Introduction
Mantle cell lymphoma (MCL) is a subtype of B-cell
non-Hodgkin lymphoma (NHL) that accounts for 3–10% of NHL cases,
typically involving the lymph nodes (1). The majority of patients with MCL are
diagnosed at an advanced stage, with bone marrow involvement and
lymph node spread (1,2). The gastrointestinal tract is impacted
in 15–30% of patients with MCL (1,3).
Primary MCL in the gastrointestinal tract is rare, accounting for
4–9% of all reported cases of gastrointestinal NHL (1) Primary gastrointestinal tract MCL is
also referred to as lymphomatous polyposis and it typically
manifests as single or multiple polypoid lesions, ulcerative
lesions or thickening of the gastrointestinal wall (1,3). Most
commonly the stomach and ileocecal regions are affected but any
part of the gastrointestinal tract can be involved (3,4);
however, complete involvement of the gastrointestinal tract in MCL
is rare (5). In the present report,
a case of MCL that manifested as numerous diffuse polypoid lesions
along the entirety of the digestive tract is described.
Case report
A previously healthy 56-year-old male patient was
admitted to the Department of Gastroenterology of Hunan Provincial
People's Hospital (Changsha, China) in August 2023 with abdominal
pain that had lasted for >6 months and had worsened over the
past month. The patient complained of upper abdominal distension
and pain accompanied by acid reflux, especially at night, with
occasional diarrhea. No other symptoms of discomfort were reported.
Since the onset of symptoms, the patient had a poor mental state
and sleep quality, moderate appetite, changes in bowel movements
(low fecal volume and difficulty in defecation), normal urination,
and no significant change in weight. The patient denied any
familial genetic history of malignant tumors of the digestive
tract, and their spouse and child were healthy.
At admission, the body temperature of the patient
was 36.4°C, the heart rate was 80 bpm and the blood pressure was
134/80 mmHg. Physical examination revealed mild tenderness of the
upper abdomen and superficial lymph node enlargement in the neck,
subclavian region and groin. Based on the clinical symptoms alone,
the patient could easily be diagnosed with chronic gastritis. CT
(Fig. 1A) showed multiple
thickenings of the gastric and intestinal walls and numerous
enlarged lymph nodes throughout the body. Superficial lymph node
ultrasonography (Fig. 1B) revealed
enlarged lymph nodes in the bilateral neck, upper and lower
clavicle areas, armpits, groin and abdominal cavity. Gastroscopy
(Fig. 1C) showed numerous wide
basal and circular polypoid lesions in the entire gastric cavity,
duodenal bulb and descending part, with rich vascular networks on
the surface and a hard texture. Colonoscopy (Fig. 1D) revealed that the ileocecal valve
was swollen and uneven, and the valve opening was not visible. The
entire tract was covered with wide basal round or spindle-shaped
polypoid lesions with a size of 0.5–1.5 cm. The surfaces of the
polypoid lesions were rich in vascular networks, and a portion of
the lesions were internally erosive, fragile and prone to bleeding.
Other abnormal results included the fecal occult blood test [(+;
normal value, (−); Colloidal Gold Method] and albumin level of
29.59 g/l (normal range, 35–55 g/l; Bromocresol Green Method). The
white blood cell (WBC) count was 9.82×109/l (normal
range, 4.00–10.00×109/l). The quantitative value of
lactic dehydrogenase (LDH) was 205.4 U/l (normal range, 100.0–240.0
U/l). Based on the symptoms and examination results of the patient,
lymphoma was suspected. Samples were fixed using 10% neutral
buffered formalin at room temperature (20–26°C) for 24–48 h.
