Introduction
Lung cancer, a major factor highly associated with
cancer-related mortality worldwide, resulted in 710,000 deaths in
China alone, out of 3 million cancer-related fatalities in 2020.
Non-small cell lung cancer (NSCLC) is involved in ~80% of these
cases (1,2).
Notably, the majority of these patients are
diagnosed at an advanced stage of the disease, making them
unsuitable candidates for surgical resection. Advanced-stage lung
cancer patients are commonly treated with platinum-based
chemotherapy regimens, which exert favorable efficacy (3). However, in addition to the therapeutic
benefits of platinum-based regimens, attention should be also paid
to their hematologic and gastrointestinal toxicities, which can
potentially significantly compromise the patient's immune function
and treatment outcomes (4).
According to the cancer immunoediting theory, including immune
elimination, homeostasis and escape, indicates that immunity is
closely associated with the occurrence and progression of cancer
(5). Therefore, assessing and
improving the immune function of late-stage patients with NSCLC
following chemotherapy has become a clinical concern.
Previous studies indicated that compared with
healthy individuals, patients with lung cancer exhibited lower
count of CD4+ T and NK cells and reduced
CD4+/CD8+ ratios, accompanied by elevated
number of regulatory T cells (Tregs) (6,7).
Additionally, another study showed that patients with NSCLC, who
responded effectively to immunotherapy, experienced an increase in
the proportion of CD4+ T cells compared with the
baseline values, while no statistically significant changes were
observed in the ineffective response group (8). Furthermore, previous studies also
demonstrated that chemotherapy drugs could impair the proliferation
and function of peripheral circulating effector T cells, thus
potentially leading to the reduced proliferative activity of
cytotoxic T lymphocytes (CTLs) (9,10).
However, it has been also reported that chemotherapy drugs can
induce tumor cell apoptosis, which in turn trigger immune
responses, eventually enhancing the cytotoxicity of CTLs and
strengthening anti-tumor immunity (11). Chemotherapy drugs can also
accelerate CD4+CD25+ Treg apoptosis, thus
reducing the number of Tregs and effectively regulating tumor
immunity (12). In summary, there
is no consensus regarding the effect of chemotherapy on the immune
function of patients with cancer and the immune status on
chemotherapy outcome. Therefore, more research and immune function
assessment methods are needed to establish a conclusive
understanding.
The CD4+ T cell adenosine triphosphate
(ATP) release assay is used to measure intracellular ATP
concentration within purified CD4+ T lymphocytes
following stimulation with phytohemagglutinin-L. This assay is used
to assess peripheral immune function and emerging evidence suggests
that it has significant predictive value in predicting
post-transplant infections in organ transplant recipients and
hematopoietic stem cell recipients, as well as septic shock in
septic patients (13–17).
Some researchers have found that patients who
developed septic shock had markedly lower CD4+ T cell
ATP levels compared with septic patients who did not shock.
However, there were no significant differences in the percentages
of CD4+ and CD8+ T lymphocytes between the
two groups (17), Additionally,
Serban et al (16) conducted
a study showed that patients with decreased CD4+ T cell
ATP levels five months after allogeneic transplantation had a
higher risk of infection, while CD4+ T cell counts
remained at an increased level (16), This indicates that for patients with
similar immune cell quantities and poorer health status
sATPCD4 holds a special clinical value. Clinical trials
have been conducted to evaluate CD4+ T cells ATP release
levels in hepatocellular carcinoma, thus revealing a close
association between progression-free survival (PFS), overall
survival (OS) and cancer recurrence (18,19).
Tumor initiation and progression are intricately associated with
the host immune function. Tumor immunity represents a dynamic
process, where T cell responses are enhanced. Therefore,
maintaining adequate immune function is crucial for fostering a
beneficial cycle (20). However, to
the best of our knowledge, the association between CD4+
T cells ATP levels and NSCLC progression has not been previously
investigated. Therefore, exploring the association between
CD4+ T cells ATP release levels and NSCLC holds
potential value in understanding the interplay between immune
function and NSCLC progression.
The present study aimed to evaluate the changes in
immune function prior and after chemotherapy, as well as the
association between post-chemotherapy immune status and tumor
progression.
Materials and methods
Patients
Patients diagnosed with NSCLC, who were treated with
chemotherapy, were retrospectively reviewed between August 15 2022
and August 30 2023, at the Fifth Affiliated Hospital of Guangzhou
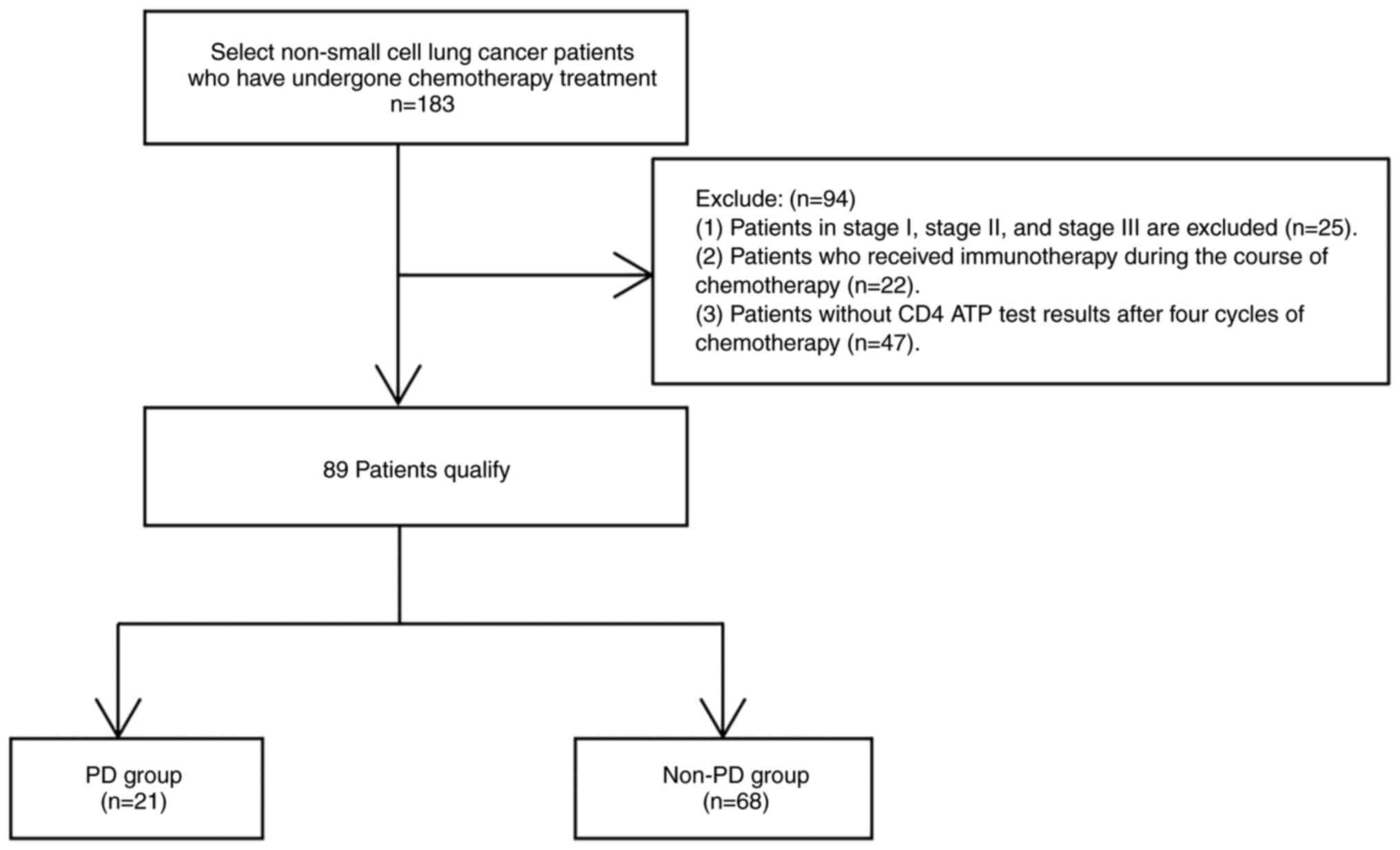
Medical University Hospital, Guangzhou, China (n=183). Among a
total of 183 patients, 89 patients were screened and allocated into
the disease progression (PD, n=21) and disease stability (non-PD;
n=68; Fig. 1) groups, males
accounted for 52.8% of the population, with patient ages ranging
from 33 to 78 years (mean age 66.2±10.2). A total of 10 and 20
patients in the PD and non-PD groups, respectively, underwent
immunocellular function assay before chemotherapy. The present
study received approval from the Ethics Committee of the Fifth
Affiliated Hospital of Guangzhou Medical University (Guangzhou,
China) dated 08-07-2022 of and approval no. KY01-2022-07-08.
