Introduction
Human epidermal growth factor receptor 2 (HER2)
overexpression is not only an independent poor prognostic factor
(1) but also a biomarker for
guiding the use of trastuzumab-based anti-HER2 systemic therapy in
operable primary breast cancer. Trastuzumab-based anti-HER2 therapy
is the first option for node-negative (pN0), HER2-overexpressing
breast cancer with an invasive size >1 cm (2). Although in the APT trial, among
patients with small (≤3 cm), negative nodes, and HER2-positive
breast cancer that received adjuvant paclitaxel and trastuzumab,
the 7-year DFS of hormone receptor (HR) negative tumors was worse
than that of positive tumors, evidence on the effectiveness and
indication of trastuzumab-based therapy for pN0,
HER2-overexpressing cases with invasive size ≤1.0 cm (pT1a/b) is
lacking (2,3). Systemic treatment, including
trastuzumab, has been reported to be unnecessary for the clinical
management of pT1a HER2-positive tumors (4). The recurrence rate of pT1a/b pN0,
HR-positive HER2-positive tumors has been reported to be 5–25% at 5
years, which is significantly higher than that of pT1a/b pN0,
HR-positive HER2-negative tumors (3,5,6). The
wide range in reported relapse rates is presumably due to
variations in the ratio of induction adjuvant chemotherapy,
including trastuzumab (3,5,6).
Despite being rare, cases of pT1a pN0 HER2-positive breast cancer
with recurrence have been reported. This report presents a case of
pT1a HR-positive HER2-positive breast cancer with multiple
metastases to the axillary lymph nodes and liver within one year
after radical surgery. The purpose of this case report is to
address the necessity to identify pT1a pN0 HER2-positive breast
cancer patients who are expected to benefit from adjuvant
chemotherapy, including trastuzumab.
Case report
A 58-year-old woman was referred to the Department
of Surgery, National Defense Medical College, Tokorozawa, Japan, by
her family doctor due to a 20-mm breast mass. Her medical history
was unremarkable. She went through menopause at the age of 51. She
has one child and no family history of breast cancer. Physical
examination revealed a 20-mm tumor palpated in the upper external
quadrant of the left breast. Serum tumor markers were within normal
ranges. Ultrasonography (US) showed hyperechoic lesions in the
dilated mammary ducts up to 5 mm in diameter. US-guided core needle
biopsy was performed, and the tumor was histologically diagnosed as
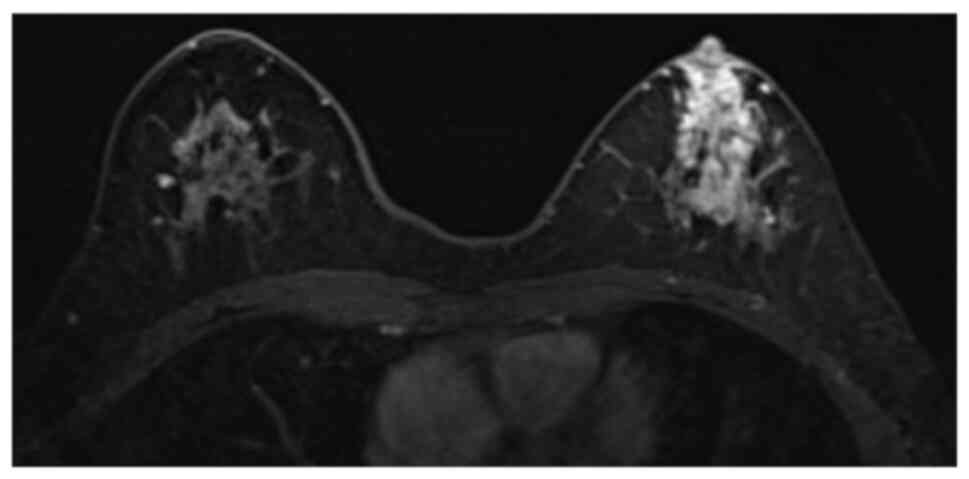
ductal carcinoma in situ (DCIS). Magnetic resonance imaging
(MRI) showed a non-mass-enhancing lesion measuring 51×21 mm,
extending to the nipple in the left breast (Fig. 1). US or MRI showed no obvious lymph
node enlargement. Preoperative workup with fluorine-18
fluorodeoxyglucose positron emission tomography/computed tomography
(18F-FDG PET/CT) showed uptake in the left breast lesion
but no evidence of distant metastasis. Based on the diagnosis of
DCIS (cTis cN0) stage 0 (7), left
total mastectomy and sentinel lymph node biopsy were performed.
