Introduction
Breast cancer (BC) has surpassed lung cancer as the
most widespread malignant tumor worldwide, particularly among
women. This poses a substantial risk to their physical and mental
well-being and quality of life (1,2). With
increased health awareness and the implementation of BC screening,
more patients with early breast cancer (EBC) are being identified.
Axillary lymph nodes (ALN) are the primary pathway for BC
metastasis and dissemination. Identifying ALN metastasis (ALNM) is
essential not only for accurately determining the tumor stage but
also for determining the appropriate degree of axillary dissection
to prevent tumor metastasis and spread (3).
Sentinel lymph node biopsy (SLNB) is the standard
approach for axillary staging in patients with EBC and clinically
negative axillary lymph nodes (cN0) who have not undergone
neoadjuvant chemoradiotherapy (4).
Nonetheless, >70% of patients with EBC and cN0 do not exhibit
ALNM (4,5). Moreover, SLNB is an invasive procedure
and can result in complications, such as infections in the wound,
hematomas and abnormalities in sensory perception (6,7). Liu
et al have suggested that SLNB might be an overtreatment for
most patients with EBC and cN0 (8).
Recent studies have increasingly focused on the possibility of
identifying patients with low risk of developing ALNM among those
with EBC having cN0 to avoid unnecessary SLNB (9,10).
Therefore, developing a convenient and effective method to predict
the ALN status in patients with EBC and cN0 is necessary, which
could greatly assist in devising individualized treatment
strategies. Predicting the preoperative ALN status can help
eliminate unnecessary SLNB and minimize surgical trauma.
Ultrasound (US) is preferred for assessing ALN
status. ALNM prediction is based on morphological alterations of
the size, cortical thickness, blood flow, lymphatic portal
structure and boundary characteristics of the ALN (11–13).
During the first phases of metastasis, there are minimal
alterations in the size and structure of ALN. As a result, the US
features of metastatic and reactive lymph nodes frequently exhibit
similarities (13,14). Therefore, the sensitivity,
specificity, and accuracy of US alone for ALNM diagnosis remain
suboptimal (15,16).
In the era of precision medicine, constructing a
more practical, reliable and accurate clinical decision-making tool
for ALNM risk prediction carries great significance. Therefore, the
present study aimed to develop a nomogram model for predicting risk
of ALNM, utilizing readily available axillary US findings and
clinicopathological features of tumors.
Patients and methods
Patients
The present study included data from a total of
1,799 patients with BC admitted to the Department of Breast
Diseases of Jiaxing Maternity and Child Health Care Hospital
(Jiaxing, China) between January 1st, 2014 and September 10th,
2023. The inclusion criteria were as follows: i) Having
histologically confirmed early-stage (T1-T2) invasive ductal
carcinoma; ii) in cases of SLN metastasis, the metastatic lesion
was ≥2 mm with SLNB performed intraoperatively (17); iii) preoperative US examination was
conducted; iv) preoperative clinical absence of ALN involvement;
and v) availability of complete clinical data. The exclusion
criteria were as follows: i) Male patients; ii) incomplete clinical
data; iii) prior systemic neoadjuvant chemoradiotherapy; iv)
non-invasive ductal carcinoma; v) recurrent or bilateral BC; vi)
other concurrent malignant tumors; and vii) preoperative clinical
positivity for ALN involvement. The present study was approved
(approval no. KY-2023-132) by the Research Ethics Committee of
Jiaxing Maternity and Child Health Care Hospital (Jiaxing,
China).
Patient screening process
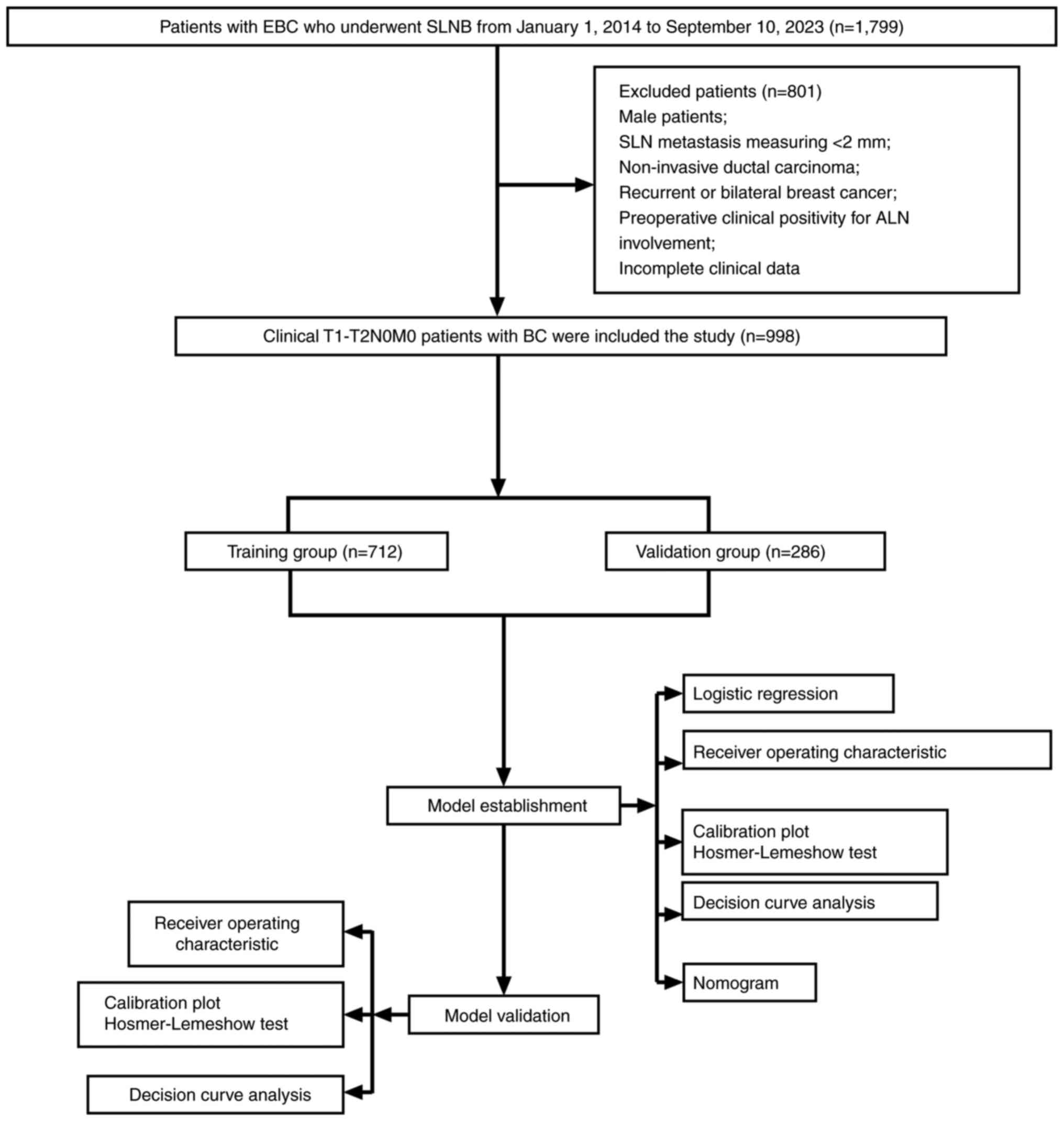
After applying the inclusion and exclusion criteria,
a total of 998 patients, ranging from 21–87 years old were enrolled
in the study and randomly divided into a training and validation
group in a 7:3 ratio. The 7:3 split aims to balance between having
enough training data and enough validation data to reliably
estimate model performance on unseen data. The axillary US findings
and clinicopathological features of tumors of the enrolled patients
were then retrospectively analyzed. Logistic regression analysis
was performed to identify independent risk factors for ALNM. Based
on the results, a nomogram model was constructed and was
subsequently validated (Fig.
1).
