Introduction
Ovarian cancer is an important cause of
gynaecological tumour-related death. Cancer of the ovary, brain,
pancreas, oesophagus and stomach had the highest annualized initial
treatment costs at ~80,000, 100,000, 90,000, 80,000 and 70,000
dollars, respectively, in the United States in 2010. Conversely,
melanoma, prostate cancer and breast cancer had the lowest
annualized initial costs at 5,000, 20,000 and 23,000 dollars,
respectively. The cost of ovarian cancer can increase to 100,000
dollars in the final year. The financial burden of treatment
represented by these costs highlights that early detection and
prevention of ovarian cancer is an economic and cost-effective
strategy (1,2). Epithelial ovarian cancer occurs
through two carcinogenic pathways, type I and type II. Common
high-grade serous tumours arising through the type II pathway
harbour tumor protein p53 and BRCA DNA repair associated (BRCA)
mutations (3–5). Numerous patients are diagnosed with
distant metastases when visiting a doctor for unrelated reasons. At
present, proteomics techniques, such as mass spectrometry and
protein array analysis, play an important role in the diagnosis and
treatment of ovarian cancer (6).
Common metastatic sites of ovarian cancer include the liver,
spleen, lungs, pleura and lymph nodes (7,8). The
incidence of skin metastasis in ovarian cancer, including Sister
Mary Joseph nodule (SMJN) and non-SMJNs, is low at 0.9–5.8% in
specific regions, including the following areas: California, USA;
Chiba, Japan; Bari, Italy; Hangzhou, China. SMJNs are more common
at the first visit, and non-SMJNs are more common at recurrence,
with a median survival of 12 months for both (7,9). At
present, the best treatment for skin metastases from ovarian cancer
is still unclear. Surgery, radiotherapy, chemotherapy, targeted
therapy, immunotherapy and other combined therapies have all been
considered. Owing to individual differences, not all patients
receive comprehensive treatment. Surgery, chemotherapy and targeted
therapy have been found to be effective in extending survival, even
in older patients (10,11). The current study presents a case of
advanced ovarian cancer with a BRCA1 mutation and skin metastasis,
showing the rare clinical manifestation of severe anal distension
due to rectal wall thickening, and reporting its response to
chemotherapy, angiogenesis therapy and poly(ADP ribose) polymerase
(PARP) inhibitors.
Case report
Initial diagnosis and treatment
A 49-year-old woman was admitted to the Affiliated
Hospital of Southwest Medical University (Sichuan, China) in
November 2019 after finding a navel nodule. Pathological biopsy
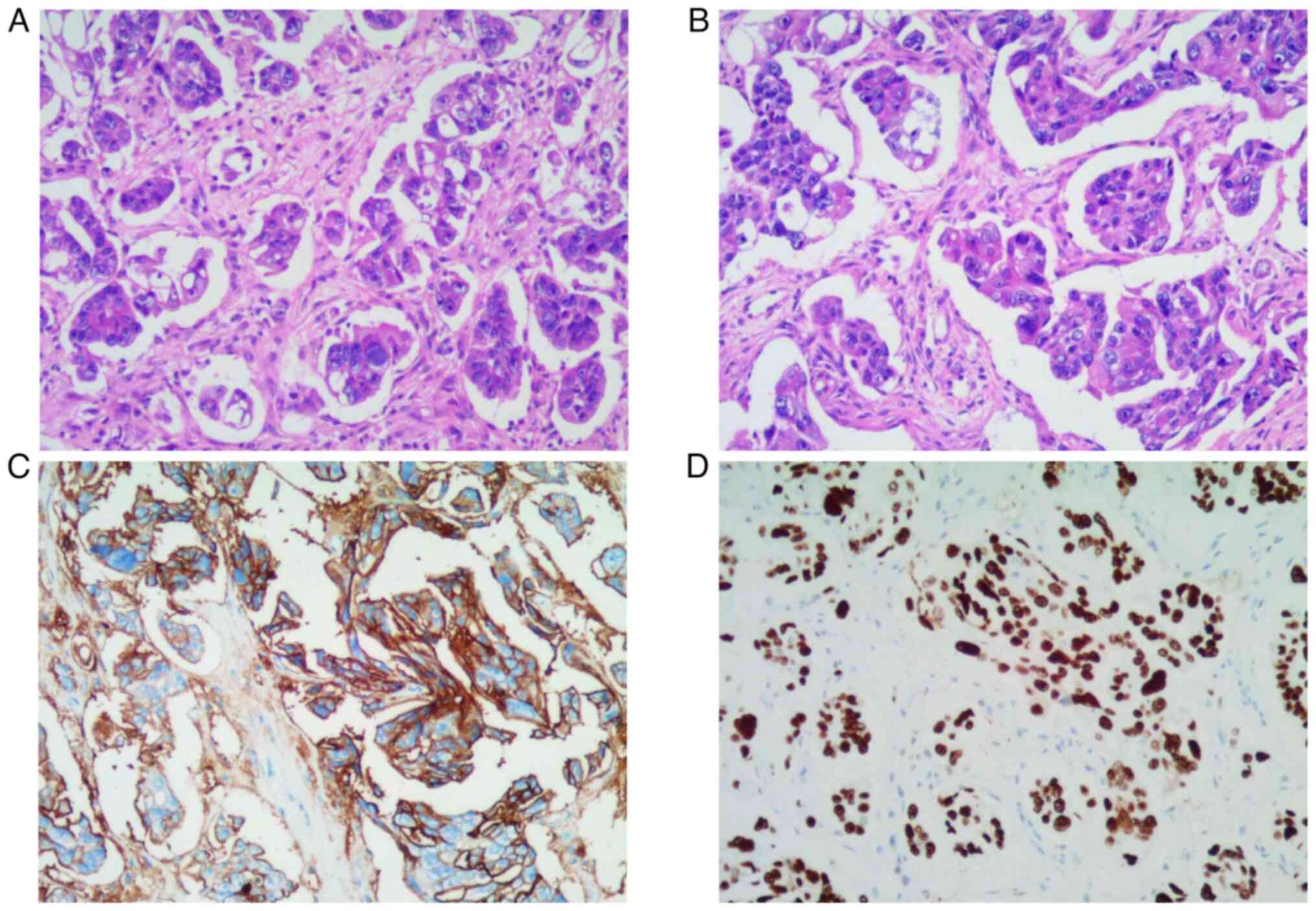
revealed the nodule to be cancer antigen 125 (CA125)(+) and p53 (+,
90%), as shown in Fig. 1. 4%
paraformaldehyde was fixed at room temperature for 24 h, 4 micron
thick sections were stained at room temperature, stained with
hematoxylin for 10 min, stained with eosin for 1 min, and observed
under a light microscope at a magnification of 200 times. IHC
details: Tissues were embedded in paraffin, fixed in 4%
paraformaldehyde for 24 h at room temperature and sectioned at 4
micron thickness. P53 was 1:500, cat. no. MAB-0674, the dilution
ratio of CA125 was 1:500, cat. no. MAB-0830, the supplier was
Fuzhou Maisin Biotechnology Development Co., LTD., and the
incubation time was 20 min at 32°C, the secondary antibody dilution
ratio was 1:500. The cat. no. DS-0003, the supplier was Beijing
Zhongshan Jinqiao Biotechnology Co., LTD. (peroxidase/phosphatase),
and the incubation time was 20 min at 32°C. The chromogenic
detection reagent was EnVision FLEX, High pH (Dako Omnis). The
light microscope was used for observation at a magnification of
200. The patient was diagnosed with stage IVB ovarian
adenocarcinoma, considering high-grade serous adenocarcinoma of the
ovary with navel metastasis. Serum tumour markers showed elevated
levels of CA125 (5,304.07 U/ml; reference range, 0–35 U/ml) and
human epididymis protein 4 (HE4: 337.80 pmol/l; reference range,
<92.1 pmol/l in premenopausal women and <121 pmol/l in
postmenopausal women). Two cycles of chemotherapy [paclitaxel (175
mg/m2) on day 1 + carboplatin (area under the curve (AUC)=5) on day
1, every 21 days] were administered from November to December 2019,
after which the patient developed grade IV myelosuppression. The
patient had a white blood cell count of 0.86×109/l (reference range
3.5–9.5×109/l), a neutrophil count of 0.17×109/l (reference range
1.8–6.3×109/l), and a platelet count of 65×109/l (reference range
125–350×l109/l). Because the patient needed a long time to recover
white blood cells and platelets to the normal range, the treatment
time was delayed, so the chemotherapy regimen was changed to
paclitaxel + cisplatin. Three cycles of chemotherapy [paclitaxel
(175 mg/m2) on day 1 + cisplatin (75 mg/m2) on day 2, every 21
days] were administered between January and March 2020, and the
response evaluation was partial response (sum of target lesion
diameters was reduced by more than 30% from baseline). After a
multidisciplinary team discussion, tumour cell resection (graded R0
following microscopic examination) was performed in March 2020.