Intraoperative frozen section of the sentinel node was negative for
metastasis. The negativity of the sentinel node for metastasis was
also confirmed by the sections of the formalin-fixed and
paraffin-embedded material. A 50×40 mm multinodular lesion was
observed in the resected specimen (Fig.
2A). The histological diagnosis was invasive ductal carcinoma
with a predominantly intraductal component. The DCIS component was
of solid type with comedo necrosis and was accompanied by
approximately 10 invasive foci up to 2.5×2.5 mm in diameter with
invasion to the adipose tissue (Fig. 2B
and C). The nuclear grade was 3 in both the DCIS and invasive
components. No lymphatic or venous invasion was observed. The
surgical margin was free of cancer cells. The invasive carcinoma
component was immunohistochemically positive for estrogen receptor
(Allred score: 4) (Fig. 2D),
negative for progesterone receptor (Allred score: 2), and positive
for HER2 overexpression (score 3+) (Fig. 2E). The Ki-67 labeling index was
37.1%, which corresponds to the luminal B-like subtype. The tumor
stage was pT1a pN0 (stage IA).
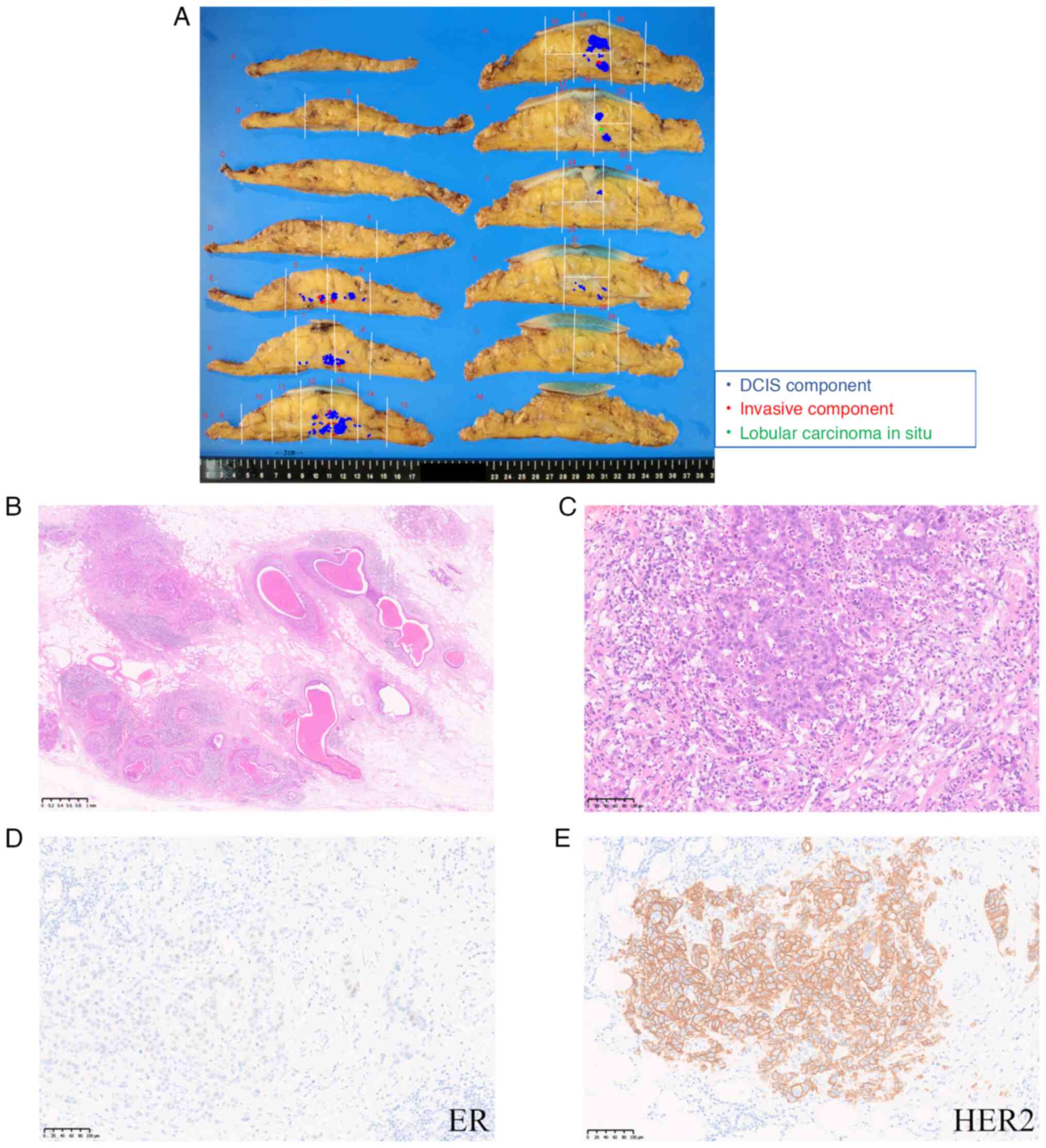 | Figure 2.Macroscopic and microscopic findings.
(A) A multinodular lesion measuring 30 mm in the upper external
quadrant of the left breast. Blue dots indicate the DCIS component,
and red dots indicate 10 foci of the invasive ductal carcinoma