Sections (3-µm thick) were stained with hematoxylin staining
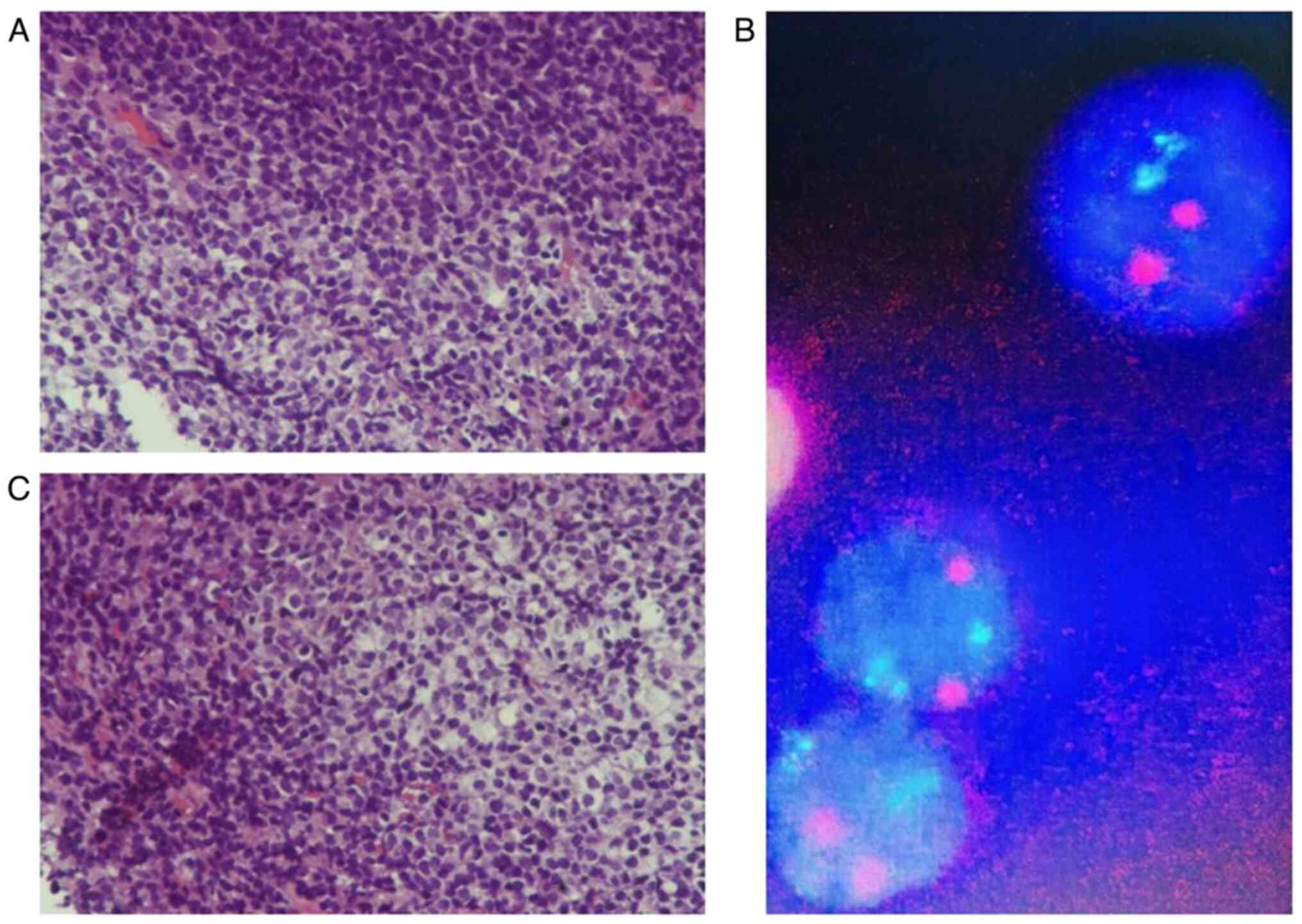
solution at room temperature for 1 min. The results (Fig. 2A and C); showed patchy lymphocyte
infiltration in the mucosa, with local follicular structures.