Indicators
The evaluation indicators for the present study were
categorized into two groups: i) Axillary US findings and ii)
clinicopathological features of tumors. The morphological
characteristics of ALN play a crucial role in determining ALNM via
US. In healthy individuals, ALNs have an elliptical shape (18). However, when metastatic tumor cells
infiltrate, the structure of ALNs becomes disrupted, leading to
enlargement, thickening of the cortical layer, increased blood
flow, expansion in the lateral direction and a decrease in the
aspect ratio (19,20). A comprehensive review of the US
findings for the enrolled patients was performed to assess ALN
characteristics, including number, size, shape, aspect ratio,
internal echogenicity, cortical thickness, lymphatic portal
structure and blood flow patterns. Suspicious metastasis (positive)
was considered when more than two metastatic features were present
(21–23).
Information regarding the clinicopathological
characteristics of tumors was obtained from the electronic medical
record system. The data included variables such as age, menopausal
status, pathological type, maximum diameter (MD), tumor location,
lymphovascular invasion (LVI), estrogen receptor (ER) status,
progesterone receptor (PR) status, human epidermal growth factor
receptor-2 (HER-2) status, Ki-67 expression, histological grade,
molecular subtype and ALN status. Several lesions were observed,
measurements were obtained for each lesion, and the largest MD was
selected. The tumor location was categorized into upper outer and
other quadrants. Histological grade was stratified into grades I/II
and III. The positive threshold for ER and PR immunohistochemistry
(IHC) was set at ≥1%, with an ER/PR expression of ≥1% classified as
hormone receptor (HR)-positive (24). Initially, the HER-2 status was
evaluated via IHC, where an IHC score of 3+ indicated HER-2
positivity, while an IHC score of 0 or 1+ indicated HER-2
negativity. Subsequently, IHC 2+ was further verified through
fluorescence in situ hybridization (25,26).
The molecular subtype was divided into three categories based on
the 2013 St. Gallen conference guidelines: i) Triple-negative BC
(TNBC) [HR (−), HER-2 (−)]; ii) HER-2-positive BC [HR (−)/HR (+),
HER-2 (+)] and luminal BC [HR (+), HER-2 (−)] (27).
Statistical analysis
Statistical Package for the Social Sciences (SPSS;
version 26.0; IBM) and R (v.4.2.3; http://www.r-project.org/) software were used for data
analysis. Receiver operating characteristic (ROC) curve analysis
was used to convert continuous measurement data into binary
classification countable data. These countable data were presented
as frequencies (percentages) and analyzed using the chi-square
test. To develop a nomogram model, logistic regression analysis was
conducted using the ‘glm’ function (R v.4.2.3; http://www.r-project.org/). The findings were
presented as odds ratios (OR) and 95% confidence intervals (CIs).
The Akaike Information Criterion (AIC) was used to select the final
model, that is, the model with the lowest AIC. To evaluate the
presence of multicollinearity among the predictive factors, the
variance inflation factor (VIF) was computed for each variable. A
VIF value <5 indicated the absence of significant
multicollinearity. The ‘pROC’ package (R v.4.2.3; http://www.r-project.org/) was utilized to evaluate
the performance of the model by generating the ROC curve and
computing the corresponding area under the curve (AUC). Calibration
curves were generated, and the nomogram was constructed using the
‘rms’ package (R v.4.2.3; http://www.r-project.org/). The calibration quality
was evaluated using the Hosmer-Lemeshow test, which was applied
using the ‘ResourceSelection’ package (R v.4.2.3; http://www.r-project.org/). The lower the P-values
from this test, the poorer the calibration. The ‘rmda’ package (R
v.4.2.3; http://www.r-project.org/) was used
to conduct decision curve analysis (DCA) to gauge the clinical
utility of the model (28).
Moreover, the internal validation was carried out using the
Bootstrap resampling method with 1,000 iterations. P<0.05 was
considered to indicate a statistically significant difference.
Results
Determination of cutoff thresholds for
continuous data
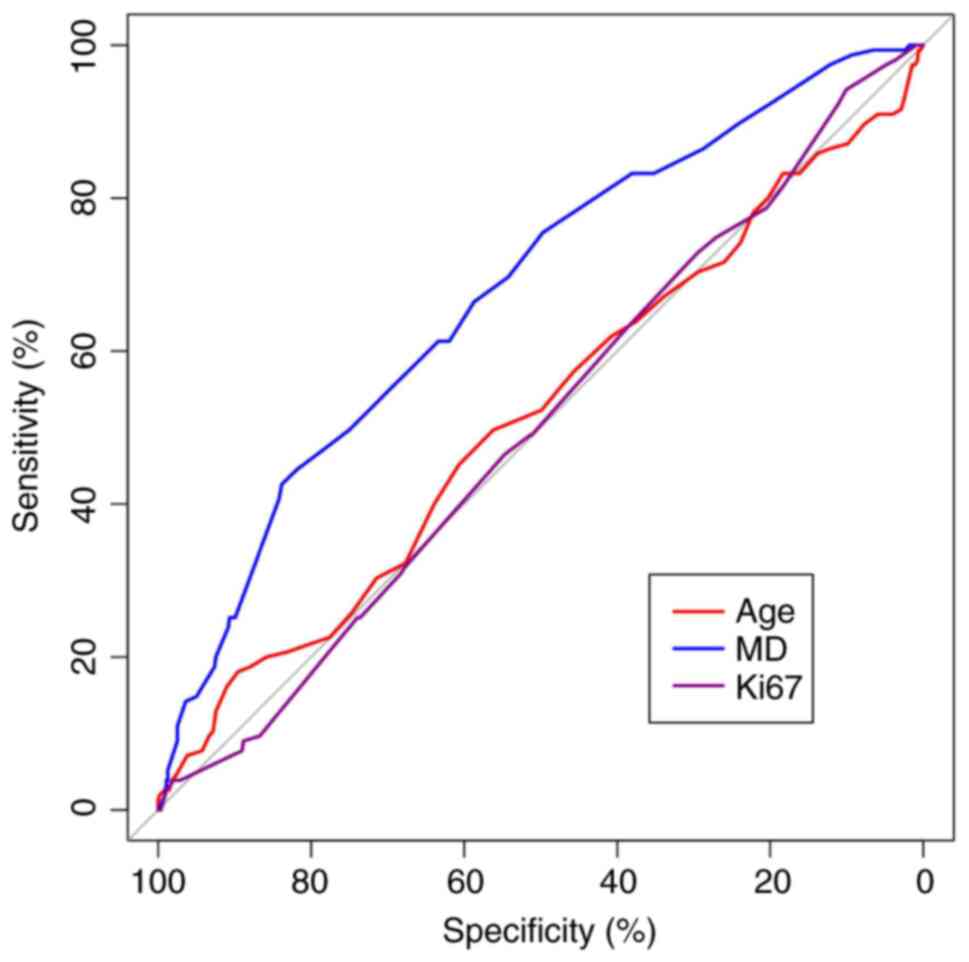
The ROC curve for continuous data was based on the
training group, and significant differences were observed in the
ROC curve analysis for MD (P<0.05). Conversely, the ROC curve
analysis for Ki-67 and age were not significantly different
(P>0.05; Fig. 2). The continuous
data with significant ROC curve differences were categorized into
high and low groups according to the maximum Youden index values
(29), which were used to determine
the cutoff values for the variables. MDs measuring <2.35 and
≥2.35 cm were divided into two groups. Furthermore, the continuous
measurement data, which exhibited no significant differences in the
ROC curve, were separated into two groups based on the median
value. Ki-67 was categorized as <30 and ≥30%, while age was
divided into <52 and ≥52, respectively.
Evaluating clinicopathological
features of tumors and axillary US findings in the training and
validation groups
The present study included a total of 998 patients
ranging from 21–87 years old. They were randomly allocated into
training and validation groups in a 7:3 ratio. Overall, the
distribution of variables between the two groups was fundamentally
similar, with only slight differences observed in histological
grading, making them suitable for constructing and validating a
nomogram model. In the training group, the incidence rate of ALNM
was 21.8%, whereas in the validation group, the rate was 25.5%.
There was no significant difference in the incidence rate of ALNM
(P=0.201; Table I). Significant
statistical differences were observed within the training group in
factors such as LVI, tumor location, US, MD and histological
grading (P<0.05). These findings are essential for selecting
variables when developing the nomogram model. Similarly, the
validation group exhibited significant differences in LVI, tumor
location, US and MD (P<0.05), confirming the significance of
these variables in the model construction (Table II).
 | Table I.Baseline characteristics of the
training and validation groups. |
Table I.