During surgery, it was found that the left round ligament of uterus
entering the groin was significantly thickened and hardened. Three
cycles of adjuvant chemotherapy [paclitaxel (175 mg/m2) on day 1 +
carboplatin (AUC=5) on day 1, every 21 days] were administered
between April and June 2020. The patient was followed up regularly,
with CA125 levels of 8 U/ml and the HE4 levels of 44.8 pmol/l at
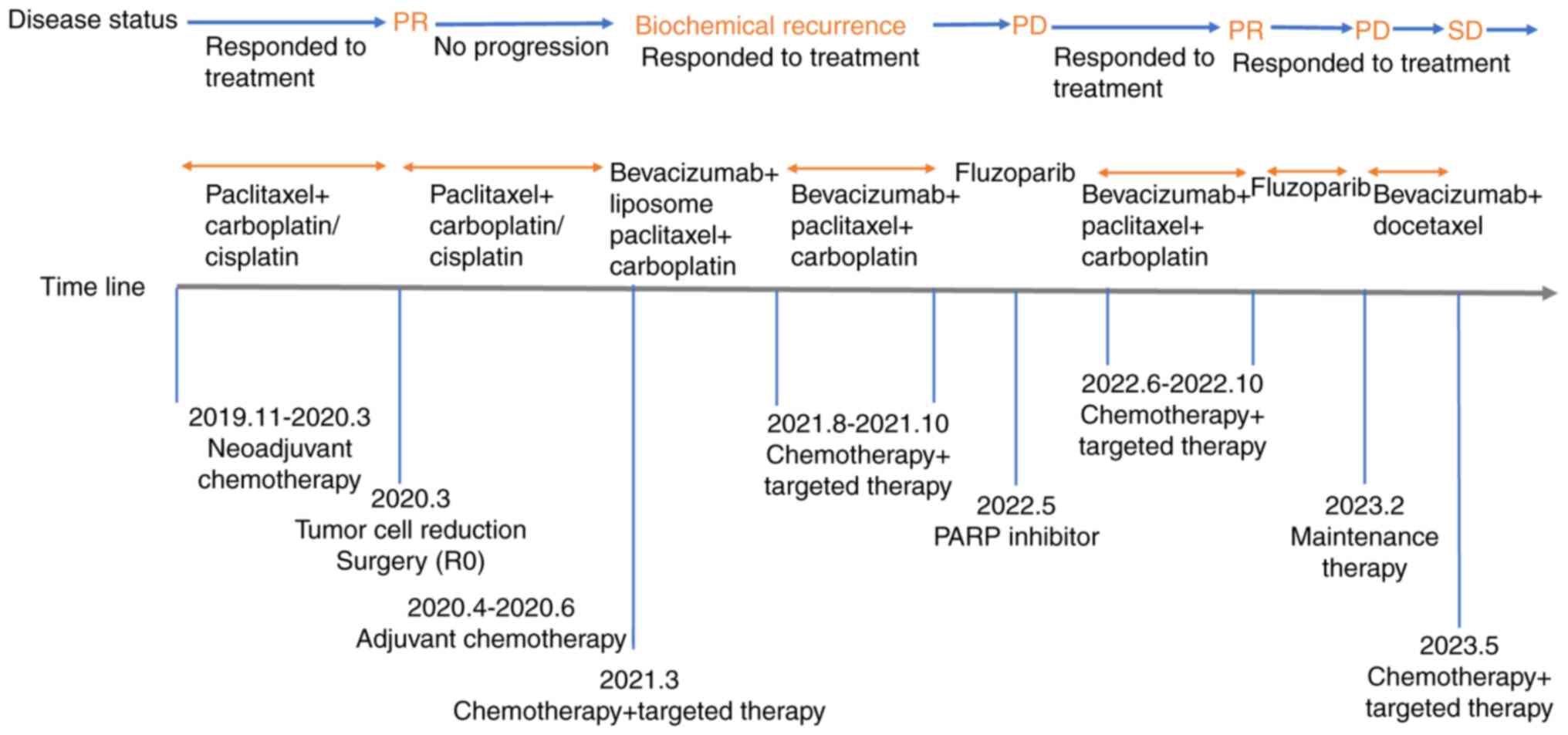
the end of August 2020. The treatment timeline is shown in Fig. 2.
Diagnostic assessment of
recurrence
In March 2021, the patient's CA125 levels were
425.56 U/ml and HE4 levels were 62.98 pmol/l, indicating possible
biochemical recurrence. One cycle of treatment [bevacizumab (7.5
mg/kg) + liposome paclitaxel (175 mg/m2) on day 1 + carboplatin
(AUC=5) on day 1, every 21 days] was administered 1 week later.
CA125 levels at follow-up were 108.53 U/ml, and the patient
discontinued chemotherapy due to a severe gastrointestinal reaction
after chemotherapy. At the end of July 2021, the follow-up results
showed CA125 levels of 577.33 U/ml, with no clear lesions found on
imaging. Following this, four cycles of treatment [bevacizumab (7.5
mg/kg) + paclitaxel (175 mg/m2) on day 1 + carboplatin (AUC=5) on
day 1, every 21 days] were administered from August to October
2021, after which the patient refused to continue chemotherapy.
However, after communication with the patient, timely adjustments
were made to the treatment plan; after two cycles of paclitaxel and
carboplatin chemotherapy from November to December 2019, the
patient had grade IV myelosuppression that did not recover for a
long time, so the chemotherapy regimen was changed to paclitaxel
and cisplatin, and the patient's chemotherapy-related side effects
were actively managed.
