Introduction
Small bowel cancer is rare, accounting for less than
5% of all gastrointestinal cancers, although the small intestine
accounts for 75% of the digestive tract length and 90% of the tract
mucosal surface area. Small bowel adenocarcinoma (SBA) accounts for
approximately 40% of all small bowel cancers (1). The prognosis is poor, with a 5-year
survival rate of 14–33% for all patients and 40–60% for those whose
tumors are curatively resectable (1,2).
Although SBA is considered to have a molecular biology distinct
from that of other gastrointestinal cancers, owing to its rarity,
the research infrastructure is inadequate, making research on SBA
difficult. Regarding treatment, folinic acid, fluorouracil, and
oxaliplatin (FOLFOX) therapy for advanced SBA is covered by
insurance, and phase III trials (JCOG1502C; J-BALLAD) on
capecitabine-oxaliplatin (CAPOX) as an adjuvant therapy are ongoing
in Japan. However, these studies are based on the findings of
colorectal cancer treatment. Overall, a new research
infrastructure, including cell lines, needs to be established to
explore disease-specific biological characteristics and provide
evidence for treatment.
Although obtaining cell lines from fresh tumors is
not technically easy, Dangles-Marie et al reported that
prior xenografting leads to efficient establishment of colon cancer
cell lines (3). In this study, we
established and biologically characterized a novel SBA cell line,
designated SiCry-15X, using patient-derived xenografts (PDXs) of
SBA.
Materials and methods
Materials
SBA tissues were obtained from the primary tumor of
a 75-year-old male patient at Chiba University Hospital (affiliated
with Chiba University Graduate School of Medicine) with no history
of Lynch syndrome, celiac disease, and inflammatory bowel disease,
nor family history of inherited disease. The patient underwent
partial jejunectomy and the tumor was located in the jejunum,
approximately 5 cm from the ligament of Treitz. The
histopathological diagnosis was por >>tub1 >tub2,
according to the Japanese Classification of Colorectal Carcinomas,
and pT4, pN2, pM0, and pStage IIIB, according to the eighth edition
of the Union for International Cancer Control Staging System.
Immunohistochemistry
Immunohistochemical analysis of p53 and Ki-67
expression was performed on formalin-fixed paraffin-embedded
sections of the original tumor using mouse anti-p53 (DO-7) antibody
(518-102364; Roche, Basel, Switzerland) and mouse anti-human Ki-67
(MIB-1) antibody (GA62661-2; Agilent Technologies, CA, USA).
Patient-derived xenograft
Six-week-old female BALB/c nu/nu mice were purchased
from Japan SLC, Inc. (Shizuoka, Japan). Small pieces, 3 mm in
diameter, were taken from the surgical specimens and transplanted
subcutaneously into the backs of the mice. The mice were
anesthetized with a mixture of 0.3 mg/kg of medetomidine
hydrochloride (Fujita Pharmaceutical. Co., Ltd., Tokyo, Japan), 4.0
mg/kg of midazolam (Sandoz K. K., Tokyo, Japan), and 5.0 mg/kg of
butorphanol tartrate (Meiji Animal Health Co., Ltd., Tokyo, Japan)
(4). This dosage is consistent with
a previous report, following the ARRIVE 2.0 guidelines and the
Guide for the Care and Use of Laboratory Animals (5). 0.3 mg/kg of atipamezole hydrochloride
(Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan) was used as an
antagonist. The anesthetic and antagonist were administered to mice
by subcutaneous injection at a volume of 0.01 ml/g of body weight.
The tumor volume was calculated using the following formula: long
diameterx(short diameter)2/2. Passages were performed when the
tumor diameter was ≥15 mm, and 3 mm diameter pieces were
re-implanted. The tumor tissues were fixed in 10% formalin and
subjected to histological analysis by hematoxylin and eosin (HE)
staining at each passage.
Establishment of PDX-derived
cells
After at least three passages, resected PDX tissues
were washed with phosphate-buffered saline (PBS; Nacalai Tesque,
Kyoto, Japan) containing 1% penicillin-streptomycin (Gibco-Life
Technologies, Grand Island, NY, USA) and minced into 1–2 mm-sized
small pieces. The samples were centrifuged at 1,500 rpm for 5 min,
and the supernatant was removed. The collected cells were
resuspended in Dulbecco's modified Eagle's medium (DMEM) + Ham's
F12 medium (Nacalai Tesque) containing 1% penicillin-streptomycin
and 10% fetal bovine serum (FBS; Gibco-Life Technologies) and
seeded into 6-well microplates. Once colony formation was
confirmed, the cells were transferred to a larger dish or flask.
For passaging and stromal cell removal, trypsin treatment was
performed using 0.25% trypsin-1 mmol/l ethylene diamine tetraacetic
acid (trypsin-EDTA; FUJIFILM Wako Pure Chemical Co., Osaka, Japan).
For stromal cell removal, trypsin-EDTA, diluted three-fold in PBS,
was used. The following criteria were defined for establishment:
long-term culturability, tumorigenicity, and similarity with the
original tumor. After more than two months of culture, the cells
were evaluated for tumorigenicity and similarity with the original
tumor.
Cell culture
Cells from the SBA (SiCry-15X), as well as gastric
cancer (MKN74; RRID: CVCL_2791, and KATO III; RRID: CVCL_0371),
colorectal cancer (HT29; RRID: CVCL_3230, and LoVo; RRID:
CVCL_0399), and mouse squamous cell carcinoma (SCC VII; RRID:
CVCL_V412) lines were used in this study. MKN74 (JCRB No. 0255) and
KATO III (JCRB No. 0611) cells were obtained from Health Science
Research Resources Bank/Japanese Collection of Research
Bioresources (Osaka, Japan). The HT29 (58483218) cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA). LoVo (RCB1639) cells were obtained from Riken Bioresource
Center Cell Bank (Tsukuba, Japan). The SCC VII cell line was kindly
provided by Professor Yuta Shibamoto (Department of Quantum
Radiology, Nagoya City University, Nagoya, Japan). The HT29 cell
line was authenticated using short tandem repeat (STR) profiles,
and the STR profiles consistent with the previous reports (6–8).
Mycoplasma infection was examined using the VenorGeM OneStep
Mycoplasma Detection Kit for Endpoint PCR (Minerva Biolabs GmbH,
Berlin, Germany).
All cells were cultured in DMEM + Ham's F12 medium
containing 1% penicillin-streptomycin and 10% FBS in a humidified
atmosphere containing 5% CO2 at 37°C.
Cell proliferation assay
The cells were seeded in flat-bottom 96-well
microplates at 1×103 cells per well in 100 µl of medium. After 48 h
of pre-culture, the cells in six wells were counted every 24 h
using a cell counting kit-8 (Dojindo Laboratories, Kumamoto, Japan)
in accordance with the manufacturer's recommended protocol.
Doubling time of the cell population was determined based on the
exponential phase of the growth curve.
DNA extraction
DNA was extracted from the cell lines and 10
mm-sized paraffin-embedded SBA samples using the DNeasy Blood &
Tissue Kit and QIAamp DNA FFPE Tissue Kit, respectively (QIAGEN,
Hilde, Germany). All procedures were performed according to the
manufacturer's instructions.