Baseline characteristics of the
training and validation groups.
|
Characteristics | Training group
(%) | Validation group
(%) | P-value |
|---|
| ALNM |
|
| 0.201 |
|
Non-ALNM | 557 (78.2) | 213 (74.5) |
|
|
ALNM | 155 (21.8) | 73 (25.5) |
|
| Age at diagnosis
(years) |
|
| 0.191 |
|
<52 | 321 (45.1) | 142 (49.7) |
|
|
≥52 | 391 (54.9) | 144 (50.3) |
|
| Menopausal
status |
|
| 0.336 |
|
Premenopausal | 342 (48.0) | 147 (51.4) |
|
|
Postmenopausal | 370 (52.0) | 139 (48.6) |
|
| Lymphovascular
invasion |
|
| 0.696 |
|
Negative | 568 (79.8) | 225 (78.7) |
|
|
Positive | 144 (20.2) | 61 (21.3) |
|
| Tumor location |
|
| 0.922 |
| Upper
outer quadrant | 341 (47.9) | 136 (47.6) |
|
|
Others | 371 (52.1) | 150 (52.4) |
|
| Ultrasound |
|
| 0.542 |
|
Negative | 586 (82.3) | 240 (83.9) |
|
|
Positive | 126 (17.7) | 46 (16.1) |
|
| Maximum diameter
(cm) |
|
| 0.747 |
|
<2.35 | 556 (78.1) | 226 (79.0) |
|
|
≥2.35 | 156 (21.9) | 60 (21.0) |
|
| Histological
grade |
|
| 0.048 |
|
I/II | 444 (62.4) | 159 (55.6) |
|
|
III | 268 (37.6) | 127 (44.4) |
|
| Ki-67(%) |
|
| 0.114 |
|
<30 | 348 (48.9) | 124 (43.4) |
|
|
≥30 | 364 (51.1) | 162 (56.6) |
|
| Molecular
subtype |
|
| 0.074 |
|
TNBC | 101 (14.2) | 57 (20.0) |
|
|
Luminal | 462 (64.9) | 170 (59.4) |
|
| HER-2
positive | 149 (20.9) | 59 (20.6) |
|
 | Table II.Comparison of axillary ultrasound
findings and clinicopathological features of tumors between ALNM
and non-ALNM in the training and validation groups. |
Table II.
Comparison of axillary ultrasound
findings and clinicopathological features of tumors between ALNM
and non-ALNM in the training and validation groups.
|
| Training group
(%) |
| Validation group
(%) |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Non-ALNM | ALNM | P-value | Non-ALNM | ALNM | P-value |
|---|
| Age at diagnosis
(years) |
|
| 0.194 |
|
| 0.118 |
|
<52 | 244 (43.8) | 77 (49.7) |
| 100 (46.) | 42 (57.5) |
|
|
≥52 | 313 (56.2) | 78 (50.3) |
| 113 (53.1) | 31 (42.5) |
|
| Menopausal
status |
|
| 0.234 |
|
| 0.042 |
|
Premenopausal | 261 (46.9) | 81 (52.3) |
| 102 (47.9) | 45 (61.6) |
|
|
Postmenopausal | 296 (53.1) | 74 (47.7) |
| 111 (52.1) | 28 (38.4) |
|
| Lymphovascular
invasion |
|
| <0.001 |
|
| <0.001 |
|
Negative | 513 (92.1) | 55 (35.5) |
| 194 (91.1) | 31 (42.5) |
|
|
Positive | 44 (7.9) | 100 (64.5) |
| 19 (8.9) | 42 (57.5) |
|
| Tumor location |
|
| 0.012 |
|
| 0.002 |
|
Others | 304 (54.6) | 67 (43.2) |
| 123 (57.7) | 27 (37.0) |
|
| Upper
outer quadrant | 253 (45.4) | 88 (56.8) |
| 90 (42.3) | 46 (63.0) |
|
| Ultrasound |
|
| <0.001 |
|
| 0.002 |
|
Negative | 492 (88.3) | 94 (60.6) |
| 187 (87.8) | 53 (72.6) |
|
|
Positive | 65 (11.7) | 61 (39.4) |
| 26 (12.2) | 20 (27.4) |
|
| Maximum diameter
(cm) |
|
| <0.001 |
|
| 0.004 |
|
<2.35 | 467 (83.8) | 89 (57.4) |
| 177 (83.1) | 49 (67.1) |
|
|
≥2.35 | 90 (16.2) | 66 (42.6) |
| 36 (16.9) | 24 (32.9) |
|
| Histological
grade |
|
| 0.010 |
|
| 0.699 |
|
I/II | 361 (64.8) | 83 (53.5) |
| 117 (54.9) | 42 (57.5) |
|
|
III | 196 (35.2) | 72 (46.5) |
| 96 (45.1) | 31 (42.5) |
|
| Ki-67(%) |
|
| 0.965 |
|
|
|
|
<30 | 272 (48.8) | 76 (49.0) |
| 89 (41.8) | 35 (47.9) | 0.359 |
|
≥30 | 285 (51.2) | 79 (51.0) |
| 124 (58.2) | 38 (52.1) |
|
| Molecular
subtype |
|
| 0.054 |
|
| 0.484 |
|
TNBC | 88 (15.8) | 13 (8.4) |
| 46 (21.6) | 11 (15.1) |
|
|
Luminal | 352 (63.2) | 110 (71.0) |
| 124 (58.2) | 46 (63.0) |
|
| HER-2
positive | 117 (21.0) | 32 (20.6) |
| 43 (20.2) | 16 (21.9) |
|
Analysis of ALNM risk factors in the
training group
Univariate logistic regression analysis revealed
that LVI, tumor location, US, MD, histologic grading and molecular
subtype exhibited statistically significant differences between the
ALNM and non-ALNM groups (P<0.05). Conversely, age, menopausal
status and Ki-67 did not demonstrate significant differences
(P>0.05). Multivariate logistic regression analysis revealed
that LVI, US, MD and molecular subtype remained independent risk
factors for ALNM (P<0.05) (Table
III).
 | Table III.Univariate and multivariable logistic
regression analyses for the prediction of axillary lymph node
metastasis. |
Table III.
Univariate and multivariable logistic
regression analyses for the prediction of axillary lymph node
metastasis.
|
Characteristics | Univariate
analysis | P-value | Multivariate
analysis | P-value |
|---|
| Age at diagnosis
(years) |
| 0.194 |
|
|
|
<52 | 1 |
|
|
|
|
≥52 | 0.789
(0.552–1.128) |
|
|
|
| Menopausal
status |
| 0.234 |
|
|
|
Premenopausal | 1 |
|
|
|
|
Postmenopausal | 0.805
(0.563–1.150) |
|
|
|
| Lymphovascular
invasion |
| <0.001 |
| <0.001 |
|
Negative | 1 |
| 1 |
|
|
Positive | 21.198
(13.622–33.588) |
| 17.741
(11.019–29.143) |
|
| Tumor location |
| 0.012 |
| 0.372 |
|
Others | 1 |
| 1 |
|
| Upper
outer quadrant | 1.578
(1.103–2.264) |
| 1.234
(0.775–1.961) |
|
| Ultrasound |
| <0.001 |
| <0.001 |
|
Negative | 1 |
| 1 |
|
|
Positive | 4.911
(3.250–7.438) |
| 3.744
(2.183–6.434) |
|
| Multifocality |
| 0.112 |
|
|
| No | 1 |
|
|
|
|
Yes | 1.958
(0.819–4.387) |
|
|
|
| Maximum diameter
(cm) |
| <0.001 |
| <0.001 |
|
<2.35 | 1 |
| 1 |
|
|
≥2.35 | 3.847
(2.604–5.688) |
| 3.110
(1.853–5.229) |
|
| Histological
grade |
| 0.010 |
| 0.283 |
|
I/II | 1 | 1 |
|
|
|
III | 1.597
(1.113–2.290) |
| 1.308
(0.798–2.135) |
|
| Ki-67 (%) |
| 0.965 |
|
|
|
<30 | 1 |
|
|
|
|
≥30 | 0.992
(0.694–1.417) |
|
|
|
| Molecular
subtype |
|
|
|
|
| TNBC | 1 |
| 1 |
|
| Luminal | 2.115
(1.174–4.101) | 0.017 | 2.469
(1.141–5.732) | 0.027 |
| HER-2 positive | 1.851
(0.936–3.846) | 0.085 | 1.788
(0.757–4.434) | 0.194 |
Multicollinearity test
A multicollinearity test performed on the four
independent risk factors revealed that the tolerance values for
LVI, US, MD and molecular subtype were 0.939, 0.942, 0.994 and
0.979, respectively, all of which were >0.1. Moreover, the
tolerance values for VIF were 1.065, 1.061, 1.006 and 1.021,
respectively, all of which were <5 (30) (Table
IV). Hence, it was concluded that there was no
multicollinearity.
 | Table IV.Multicollinearity test. |
Table IV.