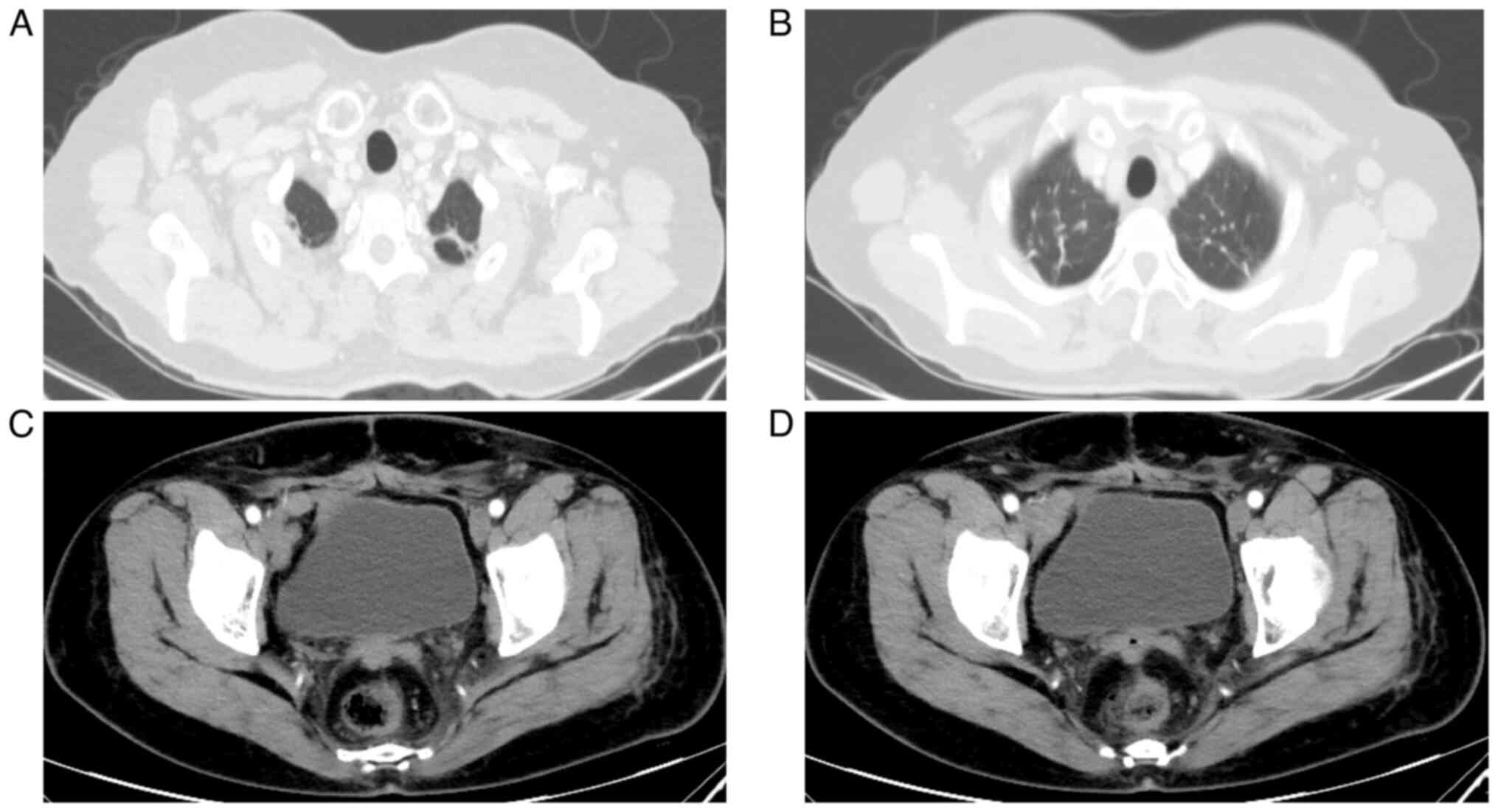
In April 2022, the CA125 levels were 506.18 U/ml,
and computed tomography indicated enhanced nodules on the posterior
wall of the vaginal stump and enlarged bilateral axillary lymph
nodes, as shown in Fig. 3. An
excisional biopsy of the right axillary lymph node was performed in
May 2022. Pathological biopsy revealed metastatic adenocarcinoma.
Genetic testing revealed a somatic BRCA1 gene mutation by employing
the following methodology: Target region capture combined with next
generation sequencing technology was used to analyse the somatic
and germline BRCA1 gene exon 2 and its adjacent ±20-bp intronic
region, including point mutations, deletions and insertions within
20 bp, and the genetic testing was performed at Shenzhen Elyland
Life Technology Investment Co., LTD., China. The patient refused
chemotherapy and was treated with oral fluzoparib monotherapy (150
mg po bid).
Diagnostic assessment of skin
metastasis
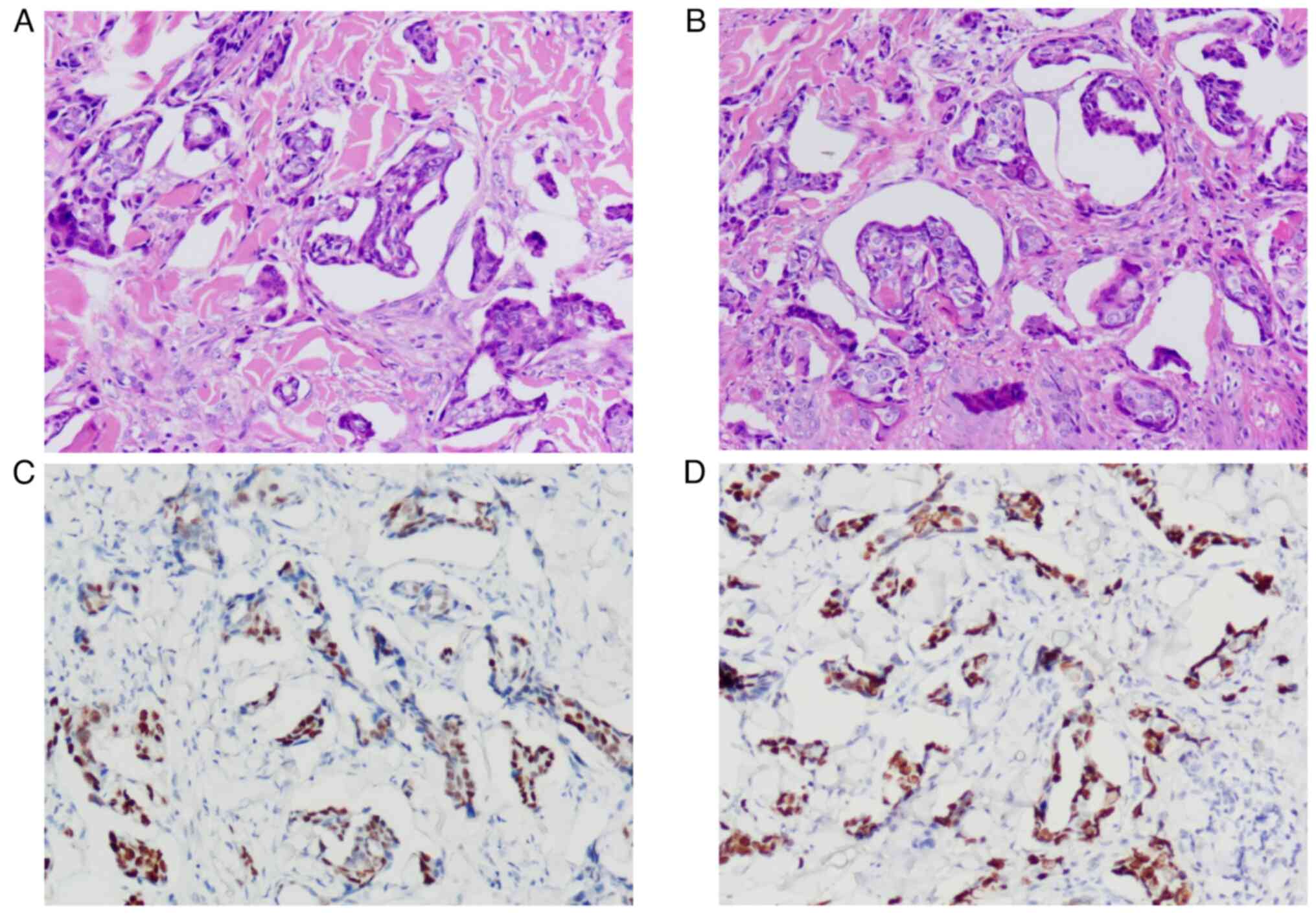
In late May 2022, the patient developed a hip skin
rash, surface swelling and redness, accompanied by severe pruritus
and pain. A hip skin biopsy was performed and immunohistochemical
results were paired box protein Pax-8 (+) and p53 (+, 90%). Tissue
samples were prepared as aforementioned. Dilution ratio of PAX-8
(1:500, cat. no. RMA-0817; Fuzhou Maishin Biotechnology Development
Co., LTD., and the incubation time was 20 min at 32°C, the
secondary antibody dilution ratio was 1:500, cat. no. DS-0004, the
supplier was Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.)