component. Green dots indicate lobular carcinoma in situ.
The resected specimens were sliced for histopathological
examination, and each was assigned a letter. Numbers were assigned
based on the site from which the histopathological specimen was
obtained. (B) Representative section of DCIS with comedo necrosis
(magnification, ×20; scale bar, 1 mm). (C) Higher magnification
view of the invasive carcinoma component. The diameter of the
invasive component was 2.5 mm (magnification, ×200; scale bar, 100
µm). (D) The invasive carcinoma component was partially positive
for ER (Allred score, 4; magnification, ×200; scale bar, 100 µm).
(E) The invasive carcinoma component was positive for HER2
(magnification, ×200; scale bar, 100 µm). DCIS, ductal carcinoma
in situ; ER, estrogen receptor; HER2, human epidermal growth
factor receptor 2. |
Based on the latest Japanese Breast Cancer Society
and National Comprehensive Cancer Network guidelines (2), an aromatase inhibitor (letrozole) was
decided to be administered as postoperative endocrine therapy for
10 years after surgery. Eleven months after surgery, serum
hepatobiliary enzyme levels were elevated (aspartate
aminotransferase, 156 U/l; alanine aminotransferase, 163 U/l;
γ-glutamyl transferase, 332 U/l), and tumor marker levels were
elevated (breast cancer antigen 225, 539 U/ml; carbohydrate antigen
15-3, 1,735 U/ml; carcinoembryonic antigen, 106 ng/ml).