Polymerase chain reaction (PCR) for
identification of animal species
To identify the cell and tissue origins of the
animal species, animal-specific mitochondrial DNA (mtDNA) sequences
were detected by PCR. PCR was performed with TaKaRa Ex Taq Hot
Start Version (Takara Bio Inc., Otsu, Japan) using the following
program: one cycle at 94°C for 5 min, 30 cycles at 94°C for 45 sec,
60°C for 30 sec, and 72°C for 90 sec, and one cycle at 72°C for 10
min, and the products were stored at 4°C. The primer sequences and
predicted product sizes were based on those from RIKEN BioResource
Research Center (https://cell.brc.riken.jp/ja/quality/pcr) and a
previous report (9) as follows:
human mtDNA, sense 5′-CTCCTATTCTTGCACGAAAC-3′ and antisense
5′-GATGGGGATTATTGCTAGGATG-3′ (330 bp); mouse mtDNA, sense
5′-GCACTGAAAATGCTTAGATGGATAATTG-3′ and antisense
5′-CCTCTCATAAACGGATGTCTAG-3′ (948 bp); 18S ribosomal RNA (rRNA),
sense 5′-CGGGGAATYAGGGTTCGATTC-3′ and antisense
5′-GCCTGCTGCCTTCCTTKGATG-3′ (70 bp). Subsequently, 5 µl each of the
PCR products were electrophoresed on a 1.5% agarose gel
(Sigma-Aldrich, St. Louis, MO, USA) containing Midori Green Advance
(Nippon Genetics Co., Ltd., Tokyo, Japan) as the staining solution
with 0.5% Tris-borate EDTA buffer. After electrophoresis, the
samples were visualized under UV light (Ez-Capture MG; ATTO Co.,
Tokyo, Japan) and photographed. A 100-bp DNA ladder (Nippon
Genetics Co., Ltd.) was used as the size marker.
STR analysis
The GenePrint 10 System (Promega, Madison, WI, USA)
was used for STR analysis, and all procedures were performed
according to the manufacturer's instructions. The following
autosomal STR loci were analyzed: TH01, D21S11, D5S818, D13S317,
D7S820, D16S539, CSF1PO, vWA, TPOX, and Amelogenin for sex
identification. The samples were processed using an Applied
Biosystems 3500×L Genetic Analyzer (Thermo Fisher Scientific).
Similarities between the STR profiles were evaluated using
evaluation values (EVs), and the profiles were considered identical
at values of 0.8 or above. The EV was calculated as follows:
(number of coincidental peaks)x2/total number of peaks in sample A
+ total number of peaks in sample B. CLASTR (version 1.4.4; RRID:
SCR_024863) was used for matching against other cell lines.
In vivo experiments
To evaluate tumorigenicity and determine the
appropriate number of cells for the cell-derived xenograft (CDX)
model, different numbers of SiCry-15X cells were suspended in 200
µl of PBS and injected subcutaneously into the backs of
six-week-old female BALB/c nu/nu mice (Japan SLC, Inc.). The tumor
diameters and body weights were measured once per week. The CDXs
formed were excised after six weeks, fixed in 10% formalin, and
subjected to histological analysis after HE staining. Mice were
euthanized promptly after the end of the study by exsanguination
under anesthesia.
Sensitivity to anticancer drugs
The cells were seeded in flat-bottom 96-well
microplates at 2×103 cells per well in 100 µl of medium. After 72 h
of pre-culture, the medium was removed, and 5-fluorouracil (5-FU;
FUJIFILM Wako Pure Chemical Co.), paclitaxel (PTX; FUJIFILM Wako
Pure Chemical Co.), irinotecan (CPT-11; Cayman Chemical Company,
Ann Arbor, MI, USA), oxaliplatin (L-OHP; Tokyo Chemical Industry
Co., Ltd., Tokyo, Japan), and cisplatin (CDDP; FUJIFILM Wako Pure
Chemical Co.) were added at various concentrations. After 96 h of
drug treatment, the cells were counted using a cell counting kit-8
(Dojindo Laboratories) in accordance with the manufacturer's
instructions. Each cell line was examined in triplicates, and 50%
inhibitory concentrations (IC50) were calculated.
Assays for tumor markers in the
conditioned medium and mouse serum samples
Carcinoembryonic antigen (CEA) and carbohydrate
antigen 19-9 (CA19-9) levels in culture supernatants (used for 96-h
culture of 5×105 cells) and mouse serum samples were measured using
chemiluminescence immunoassay (CLIA). To measure CEA and CA19-9
levels, 0.25–1 ml of each sample was used.
Statistical analysis
Cell viability in response to anticancer drugs and
tumor marker levels in culture supernatants were presented as
average ± standard deviation. The one-way analysis of variance with
Tukey's post hoc test was performed to compare the differences
tumor marker levels in culture supernatants in more than three cell
lines. Statistical significance was considered to exist at P-values
<0.05. All data were statistically analyzed using JMP Pro v.17
software (SAS institute Inc).
Results
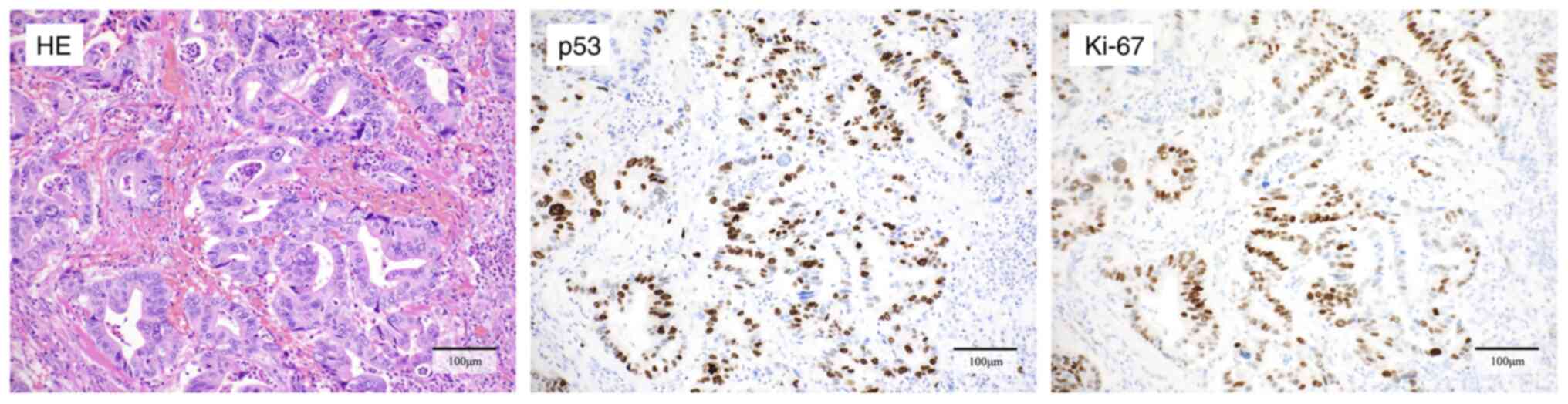
Immunohistochemical profile of
original tumor
The original tumor was a primary jejunal
adenocarcinoma, and the histopathological findings are shown in
Fig. 1. Immunohistochemical
staining showed discontinuous staining in cancer cell nuclei for
p53 and evaluated as p53 wild type. Additionally, a large number of
Ki-67 positive cells were identified, with an MIB-1 index of
58%.