(peroxidase/phosphatase), and the incubation time was 20 min at
32°C. The chromogenic detection reagent was EnVision FLEX, High pH
(Dako Omnis). The light microscope was used for observation at a
magnification of 200. This led to the consideration of skin
metastasis of high-grade serous adenocarcinoma, as shown in
Fig. 4. Six cycles of treatment
[bevacizumab (7.5 mg/kg) + paclitaxel (175 mg/m2) on day 1 +
carboplatin (AUC=5) on day 1, every 21 days] were administered
between June and October 2022, and the patient's skin pruritus,
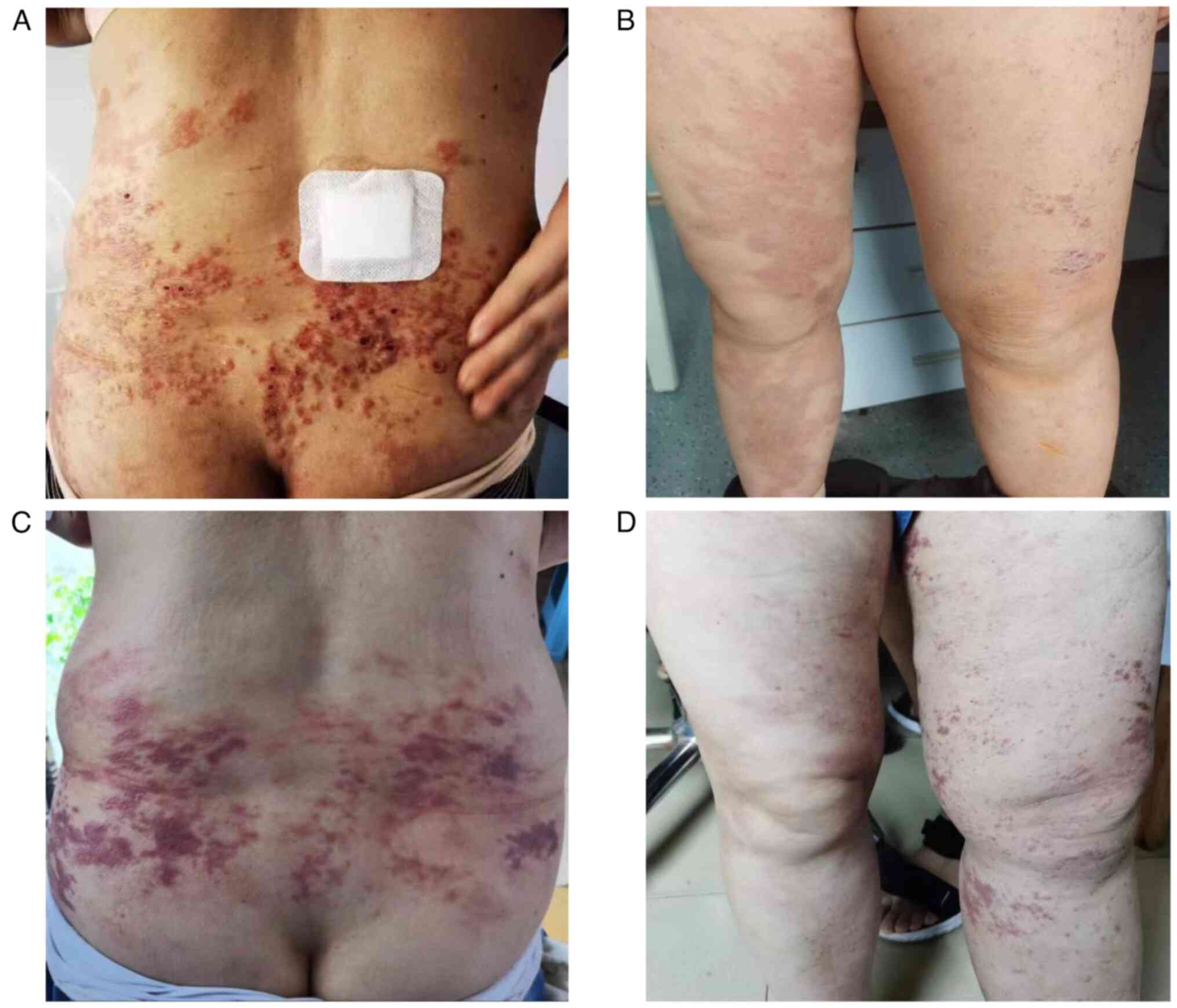
pain and lesions improved. A comparison of skin metastases before
and after treatment is shown in Fig.
5. A total of 21 days after the sixth chemotherapy cycle, oral
fluzoparib maintenance therapy at the aforementioned dose was
resumed.
Recent status
In January 2023, the patient developed erythema,
papules, blisters and pruritus on the lower back, accompanied by
erythema and blisters in the perineum. Furthermore, the patient
presented with severe pain secondary to anal distension. A
colonoscopy performed in February 2023 indicated intestinal mucosal
congestion and oedema observed from ~5 cm from the anal opening,
with nodular changes and easy bleeding when touched. A pathological
biopsy of the rectum revealed chronic active inflammation.