Fluorescence in situ hybridization (FISH; Fig. 2B) indicated that the lesion was
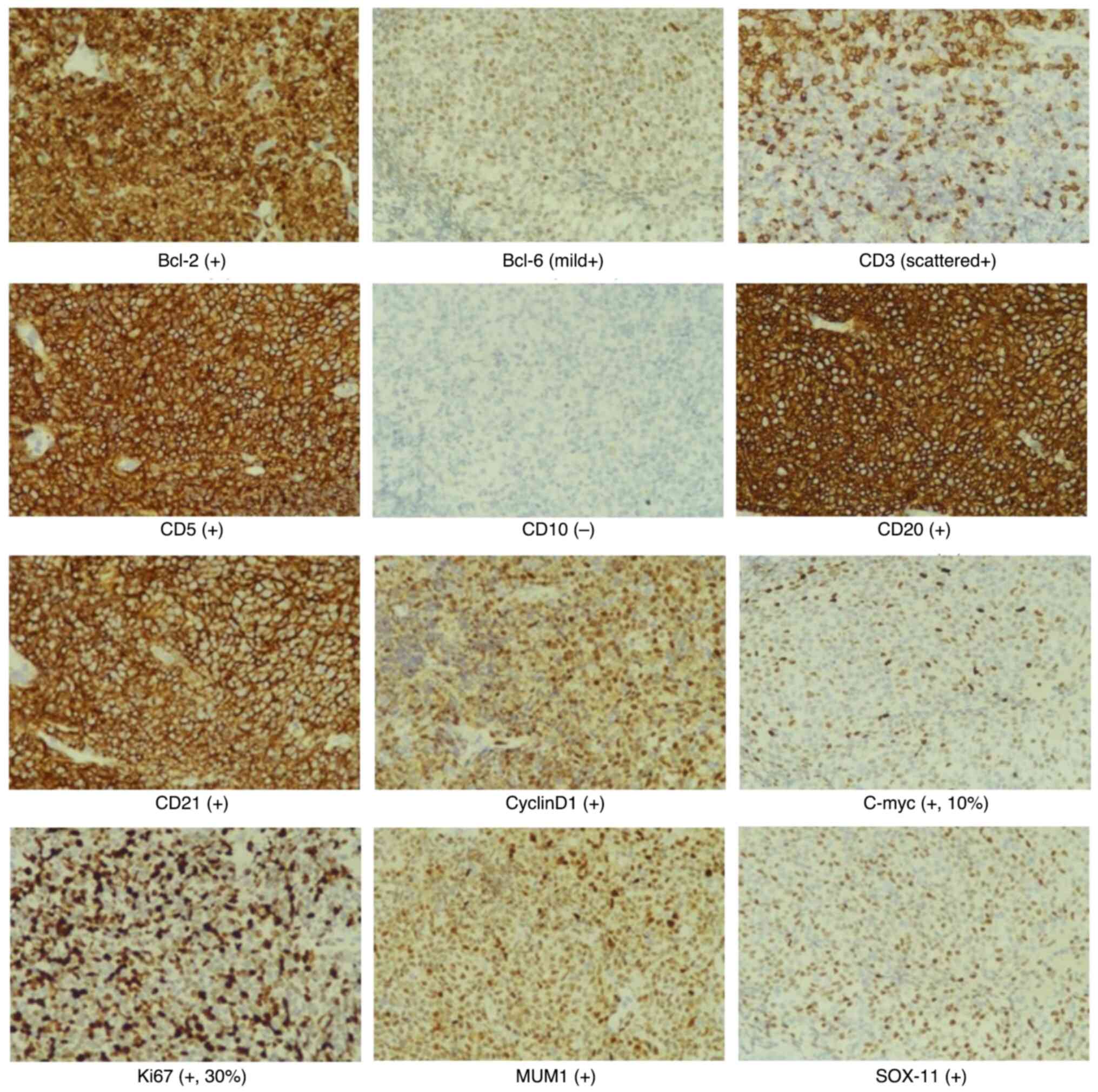
CCND1/IGH (−), and immunohistochemical staining (Fig. 3) indicated that the lesion was CD21
(+), CD20 (+), Ki67 (+; 30%), Bcl-2 (+), Bcl-6 (mild+), CD5 (+),
CyclinD1 (+) and SOX-11 (+). Therefore, the patient was diagnosed
with MCL stage IV and scheduled to be transferred to the Department
of Hematology of Hunan Provincial People's Hospital for
chemotherapy. After completing tumor assessment, the patient
received rituximab in combination with cyclophosphamide,
doxorubicin, vincristine and prednisone chemotherapy (R-CHOP).