Written informed consent was obtained from all participants,
allowing the use of their clinical data in this study. The research
adhered to the principles outlined in the Declaration of
Helsinki.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Patients
with pathological diagnosis of NSCLC; ii) with locally advanced
disease, medically or technically inoperable; iii) those treated
with periodic chemotherapy (pemetrexed, cisplatin, carboplatin,
paclitaxel, gemcitabine); iv) with Eastern Cooperative Oncology
Group (ECOG) score of 0–2 (ECOG score 0: Normal activity without
symptoms, fully active and able to carry on all pre-disease
performance without restriction; ECOG score 1: Symptomatic, but
completely ambulatory, restricted in physically strenuous activity,
but able to perform light or sedentary work; ECOG score 2:
Symptomatic, in bed <50% of waking hours and capable of limited
self-care and confined to bed or chair >50% of waking hours)
(21). Patients were staged
according to the 8th edition TNM staging system (22). The exclusion criteria were the
followings: i) Patients with stage I, II or III NSCLC; ii) those
who were treated with immunotherapy; iii) HIV-positive individuals;
and iv) those whose ATP release levels from CD4+ T
lymphocytes were not recorded after four cycles of chemotherapy.
The study conformed to the principles outlined in the Declaration
of Helsinki (approval no. KY01-2022-07-08).
Study assessment
Neck, chest, whole abdomen and pelvic computed
tomography (CT) imaging examinations were conducted prior to
initial treatment and at 20 days following the completion of four
cycles of treatment. Treatment efficacy was evaluated according to
the Response Evaluation Criteria for Solid Tumors (RECIST) 1.1
criteria (23) and categorized as
PR (partial response), SD (stable disease) or PD. Patients who were
treated with chemotherapy for four cycles and had no progression at
two consecutive radiological assessments were allocated into the
non-PD group (PR + SD), while those who did not meet the
aforementioned criteria were included in the PD group. The current
analysis was performed to assess the association between peripheral
blood CD4+T cells ATP expression and treatment efficacy
after four cycles of therapy.
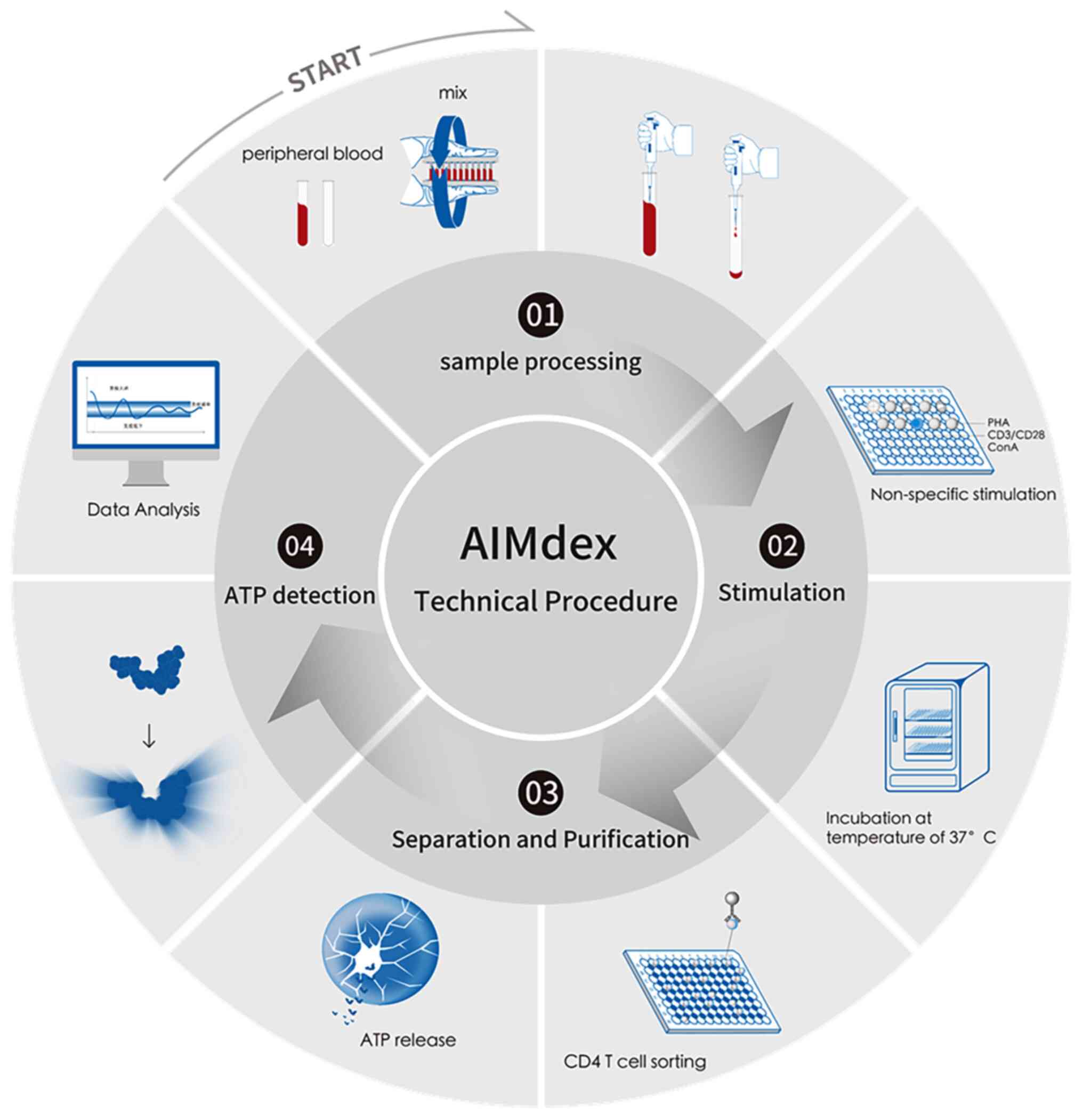
Immunocellular function assay
The levels of ATP released by stimulated
(sATPCD4) and non-stimulated (nATPCD4) CD4 T
lymphocytes were assessed through a luciferin-luciferase reaction,
according to the manufacturer's instructions (AIMdex®;
Leide Biosciences Co., Ltd.) (24).
Briefly, 100 µl of 4-fold diluted whole blood was incubated with
8.75 ng/ml sATP or without phytohaemagglutinin (PHA; nATP) for
15–18 h,37°C. CD4+ T cells were isolated using magnetic
beads (900-1, 100 µg/well; Leide Biosciences Co., Ltd.) and ATP was
released using a lysis buffer. Subsequently, the mixture was
supplemented with luciferin/luciferase and bioluminescence was
measured using a luminometer (JR-I; Weihai Weigao Biotechnology
Co., Ltd). The results were analyzed using the corresponding
software (JR-1 v2.3.8.8.181120/1.2.0; Weihai Weigao Biotechnology
Co., Ltd). A standard curve with ATP calibrators (0, 50, 100, 200,
400 and 800 ng/ml) was constructed. ATP release was determined by
comparing ATP levels between CD4+T cell- stimulated and
non-stimulated samples (Fig. 2) and
validated through clinical repeatability assessment, meeting
clinical standards.