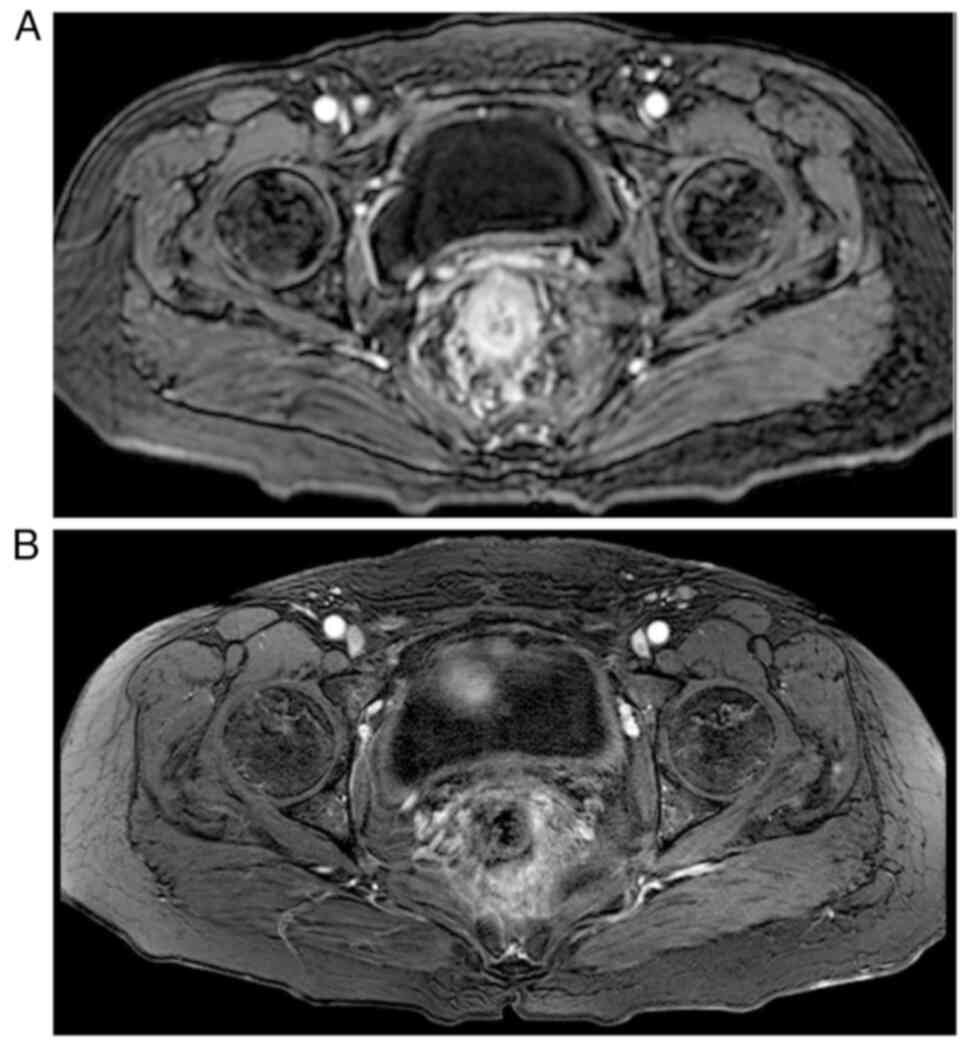
Pelvic-enhanced magnetic resonance imaging (MRI) revealed uneven
thickening of the intestinal wall in the middle and lower rectum,
and possible inflammatory changes, as shown in Fig. 6.
Considering a recurrence of platinum resistance, the
patient was treated with 7.5 mg/kg bevacizumab + 75 mg/m2 docetaxel
(both on day 1) in February 2023. After one cycle (21 days) of
treatment, the patient's skin lesions improved markedly. In
addition, the symptom of anal distension showed improvement. In May
2023, a re-examination using MRI indicated that the intestinal wall
thickening had improved. Follow-up was performed every 2–3 months
according to the NCCN guidelines. Unfortunately, the patient
ultimately lost confidence in treatment, chose hospice care, and
died due to disease progression in February 2024.
Discussion
Skin metastases are more common in breast, kidney
and lung cancer, and the incidence of skin metastasis is as high as
23.9% in female breast cancer (12). Notably, skin metastases in ovarian
cancer are relatively rare, accounting for ~10% of all skin
metastases experienced by women with cancer (7,13). The
use of surgery, chemotherapy, anti-vascular targeted therapy and
immunotherapy for ovarian cancer has extended patient survival
time, which may have led to an increase in skin metastasis. This
may be related to the PI3K/AKT/mTOR pathway, which is frequently
upregulated in epithelial ovarian cancer and is associated with
cell survival, tumour metastasis, chemotherapy resistance and a
poor prognosis (5,14,15).
Ovarian cancer is the fourth most common source of skin metastasis
after carcinoma of the breast, kidney, and lung (16,17),
which is unfavourable to the long-term prognosis of patients
(18).
The key words of literature review were ovarian
cancer and skin metastasis, and the searched databases included
PubMed, Embase and Web of Science. The inclusion criteria were
pathological diagnosis of ovarian cancer, reported location of skin
metastasis, interval between skin metastasis and initial diagnosis,
treatment methods, and PFS or survival time after skin metastasis.
Those who did not meet the inclusion criteria were excluded. The
ages of the patients in Table I
range from 34 to 70 years, indicating that skin metastases from
ovarian cancer can occur over a wide range of ages. A total of 6
patients were diagnosed with stage III–IV, and their survival time
ranged from 2 months to 1 year, indicating a shorter survival time
after skin metastasis from advanced ovarian cancer.
 | Table I.Basic characteristics of 11 patients
with skin metastasis from ovarian cancer. |
Table I.
Basic characteristics of 11 patients
with skin metastasis from ovarian cancer.
| First author,
year | Age, years |
Staging/pathology | Skin metastasis
site | BRCA gene
mutation | Transfer interval
time, months | Treatment | PFS/time,
months | (Refs.) |
|---|
| Demirci et
al, 2010 | 43 | IIIC, serous
papillary cystadenocarcinoma | Abdominal wall | Not mentioned | 72 | Radiotherapy | 7 | (19) |
| Coco and Leanza,
2020 | 65 | IB, clear cell
carcinoma | Navel | Not mentioned | 65 | Surgery +
radiotherapy | 12 | (23) |
| Wiechert et
al, 2012 | 54 | IIC, endometrioid
ovarian adenocarcinoma | Groin + vulva | Not mentioned | 37 | Chemotherapy +
radiotherapy | 10 | (48) |
| Charalampidis et
al, 2016 | 34 | III, Serous
papillary carcinoma of ovary | Chest, abdomen,
upper and lower limbs | Not mentioned | 24 | Chemotherapy | 12 | (18) |
|
| 64 | Grade II serous
papillary carcinoma of the ovary | Back | Not mentioned | 96 | Radiotherapy | 24 |
|
| Oh et al,
2017 | 49 | IIIC, serous
mucinous adenocarcinoma | Left upper abdomen
and left upper arm | Mutation | 60 | Chemotherapy | 2 | (25) |
| Achimaş-Cadariu
et al, 2015 | 49 | IIIC, serous
papillary ovarian cancer | Anterior chest +
lower abdomen + vulva + lower limb | Not mentioned | 21 | Chemotherapy | 2 | (20) |
| Kim et al,
2012 | 60 | IIIC, serous
papillary ovarian cancer | Lower limbs | Not mentioned | 42 | Surgery +
chemotherapy | 5 | (10) |
| Traiman et
al, 1994 | 37 | Serous papillary
cystadenocarcinoma | Inferior abdominal
wall | Not mentioned | 72 | Chemotherapy +
radiotherapy + surgery | 72 | (34) |
| Kanyilmaz et
al, 2016 | 51 | IB, ovarian
endometrial adenoid carcinoma | Chest wall | Not mentioned | 34 | Radiotherapy +
chemotherapy | 4 | (12) |
| Hastings et
al, 2020 | 70 | IVB, serous
adenocarcinoma | Thighs + lower
abdomen | Negative | 5 | Chemotherapy +
surgery + targeting + immunotherapy | 6 | (36) |
The time to skin metastasis from ovarian cancer can
vary from 4 months to 10 years and may be influenced by factors
such as tumour type and prior treatment plans (10–12,17,19–22).