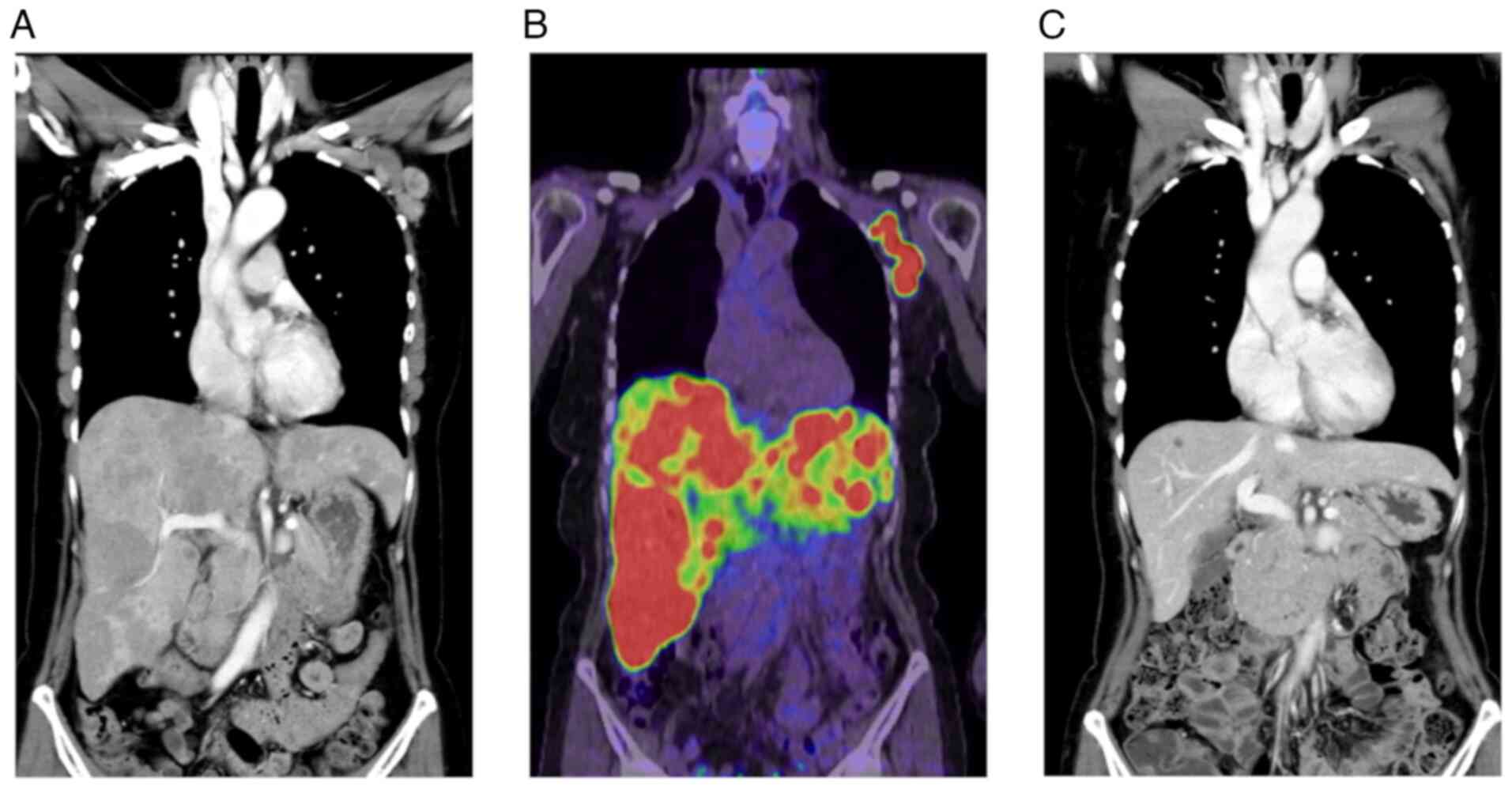
Contrast-enhanced computed tomography (CT) showed multiple
low-density lesions in the bilateral lobes of the liver and
multiple lesions in the left axilla (Fig. 3A). 18F-FDG PET/CT showed
abnormal uptake in these lesions, suggesting metastases from the
left breast cancer (Fig. 3B).
Biopsy specimens from one of the axillary lesions showed carcinoma
consistent with metastatic invasive ductal carcinoma of the
ipsilateral breast. The metastatic focus was negative for estrogen
receptor (Allred score: 0) and progesterone receptor (Allred score:
0) and positive for HER2 (score 3+). The Ki-67 labeling index was
41.4%.