Blood parameter assessment
White blood cell (WBC), granulocyte and lymphocyte
counts were measured using a fully automated hematology analyzer
(Original Mindray BC-5180 CRP; Mindray).
Treatment and data collection
The relevant clinical and laboratory data were
collected from the electronic medical records system. The clinical
characteristics of patients, such as age, sex, smoking and drinking
history, underlying medical conditions and histology were collected
and summarized (Table I). Baseline
measurements were defined as those after chemotherapy.
 | Table I.Comparison of baseline
characteristics between PD group and non-PD group. |
Table I.
Comparison of baseline
characteristics between PD group and non-PD group.
| Variables | PD (n=21) | non-PD (n=68) |
χ2/Z/t | P-value |
|---|
| Sex |
|
| 1.092 | 0.296 |
| Male, n
(%) | 9 (42.9) | 38 (55.9) |
|
|
| Female,
n (%) | 12 (57.1) | 30 (44.1) |
|
|
| Age, years
(interquartile Range) | 64.0
(54.0–71.5) | 67.5
(57.0–72.0) | 0.119 | 0.174 |
| BMI,
(kg/m2) | 22.4±3.7 | 21.4±3.1 | 1.178 | 0.258 |
| Smoking
history |
|
| 0.028 | 0.867 |
| Yes, n
(%) | 5 (23.8) | 15 (22.1) |
|
|
|
pack-year (≥30), n (%) | 5 (100) | 15 (100) |
|
|
| No, n
(%) | 16 (76.2) | 53 (77.9) |
|
|
| Drinking
history |
|
| 0.104 | 0.747 |
| Yes, n
(%) | 2 (9.5) | 5 (7.4) |
|
|
| Every
day, n (%) | 2 (100) | 5 (100) |
|
|
| No, n
(%) | 19 (90.5) | 63 (92.6) |
|
|
| Diabetes |
|
| 0.104 | 0.747 |
| Yes, n
(%) | 2 (9.5) | 5 (7.4) |
|
|
|
Type1 | 0 | 0 |
|
|
| Type2,
n (%) | 2 (100) | 5 (100) |
|
|
| No, n
(%) | 19 (90.5) | 63 (92.6) |
|
|
| Hypertensive |
|
| 1.057 | 0.304 |
| Yes, n
(%) | 3 (14.3) | 17 (25.0) |
|
|
| Stage
1, n (%) | 1 (33.3) | 2 (11.8) |
|
|
| Stage
2, n (%) | 2 (66.7) | 10 (58.8) |
|
|
| Stage
3, n (%) | 0 | 5 (29.4) |
|
|
| No, n
(%) | 18 (85.7) | 51 (75.0) |
|
|
| Histology |
|
| 0.338 | 0.561 |
| Non
squamous carcinoma, n (%) | 19 (90.5) | 64 (94.1) |
|
|
|
Squamous carcinoma, n (%) | 2 (9.5) | 4 (5.9) |
|
|
| Distant
metastasis |
|
| 0.002 | 0.962 |
| Yes, n
(%) | 18 (85.7) | 58 (85.3) |
|
|
| No, n
(%) | 3 (14.3) | 10 (14.70) |
|
|
| EGFR mutation |
|
| 3.760 | 0.052 |
|
EGFR+ | 18 (85.7) | 43 (63.2) |
|
|
| L858R,
n (%) | 3 (16.6) | 6 (14.0) |
|
|
|
Deletion 19, n (%) | 0 | 1 (2.3) |
|
|
| EGFR18,
n (%) | 1 (5.6) | 2 (4.6) |
|
|
| EGFR20,
n (%) | 3 (16.6) | 6 (14.0) |
|
|
| EGFR21,
n (%) | 1 (5.6) | 6 (14.0) |
|
|
| Other,
n (%) | 10 (55.6) | 22 (51.1) |
|
|
|
EGFR−, n (%) | 3 (14.3) | 25 (36.7) |
|
|
| KRAS mutation |
|
| - | - |
|
KRAS+, n (%) | 2 (9.5) | 5 (7.4) |
|
|
|
pG12C+, n (%) | 2 (100) | 2 (40) |
|
|
|
pG12D+, n (%) | 0 | 3 (60) |
|
|
| Not
detected, n (%) | 19 (90.5) | 63 (92.6%) |
|
|
| Treatment
regimen |
|
| 0.884 | 0.829 |
|
Chemotherapy only, n (%) | 4 (19.0) | 9 (13.2) |
|
|
|
Chemotherapy + anti-EGFR, n
(%) | 6 (28.6) | 21 (30.8) |
|
|
|
Chemotherapy + anti-VEGF, n
(%) | 9 (42.9) | 34 (50.0) |
|
|
|
Chemotherapy + anti-EGFR +
anti-VEGF, n (%) | 2 (9.5) | 4 (5.9) |
|
|
| Chemotherapy
regimen |
|
| 4.160 | 0.125 |
|
PemC/PemP, n (%) | 11 (52.4) | 49 (72.1) |
|
|
| GP, n
(%) | 9 ((42.9) | 14 (20.6) |
|
|
| TC, n
(%) | 1 (4.8) | 5 (7.4) |
|
|
|
Chemotherapy + anti-EGFR +
anti-VEGF, n (%) | 2 (9.5) | 4 (5.9) |
|
|
Statistical analysis
All statistical analyses were performed using SPSS
26.0 software (IBM Corp.). The clinical and demographic data are
expressed as the mean ± standard deviation (SD) or medians
[interquartile ranges (IQRs)] for continuous variables, depending
on their distribution (normally and non-normally distributed).
Categorical variables are expressed as percentages. The differences
between categorical, normally distributed and non-normally
distributed continuous variables were compared using chi-square,
Student's t-test (Between-group comparisons were performed using
independent t-tests, while within-group comparisons before and
after treatment were conducted using paired t-tests) and
Mann-Whitney U test, respectively. The association between
peripheral blood biomarkers and PD was assessed by logistic
regression analysis, while the predictive values of the baseline
peripheral blood parameters were evaluated using receiver operating
characteristics (ROC) curves. In addition, the optimal cut-off
values were determined using the Youden's index, which was
calculated using the following formula: Youden's index=Sensitivity
+ Specificity-1. The maximum Youden's index value was considered to
indicate the optimal cut-off point. The differences in area under
the curve (AUC) values were analyzed using DeLong test. All graphs
were generated using GraphPad Prism 8.0 software (Dotmatics).
Results
Patient characteristics
In the present study, a total of 89 patients were
allocated into the non-PD (n=68; including three PR and 65 SD
cases) and PD (n=21 cases) groups. The baseline characteristics of
patients are summarized in Table I.
No significant differences were recorded in terms of sex (P=0.296;
χ2=1.092), age (P=0.174; Z=0.119), body mass index (BMI;
P=0.258; t=1.178), smoking history (P=0.867; χ2=0.028),
drinking history (P=0.747; χ2=0.104), diabetes (P=0.747;
χ2=0.104), hypertensive (P=0.304; χ2=1.057),
histology (P=0.561; χ2=0.228), distant metastasis
(P=0.962; χ2=0.002), EGFR mutation status (P=0.052;
χ2=3.760), treatment regimen (P=0.829;
χ2=0.884) and chemotherapy regimen (P=0.125;
χ2=4.160) between the PD and non-PD groups.