Multicollinearity test.
|
| Collinearity
Statistics |
|---|
|
|
|
|---|
|
| Tolerance | VIF |
|---|
| Lymphovascular
invasion | 0.939 | 1.065 |
| Ultrasound | 0.942 | 1.061 |
| Molecular
subtype | 0.979 | 1.021 |
| Maximum
diameter | 0.994 | 1.006 |
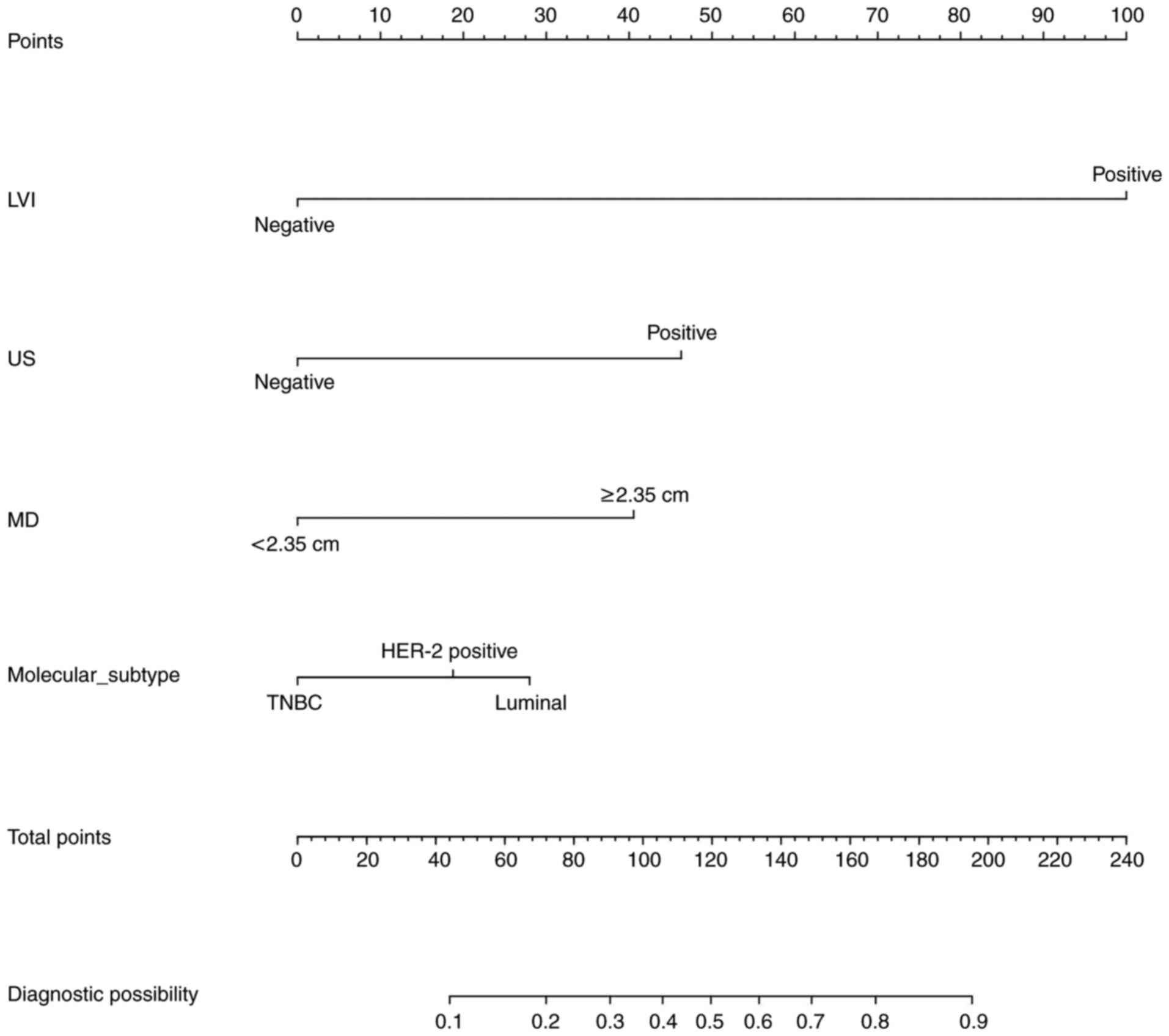
Development of a nomogram model
The model with the lowest AIC was selected. The
variables LVI, US, MD and molecular subtype were predictors. These
variables were then used to generate a visual nomogram representing
their respective weights (Fig. 3).
The variable values for each predictor are shown on the
corresponding line segments, with the length of the line segment
representing the variable's influence weight on ALNM. The higher
the weight, the higher the score.
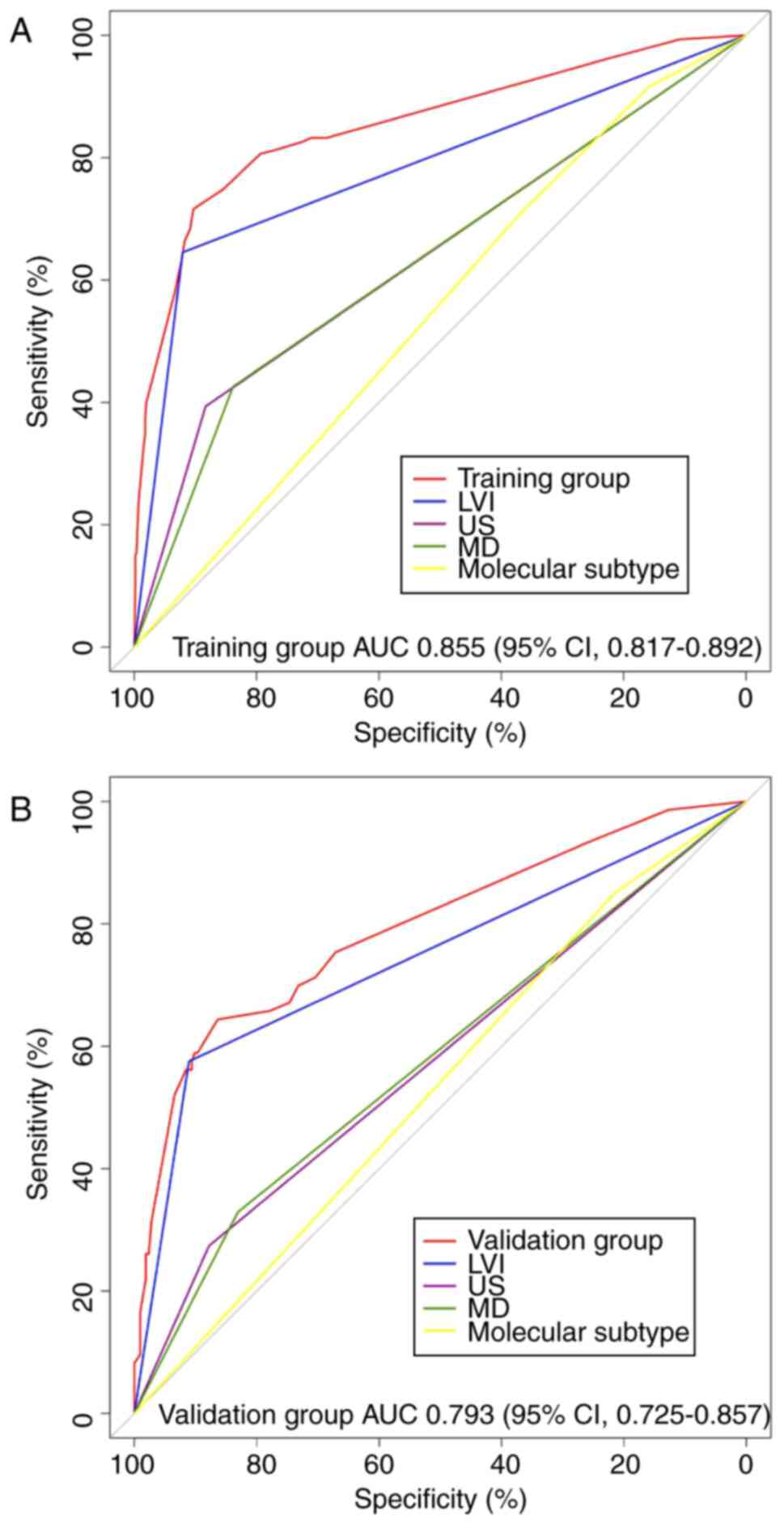
Assessment and verification of the
nomogram model
Notably, two criteria, differentiation and
calibration, were utilized to thoroughly evaluate and validate the
nomogram model. Differentiation was quantified using the AUC. The
AUCs for the training and validation groups were 0.855 (95% CI,
0.817–0.892; Fig. 4A) and 0.793
(95% CI, 0.725–0.857; Fig. 4B),
respectively. Both AUC values exceeded 0.70, indicating a favorable
degree of differentiation (31).