The longer the interval between the first surgery and the
appearance of skin metastases, the longer the patient is expected
to survive (23).
Moreover, the late recurrence of tumours may be
related to the absence of BRCA gene mutations (24). The patient in the present case had a
BRCA1 gene mutation, and recurrence was observed nearly 2 years
after the initial treatment. Oh et al (25) reported a case of ovarian cancer with
a BRCA1 gene mutation and a recurrence time of 5 years, which was
much longer than that observed in the present case. The potential
association of earlier or later recurrence with BRCA gene mutations
requires further study. Germline mutations in BRCA1 and BRCA2 are
the strongest known genetic risk factor for epithelial ovarian
cancer, occurring in 6–15% of patients with the disease. Patients
with epithelial ovarian cancer who are carriers of BRCA1 and BRCA2
have better responses to platinum-based chemotherapy than
non-carriers (26,27). Furthermore, the presence or absence
of a mutation in BRCA1/2 can be used to interpret patient
counselling regarding expected survival (26,27).
At present, the molecular characteristics of tumours have an
important role in the diagnosis, treatment and prediction of
tumours. Several studies have demonstrated that the combination of
PARP inhibitors and immunotherapy, such as anti-cytotoxic T
lymphocyte associated protein 4 and programmed cell death protein
1/programmed death ligand 1 (PD-L1), is an alternative treatment
strategy (28–33). This has partly been based on the
hypothesis that BRCA1/2 and wild-type BRCA1/2 homologous
recombination (HR) deficiency tumours display a higher neo-antigen
load than HR-proficient cancers, thereby producing a more effective
antitumour immune response. In addition, there is evidence that
BRCA deficiency may induce a stimulator of interferon
genes-dependent innate immune response, by inducing type I
interferon and pro-inflammatory cytokine production. Notably,
clinical models have also demonstrated that PARP inhibition
inactivates GSK3 and upregulates PD-L1 in a dose-dependent manner.
Consequently, T-cell activation is being suppressed, resulting in
enhanced cancer cell apoptosis. However, the combination of PARP
inhibitors and immunotherapy requires further clinical research
data (28–33). Traiman et al (34) reported that skin metastasis in
patients with ovarian cancer tended to occur 6 years after the
initial diagnosis, and the survival time of patients was 6 years
after radiotherapy and chemotherapy combined with surgery. Further
clinical trials and research are needed to determine whether the
survival time of patients is lengthened in cases with late onset of
skin metastasis compared with early onset of skin metastasis.
As in the present case, patients with ovarian cancer
skin metastases are often initially seen by a dermatologist owing
to skin lesions and pruritus. Skin metastases from ovarian cancer
are mostly located near the primary tumour, such as in the
abdominal wall (35). Table I shows that skin metastases were
most frequently found in the chest, abdomen and limbs; however,
they also occurred in rare locations such as the face, scalp, nasal
alar, vulva and breast (11,36–38).
If skin metastasis is suspected in rare locations, clinicians need
to be vigilant during examination and strive for early detection
and treatment. Whether the site of metastasis is related to
prognosis requires further investigation.
Skin metastasis is the first symptom in some
patients with ovarian cancer, and ovarian tumours are not found in
~40% of patients with skin metastasis (7,39,40).
Skin metastases may appear at the initial visit or at recurrence
(11). The case reported in the
present study was similar to a number of previous studies; the
majority of patients who developed skin metastases at the initial
visit were sensitive to paclitaxel and platinum-based chemotherapy
(7). However, 11 patients
experienced skin metastases during cancer recurrence (Table I). Most skin metastases from ovarian
cancer have been reported to be ovarian serous papillary
cystadenocarcinoma (17). Moreover,
high-grade serous carcinoma is the most common histological type of
ovarian cancer, accounting for ~70% of ovarian cancers, and is
prone to intraperitoneal metastasis, such as umbilical metastasis
and incision recurrence (41). In
Table I, 8 of the 11 patients
listed had serous carcinomas, accounting for 72.7%. Further
clinical research is required to determine the pathological types
that are sensitive to treatment, and whether these affect patient
prognosis.
Studies have found that genetic predisposition, lack
of fertility, benign inflammatory disease, persistent ovulation
hypothesis, changes in sex hormones, continuous morphological
fallopian tube changes and dysplasia are associated with the
development of ovarian cancer (42–44).
The first step of ovarian cancer metastasis is the spread of tumour
cells, which mainly includes lymphatic, implantation and
haematogenous metastases, adjacent spread and extra-nodular
invasion. The second step of ovarian cancer metastasis to the skin
is the proliferation of tumour cells at the site, which is related
to wound healing, inflammation and the presence of adipose tissue
(7).
Skin metastasis from ovarian cancer may be related
to patient age, obesity, surgical treatment and tumour pathological
type (7). The previous surgical
treatment of patients with ovarian cancer may be related to the
occurrence of skin metastases, and the incidence of abdominal wall
metastases in laparoscopic surgery may be higher than that in open
surgery (7,45). Therefore, more thorough and
meticulous ovarian cancer surgeries should be conducted to minimise
the risk of surgery-related skin metastases. Umbilical metastasis
is often accompanied by peritoneal dissemination; lymphatic or
blood transmission is also involved (9), and patients are more likely to have
recurrent metastasis due to the involvement of multiple modes of
metastasis. In the present case, skin metastasis occurred after
treatment; this may have been related to the umbilical skin
metastasis observed at the first visit.
Postoperative adjuvant chemotherapy for ovarian
cancer can reduce the risk of scar recurrence and metastasis around
the surgical incision (7). The
administration of three or more cycles of neoadjuvant chemotherapy
before cytoreductive surgery and adjuvant chemotherapy is an
alternative for selected patients, providing an opportunity to
detect early chemotherapy sensitivity and identify patients at
increased risk of recurrence (46,47).
Notably, for local metastatic lesions, surgical excision combined
with adjuvant chemotherapy seems to have greater efficacy than
adjuvant chemotherapy alone (17).