Comparison of peripheral blood
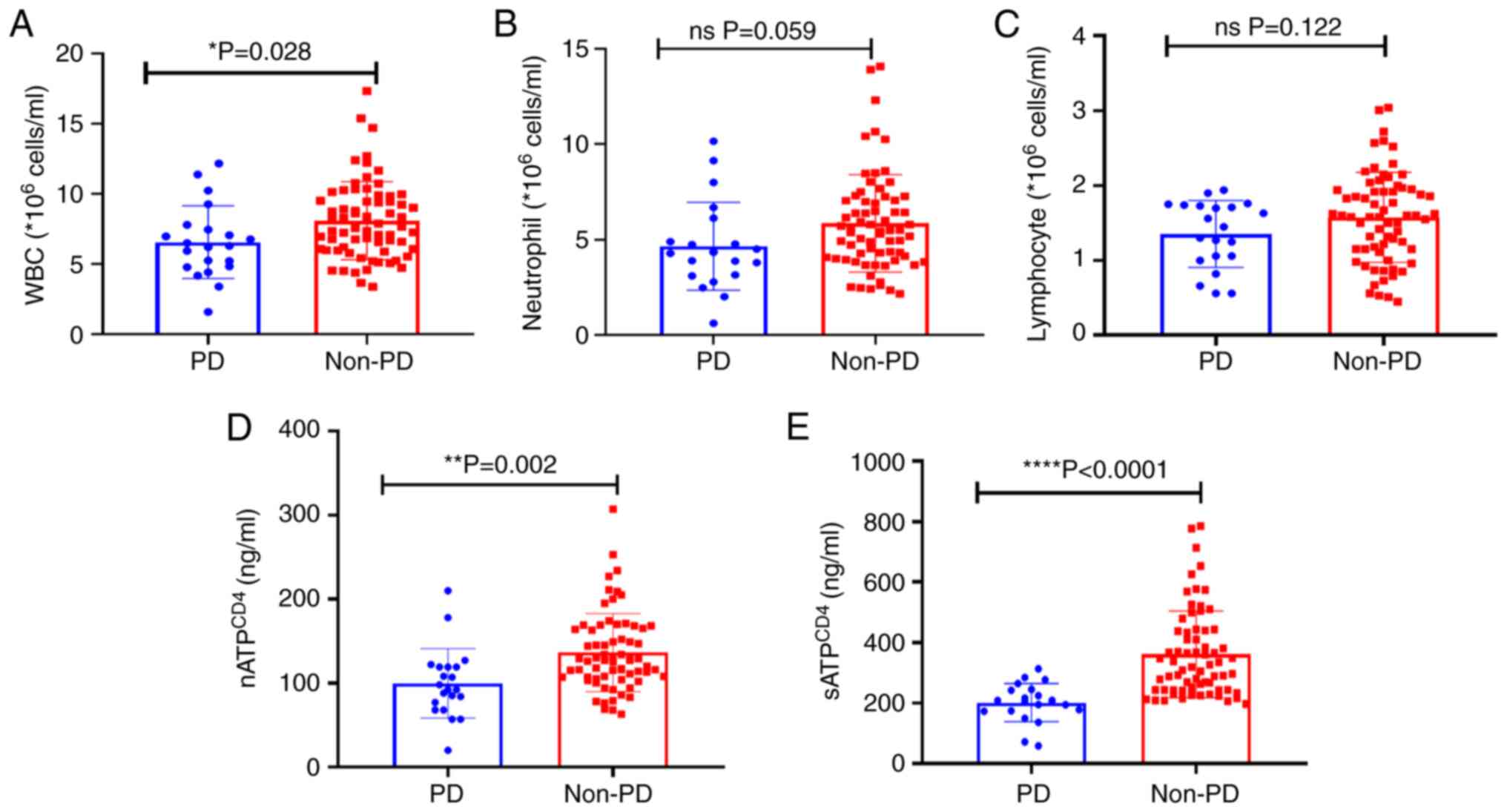
indicators between the PD and non-PD groups
Peripheral blood indicators were compared between
the PD and non-PD groups (Fig. 3).
The analysis revealed a significant reduction in WBC count
(P=0.028; Fig. 3A) and
nATPCD4 (P=0.002; Fig.
3D) and sATPCD4 (P<0.001; Fig. 3E) levels between the PD and non-PD
groups (Fig. 3A). Additionally, a
slight decrease in neutrophil (P=0.059; Fig. 3B) and lymphocyte counts (P=0.122;
Fig. 3C) was also recorded.
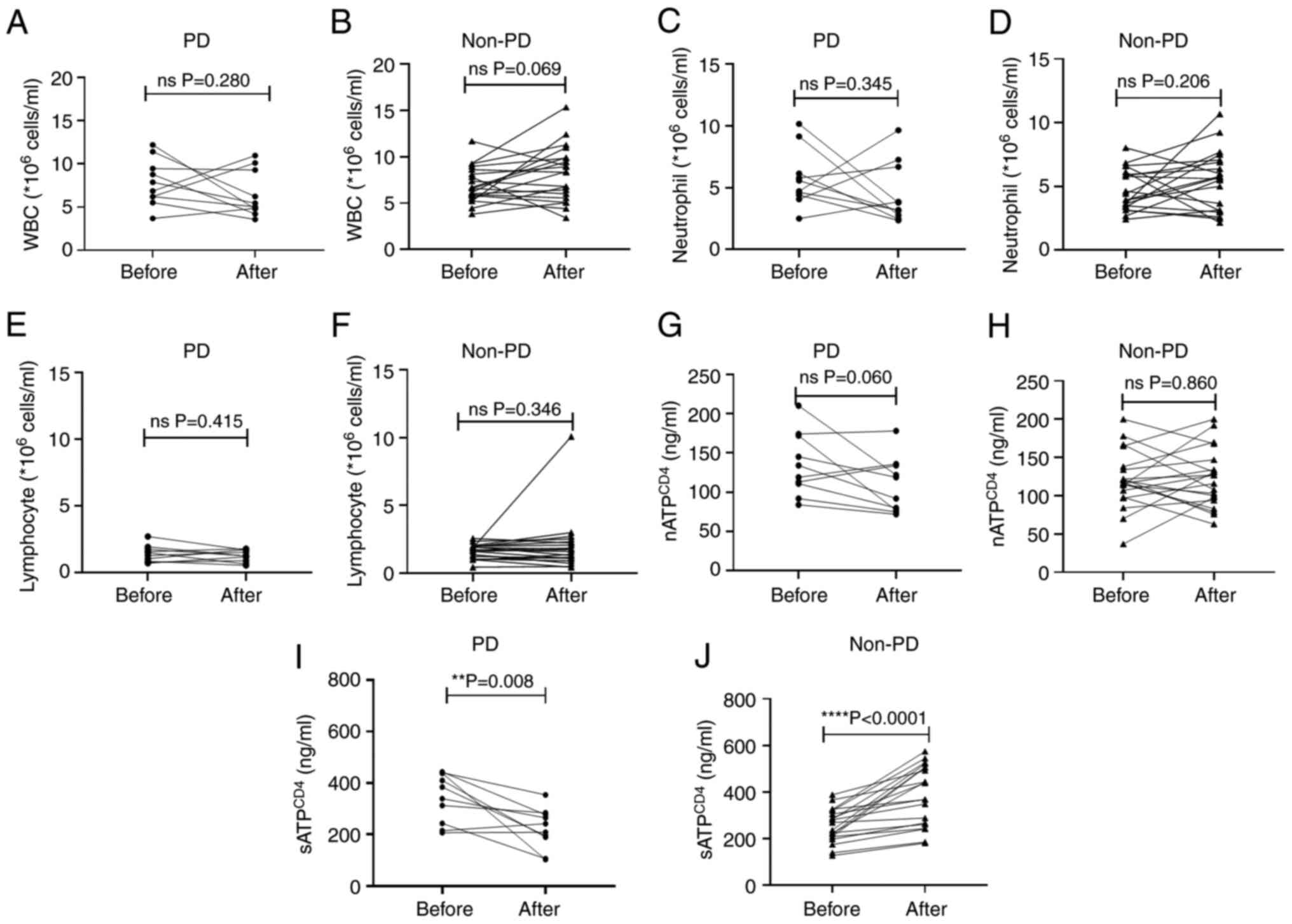
Changes in the peripheral blood
indicators after chemotherapy
To comprehensively assess the alterations in
peripheral blood indicators following chemotherapy, the levels of
the relevant indicators prior and after four cycles of treatment
were compared. Data from both the pre- and post-treatment periods
were available for 10 PD and 20 non-PD cases. No significant
changes in WBC, neutrophil and lymphocyte counts and
nATPCD4 levels were obtained after chemotherapy in both
the PD and non-PD groups (P>0.05; Fig. 4A-H). However, sATPCD4
levels were notably reduced in the PD group after chemotherapy
(P=0.008; Fig 4I), while they were
significantly enhanced in the non-PD group (P<0.0001; Fig 4J).