Based on these results, taxane therapy (paclitaxel
80 mg/mm2, administered weekly) was initiated in
combination with anti-HER2 therapy (pertuzumab 840 mg as a loading
dose, which was reduced to 420 mg for subsequent cycles
administered every 3 weeks, and trastuzumab 8 mg/kg as a loading
dose, which was reduced to 6 mg/kg for subsequent cycles
administered every 3 weeks). Follow-ups were performed every three
months. After 6 months, nine courses of taxane and anti-HER2
therapy were completed, CT images showed a complete response of
both the left axillary and hepatic lesions (Fig. 3C). The patient received 22 courses
of anti-HER2 therapy (pertuzumab 420 mg and trastuzumab 6 mg/kg
every 3 weeks) for one year. Anti-HER2 therapy was completed 6
months after no recurrence was confirmed. A complete response of
liver metastasis sites and left axillary lesions has been
maintained 4 years after the anti-HER2 therapy initiation.
Discussion
The prognostic benefit of anti-HER2 therapy plus
chemotherapy has been controversial in pT1a/b pN0 HER2-positive
breast cancer. Furthermore, inducing adjuvant therapy for pT1a/b
pN0 HER2-positive breast cancer has been dependent on the
clinician's decision in Japan. The prognosis of patients with pT1a
pN0 HER2-positive breast cancer is excellent. van Ramshorst et
al (8) reported an 8-year
breast cancer-specific survival rate exceeding 95%, and Kubo et
al (4) reported a 7-year breast
cancer-specific survival rate exceeding 98%. A previous
meta-analysis showed no benefit of adjuvant anti-HER2 therapy for
pT1a HER2-positive breast cancer regardless of HR status (4). In this case, pT1a pN0 HER2-positive
breast cancer relapsed in multiple organs in the early
postoperative period, which indicates the importance of identifying
high-risk patients with pT1a HER2-positive breast cancer to whom
standard postoperative adjuvant anti-HER2 therapy is necessary.
Some studies have reported factors associated with
the aggressive tumor biology of pT1a pN0 HER2-positive breast
cancer (9–11). The administration of trastuzumab is
currently considered based on existing prognostic factors, such as
tumor grade, Ki-67 labeling index, and HR status. Colleoni et
al (11) reported that Ki-67
was the most significant prognostic factor for pT1a/b pN0 breast
cancer, and the recurrence rate in patients with pT1a/b with high
Ki-67 (>20%) was 6.7%, which is approximately 10 times higher
than that in patients with pT1a/b pN0 with low Ki-67 (≤20%) (0.8%).
Ki-67 is reported to be inversely correlated with ER status
(12). In this case, ER was weakly
positive and Ki-67 was high, suggesting a strong proliferative
capacity. HR status can be a prognostic factor for pT1a
HER2-positive cancers (13).
Curigliano et al (14)
reported that, in pT1mi/a HER2-positive breast cancer, the 5-year
recurrence rate of the HR-negative subgroup was 12%, which is
approximately 1.7 times higher than that in the HR-positive
subgroup (7%). However, in these pT1mi/a HER2-positive cases, the
ER-positive PR-negative subgroup had a worse prognosis than the
ER-positive PR-positive subgroup (15). PR negativity has been reported to
indicate impaired growth factor signaling via the PI3K-Akt-mTOR
pathway, resulting in resistance to endocrine therapy (15). In this case, the patient was
ER-positive but PR-negative, which may have contributed to her poor
prognosis. Furthermore, histopathological findings revealed nuclear
grade 3 with comedo necrosis, which are risk factors for DCIS
(16).
This case had two poor prognostic factors: high
Ki-67 labeling index (37.1%) and nuclear grade 3 (17,18).
Based on the ‘Predict’ tool, this case would be expected to have a
cancer-related death rate of 6% 10 years after surgery with
adjuvant endocrine therapy (19).