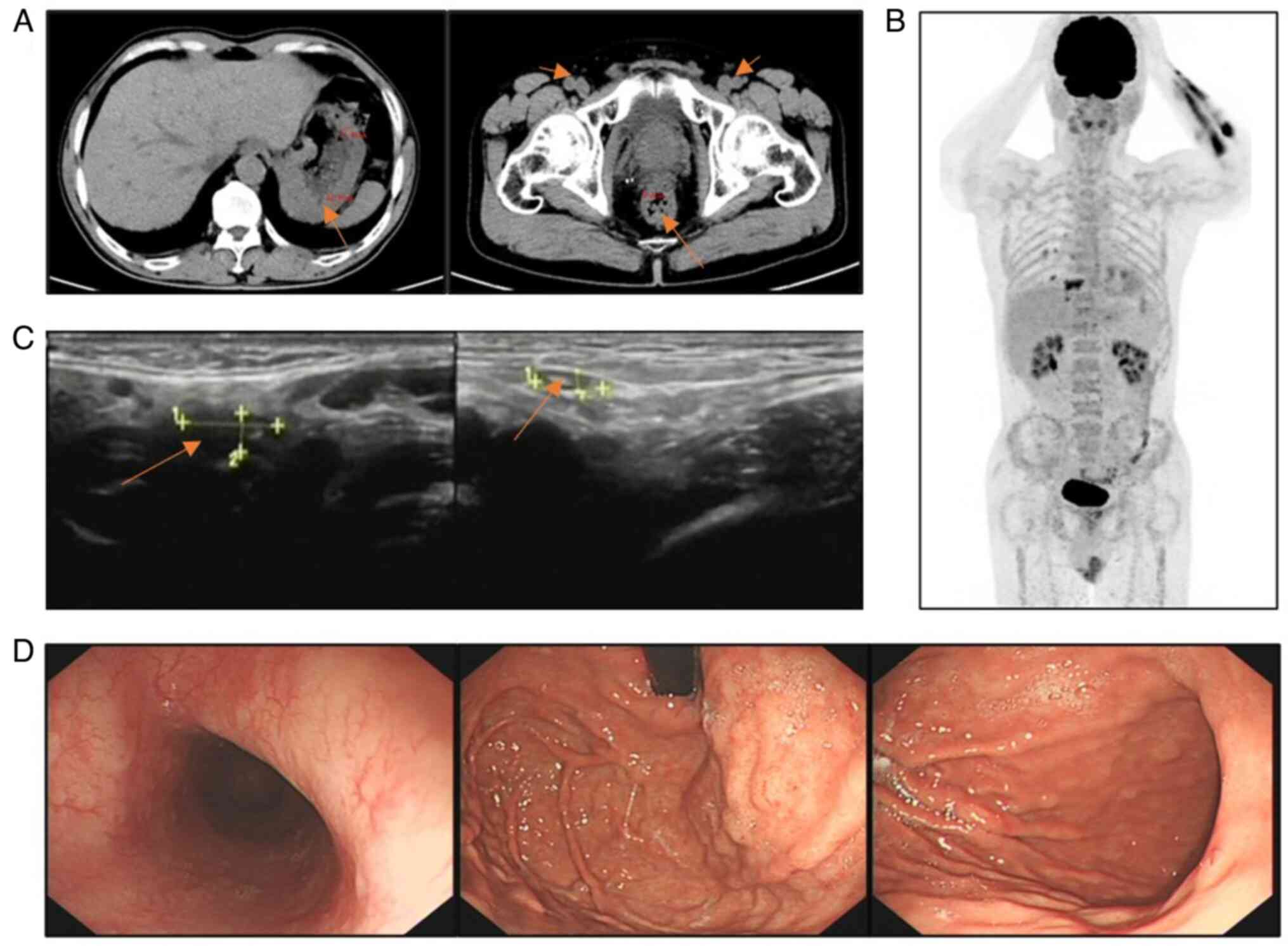
After two cycles of R-CHOP (Table
I), a CT scan (Fig. 4A) showed
a marked reduction in lymph nodes in multiple areas and less
thickening of the gastrointestinal wall compared with previous
scans, but did not demonstrate complete response (CR) because there
were still abnormalities. Treatment was changed to rituximab
combined with etoposide, oxaliplatin and ifosfamide, with the
addition of ibrutinib capsules (Table
I). After completion of cycle 6 of the treatment, the patient
underwent superficial lymph node ultrasonography, gastroscopy and
positron emission tomography (PET)-CT in February 2024. The PET-CT
scan (Fig. 4B) showed no
significant hypermetabolic lesions in the gastrointestinal wall and
the lymph nodes throughout the body. The superficial lymph node
ultrasonography (Fig. 4C) did not
reveal obvious lymph node enlargement. The gastroscopy (Fig. 4D) indicated that the polypoid
lesions in the stomach had virtually disappeared. These results
showed that the patient had achieved CR. The patient was followed
up every 21 days with the medical record system of the
hospital.
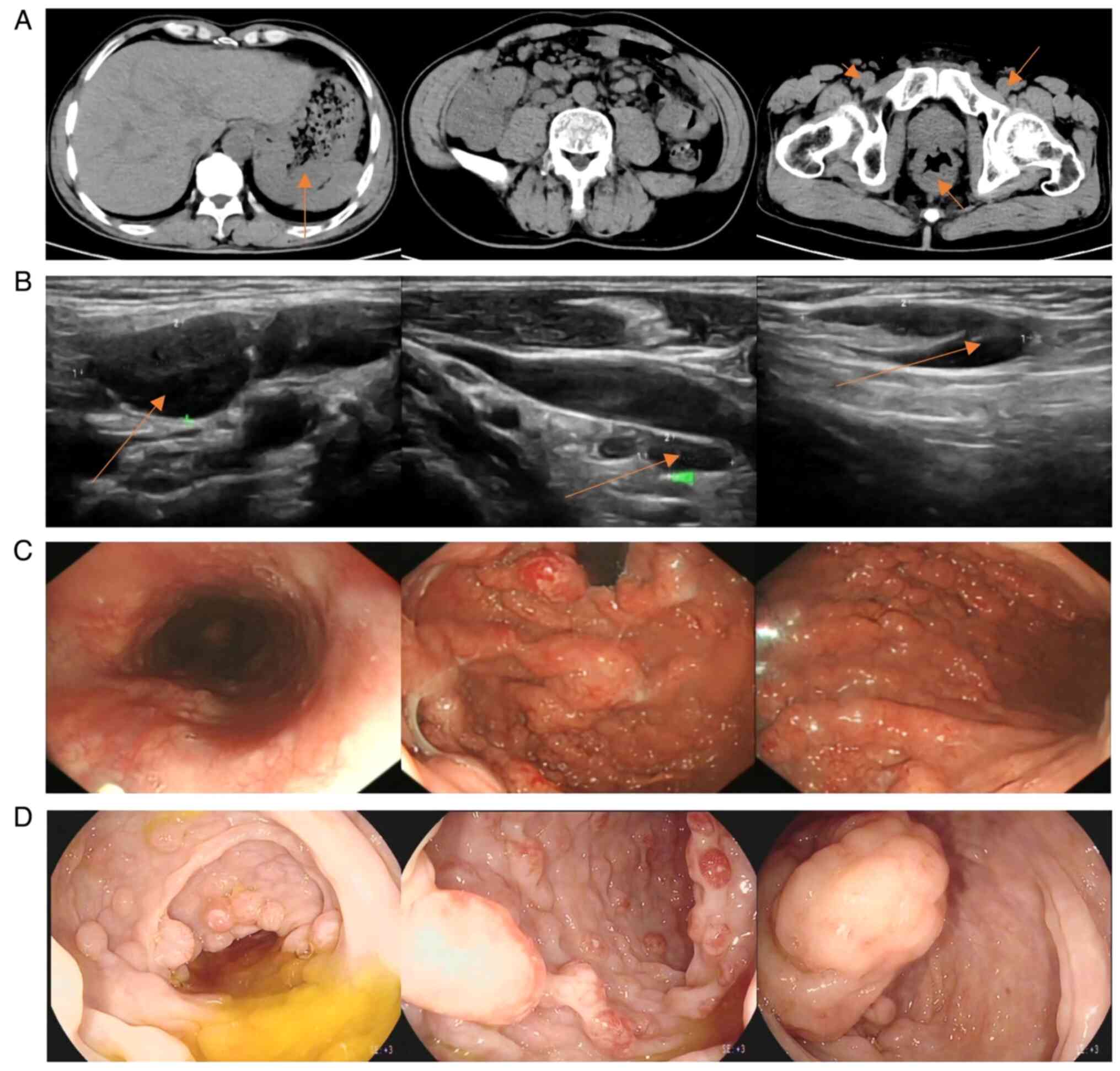 | Figure 1.(A) CT imaging demonstrating evident
thickening of the gastric wall (arrow point) at multiple locations,