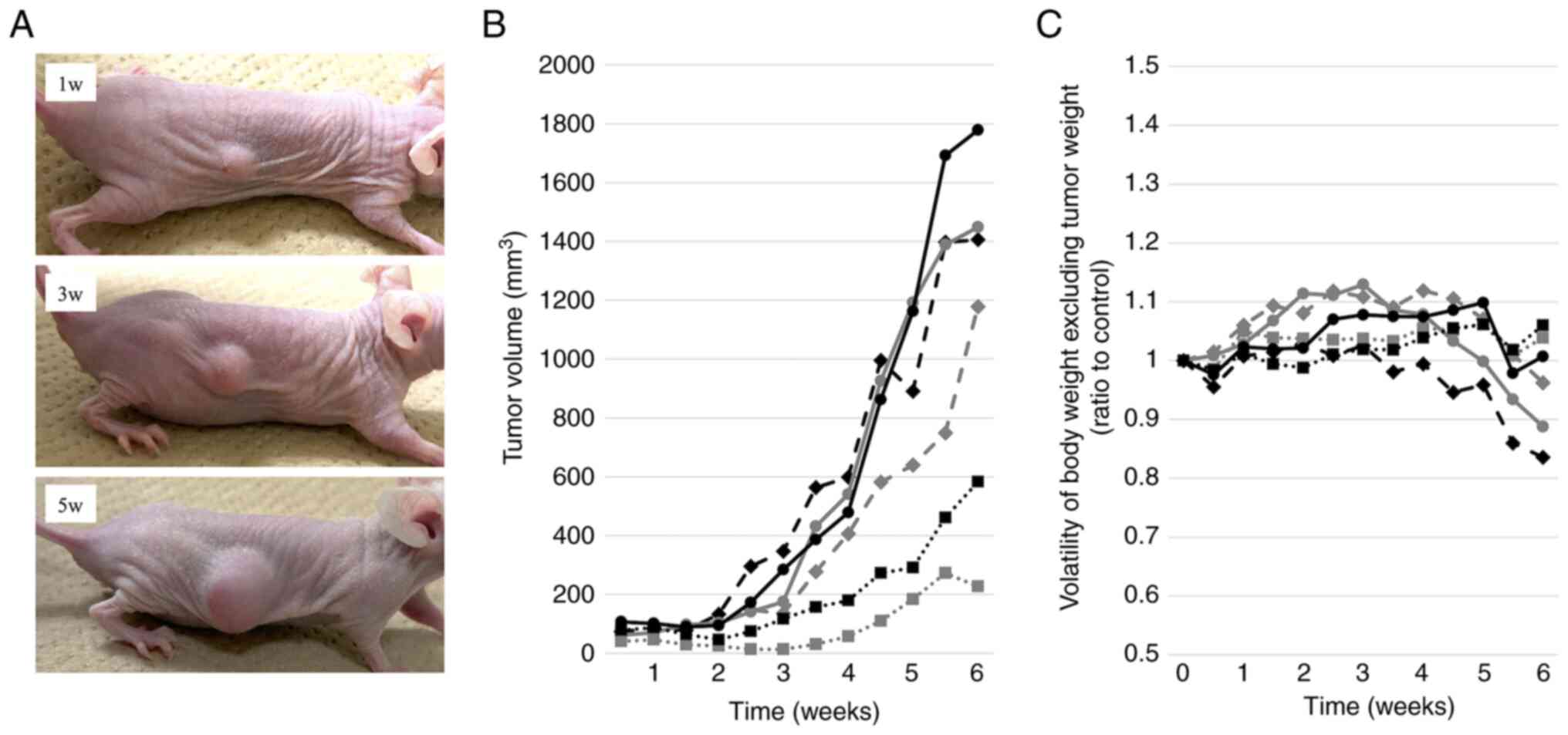
Establishment of PDXs and PDX-derived
cell line, SiCry-15X
Patient-derived tumor tissues were subcutaneously
transplanted into three mice, all of which showed viable growth.
Two or more passages were performed and designated as PDX
establishment. Fig. 2 shows the
progress of PDXs and the weight transition at passage 9.
PDX-derived cells formed colonies surrounded by
fibroblasts on the fifth day of primary culture. The first passage
was performed on the 13th day of primary culture. Fibroblasts were
eliminated by a weak trypsin treatment. The cell line was
designated as SiCry-15X and had undergone more than 50
passages.
The SiCry-15X cells adhered and grew as monolayers
(Fig. 3A). The population doubling
time of SiCry-15X was approximately 37 h. PCR amplification of
animal species-specific mtDNA indicated that the SiCry-15X cells
were of human origin. In contrast, the PDX tissue contained both
human and mouse components (Fig.
3B). Limiting dilution analysis confirmed that SiCry-15X cells
formed colonies from single cells. PCR analysis revealed that the
cells were free from mycoplasma infection.
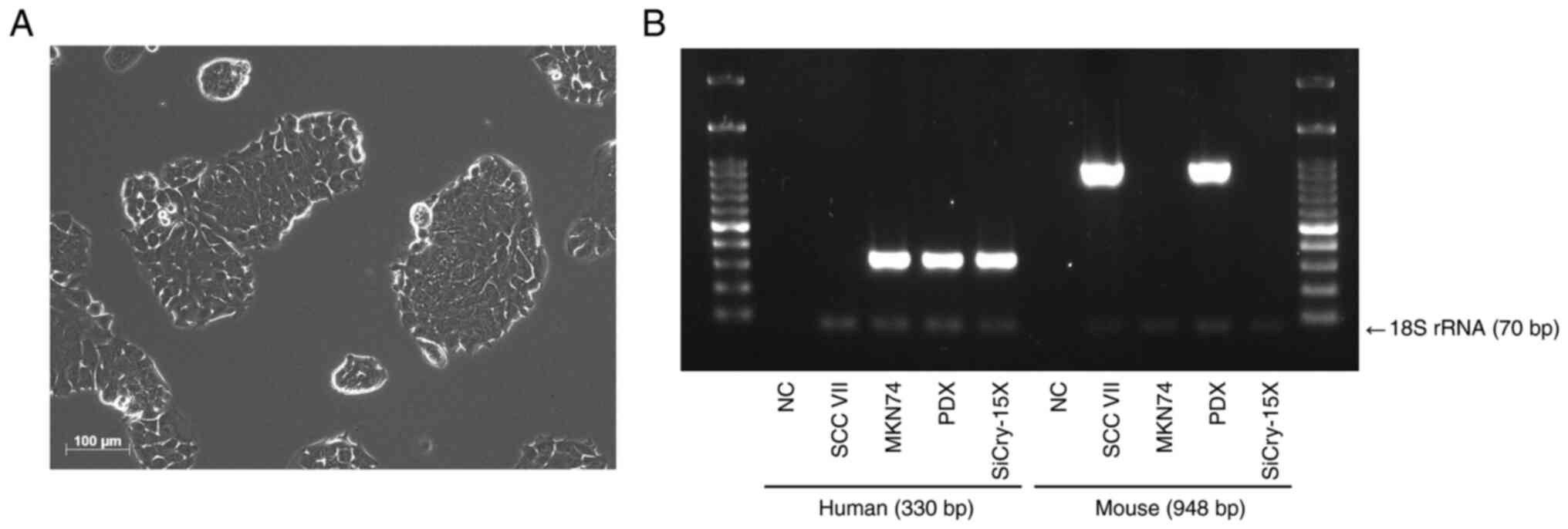 | Figure 3.Morphology and origin of SiCry-15X
cell line. (A) Microscopic image of SiCry-15X (scale bar, 100 µm).