This tool provides a prognosis of invasive breast cancer based on
age, menopausal status, ER status, HER2 status, Ki-67 status,
invasion tumor size, tumor grade, cancer detection opportunity, and
the presence or absence of lymph node metastasis. However, the
current version is based on cases diagnosed from 1999–2003 and does
not incorporate the benefits or harms of radiotherapy (20). This tool also reports the effects of
hormonal therapy and the addition of anti-HER2 therapy. In the
present case, with adjuvant chemotherapy with trastuzumab and
endocrine therapy, the cancer-related death rate was predicted to
be 3%. Although this value may be controversial as a guideline for
postoperative anti-HER2 therapy, the use of adjuvant chemotherapy
in combination with trastuzumab should be reconsidered for pT1a pN0
HER2-positive breast cancer with high-risk factors. Waks et
al (21) reported that the
HER2DX assay, which is a classifier derived from gene expression
and limited clinical features, predicts pCR following treatment
with neoadjuvant paclitaxel with trastuzumab and pertuzumab in
patients with early-stage HER2-positive breast cancer. Although
genetic testing was not performed in this case, genetic tests, such
as the HER2DX assay, may help inform the decision to induce
postoperative anti-HER2 therapy for pT1a pN0 HER2-positive breast
cancer cases in the future.
This case had at least 10 invasive foci that reached
the adipose tissue. The resected specimens were extensively
examined (Fig. 2A). However, larger
invasive foci might not be found on the surface of tissue blocks
subjected to histopathological diagnosis. Although the number of
invasive foci has not been reported to be related to patient
prognosis in patients with HER2-positive pT1a pN0, the larger the
number of invasive foci, the higher the probability that larger
invasive foci are overlooked. The number of tumors budding, which
refers to a single or small cluster of tumor cells detached from
the main tumor mass, is reported to be a prognostic factor for
colorectal and breast cancer (22).
We suspect that the number of invasive foci has the potential to
predict the prognosis of breast cancer and inform therapeutic
decision-making.
Chemotherapy has adverse effects, and the most
severe toxic effect of trastuzumab is congestive heart failure.
However, a recent study reported that the frequency of grade 3 to 4
cardiac dysfunction during treatment with trastuzumab was
approximately 3%, and most cases were reversible (23). Drug history of anthracycline and
taxane, medical history of cardiac disease, lower cardiac function,
and old age have been reported as risk factors for heart
dysfunction related to trastuzumab (24,25).
In this case, the patient was relatively young and had no history
of cardiac disease or chemotherapy. Therefore, no factors inhibited
trastuzumab administration as adjuvant therapy. This patient was
potentially at a higher risk of recurrence than other patients with
pT1a pN0 HER2-positive breast cancer. The balance between the
toxicity and benefit of trastuzumab should be evaluated more
carefully in patients with a higher risk of recurrence. According
to a review by Moja et al (26), assessing the balance of trastuzumab
therapy is difficult as the implication of the toxicity and
benefits may be perceived differently by patients and clinicians
and because trastuzumab-induced cardiac dysfunctions are reversible
in most cases.
Currently, it is difficult to predict the onset of
cardiac dysfunction caused by trastuzumab as it occurs
independently of the total dose of trastuzumab (27). However, it has been reported that
kallikrein5-PAR2 signaling is involved (27). If it becomes possible to predict the
adverse effects of trastuzumab in the future, it will be easier to
balance the toxicity and benefits of trastuzumab.
In conclusion, this report presents a case of pT1a
pN0 HR-positive HER2-positive breast cancer with multiple
metastases in the first year after radical surgery. Although, the
possibility that this case was an anecdotal single case cannot be
ruled out, this case suggested the necessity of future studies to
identify high-risk patients with pT1a pN0 HER2-positive breast
cancer who are expected to benefit from adjuvant chemotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TS, TE, MT, YA, KW, KK, HO, MFK, TY and YK were
involved in clinical management. TS contributed to
conceptualization, design and writing the original drafts. MT, YA,
KW, KK, HO, MFK and TY contributed to acquisition of data, and
interpretation of the manuscript. TE, HU, HT and YK contributed to
conceptualization and design, reviewed and edited the manuscript,
and confirmed the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication of case
information and images was obtained from the participant included
in the study.