thickening of the distal ileum wall and formation of ileocecal
intussusception; multiple lymph node enlargements in the left lung
hilum, bilateral armpits, retroperitoneum, mesenteric area, pelvic
cavity and bilateral inguinal region (arrow point); and thickening
of the lower rectal wall (arrow point). (B) Superficial lymph node
ultrasonography demonstrating that multiple lymph nodes were
markedly enlarged throughout the body. From left to right, the
cervical (arrow point, 21×11 mm), supraclavicular (10×5 mm) and
inguinal (arrow point, 28×8 mm) lymph nodes are shown. (C)
Gastroscopy demonstrating multiple protruding lesions with rich
vascular networks on the surface and a hard texture. (D)
Colonoscopy showing that the ileocecal valve was swollen and
uneven, had multiple protruding lesions, the entire tract was
covered with wide basal round or spindle-shaped protrusions, and
the surfaces were rich in vascular networks. |
 | Table I.Patient treatment plan. |
Table I.
Patient treatment plan.
| Cycle 1 (September
2023) | Cycle 2 (October
2023) | Cycles 3–6 (November
2023 to January 2024) | Cycle 7 (February
2024) |
|---|
| Rituximab (600 mg;
ivdrip; qd; d0); cyclophosphamide (1,200 mg; ivdrip; qd; d1);