The cells adhered and grew as monolayers. (B) Amplification of
human and mouse mtDNA. SCC VII, a mouse-derived cell line, and
MKN74, a human-derived cell line, were used as positive controls,
and purified water was used as the NC. 18S rRNA was used as the
internal control. In PDXs, both human and mouse components were
combined, whereas SiCry-15X cells consisted only of human
components. NC, negative control; rRNA, ribosomal RNA; PDX,
patient-derived xenograft. |
STR analysis for confirmation of
similarity of SiCry-15X cells with the original tumor
Table I summarizes
the main findings of STR analysis. Comparison of the STR profile of
the original tumor with those of the PDXs and SiCry-15X revealed
that the EVs were greater than 0.8, indicating that the PDXs and
cell line were similar to the original tumor. Furthermore, a CLASTR
analysis revealed that none of the STR profiles of human cell lines
matched those of the SiCry-15X line, confirming the uniqueness of
the SiCry-15X cell line.
 | Table I.Short tandem repeat profile of
SiCry-15X. |
Table I.
Short tandem repeat profile of
SiCry-15X.
| Locus | Original tumor | PDX | SiCry-15X |
|---|
| TH01 | 6, 9 | 6 | 6 |
| D21S11 | 30, 33.2 | 30, 33.2 | 30, 33.2 |
| D5S818 | 11, 12 | 11 | 11 |
| D13S317 | 9, 12 | 9, 12 | 9, 12 |
| D7S820 | 8, 11 | 8, 11 | 8, 11 |
| D16S539 | 9, 11 | 9, 11 | 9, 11 |
| CSF1PO | 10, 12 | 12 | 12 |
| AMEL | X, Y | X | X |
| vWA | 17, 18 | 17, 18 | 17, 18 |
| TPOX | 9, 11 | 9, 11 | 9, 11 |
Evaluation of tumorigenicity using the
CDX model
When different numbers of SiCry-15X cells were
injected subcutaneously into mice, tumors formed at all cell counts
at six weeks, indicating the tumorigenic potential of SiCry-15X
(Fig. 4A). HE-stained images of
CDXs formed from the SiCry-15X cells revealed that the
adenocarcinomas were similar to the original tumor and PDXs
(Fig. 4B). PCR amplification of
animal species-specific mtDNA revealed that the CDXs contained both
human and mouse components (Fig.
4C). Using a cutoff diameter of at least 5 mm after four weeks
of growth, the appropriate number of cells for the CDX model was
determined to be 2×106 cells, which was in the 1–5×106 cell range.
The CDX progression and weight transition of the CDX model mice, in
which 2×106 cells were injected subcutaneously, are shown in
Fig. 4D and 4E. All mice met the defined criteria, none