Competing interests
TY received honoraria from Eli Lilly Japan K.K. The
other authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
HER2
|
human epidermal growth factor receptor
2
|
|
HR
|
hormone receptor
|
|
US
|
ultrasonography
|
|
DCIS
|
ductal carcinoma in situ
|
|
MRI
|
magnetic resonance imaging
|
|
18F-FDG PET/CT
|
fluorine-18 fluorodeoxyglucose
positron emission tomography/computed tomography
|
References
|
1
|
Hudis CA: Trastuzumab-mechanism of action
and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Comprehensive Cancer Network, .
http://www.nccn.org2019 December 10–2023
|
|
3
|
Tolaney SM, Guo H, Pernas S, Barry WT,
Dillon DA, Ritterhouse L, Schneider BP, Shen F, Fuhrman K, Baltay
M, et al: Seven-year follow-up analysis of adjuvant paclitaxel and
trastuzumab trial for node-negative, human epidermal growth factor
receptor 2-positive breast cancer. J Clin Oncol. 37:1868–1875.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kubo M, Kawai M, Kumamaru H, Miyata H,
Tamura K, Yoshida M, Ogo E, Nagahashi M, Asaga S, Kojima Y, et al:
A population-based recurrence risk management study of patients
with pT1 node-negative HER2+ breast cancer: A national clinical
database study. Breast Cancer Res Treat. 178:647–656. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma SJ, Oladeru OT and Singh AK:
Association of survival with chemoendocrine therapy in women with
small, hormone receptor-positive, ERBB2-positive, node-negative
breast cancer. JAMA Netw Open. 3:e2025072020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gonzalez-Angulo AM, Litton JK, Broglio KR,
Meric-Bernstam F, Rakkhit R, Cardoso F, Peintinger F, Hanrahan EO,
Sahin A, Guray M, et al: High risk of recurrence for patients with
breast cancer who have human epidermal growth factor receptor
2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol.
27:5700–5706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours. John Wiley
& Sons; 2017
|
|
8
|
van Ramshorst MS, van der Heiden-van der
Loo M, Dackus GM, Linn SC and Sonke GS: The effect of
trastuzumab-based chemotherapy in small node-negative HER2-positive
breast cancer. Breast Cancer Res Treat. 158:361–371. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Musolino A, Falcini F, Sikokis A, Boggiani
D, Rimanti A, Pellegrino B, Silini EM, Campanini N, Barbieri E,
Zamagni C, et al: Prognostic impact of interval breast cancer
detection in women with pT1a N0M0 breast cancer with HER2-positive
status: Results from a multicentre population-based cancer registry
study. Eur J Cancer. 88:10–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Musolino A, Boggiani D, Sikokis A, Rimanti
A, Pellegrino B, Vattiato R, Sgargi P, Falcini F, Caminiti C,
Michiara M and Leonardi F: Prognostic risk factors for treatment
decision in pT1a,b N0M0 HER2-positive breast cancers. Cancer Treat
Rev. 43:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Colleoni M, Rotmensz N, Peruzzotti G,
Maisonneuve P, Viale G, Renne G, Casadio C, Veronesi P, Intra M,
Torrisi R and Goldhirsch A: Minimal and small size invasive breast
cancer with no axillary lymph node involvement: The need for
tailored adjuvant therapies. Ann Oncol. 15:1633–1639. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inwald EC, Klinkhammer-Schalke M,
Hofstädter F, Zeman F, Koller M, Gerstenhauer M and Ortmann O:
Ki-67 is a prognostic parameter in breast cancer patients: Results
of a large population-based cohort of a cancer registry. Breast
Cancer Res Treat. 139:539–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joerger M, Thürlimann B and Huober J:
Small HER2-positive, node-negative breast cancer: Who should
receive systemic adjuvant treatment? Ann Oncol. 22:17–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Curigliano G, Viale G, Bagnardi V,
Fumagalli L, Locatelli M, Rotmensz N, Ghisini R, Colleoni M,
Munzone E, Veronesi P, et al: Clinical relevance of HER2
overexpression/amplification in patients with small tumor size and
node-negative breast cancer. J Clin Oncol. 27:5693–5699. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davey MG, Ryan ÉJ, Folan PJ, O'Halloran N,
Boland MR, Barry MK, Sweeney KJ, Malone CM, McLaughlin RJ, Kerin MJ
and Lowery AJ: The impact of progesterone receptor negativity on
oncological outcomes in oestrogen-receptor-positive breast cancer.