vincristine (2 mg; iv; qd; d1); liposomal doxorubicin (40 mg;
ivdrip; qd; d1); dexamethasone (10 mg; ivdrip; qd; d1-5) | Rituximab (600 mg;
ivdrip; qd; d0); cyclophosphamide (1,200 mg; ivdrip; qd; d1);
vincristine (2 mg; iv; qd; d1); liposomal doxorubicin (40 mg;
ivdrip; qd; d1); methylprednisolone (40 mg; po; twice daily;
d1-5) | Rituximab (600 mg;
ivdrip; qd; d0); etoposide (150 mg; ivdrip; qd; day 1-3 of
chemotherapy); oxaliplatin (150 mg; ivdrip; qd; d2); ifosfamide (3
g; ivdrip; every 12 h; d2); ibrutinib capsules (560 mg; po; qd;
every day) | Rituximab (600 mg;
ivdrip; qd; d0); ibrutinib capsules (560 mg; po; qd; every
day) |
Discussion
MCL is a rare aggressive lymphoma with poor
prognosis (6). Classical MCL
accumulates in the lymph nodes, spleen and extranodal sites,
including the gastrointestinal tract (7); however, MCL is rarely diagnosed in the
gastroenterology department, as MCL belongs to the category of
hematological malignancies. MCL commonly harbors chromosomal
translocations, such as the t(11;14)(q13;q32) translocation
involving the IGH and CCND1 genes (6). The pathogenesis of MCL includes Cyclin
D1 expression upregulation, SOX-11 expression upregulation, TP53
mutations and other molecular alterations such as chromosomal
complexity and NSD2 (7).