showed excessive weight loss, and the mice were appropriate for use
as CDX models.
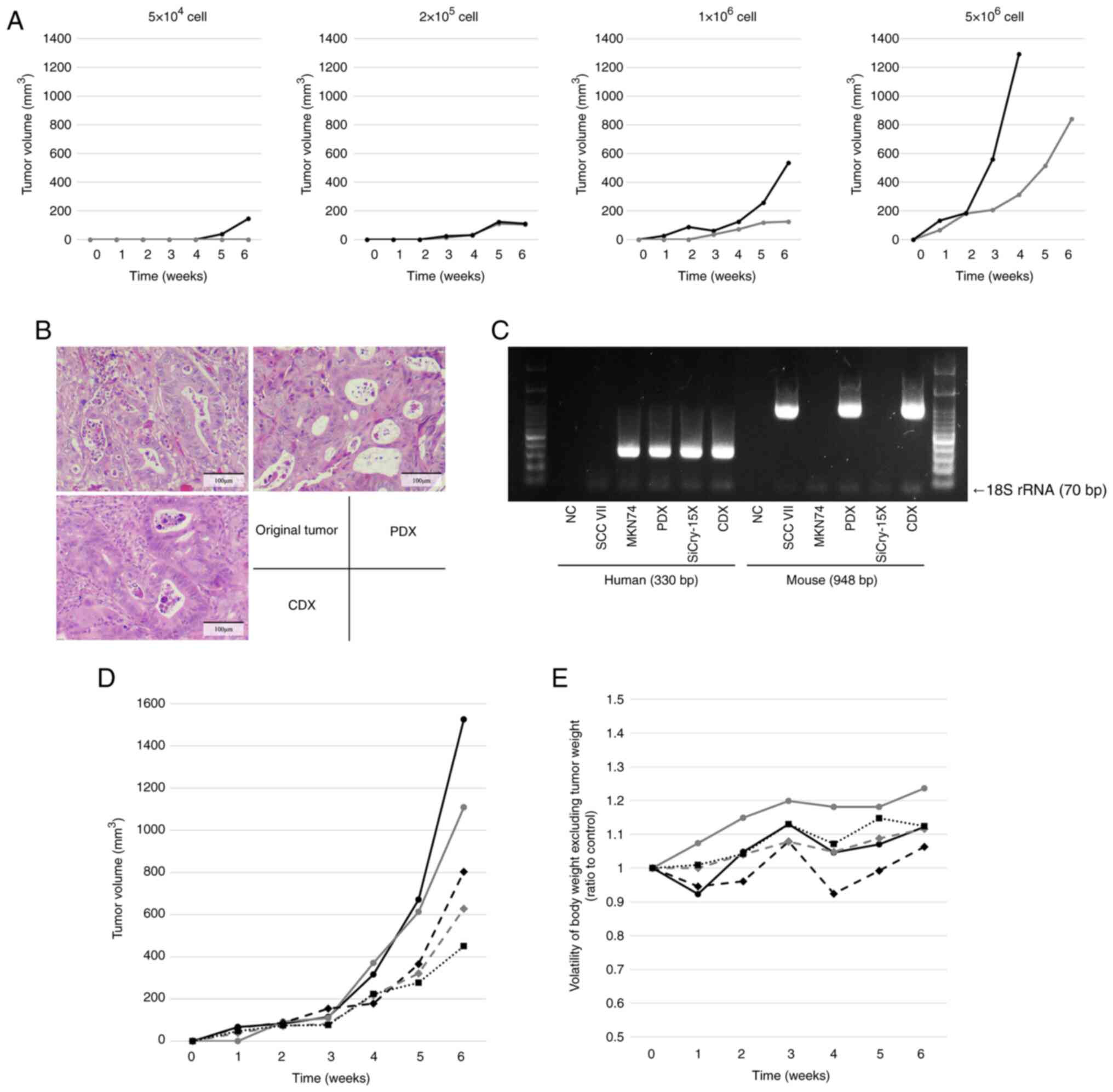 | Figure 4.In vivo experiments to evaluate
tumorigenicity and establish CDX model. (A) Plots of tumor
progression at different cell counts for cells injected
subcutaneously into nude mice (n=2). Tumors formed at all cell
counts at 6 weeks, but only one of the two mice formed a tumor when
injected with 5×104 cells. Conversely, one of the mice
injected with 5×106 cells developed a tumor with a
length that exceeded 15 mm within <6 weeks; observations for
this mouse were not recorded thereafter. (B) Hematoxylin and
eosin-stained images of the original tumor, PDX and CDX. The CDX
retained atypical glandular duct structures similar to those of the
original tumor and PDX. Scale bar=100 µm. (C) Amplification of
human- and mouse-specific mitochondrial DNA. CDX contains both
human and mouse components, similar to that observed in PDX. Plots
of (D) tumor volume progression and (E) volatility of body weight,
excluding the estimated tumor weight, for nude mice injected
subcutaneously with 2×106 cells (n=5). All mice showed
tumor growth, and none showed excessive weight loss. CDX,
cell-derived xenograft; PDX, patient-derived xenograft; NC,
negative control; rRNA, ribosomal RNA. |
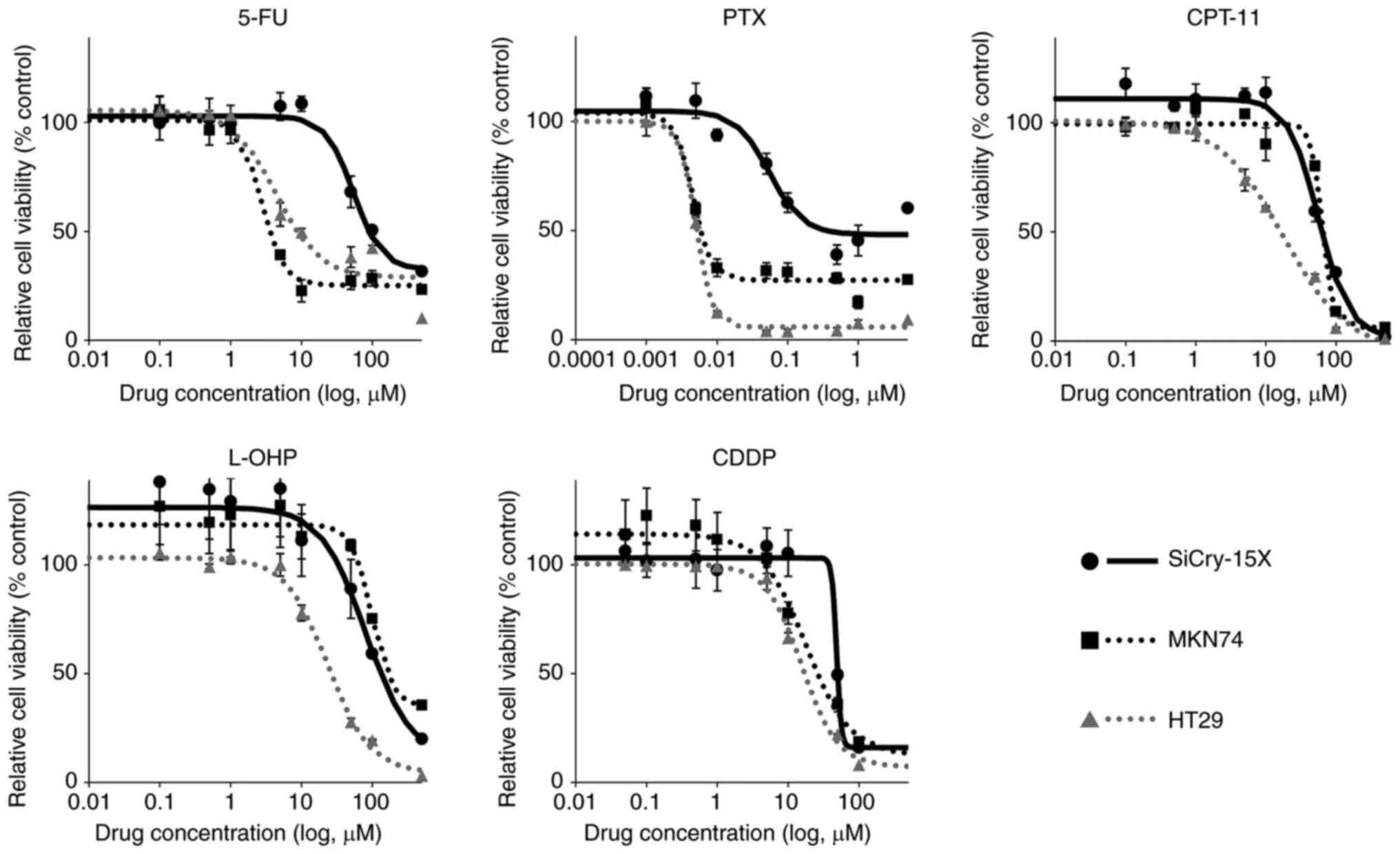
Anticancer drug sensitivity of cell
lines
The sensitivity of SiCry-15X cells to various
anticancer drugs was evaluated in vitro. The IC50 of 5-FU, PTX,
CPT-11, L-OHP, and CDDP for SiCry-15X were 104.05, 0.24, 63.3,
146.55, and 49.29 µM, respectively (Fig. 5, Table
II). For reference, similar experiments were performed using
the MKN74 and HT29 cell lines.
 | Table II.Anticancer drug sensitivity of cell
lines. |
Table II.
Anticancer drug sensitivity of cell
lines.
| Drug | Cell line | IC50
(µM) |
|---|
| 5-fluorouracil | SiCry-15X | 104.05 |
|
| MKN74 | 3.69 |
|
| HT29 | 9.68 |
| Paclitaxel | SiCry-15X | 0.24 |
|
| MKN74 | 0.0065 |
|
| HT29 | 0.0053 |
| Irinotecan | SiCry-15X | 63.30 |
|
| MKN74 | 68.51 |
|
| HT29 | 17.90 |
| Oxaliplatin | SiCry-15X | 146.55 |
|
| MKN74 | 279.85 |
|
| HT29 | 24.50 |
| Cisplatin | SiCry-15X | 49.29 |
|
| MKN74 | 28.97 |
|
| HT29 | 18.15 |
Evaluation of CEA and CA19-9
production by SiCry-15X
The clinical time courses of the serum CEA and
CA19-9 levels in the patient from whose samples the SiCry-15X line
originated are shown in Fig. 6A.