Cut-off points and association with
the occurrence of PD
The diagnostic efficacy of WBC, nATPCD4
and sATPCD4 after four treatment cycles was assessed by
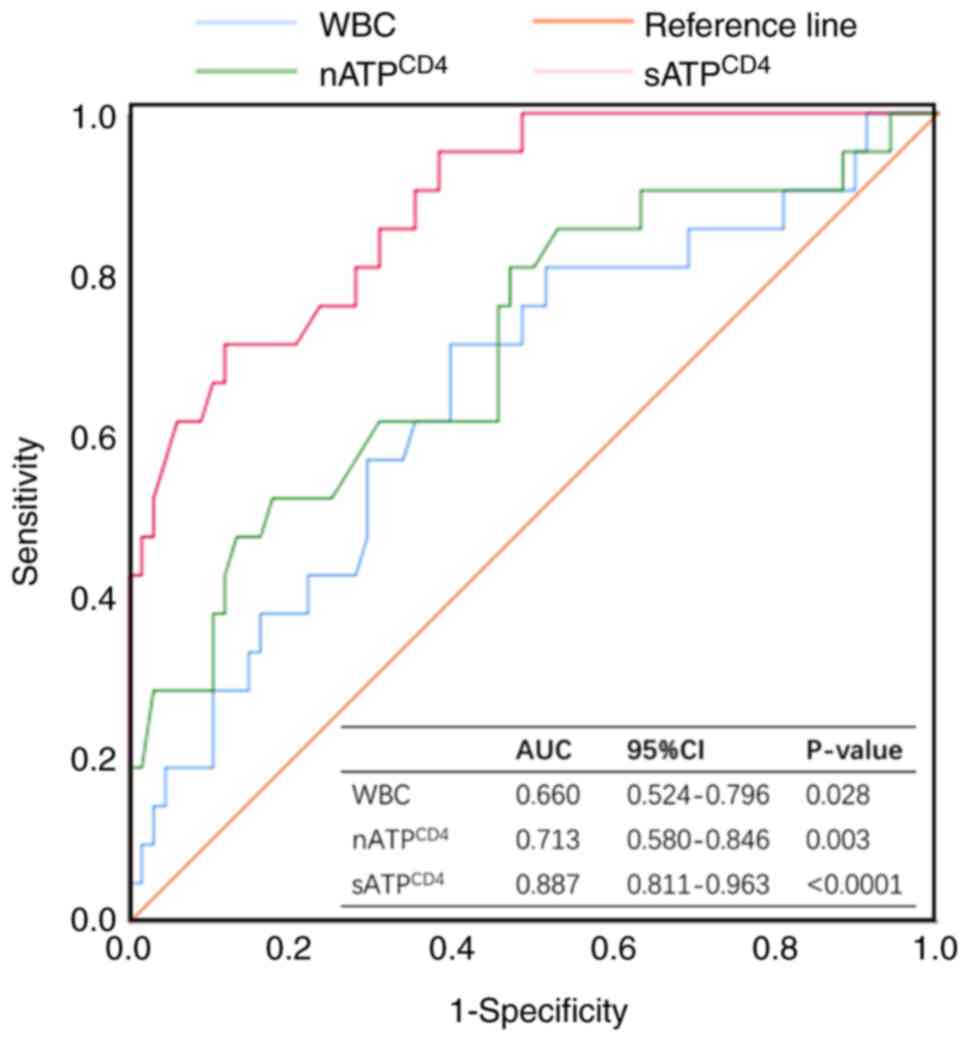
ROC curves (Fig. 5). For
sATPCD4, a cut-off value of 224.5 ng/ml [AUC=0.887; 95%
confidence interval (CI), 0.811–0.963; specificity 88.2%,
sensitivity 71.4%] displayed the highest sensitivity and
specificity for PD diagnosis. Consistently, cut-off values of 99
ng/ml (AUC=0.713; 95% CI, 0.580–0.846; specificity 82.4%,
sensitivity 52.4%) and 7.09×109 cells/l (AUC=0.660; 95%
CI, 0.524–0.796; specificity 60.3%, sensitivity 71.4%) were
obtained for nATPCD4 and WBC, respectively. The AUC
value for sATPCD4 was significantly higher compared with
that for nATPCD4 (P=0.010) and WBC (P=0.001; Table II).
 | Table II.Paired-sample area differences under
the receiver operating characteristics curves for sATPCD4, nATPCD4
and WBC. |
Table II.
Paired-sample area differences under
the receiver operating characteristics curves for sATPCD4, nATPCD4
and WBC.
| Results of the
tests | z | P-value | Difference in area
under the curve | Difference in
standard error | 95% confidence
interval |
|---|
| sATPCD4-
nATPCD4 | 2.592 | 0.010 | 0.174 | 0.326 | 0.042–0.306 |
| sATPCD4-
WBC | 3.181 | 0.001 | 0.227 | 0.329 | 0.087–0.367 |
| nATPCD4-
WBC | 0.599 | 0.549 | 0.053 | 0.371 | −0.121–0.227 |
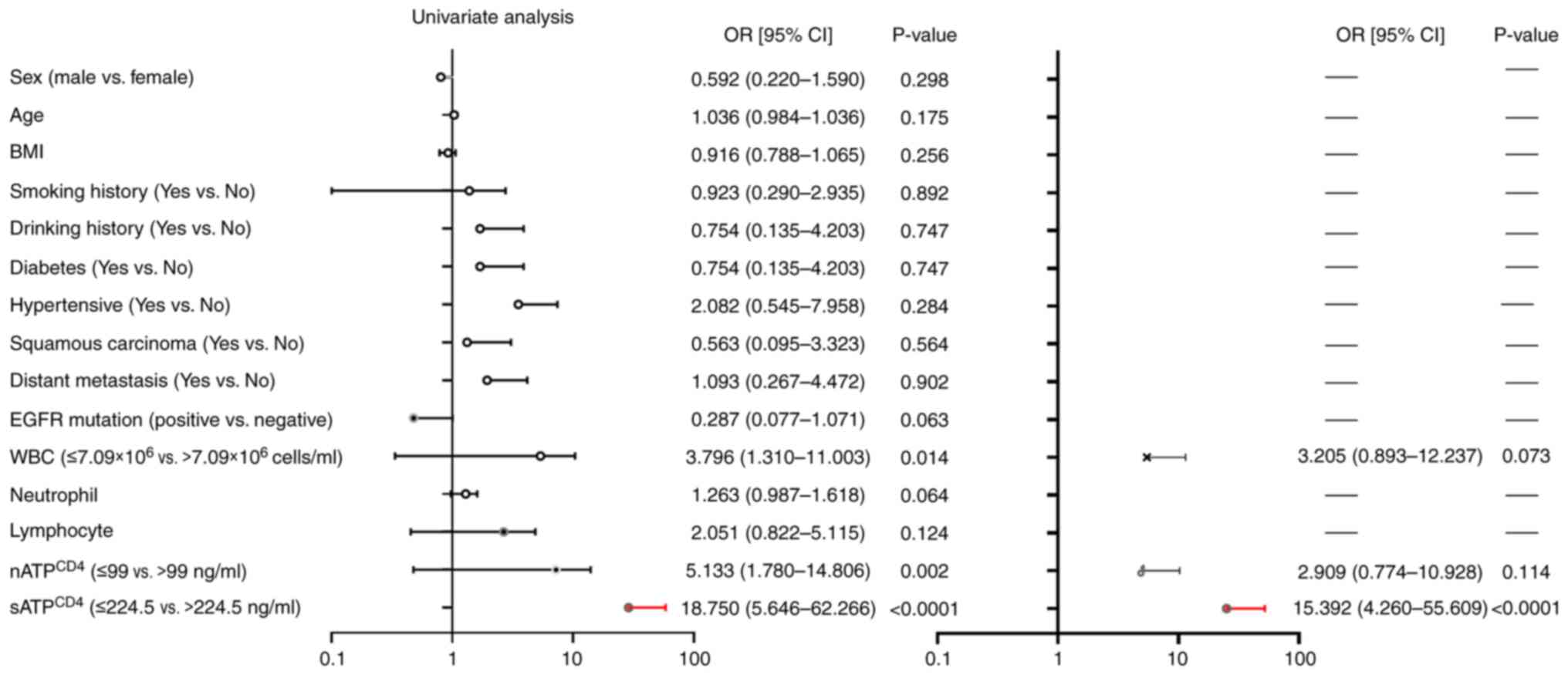
Analysis of risk factors associated
with the occurrence of PD
Univariate and multivariate logistic regression
analyses were performed to explore the association between measured
variables and PD occurrence. In univariate analysis, WBC count
(OR=3.796; 95% CI, 1.310–11.003; P=0.014) and nATPCD4
(OR=5.133; 95% CI, 1.780–14.806; P=0.002) and sATPCD4
(OR=18.750; 95% CI, 5.646–62.266; P<0.0001) levels were
significantly associated with the occurrence of PD. However, no
significant association was recorded for other factors. After
adjusting for confounders, namely WBC count and nATPCD4
and sATPCD4 levels, patients with low sATPCD4
levels (<224.5 ng/ml) displayed a 15-fold higher risk of PD
compared with those with high sATPCD4 levels (≥224.5
ng/ml; OR=15.392; 95% CI, 4.260–55.609; P<0.0001; Fig. 6).