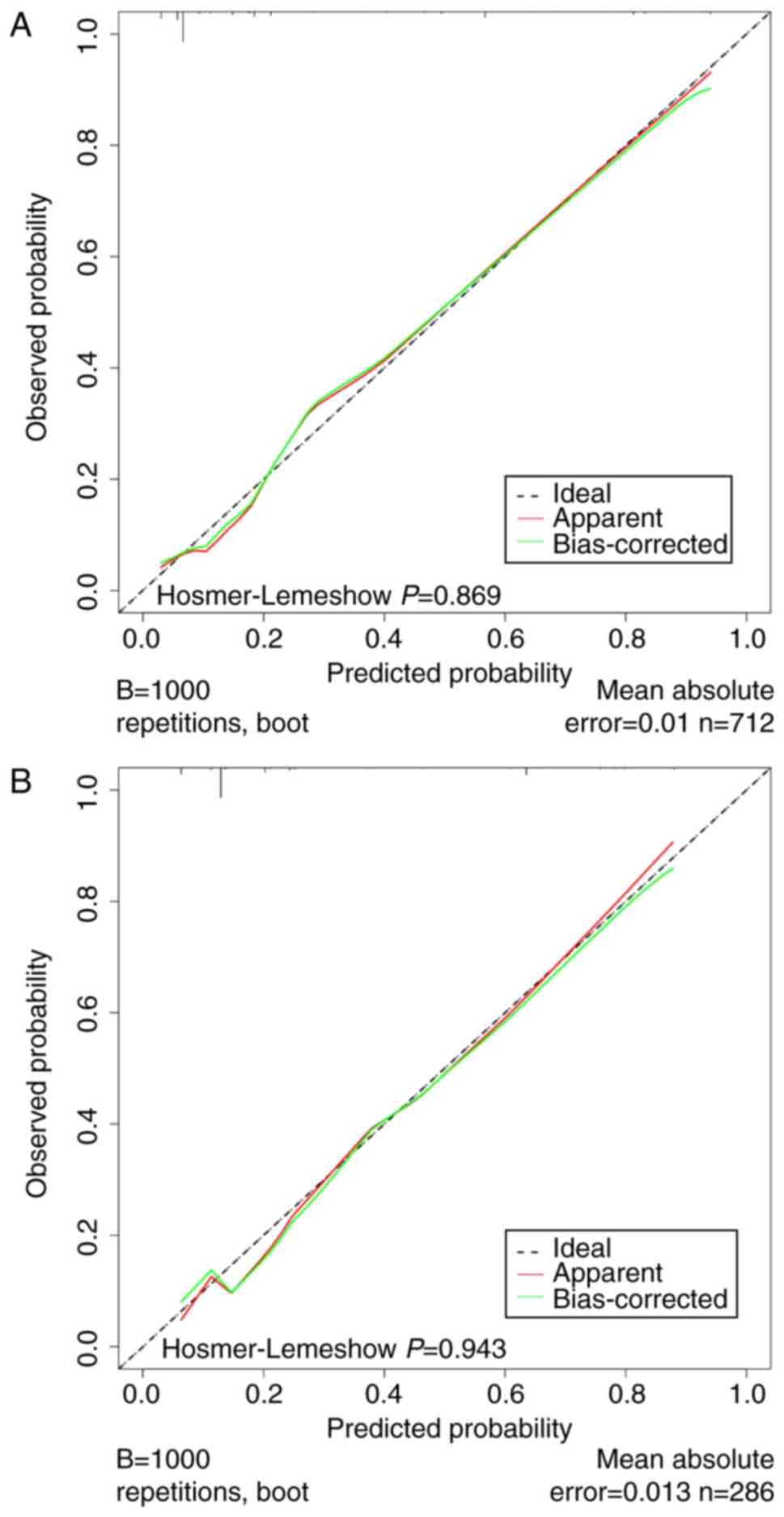
Calibration was assessed by plotting the calibration curves and
conducting the Hosmer-Lemeshow test. The calibration curves for
this model exhibited a close fit between the true and ideal ALNM
values, with an absolute error of <0.05 (Fig. 5A and B). Moreover, the P-values
obtained from the Hosmer-Lemeshow tests were 0.869 and 0.943 for
the training and validation groups, respectively (P>0.05),
indicating strong alignment between the predicted and actual
values. These analyses collectively demonstrated the robust
differentiation and calibration of the nomogram, offering valuable
insights into ALN status evaluation.
Assessment of clinical utility and
applicability
ROC curves and their corresponding AUC values are
frequently employed to evaluate the performance of prediction
models. However, this approach primarily emphasizes sensitivity and
specificity and provides limited insight into the clinical
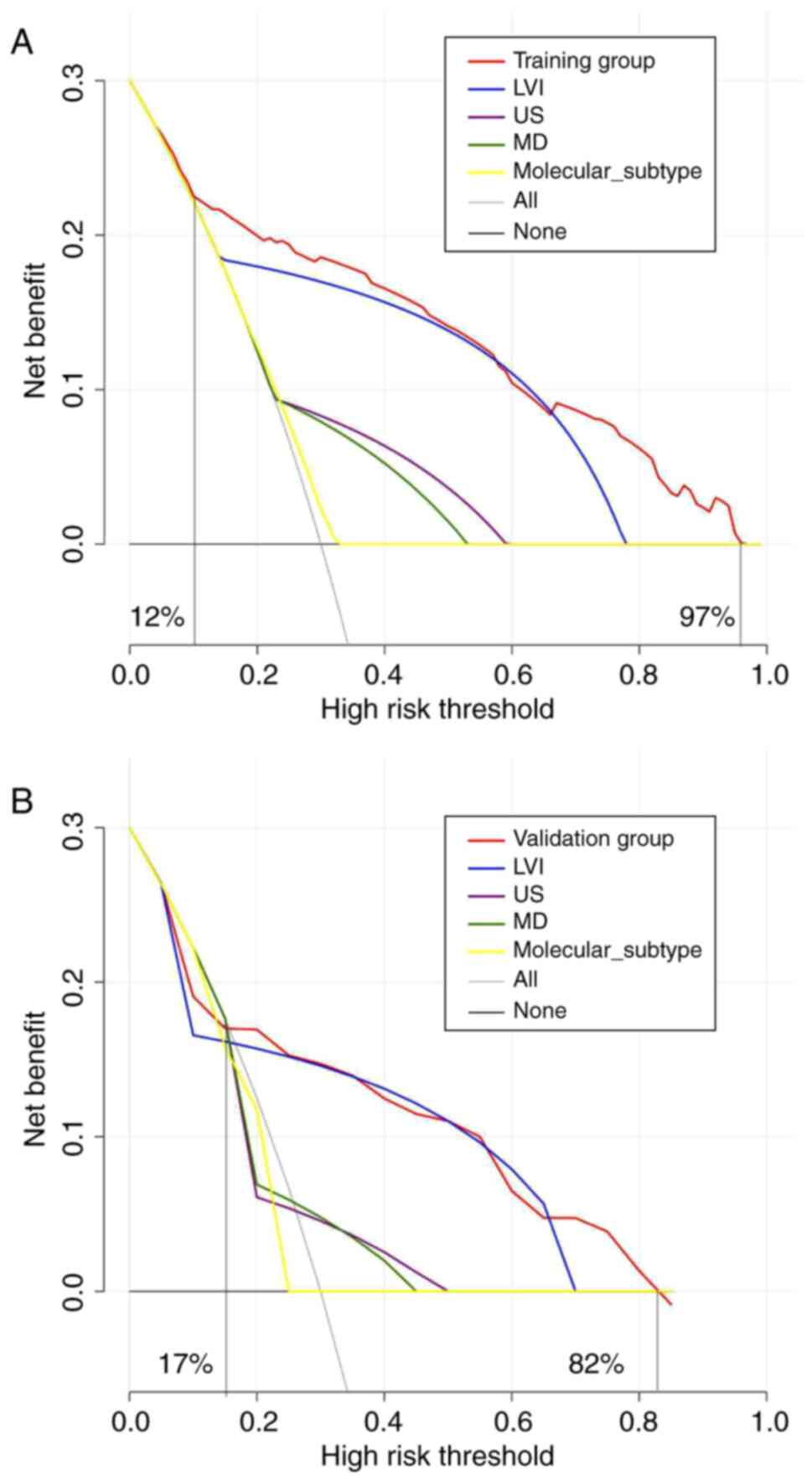
applicability of the model. Hence, DCA was also conducted to
evaluate the practical utility of the model. The DCA plots have a
black line at the bottom, which depicts a hypothetical situation
where all patients neither developed ALNM nor underwent SLNB. The
presence of ALNM in all patients is indicated by the gray diagonal
line, which necessitated SLNB for all. The greater the DCA curve
deviation from the black and gray extreme lines, the higher the net
clinical benefit rate. The red curve corresponds to the DCA curve
generated from the nomogram model. By contrast, the remaining four
curves represent the net benefit of four individual variable
models: LVI, US, MD and molecular subtype. Within the training
group, patients who treated using the nomogram model consistently
experienced a net benefit, as opposed to those who did not, over a
range of threshold probabilities from 12 to 97% (Fig. 6A). Similarly, in the validation
group, patients treated with the nomogram model showed a more
significant net benefit than those who did not while considering
threshold probabilities ranging from 17 to 82% (Fig. 6B).