The patient's CEA and CA19-9 serum levels were markedly elevated
preoperatively and decreased rapidly after surgery. Recurrence of
SBA or re-elevation of CEA or CA19-9 levels were not observed.
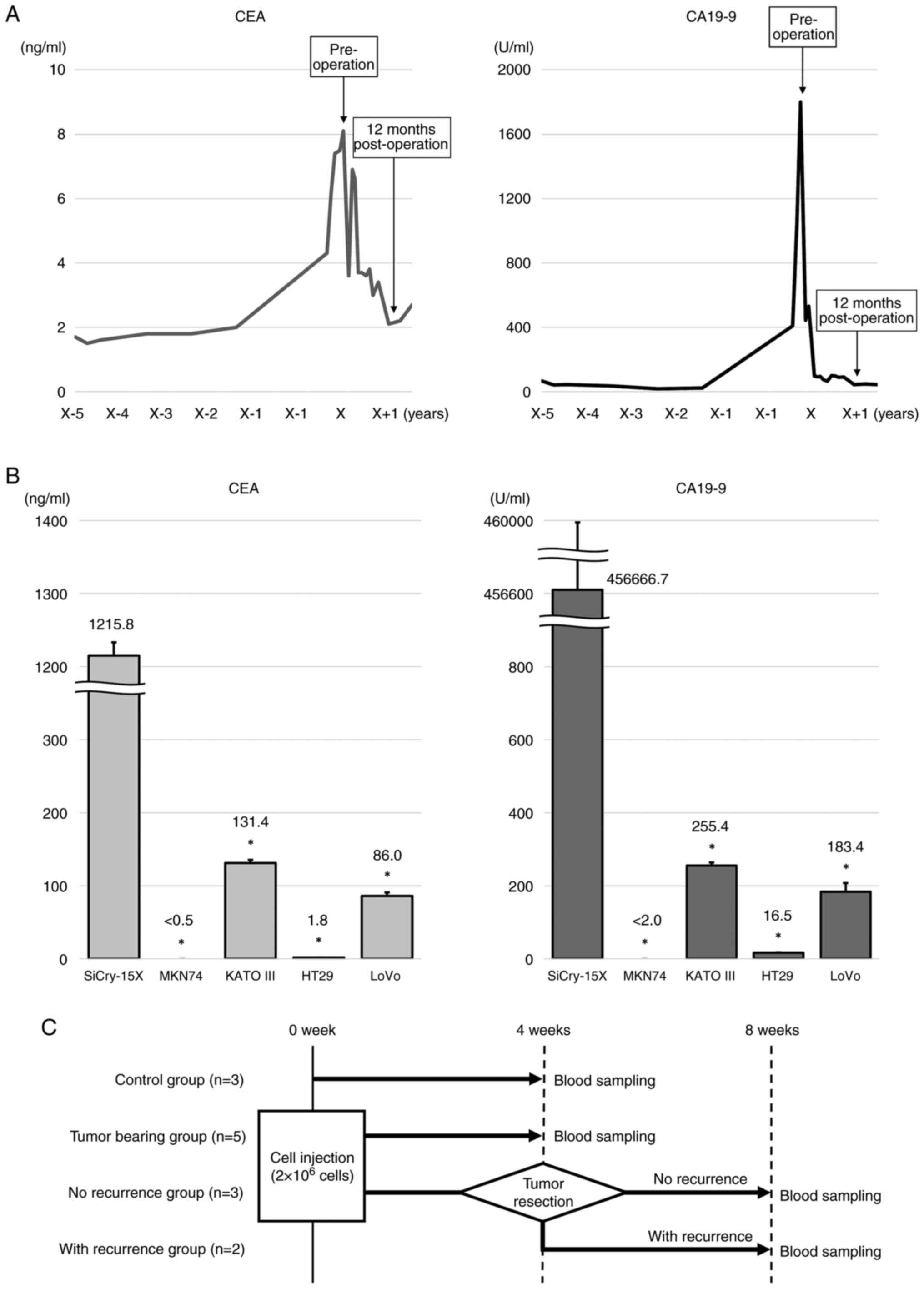 | Figure 6.Ability of SiCry-15X cells to produce
CEA and CA19-9. (A) Clinical time courses of serum CEA and CA19-9
levels, and the origin of SiCry-15X. CEA and CA19-9 levels in the
patient were monitored over several years, and the patient was
followed up for detection of complications; a marked increase in
CEA and CA19-9 levels prompted investigation for SBA. After radical
resection, both CEA and CA19-9 levels decreased rapidly. (B)
Average CEA and CA19-9 concentrations in the culture media of
different cell lines. LoVo, a CEA-producing cell line, was used as
a positive control for CEA. KATO III and MKN74, were used as
positive and negative controls for CA19-9, respectively. High
levels of CEA and CA19-9 were secreted in the culture medium of
SiCry-15X. *P<0.0001 compared with SiCry-15X. (C) Schematic
diagram of grouping and blood sampling schedule for CDX mice. The
number of SiCry-15X cells injected into the mice was adopted as the
optimal number of cells for the CDX model established in this
study. The control (n=3) and ‘tumor-bearing’ groups (n=5) underwent
blood sampling at 4 weeks. Five mice underwent tumor resection four
weeks after cell injection. Three mice that showed no recurrence 4
weeks after resection were designated the ‘no recurrence’ group,
and two mice that showed local recurrence were designated the ‘with
recurrence’ group. CEA, carcinoembryonic antigen; CA19-9,
carbohydrate antigen 19-9; SBA, small bowel adenocarcinoma; CDX,
cell-derived xenograft. |
Regarding the CEA, the concentration in the
SiCry-15X culture media was 1,215.8 ± 18.2 ng/mL. This
concentration was significantly higher than that of other cell
lines, including LoVo, a CEA-producing cell line (MKN74: <0.5 ±
0 ng/mL; KATO III: 131.4 ± 4.1 ng/mL; HT29: 1.8 ± 0.1 ng/mL; LoVo:
86.0 ± 4.9 ng/mL, P<0.0001, compared to SiCry-15X,
respectively). Similarly, the CA19-9 concentration in the SiCry-15X
culture media was 456,666.7 ± 4714.0 U/mL, and significantly
increased than other cell lines, including KATO III, which has been
reported to produce CA19-9 (10)
(MKN74: <2.0 ± 0 U/mL; KATO III: 255.4 ± 8.6 U/mL; HT29: 16.5 ±
1.0 U/mL; LoVo: 183.4 ± 24.2 U/mL, P <0.0001, compared to
SiCry-15X, respectively) (Fig.
6B).
Sera from the model mice harboring SiCry-15X were
collected at the time points shown in Fig. 6C, and CEA and CA19-9 concentrations
were measured. The average CEA and CA19-9 levels were elevated in
the ‘tumor-bearing’ (17.0 ng/mL and 15,000.0 U/mL, respectively)
and ‘with recurrence’ groups (17.8 ng/mL and 1,173.7 U/mL,
respectively), but the levels were below the detection sensitivity
level in the control and ‘no recurrence’ groups, which were
tumor-free (Table III). These
results indicate that CEA and CA19-9 were produced by SiCry-15X
cells and distributed throughout the blood. The CDX model harboring
SiCry-15X mimicked the clinical course of the change in levels of
CEA and CA19-9.
 | Table III.Serum CEA and CA19-9 in cell-derived
xenograft model mice. |
Table III.