Discussion
The treatment of NSCLC continues to face challenges.
Notably, several multiplexed detection methods, based on an
optimized nanoparticle-based laser desorption/ionization mass
spectrometry platform, have been developed. This treatment approach
is considered as an effective tool for the screening of patients
with NSCLC, potentially allowing the early diagnosis of a greater
number of patients with lung cancer (25). However, for patients with
advanced-stage lung cancer, chemotherapy remains a critical
treatment modality. Chemotherapy efficacy is a pivotal concern in
the treatment of various malignancies, since it directly affects
patient outcomes and OS. However, PD remains the leading clinical
problem (26,27). The current study aimed to identify
factors associated with PD in patients with NSCLC undergoing
chemotherapy.
The potential of immune-related biomarkers,
including N6-methyladenosine and immune-related lncRNAs, in
predicting immunotherapy response in lung squamous cell carcinoma
via delineating molecular subtypes with varying treatment efficacy
has been recently underlined (28).
However, their applicability in predicting chemotherapy response
has not been previously investigated. A previous study emphasized
the significance of the tumor microenvironment in the context of
cancer chemotherapy (29).
Nevertheless, it has been suggested that the immune
microenvironment status within the tumor site and the overall
systemic immune profiles of patients with cancer prior to treatment
can affect their response to chemotherapy (30). The association between peripheral
blood-related immunological markers, including lower peripheral
blood mononuclear cell counts and diminished cytokine expression
levels and inferior treatment responses has been widely
investigated (31,32). Considering the aforementioned
findings, in the present study, a comparative analysis of routine
peripheral blood parameters, including WBC, neutrophil and
lymphocyte counts, between the PD (n=21) and non-PD groups (n=68)
was performed. The results revealed that WBC counts were notably
reduced in patients in the PD group compared with those in the
non-PD group, with relatively stable disease status (P=0.028).
The present study unveiled new possibilities for
exploiting ATP detection as a sensitive and specific biomarker for
predicting disease progression. The majority of cellular functions
depend on ATP production and therefore intracellular ATP synthesis
serves as a marker of cell activity (33). In a previous study, Cylex's Immuknow
assay was used to evaluate the activity of CD4+ T
lymphocytes (34). Assessing the
activity of CD4+ T lymphocytes in the peripheral blood
of organ transplant recipients can enable the early identification
of potential risks associated with rejection and infection
(35). Previous studies in sepsis
revealed that sATPCD4 levels were markedly enhanced
among survivors compared with non-survivors within the first day of
intensive care unit admission (13,36).
The aforementioned divergent findings highlight the complex nature
of sATPCD4 and its potential roles in several diseases.
The current study revealed a noteworthy decline in
sATPCD4 levels within the PD group following
chemotherapy. Intriguingly, a notable increase in
sATPCD4 levels was observed in the non-PD group,
characterized by stable disease status (Fig. 4). Furthermore, both
nATPCD4 and sATPCD4 levels were significantly
diminished in patients experiencing PD compared with the non-PD
group (P=0.002; P<0.0001; Fig.
3). These results supported the ability of ATP to mirror the
effect of chemotherapy on immune function in NSCLC.
As a crucial factor of anti-tumor immunity,
peripheral blood CD4+ T cells play a pivotal role in
regulating and enhancing the priming, migratory potential and
killing activity of CTLs (37). In
the present study, ROC curve analysis demonstrated a superior
discriminatory ability for sATPCD4, as evidenced by its
higher AUC value (0.887) compared with that obtained for WBC
(AUC=0.660) and nATPCD4 (AUC=0.713). Employing a
designated cut-off value of 224.5 ng/ml for sATPCD4, the
sensitivity and specificity were estimated to be 77.4 and 88.2%,
respectively (Fig. 5). Notably,
sATPCD4 levels <224 ng/ml were indicative of an
elevated risk of PD, thus underscoring a robust association between
CD4+ T cell immune function and patient prognosis. Low
ATP levels indicate a state of immunosuppression, thus accelerating
tumor progression via the intricate immunosuppressive network
established by interactions between cancer cells and host immune
cells. This phenomenon could promote tumor growth, while
simultaneously providing a shield against immune attacks, thus
contributing to the complex dynamics of cancer progression
(38). It has been reported that
during the complex immune editing process, where several
CD4+ T cell subsets play significant roles in both tumor
promotion and rejection, sATPCD4 serves as a valuable
indicator reflecting the overall net status of immune activity
(35).
Factors that affect treatment efficacy in patients
with NSCLC include lymphocyte subpopulations (39,40),
treatment regimen (41,42) and gene mutations (42,43).
The results of the present study showed that only
sATPCD4 was a risk factor for tumor progression. This
finding could be associated with the small sample size. The results
of the current study were consistent with those reported in liver
cancer, demonstrating that patients with low sATPCD4
levels exhibited markedly lower PFS and OS compared with those in
the high ATP group (18). Another
study also suggested that adjusting the dosage of immunosuppressive
agents based on ATP levels could markedly improve the OS and reduce
infection rates in liver transplant patients (44). The present study and previous
studies demonstrated that sATPCD4 levels could reflect
immune function in patients with cancer and were associated with
treatment efficacy and prognosis. However, the mechanism underlying
the effect of sATPCD4 levels on reflecting the efficacy
of chemotherapy remains to be elucidated.
In the present study, the particular underlying
mechanism was investigated, which could be associated with how
chemotherapy could promote the death of tumor cells within a short
period. In turn, the death of tumor cells induces the release of
several tumor antigens, potentially stimulating antigen-presenting
cells to present tumor antigens to T cells, thus promoting T cell
activation and proliferation (20).
Additionally, the combination of EGFR-targeted agents could enhance
the presentation of MHC I-class tumor antigens to facilitate the
uptake of tumor material by dendritic cells and promote T cell
activation in the absence of additional immune stimulation signals
(45). Activated T cells infiltrate
tumor tissues and attack antigen-expressing tumor cells.