Discussion
Evaluating the ALN status is crucial for performing
the pathological staging and deciding on treatment options for EBC.
It also substantially impacts the locoregional recurrence rates
(32). With the latest developments
in precise-oriented BC surgery, axillary treatments have
transitioned from extensive ALN dissection to the less invasive
strategy of SLNB (4). As EBC
screening becomes more widespread, it is now possible to detect
smaller tumors in patients at the time of diagnosis. This leads to
a reduced probability of having ALNM. Thus, performing SLNB on all
patients with EBC and cN0 is no longer justifiable. Accurate
assessment and treatment of ALN and reduction of unnecessary trauma
pose significant clinical challenges at this stage. Consequently,
there has been a rise in research on alternatives for SLNB in
patients with EBC and cN0 status. Therefore, finding other methods
to detect the status of the ALNs is essential. While US-guided
needle biopsy is one option, performing biopsies on non-enlarged
ALNs can be challenging and carries a risk of vascular injury.
With advancements in imaging technology, imaging
modalities such as X-ray, computed tomography (CT), US, magnetic
resonance imaging (MRI) and positron emission tomography-computed
tomography (PET-CT) have emerged as the preferred methods for
preoperative assessment of ALN status. There are limitations to the
diagnostic utility of X-ray and CT in determining ALN status
(33). Despite their potential to
yield important information, MRIs and PET-CT scans are not
frequently performed due to their high cost and limited
practicality for routine usage in all patients (34,35).
Conversely, US scanning is a straightforward, affordable, and
non-invasive imaging technique that does not require radiation or
intravenous contrast agents, and it is commonly used to determine
the ALN status (11,12). It is important to mention that ALNM
usually does not cause major alterations in the size and structure
of ALN during the initial stages of metastasis.
Despite difficulties and challenges, substantial
efforts have been made to explore the feasibility of exempting
patients with EBC and cN0 status from SLNB. The SOUND study, for
instance, reported that there was no significant difference in
results between SLNB and the absence of axillary surgery in
patients with BC with negative preoperative axillary US findings
and an MD of ≤2 cm. For such patients, SLNB can be safely omitted
(9). The findings of the SOUND
trial established the potential for safely avoiding SLNB based on
preoperative axillary US findings. Notably, the SOUND trial
employed relatively stringent selection criteria, with a majority
(87.8%) of cases classified as luminal BC.
A number of studies have established a close
association between clinicopathological features of tumors and ALNM
(36–38). In the present study, a nomogram
model was developed to predict the risk of ALNM in patients with
EBC and cN0. The model considers the results of axillary US
examinations and the clinicopathological characteristics of the
tumors. The model aimed to reduce surgical trauma and associated
consequences in low-risk patients. All included indicators were
systematically grouped in this investigation, and univariate and
multivariate logistic regression analyses were conducted. The
results indicated that LVI emerged as an independent risk factor
for ALNM. LVI refers to the process by which tumor cells infiltrate
the lymphatic or blood arteries, acting as the main pathway for BC
to metastasize to lymph nodes or distant organs. This finding
aligns with the conclusions drawn in numerous previous studies as
well (39,40). Furthermore, a positive axillary US
also emerged as an independent risk factor for ALNM, underscoring
the need for vigilance when encountering suspicious axillary US
findings (8,41). Ding et al (42) and Orsaria et al (43) have previously reported that a larger
MD and increasingly irregular tumor boundaries are associated with
a heightened risk of developing ALNM. The results of the present
study were consistent with these observations. Out of the molecular
subtypes of EBC, there were 632 instances of luminal BC, 208 cases
of HER-2 positive BC and 158 cases of TNBC. Luminal BC constituted
approximately two-thirds of the EBC molecular subtypes. Consistent
with previous studies, the present study also identified that TNBC
had the lowest likelihood of ALMN (44,45).
Prior research has consistently found that luminal BC is more
susceptible to ALNM than TNBC and HER-2-positive BC (46–48),
which aligns with the findings of the present study. The difference
in risk of ALNM may be due to the higher vulnerability of TNBC to
distant metastasis rather than local axillary metastasis (47,49).
The limited sample size of TNBC could have influenced this result
in the present study. Furthermore, Houvenaeghel et al
(44) reported that HER-2-positive
patients exhibited a higher probability of ALNM than HER-2-negative
patients (31.9 vs. 22.9%). However, the present study did not find
a significant difference (23.07 vs. 22.78%; Table II). Age, tumor location,
histological grade and Ki-67 have also been found to be independent
risk factors for ALNM in earlier research. Nevertheless, due to
variances in sample size and population selection, these parameters
did not show significant differences in the logistic regression
analysis of the present study (37,38,50–52).
The nomogram was constructed by selecting four
independent risk factors (LVI, US, MD and molecular subtype) based
on the AIC. The feasibility of the model was cross-verified using
both the training and the validation groups. The AUCs for the
training and the validation groups were 0.855 (95% CI, 0.817–0.892)
and 0.793 (95% CI, 0.725–0.857), respectively. The Hosmer-Lemeshow
test yielded P-values of 0.869 and 0.943 for the training and
validation groups, respectively (P>0.05), indicating the best
fit. Additionally, there was exceptional alignment between the
three curves on the calibration chart. These metrics collectively
suggested that the nomogram model offers robust differentiation and
calibration, highlighting its predictive efficacy. The clinical
practicality of the prediction model was assessed by analyzing the
DCA curves. According to the DCA, the nomogram model offered a
superior net clinical benefit to patients in both the training
group and the validation group.
Previous reports have detailed the construction of
ALNM prediction models for patients with EBC and cN0 (8,36,38,53–55).
By contrast, the current study utilized four independent risk
variables, namely LVI, MD, US and molecular subtypes, which may be
acquired by either mass puncture or resection. The US is a
relatively straightforward examination method also used in less
developed regions. Based on axillary US results and
clinicopathological characteristics of tumors, the nomogram model
developed in the present study is now the most pragmatic and
well-aligned with clinical practice.