Radiotherapy can improve itching symptoms from skin metastases
(48). Chemotherapy combined with
targeted therapy can achieve better results in patients with
recurrent ovarian cancer and skin metastases, as in the present
case. Furthermore, the efficacy of immunotherapy in patients with
skin metastases from ovarian cancer has been reported (36). Chemotherapy can be administered to
patients with skin metastases with other site metastases who can
tolerate chemotherapy. Surgical treatment may be used in patients
with locally isolated skin metastases. Radiotherapy can be used in
patients with local skin metastases who cannot tolerate
chemotherapy or surgery, and immunotherapy may be an effective
treatment for skin metastases from ovarian cancer, including
chemotherapy-resistant ovarian cancer (7,49).
The survival time of patients with ovarian cancer
with skin metastases ranges from 2 to 65 months (17). Currently, chemotherapy,
radiotherapy, surgery and targeted therapy are used for skin
metastasis in ovarian cancer (23),
and the progression-free survival period can last up to 6 years
with application of multiple treatments (34). Combining various treatments is
crucial for effectively managing skin metastases in ovarian cancer.
The prognosis of ovarian cancer with skin metastasis varies from
person to person. Furthermore, it varies depending on the site of
metastasis and whether it is accompanied by metastasis from other
organs. The comprehensive application of various therapeutic
methods may be beneficial for improving the quality of life and
prolonging the survival of patients.
Patients with ovarian cancer treated with
bevacizumab experience an increased incidence of gastrointestinal
side effects, and a history of inflammatory bowel disease is
associated with an increased risk of gastrointestinal side effects.
Therefore, women with ovarian cancer suffering from inflammatory
bowel disease should be closely monitored when using bevacizumab
(50). In the present rare case,
the patient with skin metastasis was considered to have lymphatic
vessel involvement, which affected the intestinal blood supply,
resulting in anal distension and intestinal wall thickening. These
characteristics differ from those in previously reported cases. In
future clinical practice, if unexplained intestinal wall thickening
and related intestinal symptoms are found, tumour invasion should
be considered.
In the present case, the patient could not tolerate
the adverse reactions from treatment such as myelosuppression and
chemotherapy-associated gastrointestinal reactions, which resulted
in the unfinished standard second-line treatment and subsequent
treatment. Therefore, for patients with treatment-related side
effects, existing as well as new treatment methods should be used
to improve their treatment compliance and outcomes.
In the present study, the occurrence of skin
metastasis in ovarian cancer may have been related to the
occurrence of metastasis to the navel skin at the initial
diagnosis. Although the patient had a somatic BRCA1 mutation, after
first-line platinum sensitivity relapses, non-standard second-line
treatment led to rapid skin metastasis in the patient. Salvage
treatment, whether targeted combination chemotherapy or PARP
maintenance therapy, had limited effectiveness. For patients with
skin metastasis of ovarian cancer, chemotherapy, anti-vascular
targeted therapy and PARP inhibitors can bring benefits to
patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article. The raw sequencing
data has been uploaded to the public database NCBI Sequence Read
Archive under accession number RJNA1104125 or at the following URL:
https://www.ncbi.nlm.nih.gov/sra/PRJNA1104125.
Authors' contributions
JZ contributed to the study design, interpretation
of data, and drafting and revision of the manuscript. WH
contributed to manuscript writing and revision, data collection and
table production. ZZ contributed to the writing and revision of the
manuscript, image collection and screening. HD and XD performed
imaging, analyzed data and wrote the manuscript. QW and DL
contributed to the study design, patient care recommendations,
analysis of the results and writing and revision of the manuscript.
QW and DL confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Southwest Medical University (Luzhou,
China; approval no. KY2024158).
Patient consent for publication
Written informed consent was obtained from the
patient agreeing to the publication of any identifiable images or
data included in this article.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Ghose A, Bolina A, Mahajan I, Raza SA,
Clarke M, Pal A, Sanchez E, Rallis KS and Boussios S: Hereditary
ovarian cancer: towards a cost-effective prevention strategy. Int J
Environ Res Public Health. 19:120572022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mariotto AB, Yabroff KR, Shao Y, Feuer EJ
and Brown ML: Projections of the cost of cancer care in the United
States: 2010–2020. J Natl Cancer Inst. 103:117–128. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duska LR and Kohn EC: The new
classifications of ovarian, fallopian tube, and primary peritoneal
cancer and their clinical implications. Ann Oncol. 28 (Suppl
8):viii8–viii12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavlidis N, Rassy E, Vermorken JB, Assi T,
Kattan J, Boussios S and Smith-Gagen J: The outcome of patients
with serous papillary peritoneal cancer, fallopian tube cancer, and
epithelial ovarian cancer by treatment eras: 27 Years data from the
SEER registry. Cancer Epidemiol. 75:1020452021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aliyuda F, Moschetta M, Ghose A, Sofia
Rallis K, Sheriff M, Sanchez E, Rassy E and Boussios S: Advances in
ovarian cancer treatment beyond PARP inhibitors. Curr Cancer Drug
Targets. 23:433–446. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghose A, Gullapalli SVN, Chohan N, Bolina
A, Moschetta M, Rassy E and Boussios S: Applications of proteomics
in ovarian cancer: Dawn of a new era. Proteomes. 10:162022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Otsuka I: Cutaneous metastases in ovarian
cancer. Cancers (Basel). 11:12922019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Xu M, Sakandar A, Du X, He H, He
W, Li D and Wen Q: Successful treatment of a patient with brain
metastasis from ovarian cancer with BRCA wild type using niraparib:
A case report and review of the literature. Front Oncol.
12:8731982022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otsuka I and Matsuura T: Skin metastases
in epithelial ovarian and fallopian tube carcinoma. Medicine
(Baltimore). 96:e77982017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim MK, Kim SH, Lee YY, Choi CH, Kim TJ,
Lee JW, Lee JH, Bae DS and Kim BG: Metastatic skin lesions on lower
extremities in a patient with recurrent serous papillary ovarian
carcinoma: A case report and literature review. Cancer Res Treat.
44:142–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sehra D, Singhal S, Bhatla N, Meena J,
Singh A, Kumari R and Sharma S: 2022-RA-1602-ESGO Cutaneous
metastasis in epithelial ovarian cancer: Experience from a tertiary
care cancer institute. Int J Gynecol Cancer. 32 (Suppl
2):A352–A353. 2022.
|
|
12
|
Kanyilmaz G, Aktan M, Koc M and Findik S:
Cutaneous metastases of the synchronous primary endometrial and
bilateral ovarian cancer: An infrequent presentation and literature
review. Case Rep Oncol Med. 2016:45686532016.PubMed/NCBI
|
|
13
|
Tharakaram S: Metastases to the skin. Int
J Dermatol. 27:240–242. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang TT, Lampert EJ, Coots C and Lee JM:
Targeting the PI3K pathway and DNA damage response as a therapeutic
strategy in ovarian cancer. Cancer Treat Rev. 86:1020212020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dienstmann R, Rodon J, Serra V and
Tabernero J: Picking the point of inhibition: A comparative review
of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 13:1021–1031.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brownstein MH and Helwig EB: Patterns of
cutaneous metastasis. Arch Dermatol. 105:862–868. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cormio G, Capotorto M, Di Vagno G,
Cazzolla A, Carriero C and Selvaggi L: Skin metastases in ovarian
carcinoma: A report of nine cases and a review of the literature.