Furthermore, emerging evidence has suggested that cellular
metabolism plays a critical role in T cell differentiation and
function. Changes in the metabolic activity of T cells can directly
affect their function and survival, which is reflected in ATP
expression levels (46). Therefore,
higher nATPCD4 and sATPCD4 expression levels
were observed in patients with disease stabilization or remission
following chemotherapy. Conversely, in patients with PD,
chemotherapy drugs could not only promote tumor cell injury, but
they could also harm normal immune cells, thus resulting in
impaired immune function. The aforementioned processes could be
accompanied by lower nATPCD4 and sATPCD4
expression levels. Consequently, the immune cells of these patients
could fail to effectively clear tumor cells, thus leading to
PD.
However, the present study had several limitations.
First, the relatively small sample size and limited number of
events, as well as the fact that it was conducted only on Chinese
individuals without validation in other ethnic backgrounds,
constrained comprehensive multivariable analysis. Additionally, an
increasing number of studies have demonstrated that advanced NSCLC
patients with KRAS mutations exhibit markedly lower objective
response rate and potentially lower 6-month/1-year PFS rates
compared with wild-type patients after first-line chemotherapy
(43,47,48).
However, the limited number of cases with detected KRAS mutations
in this study (Table I) precludes
the calculation of whether KRAS gene mutations affect chemotherapy
efficacy. Whether KRAS mutations serve as risk factors for
sATPCD4 requires further investigation. Therefore,
further validation of the results is needed to draw definitive
conclusions. Additionally, the retrospective and single-center
nature of this study may introduce some bias, potentially resulting
in inherent deviations. Considering the primary goals of
immunotherapy, extending the observation period, treatment cycles
and longitudinally monitoring changes in sATPCD4 subsets
are crucial for investigating their association with OS and
PFS.
In summary, in the present study a noteworthy
association between low nATPCD4 and sATPCD4
levels and tumor progression was observed in patients with advanced
NSCLC treated with chemotherapy. These findings could assist
physicians in assessing immune function to clearly determine the
immunological status of patients with cancer, thus tailoring
treatment strategies to prevent disease progression. The current
study was the first to provide such findings, to the best of the
authors' knowledge. However, further prospective and multicenter
studies with larger sample sizes are needed to validate the
aforementioned results.
The present study suggested that sATPCD4
levels could serve as an indicator of the effect of chemotherapy on
immune function and holds promise for assessing the potential risk
of disease progression in patients with NSCLC.
Acknowledgements
The authors would like to thank Ms. Juanjuan Peng
and Ms. Hongxia Wei (Leide Biosciences Co., Ltd.) for their
technical guidance.
Funding
The present study was supported by the Guangzhou Characteristic
Technology Project (grant no. 2023C-TS29); and the Research Project
of Guangzhou Science and Technology Bureau (grant no.
202201010787).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WY, KH, NT, WL, ZT, QH, DY, HL, ZD, YX and GY
contributed to the study conception and design. Material
preparation, data collection and analysis were performed by WY, KH
and NT under supervision of GY. The first draft of the manuscript
was written by WY. KH and NT confirm the authenticity of all the
raw data. WL, ZT and QH reviewed and revised the first draft,
conducted domestic and international literature searches for the
discussion section and improved the discussion section. DY and HL
further reviewed and examined the data analysis and graphics
production in the article. ZD and YX formatted the final version of
the article. GY supervised the entire experiment and performed the
final review of the article. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study received approval from the Ethics
Committee of the Fifth Affiliated Hospital of Guangzhou Medical
University (Guangzhou, China; dated 08-07-2022; approval no.
KY01-2022-07-08). Written informed consent was obtained from all
participants, allowing the use of their clinical data in this
study. The research adhered to the principles outlined in The
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen X, Liu Y, Røe OD, Qian Y, Guo R, Zhu
L, Yin Y and Shu Y: Gefitinib or erlotinib as maintenance therapy
in patients with advanced stage non-small cell lung cancer: A
systematic review. PLoS One. 8:e593142013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li K, Zhang Q, Zhang Y, Yang J and Zheng
J: T-cell-associated cellular immunotherapy for lung cancer. J
Cancer Res Clin Oncol. 141:1249–1258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carlisle JW, Steuer CE, Owonikoko TK and
Saba NF: An update on the immune landscape in lung and head and
neck cancers. CA Cancer J Clin. 70:505–517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazzaschi G, Facchinetti F, Missale G,
Canetti D, Madeddu D, Zecca A, Veneziani M, Gelsomino F, Goldoni M,
Buti S, et al: The circulating pool of functionally competent NK
and CD8+ cells predicts the outcome of anti-PD1 treatment in
advanced NSCLC. Lung Cancer. 127:153–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kotsakis A, Koinis F, Katsarou A,
Gioulbasani M, Aggouraki D, Kentepozidis N, Georgoulias V and
Vetsika EK: Prognostic value of circulating regulatory T cell
subsets in untreated non-small cell lung cancer patients. Sci Rep.
6:392472016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan Y, Wang X, Liu C and Jia J:
Association of lymphocyte subsets with efficacy and prognosis of
immune checkpoint inhibitor therapy in advanced non-small cell lung
carcinoma: A retrospective study. BMC Pulm Med. 22:1662022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robaire B and Hales BF: Mechanisms of
action of cyclophosphamide as a male-mediated developmental
toxicant. Adv Exp Med Biol. 518:169–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vollmer T, Stewart T and Baxter N:
Mitoxantrone and cytotoxic drugs' mechanisms of action. Neurology.
74:S41–S46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hodge JW, Garnett CT, Farsaci B, Palena C,
Tsang KY, Ferrone S and Gameiro SR: Chemotherapy-induced
immunogenic modulation of tumor cells enhances killing by cytotoxic
T lymphocytes and is distinct from immunogenic cell death. Int J
Cancer. 133:624–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Locher C, Conforti R, Aymeric L, Ma Y,
Yamazaki T, Rusakiewicz S, Tesniere A, Ghiringhelli F, Apetoh L,
Morel Y, et al: Desirable cell death during anticancer
chemotherapy. Ann N Y Acad Sci. 1209:99–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xue F, Gao W, Qin T, Wu C, Luo Y, Chen J,
Zhou T, Feng M, Qiu B, Zhu J, et al: Immune cell function assays in
the diagnosis of infection in pediatric liver transplantation: An
open-labeled, two center prospective cohort study. Transl Pediatr.
10:333–343. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu W, Wang K, Zhao YH, Song GP, Gao W and
Li DH: Clinical relevance of a CD4+ T cell immune function assay in
the diagnosis of infection in pediatric living-donor liver
transplantation. Exp Ther Med. 18:3823–3828. 2019.PubMed/NCBI
|
|
15
|
Maidman SD, Gidea C, Reyentovich A, Rao S,
Saraon T, Kadosh BS, Narula N, Carillo J, Smith D, Moazami N, et
al: Pre-transplant immune cell function assay as a predictor of
early cardiac allograft rejection. Clin Transplant. 36:e147452022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Serban G, Whittaker V, Fan J, Liu Z, Manga
K, Khan M, Kontogianni K, Padmanabhan A, Cohen D, Suciu-Foca N, et
al: Significance of immune cell function monitoring in renal
transplantation after Thymoglobulin induction therapy. Hum Immunol.
70:882–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xian Y, Zhang KX, Bi XG, Zheng CL and Xie
D: Value of adenosine triphosphate in CD4+ T lymphocytes to the
prediction of prognosis of septic patients. Chin Pract Diagn Ther.