Although the model adequately demonstrated the
importance of each predictor variable, it has certain limitations.
First, this was a single-center, retrospective study with a limited
sample size, potentially introducing inherent selection bias that
could impact the validity and reliability of the study. Second,
using a relatively small sample size, the model only underwent
internal validation. Further validation within a multi-center,
independent cohort is imperative to assess its predictive capacity
more comprehensively. Additionally, the present study solely relied
on the review of US reports, which could introduce some errors.
Therefore, in subsequent validation studies, the US characteristics
related to ALNM should be refined, additional risk factors should
be incorporated, and the predictive performance of the model should
be further enhanced.
In conclusion, the present study constructed a
nomogram model using LVI, US, MD and molecular subtypes. The ROC,
calibration and DCA curves of both the training and validation
groups demonstrated strong predictive performance of the model. The
predictive indicators used in this model were easily accessible
clinically. The nomogram effectively and explicitly depicted the
magnitude of the weight of each predictor variable, which can be
graphically represented using a line segment image. By calculating
the weights of the different predictive variables, the magnitude of
the risk for ALNM can be obtained to improve the ability to
clinically predict the outcomes in patients with ALN metastasis
under limited conditions. Combined with clinical experience, the
nomogram model can improve the accuracy of predicting the
occurrence of ALNM in patients with EBC and cN0 to a certain extent
and has a specific application prospect in practical clinical
diagnosis and treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QJ and ZZ contributed to the conception and design
of the study. JW and XY prepared the materials, collected the data
and performed the analysis. ZZ drafted the manuscript. QJ and ZZ
confirm the authenticity of all the raw data. All authors revised
the manuscript. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
ethical standards of the institutional research committee and with
the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. The authors are accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. The study was approved (approval no.
KY-2023-132) by the Research Ethics Committee of Jiaxing Maternity
and Child Health Care Hospital (Jiaxing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID: Ziran Zhang,
orcid.org/0000-0002-7835-8788.
Glossary
Abbreviations
Abbreviations:
|
EBC
|
early breast cancer
|
|
cN0
|
clinical axillary lymph node
negative
|
|
SLNB
|
sentinel lymph node biopsy
|
|
US
|
ultrasound
|
|
ALNM
|
axillary lymph node metastasis
|
|
ROC
|
receiver operating curve
|
|
AUC
|
area under the curve
|
|
DCA
|
decision curve analysis
|
|
AIC
|
Akaike Information Criterion
|
|
BC
|
breast cancer
|
|
ALN
|
axillary lymph nodes
|
|
MD
|
maximum diameter
|
|
LVI
|
lymphovascular invasion
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER-2
|
human epidermal growth factor
receptor-2
|
|
IHC
|
immunohistochemistry
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malter W, Hellmich M, Badian M, Kirn V,
Mallmann P and Kraemer S: Factors predictive of sentinel lymph node
involvement in primary breast cancer. Anticancer Res. 38:3657–3662.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krag DN, Anderson SJ, Julian TB, Brown AM,
Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP,
Jalovec LM, et al: Sentinel-lymph-node resection compared with
conventional axillary-lymph-node dissection in clinically
node-negative patients with breast cancer: Overall survival
findings from the NSABP B-32 randomised phase 3 trial. Lancet
Oncol. 11:927–933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reimer T, Engel J, Schmidt M, Offersen BV,
Smidt ML and Gentilini OD: Is axillary sentinel lymph node biopsy
required in patients who undergo primary breast surgery? Breast
Care (Basel). 13:324–330. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langer I, Guller U, Berclaz G, Koechli OR,
Schaer G, Fehr MK, Hess T, Oertli D, Bronz L, Schnarwyler B, et al:
Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and
completion axillary lymph node dissection after breast cancer
surgery: A prospective Swiss multicenter study on 659 patients. Ann
Surg. 245:452–461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McLaughlin S: A longitudinal comparison of
arm morbidity in stage I–II breast cancer patients treated with
sentinel lymph node biopsy, sentinel lymph node biopsy followed by
completion lymph node dissection, or axillary lymph node
dissection. Breast Diseases. 22:68–70. 2011.
|
|
8
|
Liu D, Lan Y, Zhang L, Wu T, Cui H, Li Z,
Sun P, Tian P, Tian J and Li X: Nomograms for predicting axillary
lymph node status reconciled with preoperative breast ultrasound
images. Front Oncol. 11:5676482021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gentilini OD, Botteri E, Sangalli C,
Galimberti V, Porpiglia M, Agresti R, Luini A, Viale G, Cassano E,
Peradze N, et al: Sentinel lymph node biopsy vs no axillary surgery
in patients with small breast cancer and negative results on
ultrasonography of axillary lymph nodes: The SOUND randomized
clinical trial. JAMA Oncol. 9:1557–1564. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jung JG, Ahn SH, Lee S, Kim EK, Ryu JM,
Park S, Lim W, Jung YS, Chung IY, Jeong J, et al: No axillary
surgical treatment for lymph node-negative patients after
ultra-sonography [NAUTILUS]: Protocol of a prospective randomized
clinical trial. BMC Cancer. 22:1892022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cools-Lartigue J and Meterissian S:
Accuracy of axillary ultrasound in the diagnosis of nodal
metastasis in invasive breast cancer: A review. World J Surg.
36:46–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ibrahim-Zada I, Grant CS, Glazebrook KN
and Boughey JC: Preoperative axillary ultrasound in breast cancer:
Safely avoiding frozen section of sentinel lymph nodes in
breast-conserving surgery. J Am Coll Surg. 217:7–15; discussion
15–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Sui X, Zhou S, Hu L and Huang X:
Correlation of conventional ultrasound characteristics of breast
tumors with axillary lymph node metastasis and Ki-67 expression in
patients with breast cancer. J Ultrasound Med. 38:1833–1840. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marino MA, Avendano D, Zapata P, Riedl CC
and Pinker K: Lymph node imaging in patients with primary breast
cancer: Concurrent diagnostic tools. Oncologist. 25:e231–e242.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diepstraten SC, Sever AR, Buckens CF,
Veldhuis WB, van Dalen T, van den Bosch MA, Mali WP and Verkooijen
HM: Value of preoperative ultrasound-guided axillary lymph node
biopsy for preventing completion axillary lymph node dissection in
breast cancer: A systematic review and meta-analysis. Ann Surg
Oncol. 21:51–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koehler KE and Ohlinger R: Sensitivity and
specificity of preoperative ultrasonography for diagnosing nodal
metastases in patients with breast cancer. Ultraschall Med.
32:393–399. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vohra LM, Gulzar R and Saleem O: Intra
operative frozen examination of sentinel lymph node in breast
cancer. J Ayub Med Coll Abbottabad. 27:40–44. 2015.PubMed/NCBI
|
|
18
|
Andersson Y, Bergkvist L, Frisell J and de
Boniface J: Long-term breast cancer survival in relation to the
metastatic tumor burden in axillary lymph nodes. Breast Cancer Res
Treat. 171:359–369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feu J, Tresserra F, Fábregas R, Navarro B,
Grases PJ, Suris JC, Fernández-Cíd A and Alegret X: Metastatic
breast carcinoma in axillary lymph nodes: In vitro US detection.
Radiology. 205:831–835. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang WT, Chang J and Metreweli C: Patients
with breast cancer: differences in color Doppler flow and
gray-scale US features of benign and malignant axillary lymph
nodes. Radiology. 215:568–573. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bedi DG, Krishnamurthy R, Krishnamurthy S,
Edeiken BS, Le-Petross H, Fornage BD, Bassett RL Jr and Hunt KK:
Cortical morphologic features of axillary lymph nodes as a
predictor of metastasis in breast cancer: In vitro sonographic
study. AJR Am J Roentgenol. 191:646–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elmore LC, Appleton CM, Zhou G and
Margenthaler JA: Axillary ultrasound in patients with clinically
node-negative breast cancer: which features are predictive of
disease? J Surg Res. 184:234–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Q, Xing P, Dong H, Zhao T and Jin F:
Preoperative assessment of axillary lymph node status in breast
cancer patients by ultrasonography combined with mammography: A
STROBE compliant article. Medicine (Baltimore). 97:e114412018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allison KH, Hammond MEH, Dowsett M,
McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR,
Chavez-MacGregor M, Perlmutter J, et al: Estrogen and progesterone
receptor testing in breast cancer: American society of clinical
oncology/College of American pathologists guideline update. Arch
Pathol Lab Med. 144:545–563. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American society of
clinical oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, McShane LM and Dowsett M: HER2 testing in breast cancer:
American society of clinical oncology/College of American
pathologists clinical practice guideline focused update summary. J
Oncol Pract. 14:437–441. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen International expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kerr KF, Brown MD, Zhu K and Janes H:
Assessing the clinical impact of risk prediction models with
decision curves: Guidance for correct interpretation and
appropriate use. J Clin Oncol. 34:2534–2540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schisterman EF, Perkins NJ, Liu A and
Bondell H: Optimal cut-point and its corresponding Youden Index to
discriminate individuals using pooled blood samples. Epidemiology.