BJS Open. 5:zrab0402021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lagios MD, Margolin FR, Westdahl PR and
Rose MR: Mammographically detected duct carcinoma in situ.
Frequency of local recurrence following tylectomy and prognostic
effect of nuclear grade on local recurrence. Cancer. 63:618–624.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petrelli F, Viale G, Cabiddu M and Barni
S: Prognostic value of different cut-off levels of Ki-67 in breast
cancer: A systematic review and meta-analysis of 64,196 patients.
Breast Cancer Res Treat. 153:477–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuda H, Akiyama F, Kurosumi M, Sakamoto G
and Watanabe T: Establishment of histological criteria for
high-risk node-negative breast carcinoma for a multi-institutional
randomized clinical trial of adjuvant therapy. Japan national
surgical adjuvant study of breast cancer (NSAS-BC) pathology
section. Jpn J Clin Oncol. 28:486–491. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Health Service, . Predict breast
cancer. University of Cambrigde; 2023, https://breast.predict.nhs.uk/December 10–2023
|
|
20
|
Grootes I, Wishart GC and Pharoah PDP: An
updated PREDICT breast cancer prognostic model including the
benefits and harms of radiotherapy. NPJ Breast Cancer. 10:62024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Waks AG, Ogayo ER, Paré L, Marín-Aguilera
M, Brasó-Maristany F, Galván P, Castillo O, Martínez-Sáez O,
Vivancos A, Villagrasa P, et al: Assessment of the HER2DX assay in
patients with ERBB2-positive breast cancer treated with neoadjuvant
paclitaxel, trastuzumab, and pertuzumab. JAMA Oncol. 9:835–840.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salhia B, Trippel M, Pfaltz K, Cihoric N,
Grogg A, Lädrach C, Zlobec I and Tapia C: High tumor budding
stratifies breast cancer with metastatic properties. Breast Cancer
Res Treat. 150:363–371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dang C, Guo H, Najita J, Yardley D, Marcom
K, Albain K, Rugo H, Miller K, Ellis M, Shapira I, et al: Cardiac
outcomes of patients receiving adjuvant weekly paclitaxel and
trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA
Oncol. 2:29–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sengupta PP, Northfelt DW, Gentile F,
Zamorano JL and Khandheria BK: Trastuzumab-induced cardiotoxicity:
Heart failure at the crossroads. Mayo Clin Proc. 83:197–203. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guarneri V, Lenihan DJ, Valero V, Durand
JB, Broglio K, Hess KR, Michaud LB, Gonzalez-Angulo AM, Hortobagyi
GN and Esteva FJ: Long-term cardiac tolerability of trastuzumab in
metastatic breast cancer: The M.D. anderson cancer center
experience. J Clin Oncol. 24:4107–4115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moja L, Tagliabue L, Balduzzi S, Parmelli
E, Pistotti V, Guarneri V and D'Amico R: Trastuzumab containing
regimens for early breast cancer. Cochrane Database Syst Rev.
2012:CD0062432012.PubMed/NCBI
|
|
27
|
Sasaki R, Kurebayashi N, Eguchi H,
Horimoto Y, Shiga T, Miyazaki S, Kashiyama T, Akamatsu W and Saito
M: Involvement of kallikrein-PAR2-proinflammatory pathway in severe
trastuzumab-induced cardiotoxicity. Cancer Sci. 113:3449–3462.
2022. View Article : Google Scholar : PubMed/NCBI
|