Serum CEA and CA19-9 in cell-derived
xenograft model mice.
| Group | CEA, ng/ml
(average) | CA19-9, U/ml
(average) |
|---|
| Control (n=3) | <0.5 | <2.0 |
| Tumor bearing
(n=5) | 17.0 | 15,000.0 |
| No recurrence
(n=3) | <0.5 | <2.0 |
| With recurrence
(n=2) | 17.8 | 1173.7 |
Discussion
In this study, we established a novel cell line of
SBA, which is an uncommon tumor. To our knowledge, only four SBA
cell lines have been reported: SIAC1 (11), SBA-6 (12), SBA-16 (12) and one other (13), and this is the first report of a
CEA- and CA19-9-producing SBA cell line. SiCry-15X cells are
tumorigenic and capable of forming glandular duct structures at the
site of engraftment, suggesting that this cell line has
differentiation potential.
There is currently limited understanding of genetic
abnormalities that occur in SBA. In recent studies, TP53 (41–73%),
KRAS (27–54%), APC (13–27%), and PIK3CA (8–18%) were identified as
common mutations in SBA (14–16).
Schrock et al. reported that TP53 mutations are
significantly less common in SBA than in colorectal cancers, and
occur at a rate similar to that in gastric adenocarcinomas
(15). The frequency of APC
mutations is also considerably lower than that in colorectal
cancer. Furthermore, adenoma is infrequently found in the small
intestine except for the duodenum, suggesting that the
adenoma-carcinoma sequence is unlikely to be a major pathway of
carcinogenesis in SBA (16). In
addition, the frequency of BRAF mutations in SBA is comparable to
that in colorectal cancers, while V600E mutations are rare in SBA
(15). These results suggest that
genetic differences exist between SBA and colorectal cancer.
Therefore, to further clarify the genetic characteristics of small
intestinal adenocarcinoma, we believe that it is necessary to
establish appropriate research infrastructure, including cell
lines.
Cell line establishment from PDXs has been reported
in one case of SBA (13) and
several other cases of cancer, including those of the colon
(3), biliary tract (17), pancreas (18,19),
esophagus (18), and ovaries
(20). According to Dangles-Marie
et al., the success rate of cell line establishment from
xenografts in colon cancer was 47.4%, which was significantly
higher than the 9.7% reported for cell line establishment from
fresh tumor tissues. Furthermore, no dramatic changes in gene
expression were induced by the two cell line establishment
protocols: PDX-derived or fresh tissue-derived (3). In contrast, the PCR results for animal
species-specific mtDNA analysis in this study suggest that PDX
tissues contained stromal cells of host animal origin, in addition
to human tumor cells. Therefore, PDX-derived cell lines are at risk
of contamination with, and sometimes replacement by, stromal cells
derived from host animals such as mice. In this study, stromal
cells were removed by weak trypsin treatment, and animal-specific
mtDNA amplification was performed to confirm that the established
cell lines were free of cells of mouse origin. In addition, STR
analysis confirmed that the cell lines were genetically identical
to the original tumor and PDXs, thereby confirming that only human
tumor cells were extracted from the xenografts.
Serum CEA and CA19-9 levels are widely used as
diagnostic markers for various cancers. Elevated serum CEA and
CA19-9 levels are observed in 30 and 41% of patients, respectively
(21). In addition, CEA and CA19-9
could be prognostic markers in cases of radical resection of SBA
(22). However, CA19-9 is also
known to be elevated in benign diseases such as cholecystitis,
liver cirrhosis, and chronic pancreatitis (23), and this elevation may not
necessarily be due to secretion from tumor cells. The patient, from
whose samples the SiCry-15X line originated, had a hepatic cyst and
elevated CA19-9 levels. The patient therefore was followed up for
analysis of CEA and CA19-9 levels. SBA was detected because of a
remarkable increase in CEA and CA19-9 levels, and the tumor cells
were suspected to produce CEA and CA19-9. In this study, SiCry-15X
was proven to secrete high levels of CEA and CA19-9 into the
culture medium. Serum CEA and CA19-9 levels in mice injected with
SiCry-15X cells were similar to those observed during the clinical
course of the patient. Therefore, SiCry-15X may provide a model for
tumor marker transitions that reflect the clinical course of the
disease. In this model, or any model other than the subcutaneous
tumor model where tumor size cannot be directly measured, disease
status may be assessed by monitoring CEA and CA19-9 levels in the
blood during drug efficacy evaluation.
The development of appropriate and novel drug
therapies for SBA is an important issue. However, the cell line has
lost its heterogeneity, which is a limitation of this study. Drug
sensitivity should be evaluated by combining the in vivo results
obtained from analysis of cell lines and models that maintain tumor
heterogeneity, such as PDXs. In this study, the establishment of
PDXs and cell line pairs from the same tumor was a unique feature
and will contribute to further research on SBA. Another limitation
of this study is that only a single cell line was established.
Therefore, we aim to establish multiple SBA cell lines to elucidate
the disease-specific characteristics.
In conclusion, we established a cell line derived
from a PDX of SBA, named SiCry-15X. This cell line produces CEA and
CA19-9 and may provide a model that mirrors the clinical course of
the disease. In the case of SBA, the current research
infrastructure is inadequate, and thus, establishment of
patient-derived cancer models, including cell lines, is important.
Using this cell line, SBA pathogenesis can be elucidated in future
studies.
Acknowledgements
The authors thank Dr Keisuke Matsusaka (Department
of Pathology, Chiba University Hospital, Chiba, Japan) for his
expert assistance. We are grateful to Ms. Aki Komatsu (Department
of Frontier Surgery, Chiba University Graduate School of Medicine,
Chiba, Japan) for the technical advice and excellent technical
assistance.
Funding
The present study was supported by The Inohana Foundation (Chiba
University) Grant-in-Aid (grant no. IFCU-2023-4).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YN, YM, KN, SE, TT, RO TS, SI, HMo, TM, JH, AM and
HMa contributed to the conceptualization of this study. YN
performed most experiments. YN, YM, KM, SE, TT, RO, and TS
performed data collection and analysis. YM, KM, SE, TT, RO, TS, and
HMa conducted project administration. YM and HMa confirm the
authenticity of all the raw data. YM and HMa supervised the study.
YN wrote the manuscript with support from SI, HMo, TM, JH, and AM.
All authors provided critical feedback and read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Chiba University Graduate School of Medicine
(Chiba, Japan; approval nos. 1103, 1120 and 3520), and the study
was performed in agreement with the Declaration of Helsinki.
Written informed consent was obtained from the patient for
participation in the study and the use of their tissues. All animal
experiments were performed in accordance with the guidelines for
Animal Experiments at Chiba University and were approved by the
Institutional Animal Care and Use Committee of Chiba
University.