The clinical symptoms of patients with MCL are not
specific. Some patients have no symptoms, but present with lymph
node, spleen and bone marrow involvement (1). Clinical presentations of
gastrointestinal tract MCL rarely have typical characteristics;
however, abdominal pain, distension, diarrhea, melena and
hematochezia may occur (3,4). The clinical presentation in the
present case was chronic abdominal pain with occasional diarrhea
but no other discomfort was reported. Without digestive endoscopy,
gastroenterologists may misdiagnose the patient as having chronic
gastritis, and MCL can easily be missed.
MCL of the gastrointestinal tract often occurs in
the stomach and ileocecal regions (3). During endoscopic examination in the
present case, it was found that the lesions were present along the
entire digestive tract, including the stomach and colon. Endoscopic
findings included multiple polypoid masses of different sizes with
smooth surfaces. As these findings are similar to those of polyps,
it is easy for physicians without endoscopic experience to diagnose
endoscopic findings as hereditary polyposis, and thus, delay
treatment.
The patient was diagnosed on the basis of digestive
endoscopy and pathological evidence. Therefore, timely
histopathological and immunohistochemical staining after endoscopic
examination was key to distinguishing it from other diseases
(4,8). The pathological and
immunohistochemical specimens were polypoid tissues of the
gastrointestinal tract obtained during endoscopy. Pathological
examination revealed patchy lymphocyte infiltration in the mucosa
and a local follicular structure. Immunohistochemical staining
showed that the lesion was CyclinD1 (+) and SOX-11 (+). Based on
the results of the gastrointestinal endoscopy and pathological and
immunohistochemical staining, the case was confirmed as MCL
(7).
Patients with MCL at different disease stages
undergo different treatment strategies. PET/CT has a higher staging
accuracy than conventional CT, and the patient in the present study
was identified as having stage IV disease (8) on PET/CT. For patients with stage
III–IV MCL, both symptomatic and asymptomatic patients with a high
tumor burden should be treated as soon as a diagnosis is made, and
a regimen of rituximab plus chemotherapy is generally recommended
(8). After rituximab was combined
with chemotherapy and ibrutinib capsule-targeted therapy, endoscopy
indicated that the polypoid lesions were markedly reduced or even
disappeared, which was a significant improvement compared with the
first gastroscopy. Re-examination of the PET/CT scan revealed that
the patient achieved CR. In the present case, timely
gastrointestinal endoscopy facilitated immediate treatment after
diagnosis, resulting in highly effective therapeutic outcomes.
The prognosis of MCL is related to numerous factors,
such as stage, risk factors, gene mutations and chromosome
translocations (7). The
International Prognostic Index of MCL (MIPI) includes age, Eastern
Cooperative Oncology Group (ECOG) performance status, lactate
dehydrogenase (LDH) level and white blood cell (WBC) count at
initial diagnosis (8,9). According to the MIPI, risk groups
describing the prognosis of patients with MCL are divided into
low-, intermediate- and high-risk groups (8,9). The
present case involved a 56-year-old male patient with mild
limitations in physical activity. The initial diagnosis revealed
normal levels of LDH and WBC counts, indicating that the patient
was in the low-risk group (9). In
FISH, no abnormalities were detected in the IGH and CCND1 genes,
which may indicate that these two genes did not undergo the
expected mutations in this specific case. Therefore, the patient
had a favorable prognosis.
Infectious and immunological factors are considered
to be involved in the pathogenesis of lymphomas. Poor living
habits, including smoking and drinking, and hepatitis B and
Epstein-Barr virus infection can increase the risk of NHL (10,11).