37:1216–1221. 2023.(In Chinese).
|
|
18
|
Cheng JW, Shi YH, Fan J, Huang XW, Qiu SJ,
Xiao YS, Wang Z, Dai Z, Tang ZY and Zhou J: An immune function
assay predicts post-transplant recurrence in patients with
hepatocellular carcinoma. J Cancer Res Clin Oncol. 137:1445–1453.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uemura T, Riley TR, Khan A, Hollenbeak C,
Schreibman I, Ghahramani N, Reeves B, Domen RE, Zander DS and Kadry
Z: Immune functional assay for immunosuppressive management in
post-transplant malignancy. Clin Transplant. 25:E32–E37. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gridelli C, Ardizzoni A, Le Chevalier T,
Manegold C, Perrone F, Thatcher N, Van Zandwijk N, Di Maio M,
Martelli O and De Marinis F: Treatment of advanced non-small-cell
lung cancer patients with ECOG performance status 2: Results of an
European experts panel. Ann Oncol. 15:419–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Detterbeck FC, Chansky K, Groome P,
Bolejack V, Crowley J, Shemanski L, Kennedy C, Krasnik M, Peake M
and Rami-Porta R: IASLC Staging and Prognostic Factors Committee,
Advisory Boards, and Participating Institutions: The IASLC lung
cancer staging project: Methodology and validation used in the
development of proposals for revision of the stage classification
of NSCLC in the forthcoming (eighth) edition of the TNM
classification of lung cancer. J Thorac Oncol. 11:1433–1446. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou K, Ye W, Huang Q, Li W, Tan Z, Tao N,
Yang D, Lin H, Deng Z, Xia Y and Yu G: The predictive value of
peripheral blood CD4 cells ATP concentration for immune-related
adverse events in advanced non-small cell lung cancer patients. BMC
Immunol. 25:32024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Zhang M, Pan X, Zhao M, Huang L,
Hu X, Wang X, Qiao L, Guo Q, Xu W, et al: Integrative serum
metabolic fingerprints based multi-modal platforms for lung
adenocarcinoma early detection and pulmonary nodule classification.
Adv Sci (Weinh). 9:e22037862022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Griesinger F, Korol EE, Kayaniyil S, Varol
N, Ebner T and Goring SM: Efficacy and safety of first-line
carboplatin-versus cisplatin-based chemotherapy for non-small cell
lung cancer: A meta-analysis. Lung Cancer. 135:196–204. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chalabi M, Cardona A, Nagarkar D, Scala
AD, Gandara D, Rittmeyer A, Albert M, Powles T, Kok M and Herrera
FG; imCORE working group of early career investigators, : Efficacy
of chemotherapy and atezolizumab in patients with non-small-cell
lung cancer receiving antibiotics and proton pump inhibitors:
Pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol.
31:525–531. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding Z, Liu Y, Huang Q, Cheng C, Song L,
Zhang C, Cui X, Wang Y, Han Y and Zhang H: m6A-and immune-related
lncRNA signature confers robust predictive power for immune
efficacy in lung squamous cell carcinoma. View. 4:202200832023.
View Article : Google Scholar
|
|
29
|
Hanoteau A, Newton JM, Krupar R, Huang C,
Liu HC, Gaspero A, Gartrell RD, Saenger YM, Hart TD, Santegoets SJ,
et al: Tumor microenvironment modulation enhances immunologic
benefit of chemoradiotherapy. J Immunother Cancer. 7:102019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu L, Zou C, Zhang S, Chu TSM, Zhang Y,
Chen W, Zhao C, Yang L, Xu Z, Dong S, et al: Reshaping the systemic
tumor immune environment (STIE) and tumor immune microenvironment
(TIME) to enhance immunotherapy efficacy in solid tumors. J Hematol
Oncol. 15:872022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Salem ML, Atia I and Elmashad NM: Higher
cytotoxic activities of CD8+ T cells and natural killer cells from
peripheral blood of early diagnosed lung cancer patients. BMC
Immunology. 24:242023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang DH, Choi SW, Sun P, Chung C, Park D,
Lee SI, Koh JS, Kim Y and Lee JE: The rest period between
chemotherapy and immunotherapy influences the efficacy of immune
checkpoint inhibitors in lung cancer. Thoracic Cancer.
13:2346–2354. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonora M, Patergnani S, Rimessi A, De
Marchi E, Suski JM, Bononi A, Giorgi C, Marchi S, Missiroli S,
Poletti F, et al: ATP synthesis and storage. Purinergic Signal.
8:343–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kowalski R, Post D, Schneider MC, Britz J,
Thomas J, Deierhoi M, Lobashevsky A, Redfield R, Schweitzer E,
Heredia A, et al: Immune cell function testing: An adjunct to
therapeutic drug monitoring in transplant patient management. Clin
Transplant. 17:77–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rodrigo E, López-Hoyos M, Corral M,
Fábrega E, Fernández-Fresnedo G, Segundo DS, Piñera C and Arias M:
ImmuKnow as a diagnostic tool for predicting infection and acute
rejection in adult liver transplant recipients: A systematic review
and meta-analysis. Liver Transpl. 18:1244–1252. 2012. View Article : Google Scholar
|
|
36
|
Lawrence KL, White PH, Morris GP,
Jennemann J, Phelan DL, Hotchkiss RS and Kollef MH: CD4+ lymphocyte
adenosine triphosphate determination in sepsis: A cohort study.
Crit Care. 14:R1102010. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kagamu H, Kitano S, Yamaguchi O, Yoshimura
K, Horimoto K, Kitazawa M, Fukui K, Shiono A, Mouri A, Nishihara F,
et al: CD4+ T-cell immunity in the peripheral blood
correlates with response to anti-PD-1 therapy. Cancer Immunol Res.
8:334–344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xue F, Zhang J, Han L, Li Q, Xu N, Zhou T,
Xi Z, Wu Y and Xia Q: Immune cell functional assay in monitoring of
adult liver transplantation recipients with infection.
Transplantation. 89:620–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun B and Zhang Y: Overview of
orchestration of CD4+ T cell subsets in immune responses. Adv Exp
Med Biol. 84:1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vonderheide RH and Bayne LJ: Inflammatory
networks and immune surveillance of pancreatic carcinoma. Curr Opin
Immunol. 25:200–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang BB, Zhu W, Tao J, Li Y, Du CC, Chen
YX and Liu YD: Short-term efficacy of different first-line
chemotherapy regimens for advanced non-small cell lung cancer: A
network meta-analysis. Clin Trans Sci. 13:589–598. 2020. View Article : Google Scholar
|
|
42
|
Xu J, Xiong Y, Xu Z, Xing H, Zhou L and
Zhang X: From targeted therapy to a novel way: Immunogenic cell
death in lung cancer. Front Med (Lausanne). 9:11025502022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hamarsheh SA, Groß O, Brummer T and Zeiser
R: Immune modulatory effects of oncogenic KRAS in cancer. Nat
Commun. 11:54392020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ravaioli M, Neri F, Lazzarotto T, Bertuzzo
VR, Di Gioia P, Stacchini G, Morelli MC, Ercolani G, Cescon M,
Chiereghin A, et al: Immunosuppression modifications based on an
immune response assay: Results of a randomized, controlled trial.
Transplantation. 99:1625–1632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Petroni G, Buqué A, Zitvogel L, Kroemer G
and Galluzzi L: Immunomodulation by targeted anticancer agents.
Cancer Cell. 39:310–345. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kelly B and O'Neill LA: Metabolic
reprogramming in macrophages and dendritic cells in innate
immunity. Cell Res. 25:771–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Fang W, Yan Y, Wang M, Kang S,
Sheng J, Zhan J, Chen N, Hong S, Yang Y, et al: The efficacy of
first-line chemotherapy is associated with KRAS mutation status in
patients with advanced non-small cell lung cancer: A meta-analysis.
Med Oncol. 32:612015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dy GK, Govindan R, Velcheti V, Falchook
GS, Italiano A, Wolf J, Sacher AG, Takahashi T, Ramalingam SS,
Dooms C, et al: Long-term outcomes and molecular correlates of
sotorasib efficacy in patients with pretreated KRAS G12C-mutated
non-small-cell lung cancer: 2-year analysis of CodeBreaK 100. J
Clin Oncol. 41:3311–3317. 2023. View Article : Google Scholar : PubMed/NCBI
|