16:73–81. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lan A, Chen J, Li C, Jin Y, Wu Y, Dai Y,
Jiang L, Li H, Peng Y and Liu S: Development and assessment of a
novel core biopsy-based prediction model for pathological complete
response to neoadjuvant chemotherapy in women with breast cancer.
Int J Environ Res Public Health. 20:16172023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franken R, den Hartog AW, de Waard V,
Engele L, Radonic T, Lutter R, Timmermans J, Scholte AJ, van den
Berg MP, Zwinderman AH, et al: Circulating transforming growth
factor-beta as a prognostic biomarker in Marfan syndrome. Int J
Cardiol. 168:2441–2446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Canavese G, Bruzzi P, Catturich A, Tomei
D, Carli F, Garrone E, Spinaci S, Lacopo F, Tinterri C and Dozin B:
Sentinel lymph node biopsy versus axillary dissection in
node-negative early-stage breast cancer: 15-year follow-up update
of a randomized clinical trial. Ann Surg Oncol. 23:2494–2500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uematsu T, Sano M and Homma K: In vitro
high-resolution helical CT of small axillary lymph nodes in
patients with breast cancer: Correlation of CT and histology. AJR
Am J Roentgenol. 176:1069–1074. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
García Vicente AM, Soriano Castrejón Á,
León Martín A, Relea Calatayud F, Muñoz Sánchez Mdel M, Cruz Mora
MA, Jiménez Londoño GA and Espinosa Aunión R: Early and delayed
prediction of axillary lymph node neoadjuvant response by (18)F-FDG
PET/CT in patients with locally advanced breast cancer. Eur J Nucl
Med Mol Imaging. 41:1309–1318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Memarsadeghi M, Riedl CC, Kaneider A,
Galid A, Rudas M, Matzek W and Helbich TH: Axillary lymph node
metastases in patients with breast carcinomas: Assessment with
nonenhanced versus uspio-enhanced MR imaging. Radiology.
241:367–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fong W, Tan L, Tan C, Wang H, Liu F, Tian
H, Shen S, Gu R, Hu Y, Jiang X, et al: Predicting the risk of
axillary lymph node metastasis in early breast cancer patients
based on ultrasonographic-clinicopathologic features and the use of
nomograms: A prospective single-center observational study. Eur
Radiol. 32:8200–8212. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q, Li B, Liu Z, Shang H, Jing H, Shao
H, Chen K, Liang X and Cheng W: Prediction model of axillary lymph
node status using automated breast ultrasound (ABUS) and ki-67
status in early-stage breast cancer. BMC Cancer. 22:9292022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiong J, Zuo W, Wu Y, Wang X, Li W, Wang
Q, Zhou H, Xie M and Qin X: Ultrasonography and clinicopathological
features of breast cancer in predicting axillary lymph node
metastases. BMC Cancer. 22:11552022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fujii T, Yajima R, Hirakata T, Miyamoto T,
Fujisawa T, Tsutsumi S, Ynagita Y, Iijima M and Kuwano H: Impact of
the prognostic value of vascular invasion, but not lymphatic
invasion, of the primary tumor in patients with breast cancer.
Anticancer Res. 34:1255–1259. 2014.PubMed/NCBI
|
|
40
|
Karahallı Ö, Acar T, Atahan MK, Acar N,
Hacıyanlı M and Kamer KE: Clinical and pathological factors
affecting the sentinel lymph node metastasis in patients with
breast cancer. Indian J Surg. 79:418–422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu FH, Wang JX, Ye XH, Deng J, Hang J and
Yang B: Ultrasound-based radiomics nomogram: A potential biomarker
to predict axillary lymph node metastasis in early-stage invasive
breast cancer. Eur J Radiol. 119:1086582019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ding J, Jiang L and Wu W: Predictive value
of clinicopathological characteristics for sentinel lymph node
metastasis in early breast cancer. Med Sci Monit. 23:4102–4108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Orsaria P, Caredda E, Genova F, Materazzo
M, Capuano I, Vanni G, Granai AV, DE Majo A, Portarena I, Sileri P,
et al: Additional nodal disease prediction in breast cancer with
sentinel lymph node metastasis based on clinicopathological
features. Anticancer Res. 38:2109–2117. 2018.PubMed/NCBI
|
|
44
|
Houvenaeghel G, Lambaudie E, Classe JM,
Mazouni C, Giard S, Cohen M, Faure C, Charitansky H, Rouzier R,
Daraï E, et al: Lymph node positivity in different early breast
carcinoma phenotypes: A predictive model. BMC Cancer. 19:452019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu X, Lu X, Wang ZC, Iglehart JD, Zhang X
and Richardson AL: Predicting features of breast cancer with gene
expression patterns. Breast Cancer Res Treat. 108:191–201. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gangi A, Mirocha J, Leong T and Giuliano
AE: Triple-negative breast cancer is not associated with increased
likelihood of nodal metastases. Ann Surg Oncol. 21:4098–4103. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mattes MD, Bhatia JK, Metzger D, Ashamalla
H and Katsoulakis E: Breast cancer subtype as a predictor of lymph
node metastasis according to the SEER Registry. J Breast Cancer.
18:143–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou W, He Z, Xue J, Wang M, Zha X, Ling
L, Chen L, Wang S and Liu X: Molecular subtype classification is a
determinant of non-sentinel lymph node metastasis in breast cancer
patients with positive sentinel lymph nodes. PLoS One.
7:e358812012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Holm-Rasmussen EV, Jensen MB, Balslev E,
Kroman N and Tvedskov TF: Reduced risk of axillary lymphatic spread
in triple-negative breast cancer. Breast Cancer Res Treat.
149:229–236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abdel-Razeq H, Iweir S, Abdel-Razeq R,
Rahman FA, Almasri H, Bater R, Taqash A and Abdelkhaleq H:
Differences in clinicopathological characteristics, treatment, and
survival outcomes between older and younger breast cancer patients.
Sci Rep. 11:143402021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Andea AA, Bouwman D, Wallis T and Visscher
DW: Correlation of tumor volume and surface area with lymph node
status in patients with multifocal/multicentric breast carcinoma.
Cancer. 100:20–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu JL, Tseng HS, Yang LH, Wu HK, Kuo SJ,
Chen ST and Chen DR: Prediction of axillary lymph node metastases
in breast cancer patients based on pathologic information of the
primary tumor. Med Sci Monit. 20:577–581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li J, Ma W, Jiang X, Cui C, Wang H, Chen
J, Nie R, Wu Y and Li L: Development and validation of nomograms
predictive of axillary nodal status to guide surgical
decision-making in early-stage breast cancer. J Cancer.
10:1263–1274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Qiu SQ, Zeng HC, Zhang F, Chen C, Huang
WH, Pleijhuis RG, Wu JD, van Dam GM and Zhang GJ: A nomogram to
predict the probability of axillary lymph node metastasis in early
breast cancer patients with positive axillary ultrasound. Sci Rep.
6:211962016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xie X, Tan W, Chen B, Huang X, Peng C, Yan
S, Yang L, Song C, Wang J, Zheng W, et al: Preoperative prediction
nomogram based on primary tumor miRNAs signature and
clinical-related features for axillary lymph node metastasis in
early-stage invasive breast cancer. Int J Cancer. 142:1901–1910.
2018. View Article : Google Scholar : PubMed/NCBI
|