Furthermore, chronic hepatitis C infection may be related to the
occurrence of lymphoma, and direct-acting antiviral therapy can
improve the cure rate (12,13). Therefore, prevention of viral
infections, good living habits, long-term moderate physical
exercise and regular health examinations can reduce the incidence
of lymphoma to a certain extent (10).
In conclusion, endoscopy is necessary for patients
with gastrointestinal symptoms. Early endoscopy can improve the
detection and diagnosis of MCL, and obtaining histological
specimens can also help diagnose MCL as soon as possible and
evaluate effective early treatment options so that patients can
achieve the goal of a CR.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SC and LY conceived and designed the study. LY and
XW obtained the data. SC and LY analyzed the data and drafted the
manuscript. SC and LY confirm the authenticity of all raw data. SC
and LY revised the manuscript prior to submission. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed patient consent was obtained to
publish the article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Castellino A, Tun AM, Wang Y, Habermann
TM, King RL, Ristow KM, Cerhan JR, Inwards DJ, Paludo J, Ansell SM,
et al: Clinical characteristics and outcomes of primary versus
secondary gastrointestinal mantle cell lymphoma. Blood Cancer J.
11:82021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lamm W, Dolak W, Kiesewetter B,
Simonitsch-Klupp I, Puhr H and Raderer M: Gastrointestinal
involvement in patients with mantle cell lymphoma: A single center
experience of eighty-five patients. Dig Dis. 37:194–200. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohy-Ud-Din N, Guha A and Mitre M:
Complete endoscopic and histopathological remission of mantle cell
lymphoma of the gastrointestinal tract. Cureus.
11:e43502019.PubMed/NCBI
|
|
4
|
Zheng QF, Li JY, Qin L, Wei HM, Cai LY and
Nong B: Gastrointestinal involvement by mantle cell lymphoma
identified by biopsy performed during endoscopy: A case report.
Medicine (Baltimore). 97:e97992018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang PJ, Chang CL and Suk FM: Polypoid
lesions from the oesophagus to colon. Gut. 67:5522018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maddocks K: Update on mantle cell
lymphoma. Blood. 132:1647–1656. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jain P and Wang M: Mantle cell lymphoma:
2019 update on the diagnosis, pathogenesis, prognostication, and
management. Am J Hematol. 94:710–725. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dreyling M, Campo E, Hermine O, Jerkeman
M, Le Gouill S, Rule S, Shpilberg O, Walewski J and Ladetto M; ESMO
Guidelines Committee, : Newly diagnosed and relapsed mantle cell
lymphoma: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28 (Suppl 4):iv62–iv71. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eyre TA, Bishton MJ, McCulloch R, O'Reilly
M, Sanderson R, Menon G, Iyengar S, Lewis D, Lambert J, Linton KM,
et al: Diagnosis and management of mantle cell lymphoma: A British
Society for Haematology Guideline. Br J Haematol. 204:108–126.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu FD, Li XP, Xu XQ, Yu H, Luo PF and
Zhou JY: Disease burden of non-Hodgkin lymphoma in Jiangsu Province
from 1990 to 2019. Pract Prev Med. 30:284–287. 2023.(In
Chinese).
|
|
11
|
Feng J, Fei Y, Gao M, Meng X, Zeng D, Zuo
D, Ye H, Liang Y, Sun X, Liang R, et al: Treatment patterns,
clinical outcomes and gene mutation characteristics of hepatitis B
virus-associated mantle cell lymphoma. Hematol Oncol. 42:e32682024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mihăilă RG: Hepatitis C virus-Associated B
cell non-Hodgkin's lymphoma. World J Gastroenterol. 22:6214–6223.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazzaro C, Bomben R, Gragnani L, Visentini
M, Pozzato G, Pozzo F, Zucchetto A and Gattei V: Hepatitis C
virus-associated B-cell lymphomas: The importance of the new direct
antiviral agent therapy. Semin Hematol. 59:177–182. 2022.
View Article : Google Scholar : PubMed/NCBI
|