Patient consent for publication
Written informed consent was obtained from the
patient for genetic analysis, establishment of PDX and cell lines,
and the publication of all related information.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Yuri Nishioka ORCID iD: https://orcid.org/0009-0003-1479-5123; Dr Yasunori
Matsumoto ORCID iD: https://orcid.org/0000-0002-6239-6691
References
|
1
|
Aparicio T, Zaanan A, Svrcek M,
Laurent-Puig P, Carrere N, Manfredi S, Locher C and Afchain P:
Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis
and treatment. Dig Liver Dis. 46:97–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dabaja BS, Suki D, Pro B, Bonnen M and
Ajani J: Adenocarcinoma of the small bowel: Presentation,
prognostic factors, and outcome of 217 patients. Cancer.
101:518–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dangles-Marie V, Pocard M, Richon S,
Weiswald LB, Assayag F, Saulnier P, Judde JG, Janneau JL, Auger N,
Validire P, et al: Establishment of human colon cancer cell lines
from fresh tumors versus xenografts: comparison of success rate and
cell line features. Cancer Res. 67:398–407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawai S, Takagi Y, Kaneko S and Kurosawa
T: Effect of three types of mixed anesthetic agents alternate to
ketamine in mice. Exp Anim. 60:481–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakayama T, Saito R, Furuya S, Shoda K,
Maruyma S, Takiguchi K, Shiraishi K, Akaike H, Kawaguchi Y, Amemiya
H, et al: Inhibition of cancer cell-platelet adhesion as a
promising therapeutic target for preventing peritoneal
dissemination of gastric cancer. Oncol Lett. 26:5382023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lorenzi PL, Reinhold WC, Varma S,
Hutchinson AA, Pommier Y, Chanock SJ and Weinstein JN: DNA
fingerprinting of the NCI-60 cell line panel. Mol Cancer Ther.
8:713–724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu M, Selvaraj SK, Liang-Chu MM, Aghajani
S, Busse M, Yuan J, Lee G, Peale F, Klijn C, Bourgon R, et al: A
resource for cell line authentication, annotation and quality
control. Nature. 520:307–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medico E, Russo M, Picco G, Cancelliere C,
Valtorta E, Corti G, Buscarino M, Isella C, Lamba S, Martinoglio B,
et al: The molecular landscape of colorectal cancer cell lines
unveils clinically actionable kinase targets. Nat Commun.
6:70022015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ono K, Satoh M, Yoshida T, Ozawa Y, Kohara
A, Takeuchi M, Mizusawa H and Sawada H: Species identification of
animal cells by nested PCR targeted to mitochondrial DNA. In Vitro
Cell Dev Biol Anim. 43:168–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Natomi H, Sugano K, Iwamori M, Saito E,
Kubota S, Kondo Y, Osawa N and Takaku F: Production of CA19-9 in
cultured human gastric cancer cell lines. Nihon Shokakibyo Gakkai
Zasshi. 85:236–242. 1988.PubMed/NCBI
|
|
11
|
Suzuki H, Hirata Y, Suzuki N, Ihara S,
Sakitani K, Kobayashi Y, Kinoshita H, Hayakawa Y, Yamada A, Watabe
H, et al: Characterization of a new small bowel adenocarcinoma cell
line and screening of anti-cancer drug against small bowel
adenocarcinoma. Am J Pathol. 185:550–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adam L, San Lucas FA, Fowler R, Yu Y, Wu
W, Liu Y, Wang H, Menter D, Tetzlaff MT, Ensor J Jr, et al: DNA
sequencing of small bowel adenocarcinomas identifies targetable
recurrent mutations in the ERBB2 signaling pathway. Clin Cancer
Res. 25:641–651. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamano T, Kubo S and Tomita N: A
patient-derived xenograft and a cell line derived from it form a
useful preclinical model for small bowel adenocarcinoma. Cancer
Med. 9:3337–3343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laforest A, Aparicio T, Zaanan A, Silva
FP, Didelot A, Desbeaux A, Le Corre D, Benhaim L, Pallier K, Aust
D, et al: ERBB2 gene as a potential therapeutic target in small
bowel adenocarcinoma. Eur J Cancer. 50:1740–1746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schrock AB, Devoe CE, McWilliams R, Sun J,
Aparicio T, Stephens PJ, Ross JS, Wilson R, Miller VA, Ali SM and
Overman MJ: Genomic profiling of small-bowel adenocarcinoma. JAMA
Oncol. 3:1546–1553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tatsuguchi A, Yamada T, Ueda K, Furuki H,
Hoshimoto A, Nishimoto T, Omori J, Akimoto N, Gudis K, Tanaka S, et
al: Genetic analysis of Japanese patients with small bowel
adenocarcinoma using next-generation sequencing. BMC Cancer.
22:7232022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ojima H, Yoshikawa D, Ino Y, Shimizu H,
Miyamoto M, Kokubu A, Hiraoka N, Morofuji N, Kondo T, Onaya H, et
al: Establishment of six new human biliary tract carcinoma cell
lines and identification of MAGEH1 as a candidate biomarker for
predicting the efficacy of gemcitabine treatment. Cancer Sci.
101:882–888. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Damhofer H, Ebbing EA, Steins A, Welling
L, Tol JA, Krishnadath KK, van Leusden T, van de Vijver MJ,
Besselink MG, Busch OR, et al: Establishment of patient-derived
xenograft models and cell lines for malignancies of the upper
gastrointestinal tract. J Transl Med. 13:1152015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pham K, Delitto D, Knowlton AE, Hartlage
ER, Madhavan R, Gonzalo DH, Thomas RM, Behrns KE, George TJ Jr,
Hughes SJ, et al: Isolation of pancreatic cancer cells from a
patient-derived xenograft model allows for practical expansion and
preserved heterogeneity in culture. Am J Pathol. 186:1537–1546.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Thaye E, Van de Vijver K, Van der
Meulen J, Taminau J, Wagemans G, Denys H, Van Dorpe J, Berx G,
Ceelen W, Van Bocxlaer J and Wever OD: Establishment and
characterization of a cell line and patient-derived xenograft (PDX)
from peritoneal metastasis of low-grade serous ovarian carcinoma.
Sci Rep. 10:66882020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zaanan A, Costes L, Gauthier M, Malka D,
Locher C, Mitry E, Tougeron D, Lecomte T, Gornet JM, Sobhani I, et
al: Chemotherapy of advanced small-bowel adenocarcinoma: A
multicenter AGEO study. Ann Oncol. 21:1786–1793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li N, Shen W, Deng W, Yang H, Ma Y, Bie L,
Wei C and Luo S: Clinical features and the efficacy of adjuvant
chemotherapy in resectable small bowel adenocarcinoma: a
single-center, long-term analysis. Ann Transl Med. 8:9492020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuzawa K, Itabashi K, Matsuzawa S,
Tsutsumi O, Hiki Y and Kakita A: A case of large liver cyst with
extraordinally high level of CA19-9 in the serum, CA19-9 and CEA in
the cystic fluid. Nihon Shokakigeka Gakkai Zasshi. 32:2375–2379.
1999.(In Japanese).
|