. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vallois D, Dobay MP, Morin RD, Lemonnier
F, Missiaglia E, Juilland M, Iwaszkiewicz J, Fataccioli V, Bisig B,
Roberti A, et al: Activating mutations in genes related to TCR
signaling in angioimmunoblastic and other follicular helper
T-cell-derived lymphomas. Blood. 128:1490–1502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watatani Y, Sato Y, Miyoshi H, Sakamoto K,
Nishida K, Gion Y, Nagata Y, Shiraishi Y, Chiba K, Tanaka H, et al:
Molecular heterogeneity in peripheral T-cell lymphoma, not
otherwise specified revealed by comprehensive genetic profiling.
Leukemia. 33:2867–2883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiba S: Dysregulation of TET2 in
hematologic malignancies. Int J Hematol. 105:17–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muto H, Sakata-Yanagimoto M, Nagae G,
Shiozawa Y, Miyake Y, Yoshida K, Enami T, Kamada Y, Kato T, Uchida
K, et al: Reduced TET2 function leads to T-cell lymphoma with
follicular helper T-cell-like features in mice. Blood Cancer J.
4:e2642014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Solary E, Bernard OA, Tefferi A, Fuks F
and Vainchenker W: The ten-eleven translocation-2 (TET2) gene in
hematopoiesis and hematopoietic diseases. Leukemia. 28:485–496.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yue X, Lio CJ, Samaniego-Castruita D, Li X
and Rao A: Loss of TET2 and TET3 in regulatory T cells unleashes
effector function. Nat Commun. 10:20112019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haney SL, Upchurch GM, Opavska J,
Klinkebiel D, Hlady RA, Roy S, Dutta S, Datta K and Opavsky R:
Dnmt3a is a haploinsufficient tumor suppressor in CD8+ peripheral T
cell lymphoma. PLoS Genet. 12:e10063342016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dobay MP, Lemonnier F, Missiaglia E,
Bastard C, Vallois D, Jais JP, Scourzic L, Dupuy A, Fataccioli V,
Pujals A, et al: Integrative clinicopathological and molecular
analyses of angioimmunoblastic T-cell lymphoma and other nodal
lymphomas of follicular helper T-cell origin. Haematologica.
102:e148–e151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Couronné L, Bastard C and Bernard OA: TET2
and DNMT3A mutations in human T-cell lymphoma. N Engl J Med.
366:95–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ellin F, Landström J, Jerkeman M and
Relander T: Real-world data on prognostic factors and treatment in
peripheral T-cell lymphomas: A study from the Swedish lymphoma
registry. Blood. 124:1570–1577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
d'Amore F, Relander T, Lauritzsen GF,
Jantunen E, Hagberg H, Anderson H, Holte H, Österborg A, Merup M,
Brown P, et al: Up-front autologous stem-cell transplantation in
peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 30:3093–3099.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SI, Horwitz SM, Foss FM, Pinter-Brown
LC, Carson KR, Rosen ST, Pro B, Hsi ED, Federico M, Gisselbrecht C,
et al: The role of autologous stem cell transplantation in patients
with nodal peripheral T-cell lymphomas in first complete remission:
Report from COMPLETE, a prospective, multicenter cohort study.
Cancer. 125:1507–1517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mak V, Hamm J, Chhanabhai M, Shenkier T,
Klasa R, Sehn LH, Villa D, Gascoyne RD, Connors JM and Savage KJ:
Survival of patients with peripheral T-cell lymphoma after first
relapse or progression: Spectrum of disease and rare long-term
survivors. J Clin Oncol. 31:1970–1976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang JY, Briski R, Devata S, Kaminski MS,
Phillips TJ, Mayer TL, Bailey NG and Wilcox RA: Survival following
salvage therapy for primary refractory peripheral T-cell lymphomas
(PTCL). Am J Hematol. 93:394–400. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mead M, Cederleuf H, Björklund M, Wang X,
Relander T, Jerkeman M, Gaut D, Larson S and Ellin F: Impact of
comorbidity in older patients with peripheral T-cell lymphoma: An
international retrospective analysis of 891 patients. Blood Adv.
6:2120–2128. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi Y, Dong M, Hong X, Zhang W, Feng J,
Zhu J, Yu L, Ke X, Huang H, Shen Z, et al: Results from a
multicenter, open-label, pivotal phase II study of chidamide in
relapsed or refractory peripheral T-cell lymphoma. Ann Oncol.
26:1766–1771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lemonnier F, Dupuis J, Sujobert P,
Tournillhac O, Cheminant M, Sarkozy C, Pelletier L, Marçais A, Robe
C, Fataccioli V, et al: Treatment with 5-azacytidine induces a
sustained response in patients with angioimmunoblastic T-cell
lymphoma. Blood. 132:2305–2309. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruan J, Moskowitz A, Mehta-Shah N, Sokol
L, Chen Z, Kotlov N, Nos G, Sorokina M, Maksimov V, Sboner A, et
al: Multicenter phase 2 study of oral azacitidine (CC-486) plus
CHOP as initial treatment for PTCL. Blood. 141:2194–2205.
2023.PubMed/NCBI
|
|
28
|
Falchi L, Ma H, Klein S, Lue JK, Montanari
F, Marchi E, Deng C, Kim HA, Rada A, Jacob AT, et al: Combined oral
5-azacytidine and romidepsin are highly effective in patients with
PTCL: A multicenter phase 2 study. Blood. 137:2161–2170. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao C, Ding Y, Zeng C, Nan Y and Liu Y:
PB2310: Chidamide with azacitidine and chop treatment for patients
with newly diagnosed peripheral T-cell lymphoma: Interim analysis
of a prospective, single center, single-arm, phase 2 trial.
HemaSphere. 7((S3)): e75089382023. View Article : Google Scholar
|