Gynecol Oncol. 90:682–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Charalampidis C, Lampaki S, Zarogoulidis
P, Lazaridis G, Mpaka S, Kosmidis C, Tsakiridis K, Kioumis I,
Pavlidis P, Karapantzos I, et al: Fine-needle aspiration of skin
metastasis in ovarian cancer-report of two cases and review of the
literature. Ann Transl Med. 4:4472016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Demirci S, Yavas F, Ozsaran Z, Ozsaran A,
Dikmen Y, Zekioglu O, Karabulut B and Aras AB: Palliative
radiotherapy for the skin metastasis of ovarian cancer: A case
report and review of the literature. Med Oncol. 27:628–631. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Achimaş-Cadariu P, Vlad C, Fetica B, Zgaia
A and Cainap C: Unusual skin metastasis in a patient with recurrent
micropapillary serous ovarian carcinoma-a case report and review of
the literature. Clujul Med. 88:237–240. 2015.PubMed/NCBI
|
|
21
|
Vitner D, Amit A, Kerner H, Mayer E and
Lowenstein L: Cutaneous metastases from epithelial ovarian cancer.
Harefuah. 150:441–442. 4912011.(In Hebrew). PubMed/NCBI
|
|
22
|
Haughney RV, Slade RJ and Brain AN: An
isolated abdominal wall metastasis of ovarian carcinoma ten years
after primary surgery. Eur J Gynaecol Oncol. 22:102–103.
2001.PubMed/NCBI
|
|
23
|
Coco D and Leanza S: Cutaneous metastasis
in ovarian cancer: Case report and literature review. Maedica
(Bucur). 15:552–555. 2020.PubMed/NCBI
|
|
24
|
Zylberberg B, Dormont D, Madelenat P and
Daraï E: Relapse after more than 20 years of follow-up for
epithelial ovarian carcinoma. Obstet Gynecol. 103:1082–1084. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh SR, Park JW, Kwon HY and Rha SH:
BRCA1-mutated ovarian cancer with skin metastasis: A case report.
Obstet Gynecol Sci. 60:477–480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shah S, Cheung A, Kutka M, Sheriff M and
Boussios S: Epithelial ovarian cancer: Providing evidence of
predisposition genes. Int J Environ Res Public Health. 19:81132022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang S, Royer R, Li S, McLaughlin JR,
Rosen B, Risch HA, Fan I, Bradley L, Shaw PA and Narod SA:
Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected
patients with invasive ovarian cancer. Gynecol Oncol. 121:353–357.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Revythis A, Limbu A, Mikropoulos C, Ghose
A, Sanchez E, Sheriff M and Boussios S: Recent insights into PARP
and immuno-checkpoint inhibitors in epithelial ovarian cancer. Int
J Environ Res Public Health. 19:85772022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stewart RA, Pilié PG and Yap TA:
Development of PARP and immune-checkpoint inhibitor combinations.
Cancer Res. 78:6717–6725. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Chen Y, Li F, Bao L and Liu W:
Atezolizumab and bevacizumab attenuate cisplatin resistant ovarian
cancer cells progression synergistically via suppressing
epithelial-mesenchymal transition. Front Immunol. 10:8672019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding L, Kim HJ, Wang Q, Kearns M, Jiang T,
Ohlson CE, Li BB, Xie S, Liu JF, Stover EH, et al: PARP inhibition
elicits STING-dependent antitumor immunity in brca1-deficient
ovarian cancer. Cell Rep. 25:2972–2980.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palfi A, Toth A, Hanto K, Deres P,
Szabados E, Szereday Z, Kulcsar G, Kalai T, Hideg K, Gallyas F Jr,
et al: PARP inhibition prevents postinfarction myocardial
remodeling and heart failure via the protein kinase C/glycogen
synthase kinase-3beta pathway. J Mol Cell Cardiol. 41:149–159.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nero C, Ciccarone F, Pietragalla A,
Duranti S, Daniele G, Salutari V, Carbone MV, Scambia G and Lorusso
D: Ovarian cancer treatments strategy: Focus on PARP inhibitors and
immune check point inhibitors. Cancers (Basel). 13:12982021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Traiman P, de Luca LA and Bacchi CE: An
extremely large, cauliflower-type, cutaneous metastasis of ovarian
cancer associated with good prognosis. Gynecol Oncol. 53:239–241.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karpate SJ, Samal SL and Jain SM:
Recurrent ovarian malignancy presenting as cutaneous metastasis.
Indian J Dermatol. 54:380–381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hastings V, McEachron J and Kanis MJ:
Cutaneous metastasis of PD-L1 positive ovarian carcinoma. Gynecol
Oncol Rep. 33:1006072020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
António AM, Alves JV, Goulão J and Bártolo
E: Ovarian carcinoma presenting as cutaneous nasal metastasis. An
Bras Dermatol. 91 (5 suppl 1):S101–S104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stien S, Loget J, Soibinet P, Durlach A,
Fleury C and Viguier M: Ovarian cancer revealed by mammary skin
metastases and cutaneous lymphangitis. Ann Dermatol Venereol.
146:663–665. 2019.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schwartz RA: Cutaneous metastatic disease.
J Am Acad Dermatol. 33:161–186. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brownstein MH and Helwig EB: Metastatic
tumors of the skin. Cancer. 29:1298–1307. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prat J, D'Angelo E and Espinosa I: Ovarian
carcinomas: At least five different diseases with distinct
histological features and molecular genetics. Hum Pathol. 80:11–27.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thomakos N, Diakosavvas M, Machairiotis N,
Fasoulakis Z, Zarogoulidis P and Rodolakis A: Rare distant
metastatic disease of ovarian and peritoneal carcinomatosis: A
review of the literature. Cancers (Basel). 11:10442019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lheureux S, Gourley C, Vergote I and Oza
AM: Epithelial ovarian cancer. Lancet. 393:1240–1253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Erickson BK, Conner MG and Landen CN Jr:
The role of the fallopian tube in the origin of ovarian cancer. Am
J Obstet Gynecol. 209:409–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Paolucci V, Schaeff B, Schneider M and
Gutt C: Tumor seeding following laparoscopy: International survey.
World J Surg. 23:989–997. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leary A, Cowan R, Chi D, Kehoe S and
Nankivell M: Primary surgery or neoadjuvant chemotherapy in
advanced ovarian cancer: The debate continues…. Am Soc Clin Oncol
Educ Book. 35:153–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moschetta M, Boussios S, Rassy E,
Samartzis EP, Funingana G and Uccello M: Neoadjuvant treatment for
newly diagnosed advanced ovarian cancer: Where do we stand and
where are we going? Ann Transl Med. 8:17102020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wiechert AC, Garrett LA, Lin G and Goodman
A: Management of a skin metastasis in a patient with advanced
ovarian cancer. Gynecol Oncol Case Rep. 2:124–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hamanishi J, Mandai M, Ikeda T, Minami M,
Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S,
et al: Safety and antitumor activity of anti-PD-1 antibody,
nivolumab, in patients with platinum-resistant ovarian cancer. J
Clin Oncol. 33:4015–4022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bottoni C, Scambia G, Fagotti A and
Petrillo M: The safety of bevazicumab for the treatment of ovarian
cancer. Expert Opin Drug Saf. 17:1107–1113. 2018. View Article : Google Scholar : PubMed/NCBI
|